Abstract
Purpose
To evaluate whether the morphology of the sperm midpiece observed by high magnification microscopy relates to sperm centrosomal function.
Methods
Sperm selected by conventional microscopy were defined as controls. By high magnification microscopy, sperm with straight midpieces were defined as Group 1, while those with tapering midpieces were defined as Group 2. Heterologous ICSI of human sperm into bovine oocytes was used to assess human sperm centrosomal function and analysis of sperm aster formation.
Results
The total rate of sperm aster formation was 80.5% in Group 1, which was significantly higher (P < 0.05) than the rate of 33.3% seen for Group 2. Furthermore, sperm aster formation rates tended to be higher for Group 1 than for the controls.
Conclusions
This study demonstrates improvement of sperm aster formation rates by selecting sperm on the basis of midpiece morphology. The injection of selected sperm bearing morphologically straight midpieces may contribute to improved expression of sperm centrosomal function, providing a positive effect on fertilization after ICSI.
Keywords: Intracytoplasmic morphologically selected sperm injection (IMSI), Sperm midpiece, Centrosomal function, Sperm aster formation
Introduction
During most mammalian fertilization except for rodents, the centrosome derived from the sperm is used in the first cell cycle to organize the radial array of microtubules comprising the sperm aster [1, 2]. In human ICSI, aberrant microtubule organization in “fertilization failure eggs” suggests that centrosomal dysfunction may be a cause of fertilization arrest [3]. Therefore, the sperm centrosomes play a crucial role in mammalian fertilization [4, 5].
Using heterologous ICSI of human sperm into bovine oocytes to assess human sperm centrosomal function [6, 7], we reported human infertility due to the poor sperm centrosomal function [7, 8]. Globozoospermia is a special feature of teratozoospermia, characterized as round head, lack of acrosome and acrosomal enzymes, and disorganized midpiece [9, 10]. Dysplasia of the fibrous sheath (DFS), a rare form of teratozoospermia, results in infertility. DFS sperm, which are immotile due to deformities from midpiece to tail [4, 11] also exhibit sperm centrosomal dysfunction; both of these abnormalities may be causes of infertility [8, 12]. These findings suggest that a morphologically abnormal sperm midpiece is related to abnormal centrosomal function.
Therefore, developing a treatment for infertility resulting from poor human sperm centrosomal function would provide to be a novel strategy of assisted reproductive technology (ART).
Microinjection with motile sperm strictly selected by high-power magnification (Light microscope: 1,000×, Computer analysis with digital zoom: 6,000× ) and Nomarski differential interference contrast (DIC) optics, called intracytoplasmic morphologically selected sperm injection (IMSI), improves pregnancy rates in couples with repeated ICSI failure [13]. Current reports examining IMSI focused on the selection of sperm based on head morphology [14, 15]. We focused on the shape of the sperm midpiece, which contains the sperm centrosome.
We determined if sperm with excellent centrosomal function could be selected using criteria established by high magnification microscopy. We examined sperm aster formation after injecting human sperm into bovine oocytes following selection by high magnification microscopy. This study sought to verify if microinjection of sperm selected by midpiece characteristics in IMSI resulted in improved sperm centrosomal function.
Materials and methods
All procedures were performed with the approval of an internal review board of Tohoku University School of Medicine.
In vitro maturation of bovine oocytes
Bovine ovaries were obtained from a local slaughterhouse. Oocytes, recovered by aspiration from 2–8 mm follicles, were matured for 22–24 h in Medium 199 (M199; Gibco, Grand Island, NY) supplemented with 10% (v/v) fetal calf serum (FCS; Gibco), 0.12 IU/ml FSH (Antrin, Denka Pharmaceutical, Kanagawa, Japan), and 50 ng/ml recombinant human epidermal growth factor (Upstate, Lake placid, NY) at 38.5°C under 5% CO2 in air.
Cumulus cells were removed by a brief incubation with 1 mg/ml collagenase (Sigma, St. Louis, MO, USA) and 2 mg/ml hyaluronidase (Sigma) in M2 culture medium (Sigma). Oocytes paused at the second meiotic metaphase were selected for ICSI.
Human sperm preparation
Surplus sperm from three fertile donors who provided their informed consent were frozen in TEST-yolk buffer (Irvine Scientific Co., Santa Ana, USA). Sperm samples were thawed at room temperature and washed in modified HTF medium (Irvine Scientific) supplemented with 10% serum substitute supplement (Irvine Scientific). Following centrifugation at 500×g for 5 min, the sperm pellet was resuspended in modified HTF medium.
