Abstract
Anti-SmD autoantibodies are specific for systemic lupus erythematosus (SLE). In this investigation, the influence of HLA-D genes on immune responses to SmD was investigated. Mice with HLA-DR3, HLA-DR4, HLA-DQ0601, HLA-DQ0604 or HLA-DQ8 transgenes were immunized with recombinant SmD1 (SmD) and their antibody responses were analyzed. Analysis by ELISA showed that all strains responded well to SmD. However, when synthetic SmD peptides were used as substrate, DR3 mice had the highest antibody response followed by DQ8, DQ0604, DQ0601, and DR4. A similar trend was observed in Western blot analysis using WEHI 7.1 cell lysate as the substrate, with the exception that DR4 mice did not generate detectable amounts of antibodies. Only sera from DR3 and DQ0604 mice immunoprecipitated A-RNP, SmB and SmD. Intermolecular epitope spreading to A-RNP and SmB was evident in DR3 and DQ0604 mice, as sera depleted of anti-SmD antibodies were reactive with these proteins. DR3 mice also generated an immune response to C-RNP. Anti-nuclear antibodies were detected in the majority of the DR3 mice, while moderate reactivities were seen in DQ0604 and DQ8 mice. Interestingly, only DR3 mice mounted an anti-dsDNA antibody response. About half of the anti-dsDNA antibodies were cross-reactive with SmD. Antibody responses correlated with the strength of the T cell responses. Thus, HLA-DR3 appears to be the dominant HLA-D gene that determines the magnitude and quality of the anti-SmD immune response. In addition, our findings provide insights into the origin of the anti-dsDNA antibodies often detected in SLE patients.
Introduction
Systemic lupus erythematosus (SLE) is a multi-systemic disorder with protean clinical presentations. The disease is characterized by the presence of autoantibodies with diverse specificities. Among the autoantibodies, anti-Sm antibodies have been considered more specific for SLE (1). Recent evidence suggests that the generation of these lupus related autoantibodies is antigen-driven and depends on T cell responses to these antigens. This conclusion is further supported by the genetic finding that HLA-DR2 and HLA-DR3 are the major susceptibility genes in the pathogenesis of SLE (2–4). In addition, a study from multiplex families has shown that responses of anti-Sm antibodies are linked to HLA-DR3 homozygosity (2). Thus it is of interest to study the role of HLA-DR3 in the generation of anti-Sm antibodies.
Although many studies have been reported regarding levels of various autoantibodies in SLE patients and their relationship to the HLA complex (5), it is difficult to design a study to determine the roles of a specific HLA-D gene in either normals or in patients. This difficulty is applicable to other autoimmune disorders. To circumvent this difficulty, humanized mice, which express human HLA Class II antigens, have been used. These transgenic mice have been very informative as animal models for human autoimmune diseases (6, 7). In addition, mapping T cell epitopes of many autoantigens has been accomplished using these mice. Some examples are the mapping of T cell epitopes of collagen in collagen induced arthritis (8), preproinsulin and proinsulin in diabetes mellitus (9), proteolipid protein in experimental autoimmune encephalitis (10), retinal soluble antigen in experimental autoimmune uveitis (11), Ro60 (12) and La (13) in SLE.
In this investigation, several HLA-D transgenic mouse strains were used to study the role of HLA-D antigens in immune responses to SmD following immunization with recombinant SmD molecule. The data supports the conclusion that DR3 is the dominant gene in determining the magnitude and diversity of the response to SmD. In addition, the anti-SmD response may initiate the production of the anti-dsDNA antibodies, an autoantibody specificity that is thought to be of clinical significance.
Materials and Methods
Synthetic Peptides and Recombinant SmD1 Protein
A set of synthetic overlapping peptides covering the whole SmD protein (1–119) was obtained from the Biomolecular Research Core facility of the University of Virginia. The peptides were 15 amino acids long with an overlap of 12 amino acids over the previous peptide. Although the length of the peptides could have been in the range of 12–20 amino acids, the choice of the 15mers was made on the basis that 15mers in general give optimal binding to Class II molecules and TCR. This was confirmed using MHC class II binding algorithm (http://www.syfpeithi.de), wherein the core nonamer sequence is flanked by 3 N-terminal amino acids and 3 C-terminal residues. Cloning, expression and purification of 6× His-tagged recombinant SmD protein has been described before (14).
Mice and Immunizations
All experiments performed on mice were approved by the Institutional Animal Care and Use Committee. The following HLA transgenic mice were used in this study: Aβ0DR3 (DRB1*0301), Aβ0DQ0601 (DQA1*0103/DQB1*0601), Aβ0DQ0604 (DQA1*0103/DQB1*0604), Aβ0DQ8 (DQA1*0301/DQB1*0302), and Aβ0DR4 (DRB1*0401). The generation and characterization of these mouse strains has been described previously (15–18). These transgenic lines express mouse CD4 and endogenous Eα and are on B10 background. For performing in vitro lymph node cell (LNC) proliferation assays mice were immunized with 100 µg of purified recombinant SmD protein emulsified in Complete Freund’s Adjuvant (CFA) in one foot pad and the base of the tail. For antibody analysis, mice were immunized similarly with SmD and followed by additional injections on days 14 and 28 with 50µg of SmD protein emulsified in Incomplete Freund’s Adjuvant (IFA) by intraperitoneal route. Control mice were injected with only adjuvants. Mice were bled at different time points and sera stored at 20°C until use. Unless mentioned otherwise, data in this manuscript is from mice at 90 days after the first injection.
Antibody Analysis
Mouse sera were characterized for reactivity to SmD protein and SmD peptides by ELISA as described before (19). Reactivity to different cellular proteins was determined by Western blotting, using mouse WEHI7.1 cell extract as described previously (19). Reactivity to dsDNA was determined in ELISA, employing plasmid DNA as substrate by a method described previously (20). To determine antibody cross-reactivity, sera were absorbed with SmD coupled sepharose beads as previously described (14). Ability of absorbed sera to react with SmD was determined by immunoprecipitation assay using 35S-labeled SmD by a previously described method (19). Presence of ANA in immune sera (1:50 dilution) was determined by indirect immunofluorescence using methanol-fixed HeLa and 3T3 cell lines as substrates (21). Presence of antibodies reactive with A-RNP, SmB and SmD in immune sera was analyzed by immunoprecipitation assay as described previously (14). In the experiments presented in figure 1, Student’s t-test was performed and a p<0.05 was considered to be significant.
