Abstract
Purpose
To investigate the prevalence of spontaneously developed tail swellings (SDTS) in human sperm samples that are commonly encountered in the laboratory, and their influence on the hypo-osmotic swelling test (HOS-test).
Methods
Ejaculated, epididymal, and testicular sperm were evaluated for SDTS. Further, HOS-test scores were compared with those of vital stains using column washed sperm maintained in the laboratory.
Results
SDTS, at <10%, was present in all types of sperm samples. The highest and lowest occurrences of SDTS were found in cryopreserved sperm, and column-washed sperm respectively. SDTS can inflate the HOS-test score, and so lower the accuracy of the HOS-test. However, the HOS-test efficiency can be improved by assessing SDTS in the sample.
Conclusion
HOS-test and vital stain cannot be used interchangeably in all circumstances for sperm viability determination. The accuracy of the HOS-test can be enhanced by incorporating SDTS as a correction factor.
Keywords: HOS-Test, Spontaneously developed tail swellings, Vital stain
Introduction
The hypo osmotic swelling test (HOS-test) was introduced to evaluate hypo-osmotic swellings (HOS) for investigating membrane integrity of human spermatozoa [1]. Application of the test was extended further to include sperm viability assessment given the argument that sperm with damaged tails can be considered as nonviable and those with intact tails as viable [2, 3]. In assisted reproduction, the HOS-test gained wider acceptance over vital staining in identifying viable sperm to be used for intra cytoplasmic sperm injection (ICSI) [4–6]. The HOS-test thus slowly became an accepted alternative to conventional viability determination by vital stain [7, 8]. Despite rapid acceptance and wide use of the HOS-test in vitality assessment, its reliability and validity have been challenged by many studies [8–10]. According to these studies, the predictive power of the HOS-test is not as accurate as has been proclaimed. For example, low fertilization rates of ICSI using sperm selected by HOS-test raises question about the accuracy of the test in identifying viable sperm [9, 11, 12].
Better understanding of the factors that influence the HOS-test may help to assess the reliability of the test more objectively. Based on our own experience of handling human sperm in different in vitro conditions and published reports [1, 13], we realized that spontaneously developed tail swelling (SDTS) of sperm may play a role in causing variation of HOS-test results. The difference between SDTS and HOS is that both are tail swellings, but it is considered SDTS when swelling is unexpectedly exhibited in fluids holding physiological osmolarities, while HOS occurs if sperm are exposed to hypo-osmotic conditions [1, 13]. Physiological osmolarity in this context refers to the osmolarity values of body fluid like semen, and in vitro developed reagents in which human cells, including spermatozoa, maintain their cellular integrity and perform normal physiological functions [13–15]. Our study investigated the prevalence of SDTS in sperm samples that are commonly encountered in an andrology laboratory, and secondly the influence of SDTS in HOS-test results.
Materials and methods
In the first part of the study, ejaculated (n = 35), epididymal (n = 4), and testicular (n = 9) sperm samples were investigated for SDTS. Out of total 48 samples, 20 were fresh and 28 were cryopreserved. Cryopreserved sperm underwent the standard freeze-thaw procedure using enhanced sperm freeze solution (Conception Technology). Sperm in semen or in vitro reagents were handled in such a way that physiological osmolarity was not compromised [13–15]. SDTS was assessed by observing the sample under a phase contrast microscope at 400X magnification. Five microliters of sample without any treatment was spread in a Makler chamber and searched for SDTS. The graded cover glass of Makler chamber was helpful in determining relative abundances of SDTS. At least 200 sperm were scored in each sample load. Duplicate samples were analyzed for each specimen. Fresh semen sperm, column washed fresh and frozen sperm, epididymal and testicular sperm at pre and post cryopreservation were evaluated for SDTS.
In the second part of the study, gradient column recovered fresh human sperm (n = 12), maintained in an in vitro culture system, were used. In this system, the column enriched sperm were set at a concentration of 40–60 × 106/ml in 1.0 mL Hepes-HTF (human tubal fluid) media supplemented with 5% HSA (human serum albumin). The sperm culture was maintained in a tightly capped tube for 7 days so that original osmolarity (280 ± 10 mOsm/kg) could be sustained [14, 16]. The methodology for this procedure has been described in a previous study of ours [16]. Motility, SDTS, vital stain, and HOS-test values of sperm maintained in laboratory culture were determined within an hour of column wash, and subsequently every day for 7 days. A Makler chamber was used for motility evaluation [16]. SDTS in samples was assessed following the same method as described above. The vital staining method (Eosin-Nigrosin) for viability was employed following WHO-recommended procedure [13]. Viability determination by HOS-test involved evaluation of hypo-osmotic swellings [1, 16].