Selection of sperm with straight or tapering midpieces observed by high magnification microscopy
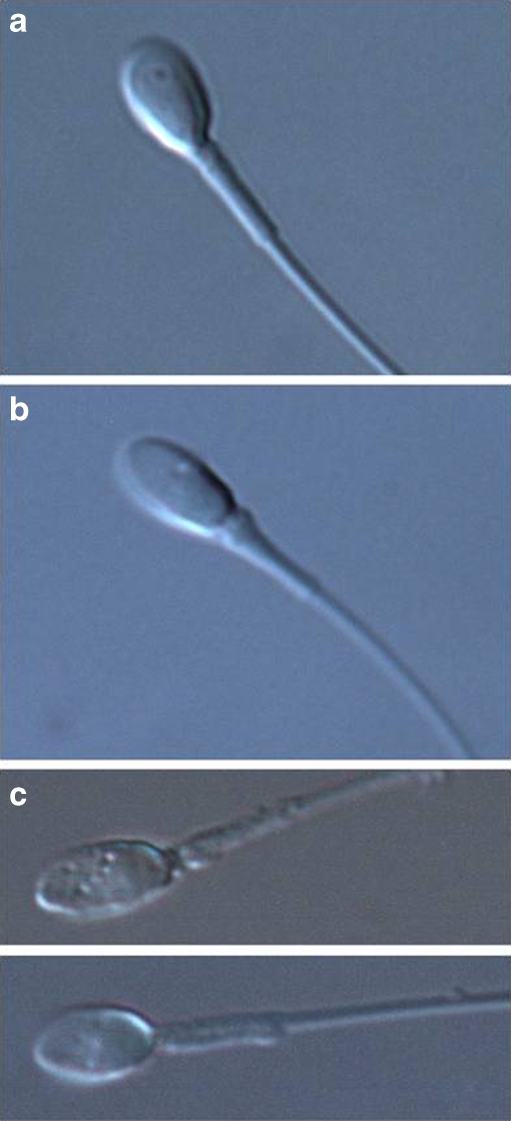
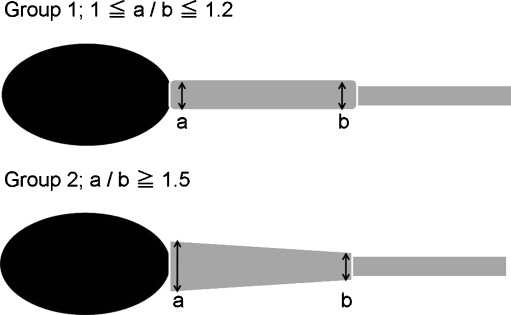
Motile sperm selected by conventional microscopy (400×; 40× dry objective lens) were used for IMSI. These sperm were classified into two groups based on midpiece shape as determined by microscopy using Nomarski differential interference contrast (DIC) optics under high magnification (Fig. 1. 1,000×; 100× oil objective lens). By using high magnification microscopy (1,000×), it was midpiece thickness that we were able to distinguish it clearly as far as we observed it. Control sperm were selected by conventional microscopy. Group 1 was comprised of sperm with straight midpieces, as determined by high magnification microscopy. Group 2 contained sperm with tapering midpieces as selected by high magnification microscopy. To define the difference between Group 1 and Group 2, we measured proximal and distal diameter of sperm midpiece. As shown in Fig. 2, Group 1 was defined when the value of proximal diameter/distal diameter is between 1 and 1.2, and Group 2 when the value is more than 1.5. A ratio of 1.5 served as an indicator to recognize the increased thickness of the midpiece by high magnification microscopy. Sperm that did not fulfill the requirements for either Group 1 or Group 2 were excluded (Figs. 1, 2). In addition, to remove influence by the vacuole of the sperm nucleus, sperm with normal shaped nucleus observed by high magnification microscopy, was chosen. Sperm with vacuoles within the sperm head were excluded from this study.
Fig. 1.
a Sperm with straight midpieces were selected by high magnification microscopy (Group 1). b Sperm with tapering midpieces were selected by high magnification microscopy (Group 2). c The sperm did not fulfill the criteria of either a or b was defined as “other”. In addition, sperm with vacuoles within the sperm head were excluded from this study
Fig. 2.