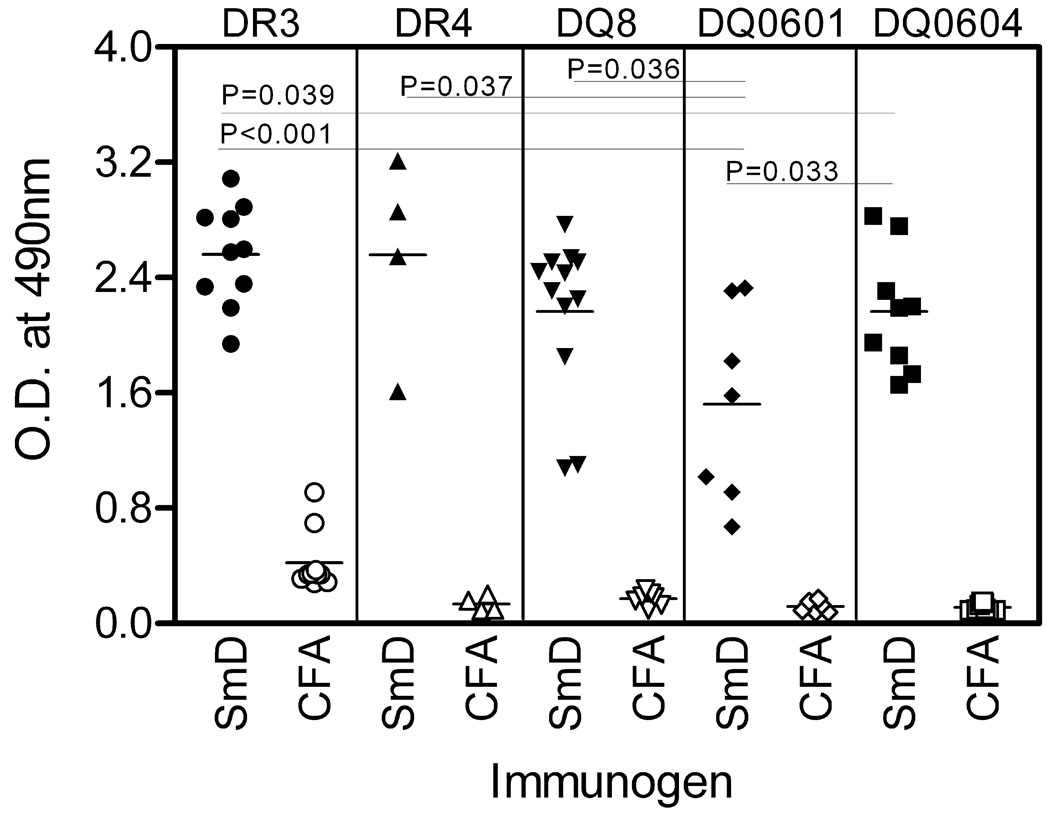
Figure 1. HLA-DR and HLA–DQ transgenic mice immunized with SmD protein generate a robust antibody response against the immunogen.
Reactivity of sera with SmD was analyzed in ELISA. Filled symbols represent individual serum samples from transgenic mice immunized with SmD. Open symbols represent sera from mice immunized only with adjuvants. Data is shown for sera obtained 90 days after immunization, at 1:300 dilution and is represented as mean duplicate O.D. at 490nm. To determine statistical significance, Student’s t-test was performed and a p<0.05 considered significant. Note mean O.D values in all strains of mice are significantly higher than the HLA-DQ0601 mice.
LNC proliferation assay
T cell epitopes on SmD were mapped by using LNC proliferation and [3H]thymidine incorporation assay as described previously (22). Briefly, draining LNCs from mice immunized either with SmD/CFA or with CFA alone, were cultured in presence of synthetic peptides and SmD protein for 4 days. During the final 16 h of culture, plates were pulsed with [3H]thymidine. Cells were harvested and incorporated radioactivity measured using a beta plate counter. The results are expressed as stimulation index, which is calculated as a ratio of mean triplicate CPM with peptide to mean triplicate CPM without peptide. A SI>2 was considered as positive response. This cutoff was based on our previous T cell epitope mapping studies for lupus-associated autoantigen Ro60 (12, 22).
Results
Anti-SmD Antibody Responses by HLA-D Transgenic Mice
Aβ0DR3, Aβ0DR4, Aβ0DQ0601, Aβ0DQ0604, Aβ0DQ8 mice on B10 background responded to immunization with recombinant SmD1 (SmD). As shown in Figure 1, at 90 days after the initial immunization, all strains generated a good antibody response to SmD as analyzed in ELISA with SmD as the substrate. Comparison amongst different strains showed that DQ0601 mice gave the lowest response. It is of note that sera from DR3 mice treated with Freund's adjuvants had some reactivity to SmD in this assay.
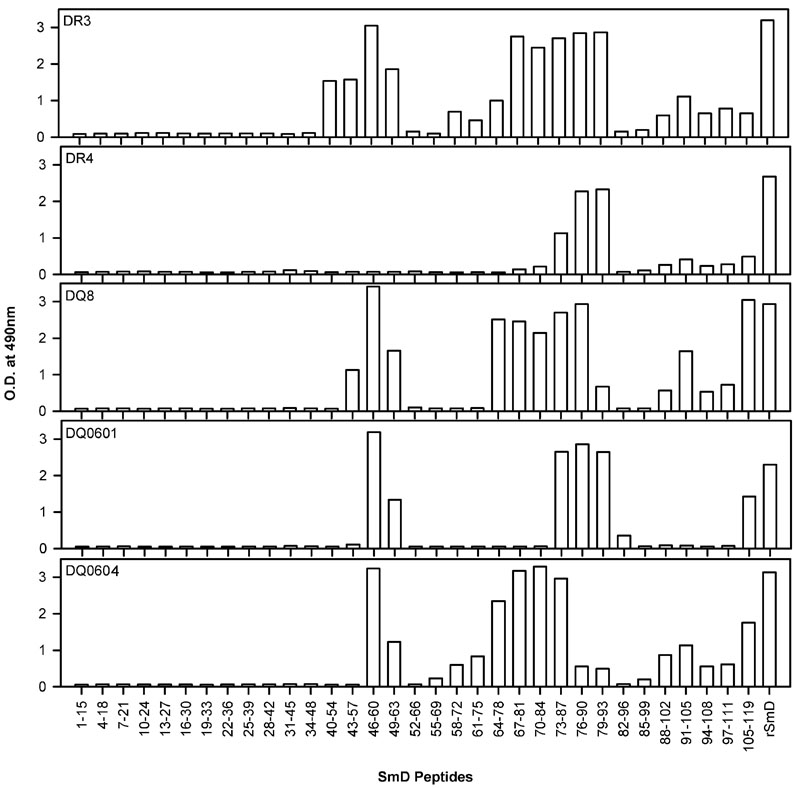
A panel of overlapping peptides covering the whole SmD molecule was used to determine the heterogeneity of the immune responses by these five HLA-D transgenic strains of mice. As shown in Figure 2, pooled anti-SmD sera from DR3 mice recognized the greatest number of peptides with the strongest reactions. The order of reactivities of the pooled sera from each of the strains were DR3>DQ8>DQ0604>DQ0601>DR4. Thus the numbers of B cell epitopes recognized by these transgenic lines vary significantly from one strain to another. These results were confirmed with another cohort of mice.
Figure 2. SmD B cell epitope specificities are different between the HLA-DR3 and HLA-DQ transgenic mice.
Sera from mice immunized with SmD were pooled and their reactivity with overlapping peptides of SmD was analyzed in ELISA. Data is shown for sera obtained 90 days post immunizations and were used at 1:100 dilution. Sera from HLA-DR3 transgenic mice recognized more SmD peptides than any other group of mice.
