Abstract
Purpose
To assess FasL mRNA levels in ejaculated sperm from adolescent patients with and without varicocele.
Methods
Semen was obtained by masturbation following 2–4 days of ejaculatory abstinence, from 14 adolescents with varicocele grades II and III (study group), and 20 adolescents without varicocele (control group). Seminal analysis was done according to World Health Organization guidelines and morphology using Kruger’s strict criteria. The Fas-ligand (FasL) gene expression was performed using reverse transcription and real-time quantitative polymerase chain reaction (RQ-PCR) analysis, according to the expression level of the housekeeping cyclophilin A gene. A Student’s t-test was applied to compare the groups, and Spearman’s rank test in order to verify possible correlations (p < 0.05).
Results
Quantitative RQ-PCR demonstrated that the expression of FasL mRNA in sperm from the varicocele group was higher than in the control group. Also, sperm concentration was higher in the controls, when compared to the varicocele group. When submitted to correlation analysis, adolescents with varicocele presented a correlation between sperm concentration and FasL gene expression levels (r = −0.470), not observed in controls.
Conclusion
Our results allow us to conclude that, in adolescents with varicocele presenting lower sperm concentration, FasL mRNA levels are higher than in adolescents without varicocele.
Keywords: Sperm, Fas-ligand, Varicocele, Adolescent, Apoptosis
Introduction
Varicocele is characterized by an abnormal dilation of the testicular veins in the pampiniform plexus. The incidence of varicocele in the general adult population is about 15–20%, and it can be considered a major cause of male infertility. Although they probably originate due to valvular absence or incompetence in the internal spermatic veins, varicoceles may occur due to a number of different mechanisms, and is thus considered a multifactorial disease [1, 2].
Varicocele is asymptomatic in most cases and the diagnosis is made during physical examination by scrotal palpation [3]. When symptoms are observed, they are usually loss of testicular mass, pain, and scrotal size asymmetry [4–6].
Varicocele is generally detected at early puberty (10 years old) and the incidence increases and reaches around 15% during in adolescence [7], although some studies have reported higher incidences [8]. It is already known that testicular development is compromised in adolescents with varicocele; this fact can adversely affect sperm quality [9, 10]. In addition, it is important to note that varicocele can induce sperm DNA damage, increased reactive oxygen species (ROS), and apoptosis rates [11, 12].
Apoptosis, or signal-induced cell death, is a process in which a genetic mechanism, responsible for a series of events related to morphologic and biochemical changes, initiates by certain stimuli and culminates in the death of a cell. It is an important mechanism through which altered or excessive cells are removed from a population, maintaining tissue integrity, differentiation, and characteristics [13, 14].
There are two major pathways that lead to apoptosis: an intrinsic, mitochondria-initiated pathway and an extrinsic pathway which initiates upon the activation of membrane death receptors [15–17]. The Fas Protein (APO-1 or CD45) and its ligand (Fas-ligand or FasL) are members of the tumor necrosis factor (TNF) family [18]. When Fas binds to FasL, a molecular complex is formed, signaling initiation of apoptosis, which involves caspase 8 activation and a subsequent cascade of events leading to DNA fragmentation and cell death [19, 20].
Spermatogenesis is characterized by a complex and dynamic process of germ cells proliferation and differentiation from spermatogonia to mature spermatozoa. A large number of germ cells are present in the testes, while Sertoli cell support is finite. Thus, apoptosis is a normal and necessary occurrence during spermatogenesis, in order to select germ cells and regulate the number of germ cells [21–26]. In some cases, apoptosis may initiate but abort, in a process known as abortive apoptosis, leading to the ejaculation of mature sperm with apoptotic traits, such as fragmented DNA and membrane-bound Fas [27, 28].
In the testis, Fas may be found in spermatogenic germ cells, and FasL in Sertoli cells [18]. On the other hand, several studies have reported that FasL may be found in sperm, suggesting that (i) it may be a protective mechanism so sperm may counteract uterine macrophages and (ii) it may present a competitive advantage over other sperm, stimulating apoptosis in adjacent cells [29].
An important characteristic of human semen that can be used as tool to identify some genes of interest in male infertility is the presence of mRNA originated from early stages of spermatogenesis [30, 31]. Studies suggest that transcripts found in sperm are important to understand its development and the events surrounding fertilization [32]. Still, there are some hypotheses to justify the presence of mRNA in human sperm; the main one concluded that before the end of spermatogenesis, a translation of mRNA into proteins could occur, which is why the mRNA in human sperm can be similar to that found in the testis, indicating accurate development of spermatogenesis [33]. Recent studies in various murine and rat tissues showed that the testis is the main source of FasL mRNA in the body [34, 35].