The sperm midpiece proximal diameter/distal diameter value showed between 1 and 1.2 was defined Group 1, and showed more than 1.5 was defined Group 2. a proximal diameter b distal diameter
ICSI with human sperm using a Piezo-micromanipulator into bovine oocytes
After selection and immobilization with a piezo-micromanipulator (MB-U; Prim Tech, Tsuchiura, Japan), sperm were loaded into a pipette. The zona pellucida was penetrated using piezo pulses. After expulsion of the segment of zona pellucida within the pipette, the immobilized sperm were moved to the tip of the injection pipette. The pipette was inserted into the ooplasm as the oolemma was punctured by the application of one piezo pulse. The sperm at the tip of the pipette was injected into the ooplasm with a minimal amount of sperm suspension medium.
After injection, oocytes were cultured at 38.5°C in M199 medium supplemented with 10% FCS under 5% CO2 in air. Oocytes were fixed 6 h after microinjection.
Each experiment was performed at least three times and all sperm selection was always performed by same observer.
Immunocytochemical detection of microtubules and DNA
Zonae pellucidae were removed from oocytes using 0.75% protease (Sigma) in M2 culture medium. After a 15 min recovery at 38.5°C, zona-free eggs and zygotes were extracted for 15 min in buffer M (25% [v:v] glycerol, 50 mM KCl, 0.5 mM MgCl2, 0.1 mM EDTA, 1 mM EGTA, 50 mM imidazole hydrochloride, and 1 mM 2-mercaptoethanol, pH 6.8) containing 5% methanol and 1% Triton X-100 detergent. Samples were then fixed in cold methanol for 10 min as described by Simerly and Schatten [16]. Oocytes were permeabilized overnight in 0.1 M PBS containing 0.1% Triton X-100.
Microtubules were labeled with a mixture of anti-β tubulin monoclonal antibodies (clone 2-28-33; diluted 1:100; Sigma) and anti-acetylated α tubulin antibodies (clone 6-11-B1; diluted 1:100; Sigma). Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (diluted 1:40; Zymed, San Francisco, CA) served as a secondary antibody. DNA was detected by staining with 10 μg/ml Hoechst 33342. Coverslips, mounted in anti-fade medium (Vectashield; Vector Labs., Burlingame, CA), were examined using a Leica DMRXA/HC (Leica Microsystems, Heidelberg, Germany) epifluorescence microscope.
Transmission electron microscopy of sample sperm
Sample sperm were fixed in 2.5% glutaraldehyde followed by 1% osmium tetroxide. The pellet was embedded in Epon Araldite and thin sections were examined and photographed in electron microscope.
Statistics
Sperm aster formation rates for the groups were compared using the chi-square test. P values less than 0.05 were considered to be statistically significant.
Results
Sperm population of straight-shaped midpiece (Group 1) and tapering-shaped midpiece (Group 2)
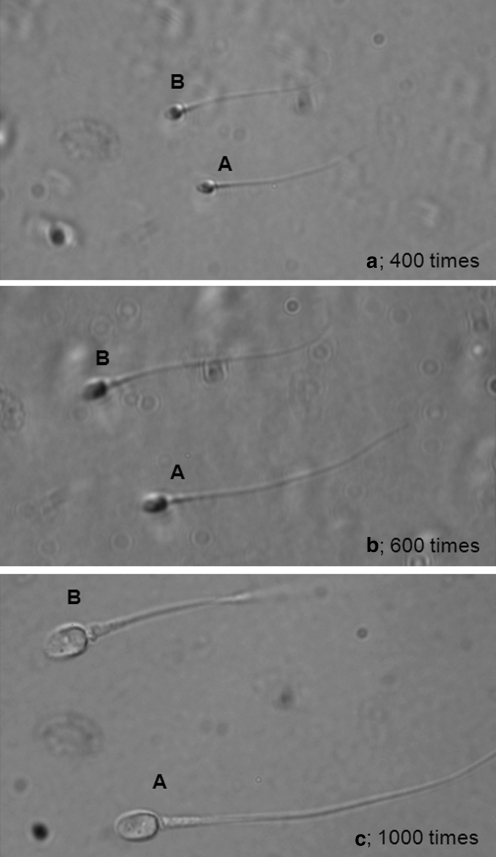
We showed three images (a–c) display the same sperm under different visualization conditions (Fig. 3). Using normal magnification microscopy, it was difficult to observe the structure of the midpiece in detail. Using high magnification microscopy, however, we were able to clearly observe small differences in midpiece morphology.