Autoantibody Diversification in HLA-D Transgenic Mice Immunized with SmD
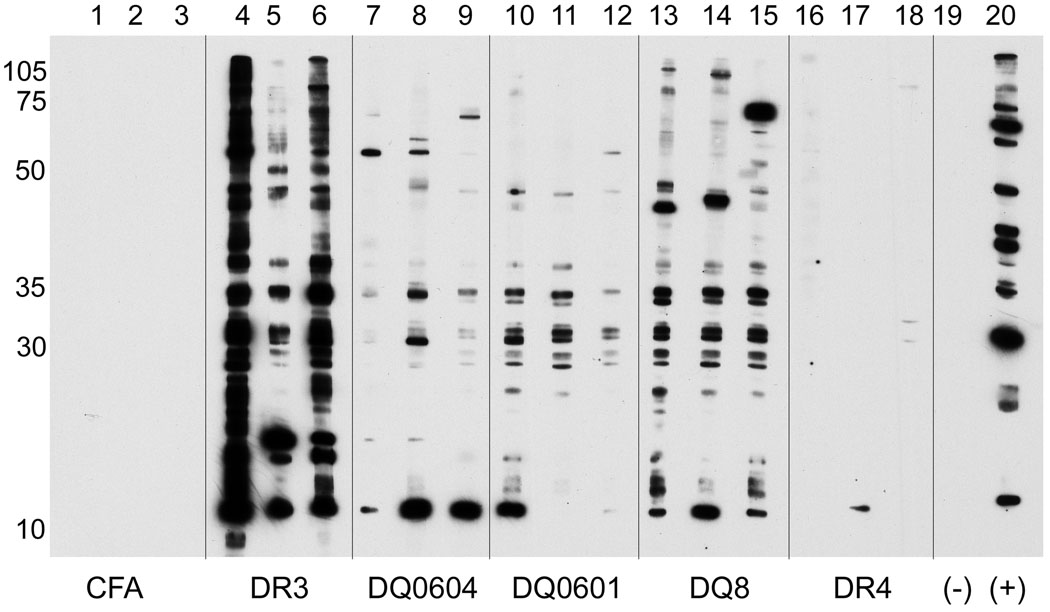
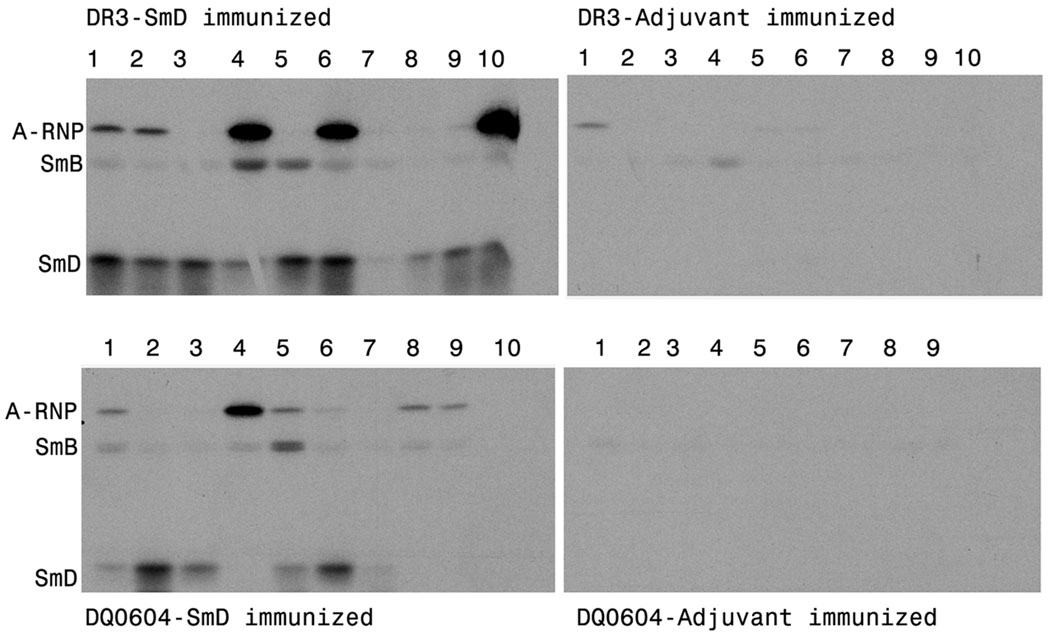
Sera at 1:100 dilutions from SmD immunized HLA-D transgenic mice (90 days) were analyzed for their reactivities against cellular antigens in the cellular extract of WEHI 7.1 by Western blot analysis. The reactivities of three of each congenic strain are shown in Figure 3. DR3 transgenic mice had the strongest reaction to various cellular constituents as evident by the very strong bands revealed by two of the three sera (Figure 3, lanes 4–6). The three immune sera from the DQ8 mice reacted with multiple bands as well (Figure 3 lanes 13–15). However the intensities were less than those seen with the DR3 immune sera. The immune sera from DQ0604 and DQ0601 were weaker than those of DR3 and DQ8 mice. Although the immune sera of DR4 mice had significant reactivity against SmD by ELISA, they hardly recognized any bands in Western blot analysis. None of the control sera from mice treated with Freund’s adjuvants recognized any bands (Figure 3, lanes 1–3).
Figure 3. Diversification of autoantibody responses in HLA-D transgenic mice immunized with SmD.
Reactivity of sera with different cellular proteins was analyzed by Western blotting using WEHI 7.1 cell extract. All sera were tested at 1:100 dilution. The figure shows reactivity of representative serum samples from different transgenic mice. As control, only reactivity of sera from CFA/IFA immunized DR3 mice are shown. Note that sera from HLA-DR3 and HLA-DQ8 mice immunized with SmD recognize the most number of cellular proteins.
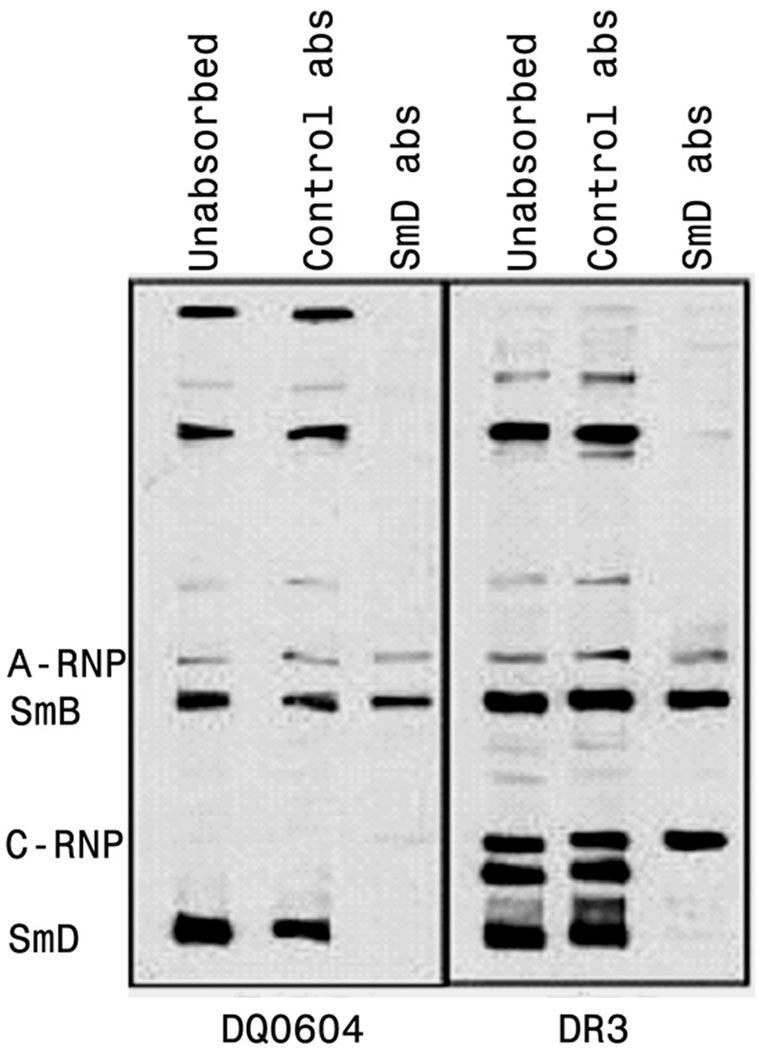
Autoantibody diversification was further documented by immunoabsorption and immunoprecipitation. For immunoabsorption, pooled immune sera were used. After absorption with SmD linked to Sepharose beads, only sera from DR3 and DQ0604 mice retain reactivities to several bands by Western blot analysis. The results are shown in Figure 4. In the case of DQ0604, the absorbed sera reacted with SmB and A-RNP (the left panel of Figure 4). The absorption abolished the sera reactivity to SmD and several bands of higher molecular weights. The DR3 sera were more reactive than the DQ0604 sera in that they recognized many more bands. The absorbed sera recognized SmB and A-RNP. In addition, they recognized a molecule with the mobility similar to that of C-RNP. This reactivity appears to be found only in immunized HLA-DR3 mice. The pattern of epitope spreading in response to SmD immunization is in accordance to that observed in patients’ sera and to A/J mice response to SmD immunization (14).