Because varicocele progressively leads to a decrease in semen quality and is associated to secondary infertility, it is important to study varicocele as it initiates during puberty onset. Moreover, varicocele leads to an increase in abortive apoptosis rates. Thus, investigation of the expression levels of genes participating in apoptosis is important in order to elucidate some aspects about varicocele. Among the many candidate genes, the Fas family participates in a number of events involved in selection of germ cells as they interact with Sertoli cells.
There is still controversy as to the role of apoptosis during spermatogenesis, both when a varicocele is present or not. Thus, the aim of this study was to compare semen quality and expression of the FasL gene in ejaculated spermatozoa of adolescents with and without varicocele.
Materials and methods
Patients
A prospective study was conducted that included 34 adolescents who were between 14 and 19 years of age. These adolescents were recruited from a local public school, and an informed written consent was signed by each adolescent and his legal guardian before participation in the study. In order to recruit the adolescents, all the adolescents at the desired age group for the study were offered an andrological evaluation and invited to participate in the study. The adolescents were divided into two groups: a control group including 20 adolescents without varicocele and a study group including 14 adolescents with varicocele grades II or III [36]. All adolescents were examined by the same urologist and testicular volume was assessed with a Prader orchidometer. The study was carried out at the Sao Paulo Federal University Human Reproduction Section, and Institutional Review Board approval was obtained from the Sao Paulo Federal University Research Ethics Committee.
Seminal samples
Seminal samples were collected by masturbation in an area adjacent to the laboratory following 2–4 days of ejaculatory abstinence. After semen liquefaction, standard semen analysis was performed according to the World Health Organization guidelines [37] and sperm morphology was evaluated by Kruger’s strict criteria.
Immediately upon collection and examination, each semen sample was subjected to a separation of the fraction of sperm with high motility. This was accomplished by Percoll density-gradient centrifugation. Briefly, 1 mL of semen was layered on discontinuous two-layer (45 and 90%) Percoll gradients (170891-01/GE Healthcare, Amersham Place, UK) and centrifuged at 600 × g for 20 min in 15 mL conical tubes. The medium used to dilute the Percoll was human tubal fluid (98270, Irvine Scientific, Santa Ana, CA, USA) supplemented with 10% bovine serum albumin (BSA) (A-6003, Sigma, St. Louis, MO, USA).
Spermatozoa collected from the bottom layer (90% layer) were resuspended in HTF medium, and the tube was centrifuged at 600 × g for 10 min. Extreme care was taken to collect the pelleted spermatozoa, avoiding the seminal plasma and interphase material containing any potentially contaminating cells. Furthermore, a small aliquot from the pelleted spermatozoa was again examined to confirm the absence of peroxidase-positive contaminating leukocytes. Immediately thereafter, the sperm pellets were flash frozen in liquid nitrogen. Samples were stored at −80°C until RNA extraction. Post-Percoll sperm motility and concentration were not assessed to avoid mRNA degradation. Thus, FasL levels were determined based on the expression of a housekeeping cyclophilin A gene, and not normalized to sperm concentration.
RNA extraction
Total RNA was extracted from the sperm pellets using TRIzol reagent (Invitrogen, Carlsbad, USA). DNase I (Invitrogen, Carlsbad, USA) treatment was performed after RNA extraction to remove any residual genomic DNA. The total extracted RNA was then quantified using the GeneQuant Pro spectrophotometer (GE Healthcare, Amersham Place, UK) and standardized into 5 μg aliquots before being reverse transcribed into cDNA. First-strand complementary DNA (cDNA) synthesis from total RNA was catalyzed by Superscript II RT (Invitrogen, Carlsbad, USA), using oligo(dT)12–18 according to the manufacturer’s protocol. Reverse transcription was performed for 60 min at 37°C. Total RNA preparation and RT-PCR analysis.
