Abstract
Background:
Antenatal Clinic-based surveillance data suggests stabilizing HIV levels in Tanzania. Data from an open demographic surveillance cohort in Northern Tanzania provide robust estimates of prevalence and incidence trends. These can help us to interpret results from national HIV surveillance.
Methods:
The Kisesa open cohort study conducted 19 rounds of household based demographic surveillance and 4 rounds of individually linked HIV serological surveys between 1994 and 2004. HIV testing was anonymous, based on informed consent without result disclosure. The effect of selective participation in sero-surveys on prevalence and incidence estimates is investigated, using longitudinal knowledge of individuals' testing histories. In addition, incidence estimates make allowance for interval censoring using a multiple imputation procedure for sero-conversion dates.
Results:
16,820 adults were interviewed and donated blood specimens for HIV testing in at least one of the four serological surveys. HIV prevalence increased steadily from 6.0% in 1994/95 to 8.3% in 2000/01, levelling out thereafter. HIV incidence increased sharply between the first and second intervals, (from 0.8% in 1994-97 to 1.2% per thousand in 1997-2000) remaining at a high level (1.1%) in 2000-03. In roadside areas incidence fell in the last inter-survey interval, especially among women, contributing to a decline in roadside prevalence, but in remote rural areas incidence (and thus prevalence) rose slightly.
Conclusion:
HIV spread is continuing in the rural part of the population suggesting a need for more intensive HIV prevention efforts and ART interventions. The levelling off in prevalence is due to a combination of high mortality among HIV infected, and a slight decrease in incidence in roadside villages.
Keywords: HIV prevalence, HIV incidence, HIV trend, Tanzania, measurement biases
Background
Our knowledge about the course of the AIDS epidemic is largely based on national HIV surveillance systems using sentinel sites. In generalized epidemics, women attending antenatal clinics are the key population group for surveillance. National household surveys with bio-marker collection have become increasingly popular: over 20 countries in sub-Saharan Africa have conducted such surveys, but no country has conducted more than one to enable trends to be assessed from this source 1. Observational cohorts (longitudinal community studies without major interventions) are rare, but provide a unique opportunity to assess long term trends in the epidemic. The longest running community study is located in rural Uganda, which furnished a valuable perspective on data on prevalence changes based on the surveillance system.
Data from the national ante-natal clinic (ANC) HIV sentinel surveillance system suggest that HIV prevalence was stabilising in Tanzania at a time when the population had little access to anti-retroviral therapy (ART) 2. From ANC data alone, we cannot tell whether stabilising prevalence is due to stable or declining incidence, or mainly due to deaths among the HIV infected exceeding new infections.3 Serial cross-sectional household surveys with HIV testing have a similar limitation.
This paper presents data from a longitudinal community study in rural northwest Tanzania, which was initiated in 19944.. The analysis presents updated estimates of HIV incidence and prevalence, using results from four surveys conducted over a decade, applying new estimation methods to obtain more robust incidence estimates and describes how the HIV epidemic has unfolded in this population over the last decade.
Methods
The study area is located 20kms from Mwanza City (Tanzania's second city) and straddles the main road to Kenya. The entire population of Kisesa ward is regularly enumerated as part of an ongoing demographic surveillance system (DSS) 4-6 which provides information on the size and structure of the population. The population grew from 19,350 in 1994, when surveillance started, to 26,330 in 2004. The contiguous area has been divided into three strata: “remote” rural villages which accounted for 57% of the total in 1994 and 53% in 2004, roadside villages and the central trading centre. The last two strata have been grouped together in this analysis. Most of the population is Sukuma, the region's dominant ethnic group, and the majority are farmers.
Since 1994, when the DSS started, there have been four community-based surveys in which blood samples were taken for anonymous HIV testing, based on informed consent without result disclosure. These surveys also obtain information about HIV awareness, sexual behaviour and reproductive health 4-7.
Awareness of HIV infection is high in this population, 98.2% of people have heard of HIV/AIDS and 95.9% have accurate knowledge about modes of transmission. Since 1995, there have been a number of HIV prevention interventions in the study population. These include a school-based education programme, mapping of high risk areas for HIV transmission, the establishment of village HIV/AIDS action committees, Health Unit based training, improved management of STDs, condom promotion and, since 2004, providing Voluntary Counselling and Testing (VCT) services in the ward. ARV therapy only became available after the last round of survey.