Figure 4. Intermolecular epitope spreading to other proteins within the snRNP complex occurs in HLA-D transgenic mice immunized with SmD.
Pooled sera from HLA-DQ0604 and HLA-DR3 mice immunized with SmD were absorbed with either SmD-coupled sepharose beads or equivalent amount of control beads. Reactivity of unabsorbed and absorbed sera with different proteins was analyzed by Western blotting. Absorption with SmD-beads completely depleted antibodies reactive with SmD and some additional proteins. However, in DQ0604 mice, reactivity to A-RNP and SmB; and in DR3 mice reactivity to A-RNP, SmB and C-RNP persisted, which is indicative of epitope spreading.
For immunoprecipitation, only pooled immune sera from HLA-DR3 and DQ0604 transgenics were positive. Thus individual immune sera from HLA-DR3 and DQ0604 mice were used (figure 5). Many of the immune sera precipitated labeled SmB and A-RNP in addition to SmD. In general, the immune sera from DR3 mice had stronger reactivity to these three proteins. It is of note that one of the DQ0604 sera had no reactivity and three of them were able to precipitate SmD and/or A-RNP without apparent reactivity to SmD. This is reminiscent of the findings when SmD peptides were used as immunogens (19).
Figure 5. Sera from HLA-DR3 and HLA-DQ0604 transgenic mice immunoprecipitate A-RNP, SmB and SmD.
To confirm the reactivity of sera with proteins within the snRNP complex, sera were used to immunoprecipitate 35S labeled-A-RNP, -SmB and SmD. Sera (50µl) obtained 3 months post immunization were used to immunoprecipitate the in vitro transcribed and translated proteins. The figure shows a representative autoradiograph.
Strong ANA and anti-dsDNA Antibody Reactivity Detected in Sera from SmD Immunized DR3 Mice
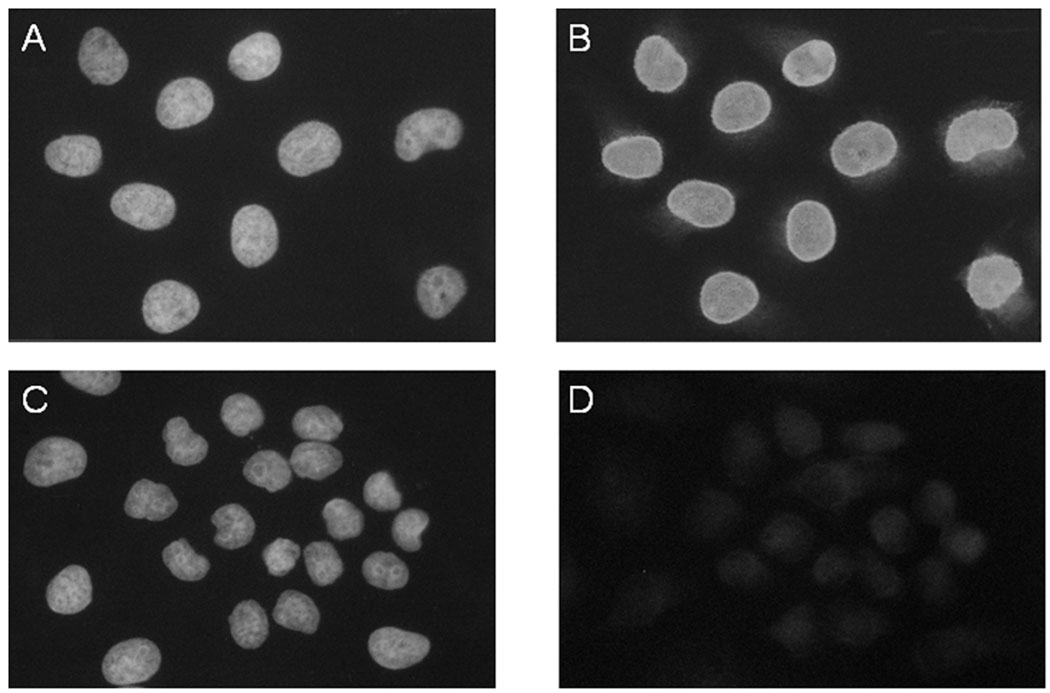
As further evidence for autoantibody diversification, some of the SmD immune sera from DR3, DQ0604 and DQ8 stained nuclei of HeLa (Figure 6) and 3T3 cell lines. For DR3, six of the ten immune sera were strongly positive for ANA. This strong reactivity was also detected in three of the nine immune sera from DQ0604 and three out of twelve DQ8 immune sera. One of the seven SmD immune sera from DQ0601 was weakly positive. No DR4 immune sera were positive in this assay. With two exceptions, none of the sera from Freund’s adjuvant immunized mice stained the nuclei. The two reactive sera were from DR3 mice and were positive without any treatment at the initiation of the experiment.
Figure 6. Anti-nuclear antibodies are generated in HLA-DR3 transgenic mice immunized with SmD.
Reactivity of representative serum samples from HLA-DR3 transgenic mice, either immunized with SmD (A and B) or only with adjuvants (C and D) is shown. Panels A and C, show nuclei stained with DAPI. Panels B shows the presence of ANA and panel D the absence of ANA using indirect immunofluorescence for antibody detection. The sera were used at 1:50 dilution.