Real-time polymerase chain reaction
The expression of each gene was analyzed by real time PCR using the ABI PRISM 7700 Sequence Detection System (Invitrogen, Carlsbad, USA). Expressions for the Fas-L and the housekeeping cyclophilin A genes were investigated. The forward primer and reverse primer for each molecule were respectively: 5′-ACA GGC AAG TCC AAC TCA AGG T-3′ (forward), 5′-TTC ACT CCA GAA AGC AGG ACA AT-3′ (reverse) (Fas-L) and 5′-CAA ATG CTG GAC CCA ACA CA-3′ (forward), 5′-TTG CCA AAC ACC ACA TGCTT-3′ (reverse) (Cyclophilin A). The PCR reaction contained 5 μL of SYBR® Green PCR Master Mix (Invitrogen, Carlsbad, USA), 4 pmol of both primers, 1.6 μL of cDNA and 2.6 μL of DEPC treated water. Amplification cycles were performed at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, 72°C for 20 s and 77°C for 20 s.
The point (designated as Ct value) at which the fluorescence intensity exceeds 10 standard deviations above the mean baseline fluorescence is a measure for the amount of cDNA, and thus mRNA, in the sample. mRNA signals for the different molecules were normalized for the cyclophilin A mRNA signal. All reactions were performed in duplicates and the Ct values were averaged for each sample. Et/Rt values were calculated, where Et is the Ct observed in the experimental sample for FasL and Rt is the Ct observed in the experimental sample for cyclophilin A.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 for Windows (SPSS, Inc., Chicago, IL). All variables were initially tested in order to determine variance homogeneity and data normality, and heteroscedastic data were transformed to their square roots. Groups were compared using Student’s T-test for unpaired samples, and correlation analysis was performed using Spearman’s rank test in untransformed values. An alpha of 5% was adopted.
Results
Results are demonstrated in Table 1. No differences were observed in age, right and left testicular volumes, sperm morphology, and sperm progressive motility. Sperm concentration was higher in the control group (p = 0.015), and expression of FasL gene were higher in the study group (p = 0.034).
Table 1.
Age, left and right testicular volume, semen analysis and FasL/cyclophilin A mRNA levels results for adolescents without varicocele (control group) and adolescents with varicocele (study group)
| Control group | Study group | p | |
|---|---|---|---|
| Age (years) | |||
| Mean; SD | 16.9; 0.9 | 16.3; 0.9 | 0.053 |
| 95% CI | [16.5; 17.3] | [15.8; 16.8] | |
| Right testicular volume (mL) | |||
| Mean; SD | 19.8; 4.3 | 18.8; 5.0 | 0.551 |
| 95% CI | [17.7; 21.8] | [15.9; 21.7] | |
| Left testicular volume (mL) | |||
| Mean; SD | 19.4; 4.2 | 17.8; 5.1 | 0.321 |
| 95% CI | [17.4; 21.4] | [14.9; 20.7] | |
| Concentration (×106/mL) | |||
| Mean; SD | 154.8; 110.6 | 52.1; 43.2 | 0.015* |
| 95% CI | [103.0; 206.6] | [27.2; 77.0] | |
| Morphology (% normal) | |||
| Mean; SD | 8.4; 5.0 | 5.9; 4.3 | 0.143 |
| 95% CI | [6.0; 10.7] | [3.4; 8.4] | |
| Motility (a + b, %) | |||
| Mean; SD | 69.3; 7.0 | 68.1; 12.9 | 0.737 |
| 95% CI | [66.0; 72.6] | [60.7; 75.6] | |
| FasL/cyclophilin A | |||
| Mean; SD | 3.43; 3.05 | 10.09; 15.08 | 0.034* |
| 95% CI | [2.00; 4.85] | [1.38; 18.80] | |
SD standard deviation
95% CI 95% confidence interval of the mean
*—statistically significant difference (p<α)
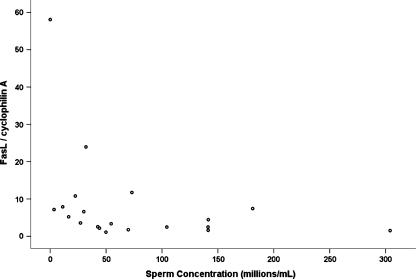
Adolescents with varicocele presented a negative correlation between sperm concentration and FasL mRNA levels (r = −0.552; p = 0.041) (Fig. 1), not observed in adolescents without varicocele (p = 0.531). No other correlations with FasL levels could be observed, in any group.
Fig. 1.