Fig. 3.
These three images (a–c) display the same sperm under different visualization conditions. It is difficult to distinguish the differences in midpiece morphology by conventional microscopy of 400× or 600× (a, b). High magnification microscopy of 1,000× (c) makes it possible to observe differences in midpiece morphology clearly
The sperm populations of three fertile donors are shown in Table 1. In sample 1, 16 out of 101 (15.8%) sperm were categorized in Group 1, while 21 of 101 sperm (20.8%) were classified as Group 2. In sample 2, 13 (12.4%) and 12 (11.4%) of 105 sperm were classified as Groups 1 and 2, respectively. In sample 3, Of 102 total sperm harvested, 17 (16.7%) and 14 (13.7%) were categorized to Groups 1 and 2, respectively.
Table 1.
Sperm population which is occupied in three fertile doners
| Group 1 | Group 2 | Others | |
|---|---|---|---|
| Sample 1 | 15.8% (16/101) | 20.8% (21/101) | 63.4% (64/101) |
| Sample 2 | 12.4% (13/105) | 11.4% (12/105) | 76.2% (80/105 |
| Sample 3 | 16.7% (17/102) | 13.7% (14/102) | 69.6% (71/102) |
Microtubule organization and chromatin configuration in bovine eggs following ICSI with human sperm
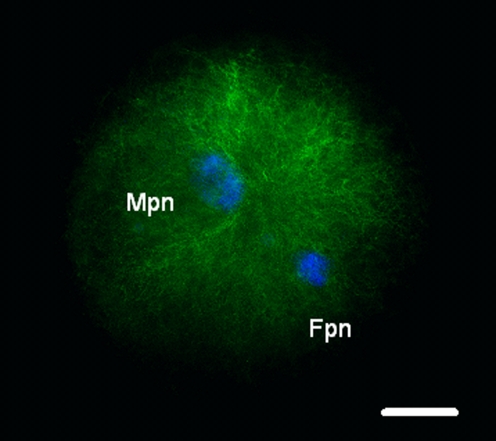
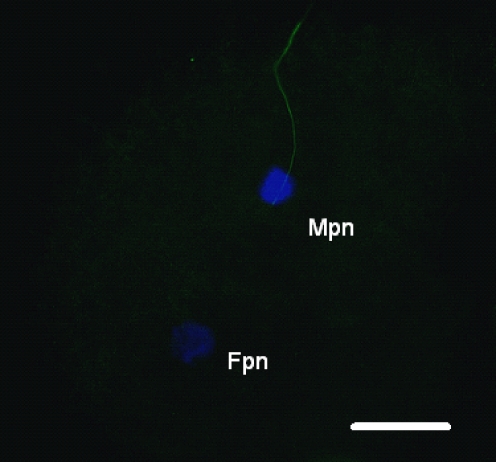
We examined the microtubule organization and chromatin configuration in bovine oocytes after ICSI. Six hours post-ICSI, oocyte activation was recognized in all bovine oocytes and the sperm aster was organized with microtubules elongating throughout the cytoplasm until contacting the developing female pronucleus, concurrently with decondensation of the male pronucleus (Fig. 4). On the other hand, some cases lacked of sperm aster formation from human sperm centrosome in bovine oocytes, even the oocyte activation and sperm pronucleus decondensation (Fig. 5).
Fig. 4.
We visualized microtubule (green) and chromatin (blue) configurations in bovine oocytes after ICSI of human sperm into bovine oocytes using a piezo-driven pipette. Six hours post-ICSI, oocytes were fixed; microtubules and chromatin were visualized by immunoflurescein staining. A radial array of microtubules (arrow, sperm aster) was organized from the sperm centrosome. Fpn, Female pronucleus; Mpn, male pronucleus. Bar: 20 μm
Fig. 5.
This figure shows the lack of sperm aster formation from human sperm centrosome in bovine oocyte, even the oocyte activation and sperm pronucleus decondensation. Bar: 20 μm
Sperm aster formation rate
The results of sperm aster formation are summarized in Table 2. For the sperm from sample 1, the sperm aster was organized in ten of 12 (83.3%) injected eggs for Group 1, but only four of 18 (22.2%) injected eggs for Group 2. For the sperm from sample 2, the sperm aster was organized in 15 of 18 (83.3%) and eight of 17 (47.1%) injected eggs from Groups 1 and 2, respectively. For the sperm from sample 3, the sperm aster was organized in eight of 11 (72.7%) injected eggs of Group 1, but only four of 13 (30.8%) injected eggs for Group 2.