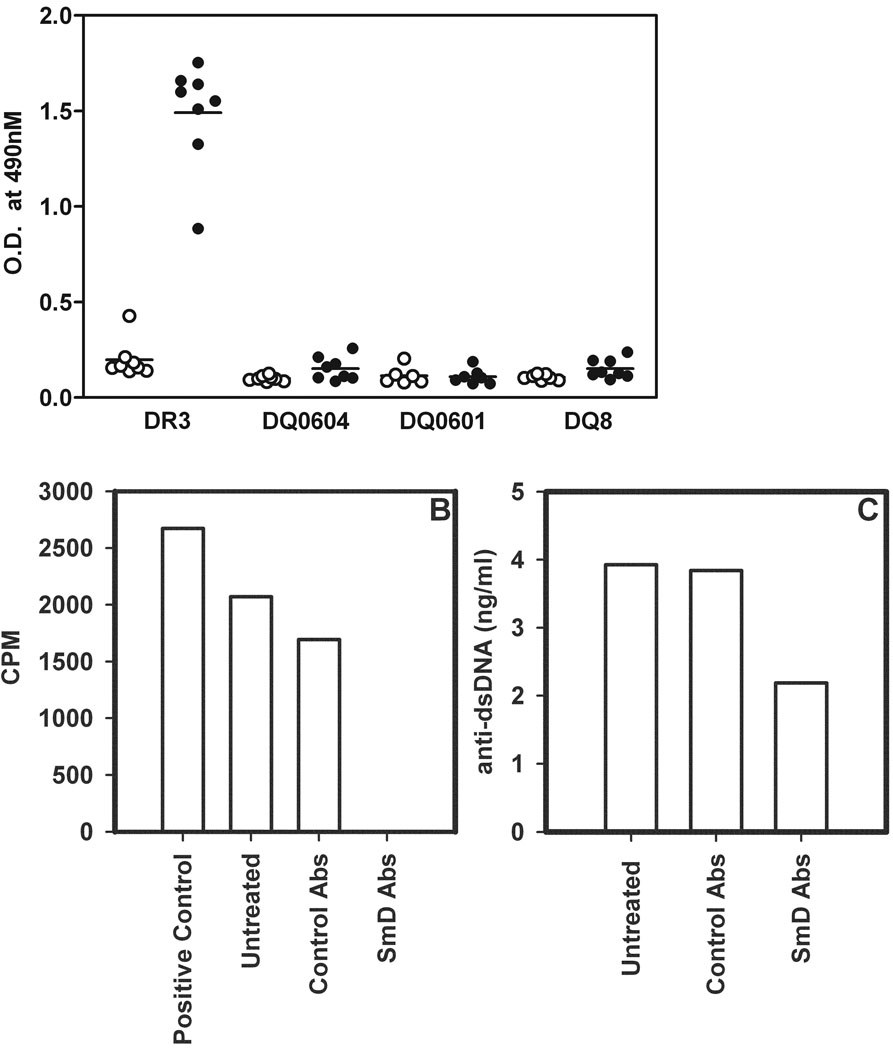
The sera from HLA-D transgenic mice immunized with SmD were assayed for anti-dsDNA antibodies by ELISA with circular plasmid DNA as the substrate. As shown in Figure 7A, DR3 immune sera were found to be strongly reactive with dsDNA while other immune sera and the vast majority of the Freund’s adjuvant immunized sera showed little such reactivity.
Figure 7. Only HLA-DR3 transgenic mice immunized with SmD generate anti-dsDNA reactive antibodies.
A) Sera from different HLA-D transgenic mice, obtained 90 days post immunization were tested for reactivity to dsDNA. Filled symbols represent sera from SmD immunized mice and open symbols represent sera from adjuvant immunized mice. B) To determine whether anti-dsDNA antibodies were cross-reactive with SmD, pooled sera from DR3 transgenic mice were absorbed with SmD-coupled beads. Absorbed sera were used to immunoprecipitate 35S-labeled SmD. The precipitated radioactivity was measured by scintillation counting and data are represented as CPM. Note all antibodies reactive with SmD were depleted following absorption. C) SmD absorbed sera were tested for their reactivity to dsDNA in ELISA. Data are represented as amount of anti-dsDNA antibody ng/ml and was calculated using a purified anti-dsDNA monoclonal antibody as standard. Note almost 50% of anti-dsDNA antibody reactivity is reduced following absorption with SmD. Control absorption (abs) was carried out with untreated Sepharose beads.
Depletion of Anti-dsDNA Antibodies in DR3 Immune Sera with SmD
Pooled DR3 immune sera were absorbed with solid phase SmD. As shown in figure 7B, anti-SmD antibodies were absorbed completely with the solid absorbent. The absorbed sera remained active against dsDNA (figure 7C). About half of the antibodies were absorbed by solid phase SmD, indicating that about half of the anti-dsDNA was cross-reactive with SmD, the immunogen.
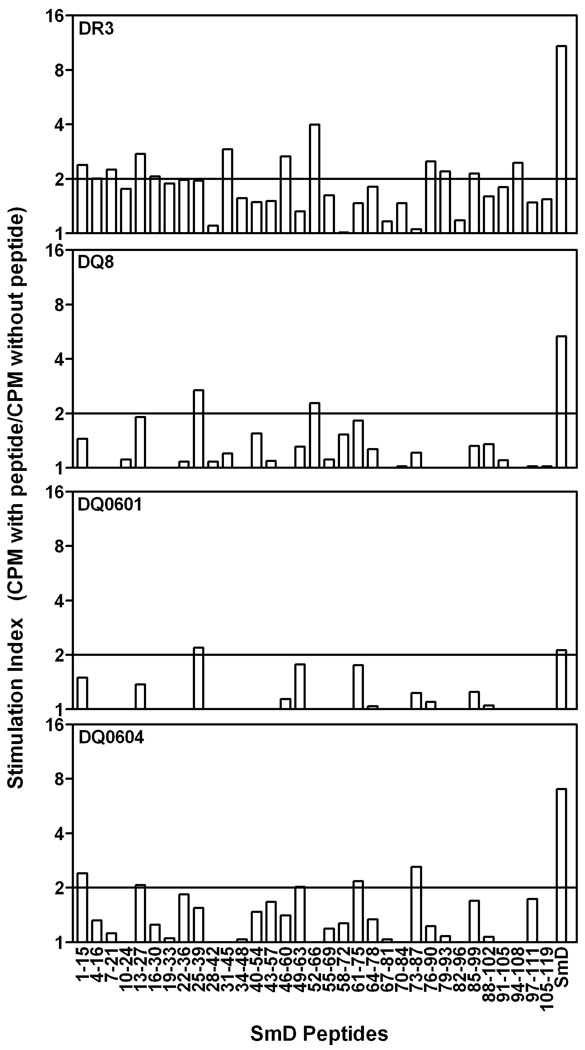
Strong Anti-SmD T cell responses in DR3 mice
As shown in Figure 8, DR3 mice mounted the strongest T cell proliferative response to SmD immunization. A SI>2.0 was considered as positive and was based on our previous mapping studies for Ro60 done in HLA-D transgenic (12) as well as non-transgenic A/J and SJL/J strains of mice (22). DR0604 and DQ8 mice mounted a moderate response and DQ0601 mice mounted a very weak response. DR4 transgenic mice had no discernable T cell response when they were immunized with SmD in a similar manner. Regarding T cell epitopes on SmD recognized by these HLA-D transgenic mice, more epitopes were recognized by DR3 mice. More limited numbers of such epitopes were recognized by DQ0604, DQ8 and DQ0601. It appears that the strengths of the B cell responses to SmD immunization correlate with the strengths of T cell responses of the HLA-D transgenic lines.
Figure 8. HLA-DR3 mice recognize more T cell epitopes on SmD protein.
Different HLA-D transgenic mice (3–5 per group) were immunized with SmD in the foot pad and the base of the tail. On day 12, draining lymph node cells were used to set up a proliferation assay. Synthetic peptides were used at a final concentration of 10µm and reactivity to SmD is shown at a concentration of 10µg/ml. Data are represented as stimulation index, which is ratio of mean triplicate CPM with peptide to mean triplicate CPM without peptide. A S.I.>2.0 was considered positive and is indicated by a solid line. Similar results were obtained in an additional cohort of mice.