Scatter plot of sperm concentration (x-axis), FasL/cyclophylin A mRNA levels (y-axis) in adolescents with varicocele (n = 14)
Discussion
Although varicocele is considered an important key factor in male infertility [38, 39], its pathophysiology has not yet been completely elucidated. Varicocele can be associated with several factors, including venous stasis, heat stress, testicular hypoxia and increased oxidative stress [40]. Some studies demonstrate an adverse effect of varicocele on spermatogenesis [41], causing alterations in sperm concentration, motility and morphology [9, 10]. Others have demonstrated that varicocele may induce DNA damage and apoptosis [11, 12]. Barqawi et al. observed increased varicocele-induced testicular apoptosis in rats. It has also been demonstrated that high levels of germ cell apoptosis may contribute to male infertility, due to testicular failure [42].
Apoptosis is a mechanism that selects sperm cells and controls the overproduction of gametes to numbers that can be supported by the Sertoli cells. It is a process naturally occurring in spermatogenesis [25]. Therefore, apoptosis may be commonly observed in spermatocytes and spermatids [26], but the presence of apoptosis in ejaculated sperm is still unresolved.
Apoptosis may be activated through extrinsic mechanisms involving the interaction of Fas and its ligand, FasL [15–17, 43], a system which controls homeostasis in various tissues [44]. Fas is a type I transmembrane protein that belongs to the tumor necrosis factor receptor superfamily. FasL is produced as a type II membrane protein and is a member of the tumor necrosis factor family of proteins [34, 45–47]. Upon binding to FasL, Fas induces apoptotic activation of intracellular caspases, effector proteins, which in turn activate the cascade of events leading to cell death [27, 41, 44].
In the testis, Fas-induced apoptosis plays an important role during spermatogenesis. Testicular germ cells which are defective or in excessive numbers externalize Fas and, when in contact with FasL from Sertoli cells, undergo apoptosis and are resorbed. Thus, apoptosis plays an important role in (i) selecting germ cells and (ii) maintaining a proper amount of germ cells available for maturation by the Sertoli cells. In some cases, apoptosis may initiate (externalization of Fas) but, due to lack of trigger signals from the Sertoli cells, abort the process and mature into abnormal sperm, in a process known as abortive apoptosis [27].
Therefore, as has been demonstrated by Fujisawa et al., apoptosis may be decreased in varicocele-induced male infertility [25]. On the other hand, excessive death signals may lead to decimation of germ cells, and thus, to altered spermatogenesis and male infertility. Excessive testicular apoptosis in varicocele has also been previously reported [48].
In any case, Sakkas et al. described the presence of Fas on ejaculated sperm, suggesting that abortive apoptosis may indeed be a major stakeholder in male infertility [27]. Other authors have suggested that the expression of Fas in ejaculated sperm may serve as a biomarker for apoptosis [49, 50]. In addition, several studies have associated the presence of DNA strand breaks with some form of apoptosis [27].
Although it is widely accepted that the Sertoli cells express FasL and the germ cells express Fas [18], early studies using Northern blot analysis and in situ hybridization demonstrated a high content of FasL mRNA and protein in meiotic spermatocytes and haploid spermatids. Moreover, FasL has also been detected on the sperm cell surface [44].
This is especially interesting because it has been previously suggested that (i) FasL may be a protective mechanism so sperm may counteract uterine macrophages and (ii) FasL may present a competitive advantage over other sperm, stimulating apoptosis in adjacent cells [29].
During the last decade, researchers discovered the presence of mRNA in sperm. mRNA found in mature spermatozoa is probably similar to mRNA found in testis, and could be indicative of the development of spermatogenesis. Moreover, it could be used as a tool for investigation of genes linked to the infertility phenotype [30, 31]. Ostermeier et al. demonstrated that mRNA from testis and spermatozoa are concordant and can reflect what occurred during gene expression during spermatogenesis [51]. Also, accumulation of a certain mRNA may indicate that translation has not occurred, suggesting that protein content for that specific gene may be lower. The association between a lower gene-to-protein turnover and infertility may assist in establishing potential targets for therapeutic intervention [52].
In our study, we analyzed semen quality and levels of the FasL gene in ejaculated sperm from adolescents with varicocele. We initially observed that sperm concentration was higher in adolescents without varicocele. Different degrees of oligozoospermia can be observed in men with varicocele [20]. Indeed, varicocele is strongly associated to a decrease in overall semen quality, probably because venous reflux and testicular temperature elevation induce testicular dysfunction [41].
Current medical practice has suggested a decrease in sperm number and motility in patients with varicocele [53, 54]. Lund et al. found an association between asymptomatic varicocele and compromised sperm quality, including sperm concentration [6]. Lipshultz and Corriere have suggested that varicocele causes a progressive decline in fertility, due to progressive testicular damage, as testicular mass and sperm counts decline with age [55]. Nevertheless, some reports have demonstrated that incidental varicocele is not associated with a decrease in sperm concentration or motility [56].