The sperm samples used in the study were collected from the male partners of infertile couples for diagnostic purposes and/ or for clinical utilization. Appropriate authorization of institutional review board was obtained to utilize the discarded portions of these samples in our study. Analysis of variance which included t-test was used for statistical analysis with Sigma Plot software, version 11.0 for Windows.
Results
SDTS was present in all 48 samples studied (Table 1). The distribution of SDTS varied considerably in every category (ejaculated, epididymal, and testicular) of sperm, but, in general remained below 10% (Table 1). The prevalence of SDTS in ejaculated sperm (fresh semen) was 5.9 ± 4.5% (mean ± SD). SDTS was the lowest (3.8 ± 3.2%) in column-washed sperm to be used for IUI or IVF and the highest (6.7 ± 3.3%) in frozen-thawed sperm (Table 1).
Table 1.
Prevalence of spontaneously developed tail swellings (SDTS) of human spermatozoa in different types of sperm samples commonly encountered in the andrology laboratory
| Sample type | Number of sample | SDTS value (in %) |
|---|---|---|
| Ejaculated sperm | ||
| Fresh semen sperm | 10 | 5.9 ± 4.5 |
| Fresh semen sperm column washed | 10 | 3.8 ± 3.2 |
| Frozen-thaw semen sperm no wash | 5 | 6.7 ± 3.3 |
| Frozen-thaw semen sperm washed | 5 | 5.2 ± 2.4 |
| Epididymal sperm | ||
| Frozen-thaw epididymal sperm no wash | 2 | 7.0 ± 5.7 |
| Frozen-thaw epididymal sperm washed | 2 | 6.0 ± 2.8 |
| Testicular sperm aspiration (TESA) | ||
| Fresh TESA sperm | 4 | 5.0 ± 4.4 |
| Frozen-thaw TESA sperm no wash | 5 | 5.8 ± 4.1 |
| Frozen-thaw TESA sperm washed | 5 | 6.4 ± 3.2 |
Values represent mean ± SD
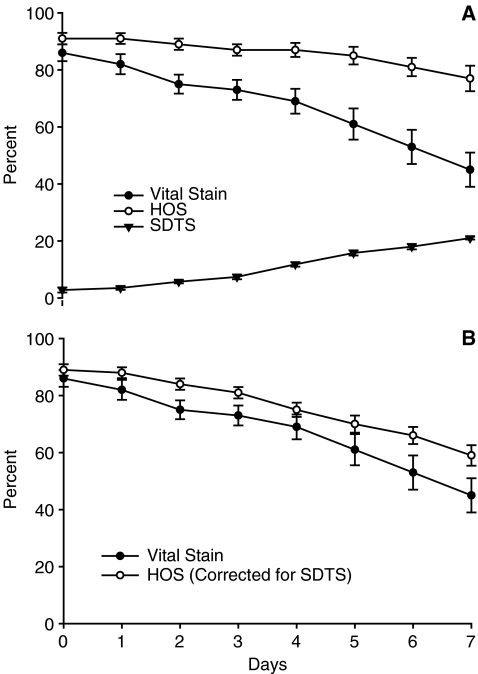
In laboratory maintained sperm (2nd part of study), the motility dropped significantly (from 81.1 ± 14.6% on day 0 to 7.5 ± 8.7% on day 7) as expected. Vital stain, HOS-test, and SDTS scores exhibited are shown in Fig. 1a. HOS-test and vital stain scores were similar on day 0 (91 ± 7% vs. 87 ± 9%, p 0.3) . However, the scores of the methods were significantly (P < 0.001) different starting on day 2 and continuing through day 7, showing consistently higher HOS-test scores than vital stains (Fig. 1a). The HOS-test scores minus vital stain scores (score difference between the tests) from day 0 to day 7 were 5.2, 7.8, 12.4, 15.4, 17.6, 24.0, 28.4, and 31.0 respectively. SDTS value in the sperm sample progressively increased with duration of maintenance (Fig. 1a). When HOS scores were corrected by subtracting SDTS from HOS, the difference between HOS-test and vital stain were less divergent at all observation points (Fig. 1b).
Fig. 1.
Similarities and differences between Vital stain and HOS-test score patterns exhibited by the laboratory maintained spermatozoa over days. a Vital stain, HOS, and SDTS scores; b vital stain scores and corrected HOS scores (original HOS minus SDTS). Values represent mean ± S.E
Discussion
Presence of SDTS in human semen was previously documented [1, 13]. In the first part of our study, we have determined the distribution of SDTS in different types of sperm samples that are commonly encountered in andrology laboratory. Our survey showed the highest occurrence of SDTS in cryopreserved sperm. This may be due to the freeze-thaw related impact on sperm tails [8, 10, 17]. The least occurrence of SDTS in IUI/IVF ready sperm compared to that of original samples (semen or cryopreserved specimen) indicates that SDTS can be reduced or eliminated from samples by laboratory techniques such as gradient column processing of semen. One of the weaknesses of the current study is that the determination of SDTS prevalence was based on a small sample size. Since SDTS has clinical significance, our findings on SDTS should be further validated by a larger sample size.