Discussion
In the present investigation, several strains of mice expressing different HLA-D antigens were used to explore their responsiveness to immunization with SmD. The DR3 transgenic mice responded with high titers in the SmD ELISA assay and their immune sera recognized the largest number of SmD peptides. In addition the immune sera contained antibodies with the most diverse specificities as revealed by Western blot analysis, and by the highest frequency of ANA. More importantly, anti-dsDNA autoantibodies were found only in the immune sera of the DR3 mice. T cells from immunized DR3 mice responded best in vitro in a recall proliferative assay. These T cells also responded to many more SmD peptides in comparison with those from other HLA-D transgenic strains. These results indicate that DR3 is the dominant gene determining the quantity and the quality of the response. They are also in congruence with the observation that HLA-DR3 is one of the major and dominant lupus susceptibility genes. Considering that mouse and human SmD have identical protein sequences, the results in this investigation support the thesis that the DR3 transgenic mouse is an excellent model for studying the pathogenesis of SLE.
In this investigation, only HLA-DR3 and HLA-DR4 transgenic mice were compared with three HLA-DQ transgenic strains. Although only two HLA-DR strains were used, significant information has been generated. HLA-DR3 and HLA-DR4 represent two very important alleles, in that HLA-DR3 is linked to SLE (3, 4) while HLA-DR4 is linked to rheumatoid arthritis (23). These two autoimmune disorders are the most prevalent rheumatic diseases with distinct serological markers. Thus the findings that only the HLA-DR3 mouse responds to recombinant SmD immunization with diverse autoantibody production and that HLA-DR4 transgenic mouse fails to do so despite its ability to generate autoantibodies to the immunogen, adds further credence to the importance of HLA-DR3 in the initiation of lupus related autoantibodies. In comparing the responsiveness of the HLA-DR3 transgenic mouse to that of the DQ transgenic mice and the previous findings of HLA-D transgenic mouse responsiveness to Ro60 (12), it may be reasonable to conclude that HLA-DQ molecule may play a secondary role in the generation of lupus related autoantibodies.
It is highly significant that HLA-DR3 and HLA-DQ0604 mice respond well to the immunization with SmD. We have previously demonstrated that mice with B6 and BALB/C background do not respond to immunization with recombinant lupus related autoantigens such as SmD (24). In contrast, SJL and A/J mice that have some autoimmune tendencies respond well with significant autoantibody diversification (24). It may be inferred from this observation that T cell epitopes presented in the context of HLA-DR3 and DQ0604 deliver a significant and strong activation signal to autoreactive T cells resulting in the generation of autoantibodies with diverse specificities. In addition, the detection of autoantibodies to the C-RNP protein in HLA-DR3 but not in HLA-DQ0604 mice suggests that HLA-DR3/SmD peptide complexes provide a stronger signal. The patterns of autoantibody diversification in the HLA-DR3 and HLA-DQ0604 transgenic mice are very similar to those observed in A/J mice (19) and in patients with SLE (2). It was also observed in this study that some of the mice despite epitope spreading to other proteins within the snRNP particle showed little response to SmD, the immunogen. This pattern of spreading was demonstrated by our previous study in A/J mice immunized with a SmD peptide, leading to the thesis that serological analysis of autoimmune sera at a given time point should not be used as evidence to suggest the nature of the initiation antigens (19). Thus the absence of anti-SmD antibodies in lupus patients’ sera with autoantibodies to SmB and/ or A-RNP does not imply that immune response to SmD is not occurring.
Several techniques were used in this investigation to document autoantibody diversification. It is of note that DR4 immune sera were found to be strongly reactive with SmD in ELISA although they did not have detectable amounts of antibodies to various autoantigens by Western blot analysis, immunoprecipitation and immunofluorescence. This pattern is very similar to the observation that ELISA assays as clinical tests for the presence of antibodies diagnostic of SLE and other autoimmune disorders are nonspecific and are of little clinical application (25). Our results add further support to the thesis that the current trend to replace the traditional immunoprecipitation methods with ELISAs using recombinant proteins should be evaluated.
Anti-dsDNA antibodies are considered an important class of autoantibodies in SLE (26–28). These antibodies are more specific for SLE. They often correlate with disease activities. Despite intense investigations, the origin of these antibodies remains controversial. The consensus is that they cannot be readily induced with purified DNA as the immunogen (29). Putterman and Diamond showed induction of anti-dsDNA antibodies in normal mice through the immunization with a peptide mimic (of an anti-dsDNA monoclonal antibody) coupled to T cell epitopes (30, 31). Riemekasten et al. (32, 33) reported that peptide SmD83–119 was able to provide T cell help for the production of anti-dsDNA antibodies. In this study, NZB/NZW F1 female mice were used. However these mice have significant numbers of plaque forming cells (PFC) with specificities for SmD or dsDNA. Therefore, in their system, the investigators dealt with augmentation of an on going response. The mechanisms for the augmentation may be multiple and were not studied further. Thus induction of anti-dsDNA antibody production following immunization with lupus autoantigens needs to be investigated in detail.
The finding herein that DR3 transgenic mice are able, as a part of response to SmD immunization to produce anti-dsDNA antibodies, some of which are cross-reactive with SmD is of considerable interest. This phenomenon is both specific for DR3 and antigen dependent. In the present investigation, it is shown that DR4, DQ0601, DQ0604 and DQ8 mice did not produce such anti-dsDNA antibodies. In the case of DQ0604 and DQ8 mice, they produced ANA without the production of anti-dsDNA antibodies. Although DR2 mice were not included in this study, they might well be able to produce anti-dsDNA antibodies as a response to SmD immunization. Our results support the thesis that the observed immune response is dependent on a specific DR gene. In a previous study, Paisansinsup et al (12) reported that ANAs were detected in all DR2, DR3 and DQ8 mice in response to recombinant human Ro60. None of them made anti-dsDNA antibodies. It is also important to stress that both the recombinant Ro60 in previous studies and the recombinant SmD in this study have the 6×-His tag. The fact that anti-DNA antibodies are only seen in HLA-DR3 transgenic mice immunized with SmD indicates that the antibodies cross-reactive with SmD and dsDNA are not likely to be due to an immune response to the 6×-His tag. The observation that no DNA was detected in the recombinant SmD suggests that the cross-reactivity is not due to the presence of dsDNA in the SmD. These observations together with the findings presented here support the thesis that the anti-dsDNA response is specific for SmD as the immunogen. The absorption experiments showing that about half of the anti-dsDNA antibodies could be absorbed by solid phase SmD requires further comments. This is reminiscent of our previous finding that a significant amount of anti-SmB and anti-A-RNP antibodies were cross-reactive with the immunogen SmD (19). The specific anti-SmB and anti-A-RNP antibodies were generated as the antigen specific T cells were expanded during the immune response (34). In this regard, it would be of interest to determine whether some of the induced anti-dsDNA antibodies are cross-reactive with SmB, A-RNP or C-RNP to which the autoimmune response to SmD are diversified. It would also be of interest to determine if anti-dsDNA antibody response is a part of the immune responses to SmB or A-RNP immunization. At the clinical level, it is of interest to note that anti-dsDNA antibodies are often found in patients with anti-Sm antibodies. Our present finding may also explain this clinical observation.