We have previously established an association between adolescent varicocele and a decrease in the total output of motile sperm [8]. We have also shown that, even when sperm concentration is not different, sperm DNA integrity is lower in adolescents with varicocele [57]. In this study, we did not set out to evaluate if varicocele does indeed cause a decrease in sperm concentration. Rather, we feel it is important to interpret FasL expression levels results in the light that sperm concentration was different. This is especially important because our study group size was limited by the number of patients we were able to recruit. Thus, we feel that it is important to discuss our results under the constraints imposed by group size. In other words, our results apply when testicular sperm output is lower.
Regarding FasL levels, new techniques are helping to show that ejaculated spermatozoa retain a complex yet specific population of RNA, and spermatozoa RNA transcripts are important to understand human spermatozoa development [58]. RNA identification in mature sperm has been performed using techniques based on the detection of RNA by means of PCR after reverse transcription [59]. Our study investigated the expression of the FasL gene in ejaculated sperm and demonstrated that expression of the FasL gene was higher in adolescents with varicocele.
In most studies, the investigators analyzed Fas in mature spermatozoa. Sakkas et al. negatively correlated the presence of Fas in the ejaculate with sperm concentration [11, 27]. In addition, the authors suggested that the Fas system is important in eliminating defective spermatozoa, but can show irregularities in sperm quality [27]. Also, Perticarari et al. explained that the presence of Fas on ejaculated sperm is due to abortive apoptosis [60].
When studying adolescents with varicocele, we have previously shown that increased RNA levels of the HSPA2 gene are associated to a higher semen quality, when comparing adolescents with varicocele and altered semen quality to adolescents with varicocele and normal semen quality [61]. It is important to note, however, that the HSPA2 gene represented the inducible form of a heat-shock protein, and higher mRNA levels for inducible genes are usually associated to higher protein levels.
In our present study, we observed higher FasL mRNA levels in sperm from adolescents with varicocele. If indeed FasL is used by mature sperm inside the female reproductive tract, it is natural to assume its expression is not inducible during spermatogenesis, at least not in testicular germ cells. Thus, FasL would assume a “constitutive” expression in sperm, and a higher mRNA content for constitutive genes would indicate a lower gene-to-protein turnover. Thus, FasL mRNA would accumulate because FasL protein is not being translated in adolescents with varicocele.
The fact that FasL mRNA and sperm concentration were negatively correlated in adolescents with varicocele in part supports this hypothesis, because the lower sperm production leads to lower translation of FasL for these sperm, thus leading to an accumulation of FasL mRNA.
Conversely, because a negative correlation was observed between sperm concentration and FasL mRNA levels, it may be the case that, under adverse testicular conditions, an increase in the expression of FasL expression may be necessary to remove altered spermatogenic germ cells. If this is true, FasL may be necessary at baseline levels, but its increase during varicocele may occur as a response to increased initial apoptotic stimuli, thus demonstrating an altered testicular environment.
New studies are necessary to better understand the presence and significance of FasL in spermatogenic germ cells of patients with varicocele, and in regular spermatogenesis in general. However, FasL does seem to play a role in normal testes from individuals without varicocele.
Thus, our results allow us to conclude that, in adolescents with varicocele presenting lower sperm concentration, FasL mRNA levels are higher than in adolescents without varicocele.
Footnotes
Capsule Adolescent varicocele leads to decreased sperm concentration concomitant with an increase in sperm Fasligand messenger RNA levels.
Contributor Information
Paula Toni Del Giudice, FAX: +55-11-55730014, Email: paula.tdg@gmail.com.
Ricardo Pimenta Bertolla, FAX: +55-11-55730014, Email: rbertolla@yahoo.com.