Table 2.
Sperm aster formation rates
| Group 1 | Group 2 | |
|---|---|---|
| Sample 1 | 83.3% (10/12)a | 22.2% (4/18)b |
| Sample 2 | 83.3% (15/18)c | 47.1% (8/17)d |
| Sample 3 | 72.7% (8/11)e | 30.8% (4/13)f |
| Total | 80.5% (33/41)g | 33.3% (16/48)h |
| Control | 69.6% (39/56)i | |
h vs i : p < 0.001
a vs b, c vs d, e vs f, g vs f: p < 0.05
g vs i : nf
The sperm aster was organized in 39 out of 56 (69.6%) injected eggs for the control group. These results gave total sperm aster formation rates of 80.5% for Group 1 and 33.3% for Group 2, demonstrating significantly higher sperm aster formation rates for Group 1 in comparison to Group 2 (P < 0.05). In comparison to controls, the sperm aster formation rates were higher for Group 1 sperm.
Analysis of transmission electron microscope of sperm midpiece morphology
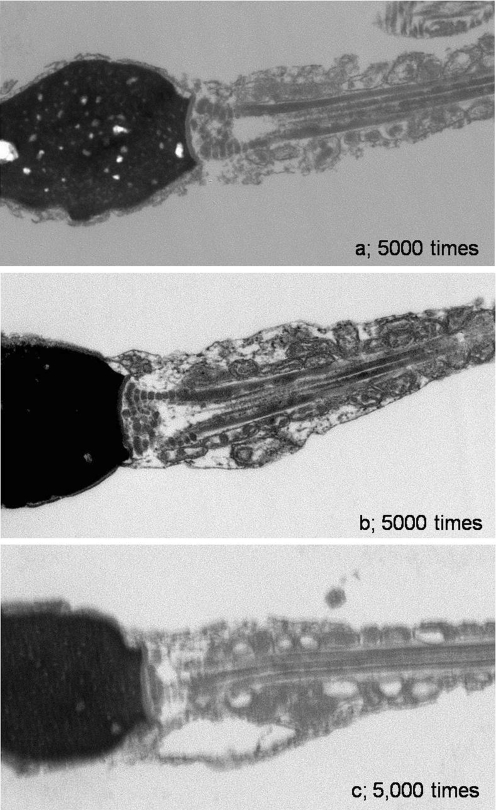
By using electron microscopy, comparison between straight midpieces (Group A) and tapered midpieces (Group B) recognized disaligned mitochondria and vacuolar structures in the second group. On the other hand, as far as we observed, the clear deference was not observed in centrosome structures of both. (Fig. 6)
Fig. 6.
The ultra-structure of the sperm midpiece by electron microscopy. As compared straight midpieces (Group A) to tapering midpieces (Group B), mitochondria disalignment and vacuole structures around midpieces were recognized in the latter. On the other hand, as far as we observed in an electron microscopy, clear difference was not observed in centrosome structures of both. a Sperm with straight midpieces (Group A). b, c Sperm with tapering midpieces (Group B)
Discussion
According to the examination of microtubule organization and DNA configuration in fertilization failure human oocytes after ICSI by Rawe et al. [3], 20% of oocytes arrested at pronuclei apposition due to lack of a sperm aster. This report indicates that proper sperm centrosomal function (sperm aster formation) is essential for fertilization [17].
We have generated a novel assay of human sperm centrosomal function using heterologus ICSI of human sperm into bovine oocytes. Sperm aster formation rates of fertile human sperm injected into bovine oocytes averaged approximately 60.0% [6]. We have reported two cases of infertility in patients with defective sperm centrosomal function; one was a case of globozoospermia [7, 18], while the other was a case of DFS [8, 12]. For both patients, the rate of sperm aster formation was lower than that of sperm from fertile men and abnormal midpiece shape was also observed in the disorganized centrosome. These findings suggest that a morphologically abnormal sperm midpiece is related to abnormal centrosomal function.