From the above discussion, it appears that anti-dsDNA antibodies are produced in a pattern very similar to certain autoantibodies as a part of diversification of the immune response to SmD immunization. This provides evidence to support the hypothesis that anti-dsDNA is generated resulting from immune responses to protein autoantigens. This observation is also congruent with the previous observations that in MRL/lpr mice the monoclonal antibodies against SmD antigen are often cross-reactive with dsDNA (35), and that antibodies to snRNP, Ribosomal P protein and other lupus autoantigens are partly cross-reactive with dsDNA (36–38). Thus our data support the thesis that our antigen induced model for anti-dsDNA antibody production resembles that observed in both spontaneous lupus-prone mice and patients with SLE. It can also be speculated further that these antibodies can be a part of the host immune responses to pathogens or other environmental antigens. This hypothesis provides an explanation that anti-dsDNA antibodies are occasionally found in normal individuals without other lupus related autoantibodies and without any clinical manifestations of SLE. It is likely that further investigations to seek support for the proposed hypothesis will settle the issue regarding the origin of anti-dsDNA antibodies found in patients and normals.
Recently, it was demonstrated in our laboratory that microbial antigens can mimic SmD peptide to induce an anti-SmD response in DR3 mice (39). This observation bears testimony to the usefulness of the DR3 mice to probe the pathogenesis of SLE. The observation adds impetus to design research to support the hypothesis that molecular mimicry among environmental antigens and autoantigens play a major role in the initiation of autoantibody responses. Evidence in the literature suggests that autoantigens are required for autoantibody diversification (22) and that TLRs play a crucial role in the amplification of these responses (40). The availability of the HLA-D transgenic strains and well defined lupus models together with translational research will undoubtedly provide further insight to the pathogenesis of this disorder.
Abbreviations Used
- ANA
anti-nuclear antibody
- CPM
counts per minute
- dsDNA
double stranded DNA
- LNC
lymph node cell
- RNP
ribonucleoprotein
- SmD
Smith D
- SLE
systemic lupus erythematosus
- snRNP
small nuclear ribonucleoprotein
Footnotes
This study was supported by grants from National Institutes of Health: P50-AR045222, R01-AI043248, R01-AR049449 (S.M.F); K01-AR051391 (U.S.D); K01-DK063065 (H.B.); and R01-AR30752 (C.S.D.)
References
- 1.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 2.Ramos PS, Kelly JA, Gray-McGuire C, Bruner GR, Leiran AN, Meyer CM, Namjou B, Espe KJ, Ortmann WA, Reichlin M, Langefeld CD, James JA, Gaffney PM, Behrens TW, Harley JB, Moser KL. Familial aggregation and linkage analysis of autoantibody traits in pedigrees multiplex for systemic lupus erythematosus. Genes Immun. 2006;7:417–432. doi: 10.1038/sj.gene.6364316. [DOI] [PubMed] [Google Scholar]
- 3.Graham RR, Ortmann WA, Langefeld CD, Jawaheer D, Selby SA, Rodine PR, Baechler EC, Rohlf KE, Shark KB, Espe KJ, Green LE, Nair RP, Stuart PE, Elder JT, King RA, Moser KL, Gaffney PM, Bugawan TL, Erlich HA, Rich SS, Gregersen PK, Behrens TW. Visualizing human leukocyte antigen class II risk haplotypes in human systemic lupus erythematosus. Am. J. Hum. Genet. 2002;71:543–553. doi: 10.1086/342290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham RR, Ortmann W, Rodine P, Espe K, Langefeld C, Lange E, Williams A, Beck S, Kyogoku C, Moser K, Gaffney P, Gregersen PK, Criswell LA, Harley JB, Behrens TW. Specific combinations of HLA-DR2 and DR3 class II haplotypes contribute graded risk for disease susceptibility and autoantibodies in human SLE. Eur. J. Hum. Genet. 2007;15:823–830. doi: 10.1038/sj.ejhg.5201827. [DOI] [PubMed] [Google Scholar]
- 5.Reveille JD. The genetic basis of autoantibody production. Autoimmun. Rev. 2006;5:389–398. doi: 10.1016/j.autrev.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Gregersen JW, Holmes S, Fugger L. Humanized animal models for autoimmune diseases. Tissue Antigens. 2004;63:383–394. doi: 10.1111/j.0001-2815.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 7.Mangalam AK, Rajagopalan G, Taneja V, David CS. HLA class II transgenic mice mimic human inflammatory diseases. Adv. Immunol. 2008;97:65–147. doi: 10.1016/S0065-2776(08)00002-3. [DOI] [PubMed] [Google Scholar]
- 8.Krco CJ, Watanabe S, Harders J, Griffths MM, Luthra H, David CS. Identification of T cell determinants on human type II collagen recognized by HLA-DQ8 and HLA-DQ6 transgenic mice. J. Immunol. 1999;163:1661–1665. [PubMed] [Google Scholar]
- 9.Congia M, Patel S, Cope AP, De Virgiliis S, Sonderstrup G. T cell epitopes of insulin defined in HLA-DR4 transgenic mice are derived from preproinsulin and proinsulin. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3833–3838. doi: 10.1073/pnas.95.7.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangalam AK, Khare M, Krco C, Rodriguez M, David C. Identification of T cell epitopes on human proteolipid protein and induction of experimental autoimmune encephalomyelitis in HLA class II-transgenic mice. Eur. J. Immunol. 2004;34:280–290. doi: 10.1002/eji.200324597. [DOI] [PubMed] [Google Scholar]
- 11.Pennesi G, Mattapallil MJ, Sun SH, Avichezer D, Silver PB, Karabekian Z, David CS, Hargrave PA, McDowell JH, Smith WC, Wiggert B, Donoso LA, Chan CC, Caspi RR. A humanized model of experimental autoimmune uveitis in HLA class II transgenic mice. J. Clin. Invest. 2003;111:1171–1180. doi: 10.1172/JCI15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paisansinsup T, Deshmukh US, Chowdhary VR, Luthra HS, Fu SM, David CS. HLA class II influences the immune response and antibody diversification to Ro60/Sjogren's syndrome-A: heightened antibody responses and epitope spreading in mice expressing HLA-DR molecules. J. Immunol. 2002;168:5876–5884. doi: 10.4049/jimmunol.168.11.5876. [DOI] [PubMed] [Google Scholar]
- 13.Dudek NL, Maier S, Chen ZJ, Mudd PA, Mannering SI, Jackson DC, Zeng W, Keech CL, Hamlin K, Pan ZJ, Davis-Schwarz K, Workman-Azbill J, Bachmann M, McCluskey J, Farris AD. T cell epitopes of the La/SSB autoantigen in humanized transgenic mice expressing the HLA class II haplotype DRB1*0301/DQB1*0201. Arthritis Rheum. 2007;56:3387–3398. doi: 10.1002/art.22870. [DOI] [PubMed] [Google Scholar]
- 14.Deshmukh US, Kannapell CC, Fu SM. Immune responses to small nuclear ribonucleoproteins: antigen-dependent distinct B cell epitope spreading patterns in mice immunized with recombinant polypeptides of small nuclear ribonucleoproteins. J. Immunol. 2002;168:5326–5332. doi: 10.4049/jimmunol.168.10.5326. [DOI] [PubMed] [Google Scholar]