References
- 1.French DB, Desai NR, Agarwal A. Varicocele repair: Does it still have a role in infertility treatment? Curr Opin Obstet Gynecol. 2008;20:269–74. doi: 10.1097/GCO.0b013e3282fcc00c. [DOI] [PubMed] [Google Scholar]
- 2.Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Updat. 2001;7:473–81. doi: 10.1093/humupd/7.5.473. [DOI] [PubMed] [Google Scholar]
- 3.Paduch DA, Skoog SJ. Current management of adolescent varicocele. Rev Urol. 2001;3(3):120–33. [PMC free article] [PubMed] [Google Scholar]
- 4.Guarino N, Tadini B, Bianchi M. The adolescent varicocele: the crucial role of hormonal tests in selecting patients with testicular dysfunction. J Pediatr Surg. 2003;38(1):120–3. doi: 10.1053/jpsu.2003.50024. [DOI] [PubMed] [Google Scholar]
- 5.Skoog SJ, Roberts KP, Goldstein M, Pryor JL. The adolescent varicocele: what’s new with an old problem in young patients? Pediatrics. 1997;100(1):112–21. doi: 10.1542/peds.100.1.112. [DOI] [PubMed] [Google Scholar]
- 6.Lund L, Rasmussen HH, Ernst E. Asymptomatic varicocele testis. Scand J Urol Nephrol. 1993;27:395–8. doi: 10.3109/00365599309180452. [DOI] [PubMed] [Google Scholar]
- 7.Steeno O, Knops J, Declerck L, Adimoelja A, Voorde H. Prevention of fertility disorders by detection and treatment of varicocele at school and college age. Andrologia. 1976;8:47–53. doi: 10.1111/j.1439-0272.1976.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 8.Mori MM, Bertolla RP, Fraietta R, Ortiz V, Cedenho AP. Does varicocele grade determine extent of alteration to spermatogenesis in adolescents? Fertil Steril. 2008;90(5):1769–73. doi: 10.1016/j.fertnstert.2007.09.052. [DOI] [PubMed] [Google Scholar]
- 9.The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil Steril. 1992;57(6):1289–93. [PubMed]
- 10.Hadziselimovic F, Herzog B, Jenny P. The chance for fertility in adolescent boys after corrective surgery for varicocele. J Urol. 1995;154:731–3. doi: 10.1016/S0022-5347(01)67147-7. [DOI] [PubMed] [Google Scholar]
- 11.Sakkas D, Moffatt O, Manicardi GC, Mariethoz E, Tarozzi N, Bizzaro D. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod. 2002;66(4):1061–7. doi: 10.1095/biolreprod66.4.1061. [DOI] [PubMed] [Google Scholar]
- 12.Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80(6):1431–6. doi: 10.1016/S0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 13.Blank M, Shiloh Y. Programs for cell death: apoptosis is only one way to go. Cell Cycle. 2007;6:686–95. doi: 10.4161/cc.6.6.3990. [DOI] [PubMed] [Google Scholar]
- 14.Liu G, Gong P, Bernstein LR, Bi Y, Gong S, Cai L. Apoptotic cell death induced by low-dose radiation in male germ cells: hormesis and adaptation. Crit Rev Toxicol. 2007;37:587–605. doi: 10.1080/10408440701493061. [DOI] [PubMed] [Google Scholar]
- 15.Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol. 2002;159:923–9. doi: 10.1083/jcb.200207071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chipuk JE, Green DR. Do inducers of apoptosis trigger caspase-independent cell death? Nat Rev Mol Cell Biol. 2005;6:268–75. doi: 10.1038/nrm1573. [DOI] [PubMed] [Google Scholar]
- 17.Lysiak JJ, Turner SD, Turner TT. Molecular pathway of germ cell apoptosis following ischemia/reperfusion of the rat testis. Biol Reprod. 2000;63:1465–72. doi: 10.1095/biolreprod63.5.1465. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Richburg JH, Shipp EB, Meistrich ML, Boekelheide K. The Fas system, a regulator of testicular germ cells apoptosis is differentially up-regulated in Sertoli versus germ cells injury of the testis. Endocrinology. 1999;140:852–8. doi: 10.1210/en.140.2.852. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs EJ, McKenna KA, Bedi A. p53-dependent DNA damage-induced apoptosis requires FAS/APO-1 independent activation of CPP32 beta. Cancer Res. 1997;57:2250–554. [PubMed] [Google Scholar]
- 20.Marmar JL. The pathophysiology of varicoceles in the light of current molecular and genetic information. Hum Reprod Updat. 2001;7(5):461–72. doi: 10.1093/humupd/7.5.461. [DOI] [PubMed] [Google Scholar]