Epidemiological sero-surveys were carried out in 1994/95, 1996/97, 1999/2000 and 2003/04. In each round, study participants provided information on behavioural and demographic characteristics and gave a blood sample for HIV testing. Residents of Kisesa ward aged over 15 years were eligible for each survey. The upper age limit was 44 years in the first survey, increased to 46 years in the second survey and with no upper age limit in the last two surveys. This allowed the oldest participants in the first survey to be included in all subsequent rounds. For each round, eligible residents were identified from the demographic surveillance data and invited to participate in the survey. In the first three rounds villages were surveyed one by one; in the fourth round two villages were surveyed simultaneously. Respondents were asked to come to central locations in their village to be interviewed and to give blood for HIV testing. Respondents who did not attend were traced and encouraged to participate. Eligible respondents and their children were offered free medical care (but not ART) at temporary clinics, which were better supplied with essential drugs than the local dispensaries. Respondents gave verbal consent for interview and consent for their blood to be tested for HIV on the understanding that the results would not be disclosed to them, and would not be linked to individuals on a named basis by the study staff. VCT was offered onsite in round 4, in earlier rounds it was available from a counsellor who followed the survey team.
All the surveys were given ethical approval by the Medical Research Co-ordinating Committee of the Tanzanian Ministry of Health.
HIV testing was carried out at a regional reference laboratory in Mwanza City. In the first survey venous blood was collected; subsequently dried blood spots from fingerpricks were used. In the first three surveys HIV testing was carried out using Vironostika HIV-MIXT 8 and Enzygnost HIV1/HIV2 9. In the fourth round Uniform 2 10 and Enzygnost HIV1/HIV2 9 were used. In all survey rounds samples for which both ELISA results were reactive were considered to be HIV positive. Western blot tests were used to discriminate between discrepant results in round 1, in subsequent rounds the two ELISA tests were repeated and if still discrepant the sample was excluded from analysis (27 samples).
Prevalence ratios are calculated for residents aged 15-44 who participated in a particular survey round, generally excluding data for those aged 45 and over that are only available in later survey rounds. Individual test results can be matched on study numbers which makes it possible to identify people who acquired HIV infection between two consecutive survey rounds as well as those who contributed person-years “at risk” but remained negative over the inter-survey intervals. Incidence rates are based on people who participated in two or more sero-surveys. In addition, the incidence analysis includes young people who had attended only one survey and had been resident in Kisesa ward when the previous round was conducted but had been too young to attend the previous round. These young people, who were all aged under 15 at the previous survey, were presumed to be uninfected at this earlier time point. The inclusion of these extra young people minimises the degree of left-censoring in the 15-19 age group, which would otherwise lead to an over-estimate of incidence in this age group.
Survival analysis was used to calculate incidence rates. Person years of exposure were calculated using time elapsed following the date of an individual's first negative HIV test, except for the young residents who had been too young to participate in their village's previous sero-survey, for whom exposure is calculated as time elapsed from their fifteenth birthday. Exposed individuals who tested positive exited at the time of sero-conversion. People who did not sero-convert are censored at the date of the last sero-survey they attended. Information on the sero-status of individuals over 45 is used to identify sero-converters and to compute person-years exposure up to age 45, but the reported incidence rates are limited to individuals aged 15-44. We have also estimated the cumulated risk of acquiring HIV infection based on the life table proportion infected by the age of 45.
Overall HIV prevalence ratios and incidence rates were standardised using direct standardisation for age and residence and are presented separately for males and females.
Since individuals can move between study villages, the intervals between consecutive survey rounds in which they participated may be quite wide (between two and three and a half years) and an approximate date for sero-conversion was imputed for each case, even for those who changed status after missing one of the survey rounds. This problem is common to most studies of HIV incidence and is often dealt with by taking the mid-point of the interval as the estimated date of sero-conversion. However this may not be an ideal solution when incidence is changing and the gap between tests is long and varies between individuals 11.