We have also compared the efficacy of the HOS-test vs. vital stain over 7 days (2nd part of the study). Maintenance of column-enriched sperm in a laboratory setting created an ideal condition and opportunity for comparing the efficacy of these two techniques. Basal (day 0) viability scores exhibited by vital stain and HOS-test were identical. This observation indicates that in a fresh sample, specifically for column enriched sperm, the viability measurement can be performed either by vital stain or HOS-test. As expected, there was a drop in viability in the laboratory maintained spermatozoa over time. However, the rate of drop as revealed by HOS test was not the same as that of vital stain. HOS-test scores were consistently higher than that of vital stains throughout the observation period, and the difference between the two steadily increased as the observation progressed. The underlying cause behind this widening difference in the score of vital stain and HOS-test is worth exploring.
First of all, it is important to realize that vital stain and HOS-test focus on two different anatomical parts, head and tail, respectively. In fresh samples, vital stain and HOS-test produce similar results. As one can expect, membrane integrity of both heads and tails will be equally well preserved in fresh conditions. However, during extended culture, the plasma membrane integrity of the head and tail are not equally maintained, thus resulting in a difference in vital stain and HOS-test scores as seen in the study. The tail, being compact, elongated, and having fibrous sheath lining, may have an advantage in sustaining membrane integrity. Furthermore, a collapse of the head’s integrity over time may have been enhanced by the collapse of the acrosome, a unique component of the sperm head [13]. Our argument of differential membranes preserving the head and tail may be true, but that probably is not the only explanation for divergent scores of HOS-test and vital stain. Our study does show that SDTS probably also plays a role in this.
In HOS-test, both HOS and SDTS are included in the same count since they are morphologically indistinguishable. Findings confusing SDTS with HOS have been reported in previous literatures [1, 13]. In our case, SDTS may have contributed to the overestimation of HOS-test scores to a certain extent. SDTS in sperm samples progressively increased with time, and these SDTS were counted as HOS in the HOS-test, causing the HOS scores to be erroneously higher. SDTS, therefore, made the HOS-test less reliable for assessment of sperm viability. It appears that the longer the duration of maintaining the sperm in the laboratory, the greater the impact of SDTS on the HOS-test. Therefore, the extent to which SDTS will affect the HOS-test can vary depending on the age of the sample. We have shown in our experiment that when a HOS score was corrected by subtracting SDTS, the difference between HOS-test and vital stain decreased. Incorporating SDTS as a correction factor toward improving the accuracy of the HOS-test can, therefore, be a viable option. We understand that the extended sperm culture that we used in our study is not a common laboratory phenomenon; however, such a setup provided a unique condition for comparing the HOS-test with the vital stain.
Despite its limitation, the HOS-test is still the method of choice in selecting viable sperm to be used in assisted reproductive procedure like ICSI. Stained sperm (as in vital stain) cannot be used in ICSI for the sake of procedural safety. The HOS-test is thus the only choice for performing ICSI with nearly and completely non-motile sperm as often seen in testicular biopsied sperm samples, and occasionally in cryopreserved regular semen sperm. In fact if the motility of such sperm cannot be activated by currently known motility activating agents (like pentoxiphyline, caffeine) then HOS-test is the last and only option that remains at the hand to perform ICSI. Further, the HOS-test is very simple and easy to perform thus can be implemented in any laboratory setup. In our study, we made an effort to address SDTS which affects the efficiency of the HOS-test. It is expected that taken into consideration of SDTS when applying HOS-test in ICSI will bring an improvement of ICSI fertilization.
Conclusion
We have shown the distribution pattern of SDTS in all types of sperm samples routinely dealt in andrology laboratory. We have also shown that progressive build up of SDTS occurs in sperm samples, which affects the accuracy of the HOS-test. The condition in our study is just one of the many laboratory treatments or conditions that human spermatozoa are subject to in vitro. We speculate that SDTS formation may also occur in sperm samples that undergo other forms of laboratory treatment or a combination of treatments and thus can also overestimate HOS-test results in those samples. In our experiment, HOS-test and vital stain produced identical test scores when applied to semen sperm and column washed sperm at hour 0 (fresh) while produced unequal scores in aged sample (e.g. laboratory maintained sperm). These observations suggest that the vital stain and the HOS-test cannot be used interchangeably in all circumstances. When the HOS-test is used for sperm viability assessment; the accuracy of the test can be improved by assessing SDTS in the sample.
Footnotes
Capsule
Incorporating SDTS as a correction factor toward improving the accuracy of the HOS-test can be a viable option.