We attempted to restore defective sperm centrosomal function for the sperm from the patient with DFS by chemical manipulation of gametes. Complicated chemical manipulation of gametes, however, could not reverse the defect in DFS sperm, which exhibit a congenital lack of normal sperm centrosomes [12]. Thus, a novel strategy is needed to treat sterility associated with poor sperm centrosomal function.
Bartoov et al. recently developed a new method, motile sperm organelle morphology examination (MSOME) with high magnification microscopy. Morphologically normal sperm nuclei, defined by MSOME, were positively associated with improved fertilization rates and pregnancy outcome [19]. Further, they compared IMSI with classic IVF-ICSI, demonstrating the obvious advantage of the former treatment in improving pregnancy outcomes [13]. Furthermore, they examined the impact of the presence of a vacuole in the sperm head, as visualized by IMSI; vacuolated sperm exhibited significantly lower pregnancy rates per cycle and significantly higher abortion rates per pregnancy in comparison to normal sperm. Thus, microinjection of vacuolated sperm appears to reduce pregnancy rates and is associated with early abortion [15]. This study is only one of several reports examining sperm nuclei by IMSI, demonstrating the efficiency of selecting morphologically good sperm using this method. As yet, however, no reports have examined the midpiece in detail.
In bovine fertilization, the centrosome is paternally derived into the oocyte, and the sperm centrosome organizes the sperm aster, as in human fertilization [20]. However, bovine oocyte activation rates after conventional ICSI were very low [21]. Recently, high fertilization and embryo development rates have been reported using the piezo-ICSI procedure [22]. We employed this technique for a heterologous ICSI system with human sperm into bovine oocytes. We think that use of piezo-ICSI is the key to oocyte activated success. In fact, oocyte activation rates were 100% in each group.
According to our study, a subset of sperm have morphologically tapering midpieces visualized by high magnification microscopy; sperm aster formation rates using these sperm were low, despite good mobility by conventional microscopy (400 times). These sperm, which comprise approximately 12% of motile sperm, are difficult to identify by conventional microscopy. We propose that it is possible to exclude these sperm by observing under high magnification microscopy. In contrast, sperm with morphologically straight midpieces, as visualized by high magnification microscopy, displayed high sperm aster formation rates. These sperm comprise approximately 15% of motile sperm. The selection of sperm with straight midpieces by high magnification microscopy may contribute to improved sperm aster formation.
Further, we investigated the ultrastructure of sperm midpeice region by electron microscopy. As compared straight midpieces (Group A) to tapering midpieces (Group B), mitochondria disalignment and vacuole structures around midpieces were recognized in the latter. On the other hand, as far as we observed in electron microscopy, differences in centrosome structure were not observed. It was guessed that these disalignments of the organelles may lead to the sperm centrosomal dysfunction. However, it remains controversial why this difference in midpiece structure would affect sperm aster formation. Additional assessment is required to elucidate the relationship between midpiece morphology and centrosomal function.
In conclusion, this study demonstrates the possibility that sperm aster formation rates can be improved by selecting for midpiece morphology using high magnification microscopy. Fine morphological observation of the midpiece during ICSI may significantly influence to sperm centrosome function. Clinically, we often expensive the difficulty of oocyte collection. In such a case, we must improve the fertilization rate by any method. We would be able to guess the success of the fertilization phenomenon after ICSI from a form of the sperm midpiece to some extent. The injection of selected sperm with morphologically straight midpieces by IMSI may contribute to improve sperm centrosomal function, positively influencing fertilization rates after ICSI.
Acknowledgements
This study was supported by Japan Society for the Promotion of Science to Y.T., and N.Y.
Footnotes
Capsule
Intracytoplasmic morphologically selected sperm injection focused on sperm midpiece was performed, and the difference of sperm aster formation rate on the morphology of sperm midpiece was recognized.