- 21.Bartke A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology. 1995;136:3–4. doi: 10.1210/en.136.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Allan DJ, Harmon BV, Roberts SA. Spermatogonial apoptosis has three morphologically recognizable phases and shows no circadian rhythm during normal spermatogenesis in the rat. Cell Prolif. 1992;25:241–50. doi: 10.1111/j.1365-2184.1992.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 23.Sinha Hikim AP, Rajavashisth TB, Sinha Hikim I, Lue Y, Bonavera JJ, Leung A, et al. Significance of apoptosis in the temporal and stage-specific loss of germ cells in the adult rat after gonadotropin deprivation. Biol Reprod. 1997;57:1193–1201. doi: 10.1095/biolreprod57.5.1193. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997;16:2262–70. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujisawa M, Hiramine C, Tanaka H, Okada H, Arakawa S, Kamidono S. Decrease in apoptosis of germ cells in the testes of infertile men with varicocele. World J Urol. 1999;17:296–300. doi: 10.1007/s003450050149. [DOI] [PubMed] [Google Scholar]
- 26.Hikim AP, Wang C, Lue Y, Johnson L, Wang XH, Swerdloff RS. Spontaneous germ cell apoptosis in humans: evidence for ethnic differences in the susceptibility of germ cells to programmed cell death. J Clin Endocrinol Metab. 1998;83:152–6. doi: 10.1210/jc.83.1.152. [DOI] [PubMed] [Google Scholar]
- 27.Sakkas D, Mariethoz E, St John JC. Abnormal sperm paremeters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathway. Exp Cell Res. 1999;251(2):350–5. doi: 10.1006/excr.1999.4586. [DOI] [PubMed] [Google Scholar]
- 28.Gorczyca W, Traganos F, Jesionowska H, Darzynkiewicz Z. Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: analogy to apoptosis of somatic cells. Exp Cell Res. 1993;207:202–5. doi: 10.1006/excr.1993.1182. [DOI] [PubMed] [Google Scholar]
- 29.Riccioli A, Salvati L, D’Alessio A, Starace D, Giampietri C, Cesaris P, et al. The Fas system in the seminiferous epithelium and its possible extra-testicular role. Andrologia. 2003;35(1):64–70. doi: 10.1046/j.1439-0272.2003.00538.x. [DOI] [PubMed] [Google Scholar]
- 30.Wykes SM, Miller D, Krawetz SA. Mammalian spermatozoal mRNAs: tools for the functional analysis of male gametes. J Submicrosc Cytol Pathol. 2000;32:77–81. [PubMed] [Google Scholar]
- 31.Miller D. Analysis and significance of messenger RNA in human ejaculated spermatozoa. Mol Reprod Dev. 2000;56:259–64. doi: 10.1002/(SICI)1098-2795(200006)56:2+<259::AID-MRD10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Yao C, Wang Z, Zhou Y, Wang Y, Liu L, et al. Characterization and quantification of mRNA transcripts in ejaculated spermatozoa of fertile men by serial analysis of gene expression. Hum Reprod. 2006;21(6):1583–90. doi: 10.1093/humrep/del027. [DOI] [PubMed] [Google Scholar]
- 33.Lambard S, Galeraud-Denis I, Martin G, Levy R, Chocat A, Carreau S. Analysis and significance of mRNA in human ejaculated sperm from normozoospermic donors: relationship to sperm motility and capacitation. Mol Hum Reprod. 2004;10(7):535–41. doi: 10.1093/molehr/gah064. [DOI] [PubMed] [Google Scholar]
- 34.Suda T, Takahashi T, Goldstein P, Nagata S. Molecular cloning and expression of the FasL, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–78. doi: 10.1016/0092-8674(93)90326-L. [DOI] [PubMed] [Google Scholar]
- 35.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, et al. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995;154:3806–13. [PubMed] [Google Scholar]
- 36.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–9. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 37.Laboratory manual for the examination of human semen and sperm–cervical mucus interaction. 4. New York: Cambridge University Press; 1999. [Google Scholar]
- 38.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–6. [PubMed] [Google Scholar]
- 39.Witt MA, Lipshultz LI. Varicocele: a progressive or static lesion? Urology. 1993;42:541–3. doi: 10.1016/0090-4295(93)90268-F. [DOI] [PubMed] [Google Scholar]
- 40.Brown JS, Dubin L, Hotchkiss RS. The varicocele as related to fertility. Fertil Steril. 1967;18:46–56. doi: 10.1016/s0015-0282(16)36184-2. [DOI] [PubMed] [Google Scholar]