Contributor Information
Amjad Hossain, Phone: +1-281-5340476, FAX: +1-281-5342770, Email: amhossai@utmb.edu.
Collin Osuamkpe, Phone: +1-281-5340476, FAX: +1-281-5342770.
Shaikat Hossain, Phone: +1-281-5340476, FAX: +1-281-5342770.
John Y. Phelps, Phone: +1-281-5340476, FAX: +1-281-5342770
References
- 1.Jeyendran RS, Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–228. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 2.Schrader SM, Platek SF, Zaneveld LJ, Perez-Pelaez M, Jeyendran RS. Sperm viability: a comparison of analytical methods. Andrologia. 1986;18:530–538. doi: 10.1111/j.1439-0272.1986.tb01822.x. [DOI] [PubMed] [Google Scholar]
- 3.Munuce MJ, Caille AM, Perfumo P, Morisoli L. Does the hypo-osmotic swelling test predict human sperm viability? Arch Androl. 2000;44:207–212. doi: 10.1080/014850100262182. [DOI] [PubMed] [Google Scholar]
- 4.Desmet B, Joris H, Nagy Z, Liu J, Bocken G, Vankelecom A, et al. Selection of vital immotile spermatozoa for intracytoplasmic injection by the hypo-osmotic swelling test Hum Reprod 1994924–31.8195347 [Google Scholar]
- 5.Casper RF, Cowan L, Lucato ML, Jarvi KA. The hypoosmotic swelling test for the selection of viable sperm for inctracytoplasmic sperm injection in men with complete asthenozoospermia. Fertil Steril. 1996;65:972–976. doi: 10.1016/s0015-0282(16)58271-5. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Tsai YL, Katz E, Compton G, Garcia JE, Baramki TA. High fertilization rate obtained after intracytoplasmic sperm injection with 100% non-motile spermatozoa selected by using a simple modified hypo-osmotic swelling test. Fertil Steril. 1997;68:373–375. doi: 10.1016/S0015-0282(97)81533-6. [DOI] [PubMed] [Google Scholar]
- 7.Smikle CB, Turek PJ. Hypo-osmotic swelling can accurately assess the viability of nonmotile sperm. Mol Reprod Develop. 1997;47:200–203. doi: 10.1002/(SICI)1098-2795(199706)47:2<200::AID-MRD11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Lin MH, Morshedi M, Srisombut C, Nassar A, Oehninger S. Plasma membrane integrity of cryopreserved human sperm: an investigation of the results of the hypoosmotic swelling test, the water tests, and eosin-Y staining. Fertil Steril. 1998;70:1148–1155. doi: 10.1016/S0015-0282(98)00351-3. [DOI] [PubMed] [Google Scholar]
- 9.Jager S, Kremer J, Mijchman J. Hypoosmotic sperm swelling test does not assess fertilizing capacity of human spermatozoa. Arch Androl. 1991;26:195–197. doi: 10.3109/01485019108987643. [DOI] [PubMed] [Google Scholar]
- 10.Esteves SC, Sharma RK, Thomas AJ, Agarwal A. Suitability of the hypo-osmotic swelling test for assessing the viability of cryopreserved sperm. Fertil Steril. 1996;66:798–804. [PubMed] [Google Scholar]
- 11.Avery S, Bolton VN, Mason BA. An evaluation of the hypo-osmotic sperm swelling test as a predictor of fertilizing capacity in vitro. Int J Androl. 1990;13:93–99. doi: 10.1111/j.1365-2605.1990.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 12.Martini AC, Estofan D, Ruiz R, Cuneo M. Improving the predictive value of the hypo-osmotic swelling test in humans. Fertil Steril. 2006;85:1840–1842. doi: 10.1016/j.fertnstert.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 13.WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4. Cambridge: Cambridge University; 1999. [Google Scholar]
- 14.Gardner DK, Weissman A, Howles CM, Shoham Z. Textbook of assisted reproductive techniques: laboratory and clinical perspectives. 1. London: Dunitz; 2001. [Google Scholar]
- 15.Rossato M, Balercia G, Lucarelli G, Mantero F. Role of seminal osmolarity in the regulation of human sperm motility. Int J Androl. 2002;37:207–218. doi: 10.1046/j.1365-2605.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 16.Hossain A, Osuamkpe C, Nagamani M. Extended culture of human spermatozoa in the laboratory may have practical value in the assisted reproductive procedures. Fertil Steril. 2007;89:237–239. doi: 10.1016/j.fertnstert.2007.01.170. [DOI] [PubMed] [Google Scholar]
- 17.Misro M, Chaki S. Development of a rapid, sensitive, and reproducible laboratory test kit for the assessment of plasma membrane integrity of human sperm. Fertil Steril. 2008;89:223–227. doi: 10.1016/j.fertnstert.2007.02.003. [DOI] [PubMed] [Google Scholar]