References
- 1.Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- 2.Simerly C, Wu GJ, Zoran S, Ord T, Rawlins R, Jones J, et al. The paternal inheritance of the centrosome, the cell’s microtubule-organizing center, in humans, and the implications for infertility. Nat Med. 1995;1:47–52. doi: 10.1038/nm0195-47. [DOI] [PubMed] [Google Scholar]
- 3.Rawe VY, Olmedo SB, Nodar FN, Doncel GD, Acosta AA, Vitullo AD. Cytoskeletal organization defects and abortive activation in human oocytes after IVF and ICSI failure. Mol Hum Reprod. 2000;6:510–6. doi: 10.1093/molehr/6.6.510. [DOI] [PubMed] [Google Scholar]
- 4.Chemes EH, Rawe YV. Sperm pathology: a step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update. 2003;9:405–28. doi: 10.1093/humupd/dmg034. [DOI] [PubMed] [Google Scholar]
- 5.Schatten H, Sun QY. The role of centrosomes in mammalian fertilization and its significance for ICSI. Mol Hum Reprod. 2009;15:531–8. doi: 10.1093/molehr/gap049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura S, Terada Y, Horiuchi T, Emuta C, Murakami T, Yaegashi N, et al. Human sperm aster formation and pronuclear decondensation in bovine eggs following intracytoplasmic sperm injection using a Piezo-driven pipette. Biol Reprod. 2001;65:1359–63. doi: 10.1095/biolreprod65.5.1359. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura S, Terada Y, Horiuchi T, Emuta C, Murakami T, Yaegashi N, et al. Analysis of the human sperm centrosomal function and the oocyte activation ability in a case of globozoospermia, by ICSI into bovine oocytes. Hum Reprod. 2002;17:2930–4. doi: 10.1093/humrep/17.11.2930. [DOI] [PubMed] [Google Scholar]
- 8.Rawe VY, Terada Y, Nakamura S, Chillik CF, Olmedo SB, Chemes HE. A pathology of the sperm centriole responsible for defective sperm aster formation, sygamy and cleavage. Hum Reprod. 2002;17:2344–9. doi: 10.1093/humrep/17.9.2344. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen H, Rebbe H. Fine structure of round-headed human spermatozoa. J Reprod Fertil. 1974;37:51–4. doi: 10.1530/jrf.0.0370051. [DOI] [PubMed] [Google Scholar]
- 10.Singh G. Ultrastructual features of round-headed human spermatozoa. Int J Fertil. 1992;37:99–102. [PubMed] [Google Scholar]
- 11.Chemes HE, Brugo S, Zanchetti F, Carrere C, Lavieri JC. Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril. 1987;48:664–9. doi: 10.1016/s0015-0282(16)59482-5. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura S, Terada Y, Rawe VY, Uehara S, Morito Y, Yoshimoto T, et al. A trial to restore defective human sperm centrosomal function. Hum Reprod. 2005;14:1–5. doi: 10.1093/humrep/deh899. [DOI] [PubMed] [Google Scholar]
- 13.Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, Lederman H, et al. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril. 2003;80:1413–9. doi: 10.1016/j.fertnstert.2003.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, et al. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod. 2005;20:185–90. doi: 10.1093/humrep/deh545. [DOI] [PubMed] [Google Scholar]
- 15.Berkovitz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006;21:1787–90. doi: 10.1093/humrep/del049. [DOI] [PubMed] [Google Scholar]
- 16.Simerly C, Schatten G. Techniques for localization of specific molecules in oocytes and embryos. Methods Enzymol. 1993;225:516–53. doi: 10.1016/0076-6879(93)25035-Z. [DOI] [PubMed] [Google Scholar]
- 17.Terada Y. Functional analyses of the sperm centrosome in human reproduction: implications for assisted reproductive technique. Soc Reprod Fertil Suppl. 2007;63:507–13. [PubMed] [Google Scholar]
- 18.Dam AH, Feenstra I, Westphal JR, Ramos L, Golde RJ, Kremer JA. Globozoospermia revisited. Hum Reprod Update. 2007;13:63–75. doi: 10.1093/humupd/dml047. [DOI] [PubMed] [Google Scholar]
- 19.Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J Androl. 2002;23:1–8. doi: 10.1002/j.1939-4640.2002.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 20.Navara CS, First NL, Schatten G. Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer: the role of the sperm aster. Dev Biol. 1994;162:29–40. doi: 10.1006/dbio.1994.1064. [DOI] [PubMed] [Google Scholar]
- 21.Goto K, Kinoshita A, Takuma Y, Ogawa K. Fertilization of bovine oocytes by the injection of immobilized, killed spermatozoa. Vet Rec. 1990;127:517–20. [PubMed] [Google Scholar]
- 22.Katayose H, Yanagida K, Shinoki T, Kawahara T, Horiuchi T, Sato A. Efficient injection of bull spermatozoa into oocytes using a Piezodriven pipette. Theriogenology. 1999;52:1215–24. doi: 10.1016/S0093-691X(99)00213-7. [DOI] [PubMed] [Google Scholar]