- 15.Strauss G, Vignali DA, Schonrich G, Hammerling GJ. Negative and positive selection by HLA-DR3(DRw17) molecules in transgenic mice. Immunogenetics. 1994;40:104–108. [PubMed] [Google Scholar]
- 16.Bradley DS, Nabozny GH, Cheng S, Zhou P, Griffiths MM, Luthra HS, David CS. HLA-DQB1 polymorphism determines incidence, onset, and severity of collagen-induced arthritis in transgenic mice. Implications in human rheumatoid arthritis. J. Clin. Invest. 1997;100:2227–2234. doi: 10.1172/JCI119760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabozny GH, Baisch JM, Cheng S, Cosgrove D, Griffiths MM, Luthra HS, David CS. HLA-DQ8 transgenic mice are highly susceptible to collagen-induced arthritis: a novel model for human polyarthritis. J. Exp. Med. 1996;183:27–37. doi: 10.1084/jem.183.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan S, Trejo T, Hansen J, Smart M, David CS. HLA-DR4 (DRB1*0401) transgenic mice expressing an altered CD4-binding site: specificity and magnitude of DR4-restricted T cell response. J. Immunol. 1998;161:2925–2929. [PubMed] [Google Scholar]
- 19.Deshmukh US, Bagavant H, Sim D, Pidiyar V, Fu SM. A SmD peptide induces better antibody responses to other proteins within the small nuclear ribonucleoprotein complex than to SmD protein via intermolecular epitope spreading. J. Immunol. 2007;178:2565–2571. doi: 10.4049/jimmunol.178.4.2565. [DOI] [PubMed] [Google Scholar]
- 20.Bagavant H, Deshmukh US, Wang H, Ly T, Fu SM. Role for nephritogenic T cells in lupus glomerulonephritis: progression to renal failure is accompanied by T cell activation and expansion in regional lymph nodes. J. Immunol. 2006;177:8258–8265. doi: 10.4049/jimmunol.177.11.8258. [DOI] [PubMed] [Google Scholar]
- 21.Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SS, Kannapell CC, Tung KS, McEwen SB, McDuffie M. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin. Immunol. 2001;100:372–383. doi: 10.1006/clim.2001.5079. [DOI] [PubMed] [Google Scholar]
- 22.Deshmukh US, Lewis JE, Gaskin F, Kannapell CC, Waters ST, Lou YH, Tung KS, Fu SM. Immune responses to Ro60 and its peptides in mice. I. The nature of the immunogen and endogenous autoantigen determine the specificities of the induced autoantibodies. J. Exp. Med. 1999;189:531–540. doi: 10.1084/jem.189.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner JH, Nepom GT. Genetics of rheumatoid arthritis: is there a scientific explanation for the human leukocyte antigen association? Curr. Opin. Rheumatol. 2002;14:254–259. doi: 10.1097/00002281-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Deshmukh US, Bagavant H, Davis S, Fu SM. Intermolecular epitope spreading within the small nuclear ribonucleoprotein complex is regulated by multiple factors. J Immunol. 2006;176:S156–S156. doi: 10.4049/jimmunol.178.4.2565. [DOI] [PubMed] [Google Scholar]
- 25.Tubb A, Ambrocio DU, Fu SM. ANA and other serological tests by enzyme-linked immunoabsorbant assays (ELISA) as screening and diagnostic tools for systemic autoimmune disorders by primary care physicians (PCP) prior to making rheumatology referrals: Lack of precision and usefulness. Arthritis Rheum. 2007;56:4294–4295. [Google Scholar]
- 26.Tan EM, Schur PH, Carr RI, Kunkel HG. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J. Clin. Invest. 1966;45:1732–1740. doi: 10.1172/JCI105479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn BH. Antibodies to DNA. N. Engl. J. Med. 1998;338:1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 28.Munoz LE, Gaipl US, Herrmann M. Predictive value of anti-dsDNA autoantibodies: importance of the assay. Autoimmun. Rev. 2008;7:594–597. doi: 10.1016/j.autrev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann M, Zoller OM, Hagenhofer M, Voll R, Kalden JR. What triggers anti-dsDNA antibodies? Mol. Biol. Rep. 1996;23:265–267. doi: 10.1007/BF00351179. [DOI] [PubMed] [Google Scholar]
- 30.Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J. Exp. Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalil M, Inaba K, Steinman R, Ravetch J, Diamond B. T cell studies in a peptide-induced model of systemic lupus erythematosus. J. Immunol. 2001;166:1667–1674. doi: 10.4049/jimmunol.166.3.1667. [DOI] [PubMed] [Google Scholar]
- 32.Riemekasten G, Kawald A, Weiss C, Meine A, Marell J, Klein R, Hocher B, Meisel C, Hausdorf G, Manz R, Kamradt T, Burmester GR, Hiepe F. Strong acceleration of murine lupus by injection of the SmD1(83–119) peptide. Arthritis Rheum. 2001;44:2435–2445. doi: 10.1002/1529-0131(200110)44:10<2435::aid-art408>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Riemekasten G, Langnickel D, Ebling FM, Karpouzas G, Kalsi J, Herberth G, Tsao BP, Henklein P, Langer S, Burmester GR, Radbruch A, Hiepe F, Hahn BH. Identification and characterization of SmD183-119-reactive T cells that provide T cell help for pathogenic anti-double-stranded DNA antibodies. Arthritis Rheum. 2003;48:475–485. doi: 10.1002/art.10762. [DOI] [PubMed] [Google Scholar]
- 34.Deshmukh US, Gaskin F, Lewis JE, Kannapell CC, Fu SM. Mechanisms of autoantibody diversification to SLE-related autoantigens. Ann. N. Y. Acad. Sci. 2003;987:91–98. doi: 10.1111/j.1749-6632.2003.tb06036.x. [DOI] [PubMed] [Google Scholar]
- 35.Retter MW, Cohen PL, Eisenberg RA, Clarke SH. Both Sm and DNA are selecting antigens in the anti-Sm B cell response in autoimmune MRL/lpr mice. J. Immunol. 1996;156:1296–1306. [PubMed] [Google Scholar]
- 36.Reichlin M, Martin A, Taylor-Albert E, Tsuzaka K, Zhang W, Reichlin MW, Koren E, Ebling FM, Tsao B, Hahn BH. Lupus autoantibodies to native DNA cross-react with the A and D SnRNP polypeptides. J. Clin. Invest. 1994;93:443–449. doi: 10.1172/JCI116980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koren E, Koscec M, Wolfson-Reichlin M, Ebling FM, Tsao B, Hahn BH, Reichlin M. Murine and human antibodies to native DNA that cross-react with the A and D SnRNP polypeptides cause direct injury of cultured kidney cells. J. Immunol. 1995;154:4857–4864. [PubMed] [Google Scholar]
- 38.Gerli R, Caponi L. Anti-ribosomal P protein antibodies. Autoimmunity. 2005;38:85–92. doi: 10.1080/08916930400022699. [DOI] [PubMed] [Google Scholar]
- 39.Deshmukh US, Sim D, Rajagopalan G, David C, Gaskin F, Fu SM. HLA-DR3 restricted T cell epitope mimics of a lupus-associated autoantigen can initiate autoimmune responses. Arthritis and Rheum. 2008;58:S872–S872. [Google Scholar]
- 40.Kim WU, Sreih A, Bucala R. Toll-like receptors in systemic lupus erythematosus; prospects for therapeutic intervention. Autoimmun. Rev. 2009;8:204–208. doi: 10.1016/j.autrev.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]