- 41.Practice Committee of the American Society for Reproductive Medicine Report on varicocele and infertility. Fertil Steril. 2006;86(5 Suppl):S93–5. doi: 10.1016/j.fertnstert.2006.07.1486. [DOI] [PubMed] [Google Scholar]
- 42.Barqawi A, Caruso A, Meacham RB. Experimental varicocele induces testicular germ cell apoptosis in the rat. J Urol. 2004;171:501–3. doi: 10.1097/01.ju.0000088775.69010.61. [DOI] [PubMed] [Google Scholar]
- 43.Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256:58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 44.D’Alessio A, Riccioli A, Lauretti P, Padula F, Muciaccia B, Cesaris P, et al. Testicular FasL is expressed by sperm cells. Proc Natl Acad Sci USA. 2001;98(6):3316–21. doi: 10.1073/pnas.051566098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 46.Oehm A, Behrmann I, Falk W, Pawlita M, Maier G, Klas C, et al. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the fas antigen. J Biol Chem. 1992;267:10709–15. [PubMed] [Google Scholar]
- 47.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 48.Barr PJ, Tomei LD. Apoptosis and its role in human disease. Biotechnology. 1994;12:487–93. doi: 10.1038/nbt0594-487. [DOI] [PubMed] [Google Scholar]
- 49.Shen HM, Dai J, Chia SE, Lim A, Ong CN. Detection of apoptotic alterations in sperm in subfertile patients and their correlations with sperm quality. Hum Reprod. 2002;17:1266–73. doi: 10.1093/humrep/17.5.1266. [DOI] [PubMed] [Google Scholar]
- 50.Paasch U, Agarwal A, Gupta AK, Sharma RK, Grunewald S, Thomas AJ, Jr, et al. Apoptosis signal transduction and the maturity status of human spermatozoa. Ann N Y Acad Sci. 2003;1010:486–8. doi: 10.1196/annals.1299.088. [DOI] [PubMed] [Google Scholar]
- 51.Ostermeier GC, Dix DJ, Miller D, Khatri P, Krawetz SA. Spermatozoal RNA profiles of normal fertile men. Lancet. 2002;360:772–7. doi: 10.1016/S0140-6736(02)09899-9. [DOI] [PubMed] [Google Scholar]
- 52.Kaufman RJ. Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem Sci. 2004;29(3):152–8. doi: 10.1016/j.tibs.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Naftulin BN, Samuels SJ, Hellstrom WJ, et al. Semen quality in varicocele patients is characterized by tapered sperm cells. Fertil Steril. 1991;56:149–51. doi: 10.1016/s0015-0282(16)54437-9. [DOI] [PubMed] [Google Scholar]
- 54.Brown JS. Varicocelectomy in the subfertile male: a ten-year experience with 295 cases. Fertil Steril. 1976;27:1046–53. [PubMed] [Google Scholar]
- 55.Lipshultz LI, Corriere JN., Jr Progressive testicular atrophy in the varicocele patient. J Urol. 1977;117:175–6. doi: 10.1016/s0022-5347(17)58387-1. [DOI] [PubMed] [Google Scholar]
- 56.Zargooshi J. Sperm count and sperm motility in incidental high-grade varicocele. Fertil Steril. 2007;88(5):1470–3. doi: 10.1016/j.fertnstert.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 57.Bertolla RP, Cedenho AP, Hassun Filho PA, Lima SB, Ortiz V, Srougi M. Sperm DNA fragmentation in adolescents with varicocele. Fertil Steril. 2006;85(3):625–8. doi: 10.1016/j.fertnstert.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 58.Miller D, Ostermeier GC, Krawetz SA. The controversy, potential and roles of spermatozoal RNA. Trends Mol Med. 2005;11:156–63. doi: 10.1016/j.molmed.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Miller D, Tang PZ, Skinner C, Lilford R. Differential RNA fingerprinting as a tool in the analysis of spermatozoal gene expression. Hum Reprod. 1994;9:864–9. doi: 10.1093/oxfordjournals.humrep.a138607. [DOI] [PubMed] [Google Scholar]
- 60.Perticarari S, Ricci G, Boscolo R, Santis M, Pagnini G, Martinelli M, et al. Fas receptor is not on ejaculated human sperm. Hum Reprod. 2008;23:1271–9. doi: 10.1093/humrep/den113. [DOI] [PubMed] [Google Scholar]
- 61.Lima SB, Cenedeze MA, Bertolla RP, Hassun Filho PA, Oehninger S, Cedenho AP. Expression of the HSPA2 gene in ejaculated spermatozoa from adolescents with and without varicocele. Fertil Steril. 2006;86(6):1659–63. doi: 10.1016/j.fertnstert.2006.05.030. [DOI] [PubMed] [Google Scholar]