To overcome the problems of interval censoring, we used a multiple imputation technique. Sero-converters were allocated a random date between their last negative test date and their first positive test date; the young Kisesa residents who tested positive at their first test were allocated a random date between the median date of the earlier survey in their village of residence (which they had been too young to attend) and their first test date. The random numbers were drawn in such a way as to produce a uniform distribution of sero-conversion dates in each interval for the population as a whole. The process of random number allocation was repeated until the average five-year age- and sex-specific incidence rates in each inter-survey interval and for both geographical strata did not change – this occurred after about 4,500 runs. The random number allocation process was continued for an additional 5,500 runs with no further changes observed. The tabulated incidence rates and estimates of lifetime risk are based on the averages of these 10,000 repetitions.
The reported variance of the incidence rate estimates is the average variance for each run plus the variance in the incidence rates across all the runs 12. Uncertainty bounds for the estimated incidence rate were derived using the t-distribution 12. The sero surveys are village censuses of the entire adult population, rather than sample surveys so the “within-run” variance associated with each incidence ratio is zero. The uncertainty bounds for the incidence rates therefore represent only the uncertainty introduced by the imputation process.
Although the prevalence ratios are not affected by sampling or imputation errors they may be affected by participation bias. We have estimated the possible effect of participation bias in three additive steps which were based on estimating the prevalence that would have been observed had non-attenders been included. First, we assumed that people who were negative at the last survey they attended had been negative in all the previous surveys for which they were eligible but did not attend, and that those who tested positive in the last survey they attended were positive in all subsequent surveys that they missed. Secondly, it was assumed that eligible non-attenders with no evidence of sero-conversion who had earlier tested negative continued to be negative at later surveys that they did not attend, and eligible non-attenders with no evidence of sero-conversion who later tested positive were already positive at earlier surveys that they did not attend. Finally, those who never tested were assigned the directly measured prevalence ratio for the sex, age and residence subgroup in each round for which they were eligible non-attenders. This last step has no effect on the specific prevalence rates in each subgroup, but allows for the effect of the age, sex and residence structure of the non-attenders on crude prevalence ratios. The bias limits given after each prevalence ratio estimate represent the highest and lowest values obtained by combining one, two or three of these steps.
Results
Attendance
In total, 28,591 interviews and 28,523 HIV tests were carried out in the four surveys among adults aged 15+. A total of 6,448 adults (55% women) came to two or more surveys and were tested for HIV on both occasions. Table 1 gives the numbers attending each survey round. 432 sero-converters were identified, of whom 46 were young Kisesa residents positive at first test, and 56 had skipped a survey round when they had become infected.
Table 1. Attendance at sero surveys in the 15-44 age range.
| Number of men who: | Number of women who: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sero- survey |
Were eligible |
Attended (as % of eligible) |
Valid test for HIV (as % of attended) |
Re-tested at later round (as % of tested this round) |
Had tested at earlier round (as % of tested this round) |
New sero- converters identified in this round* |
Were eligible |
Attended (as % of eligible) |
Valid test for HIV (as % of attended) |
Re-tested at later round (as % of tested this round) |
Had tested at earlier round (as % of tested this round) |
New sero- converters identified in this round* |
| 1994/95 | 3,770 | 2,642 | 2,628 | 1,910 | NA | NA | 3,913 | 3,030 | 3,017 | 2,198 | NA | NA |
|
| ||||||||||||
| 70.1 | 99.5 | 72.7 | 77.4 | 99.6 | 72.9 | |||||||
| 1996/97 | 4,064 | 2,802 | 2,796 | 1,546 | 1,739 | 27 | 4,207 | 3,372 | 3,368 | 1,858 | 2,024 | 35 |
| 69.0 | 99.8 | 55.3 | 62.2 | 80.2 | 99.9 | 55.2 | 60.1 | |||||
| 1999/00 | 4,134 | 2,429 | 2,425 | 1,020 | 1,443 | 67 | 4,360 | 3,221 | 3,214 | 1,360 | 1,804 | 83 |
| 58.8 | 99.8 | 42.1 | 59.5 | 73.9 | 99.8 | 42.3 | 56.1 | |||||
| 2003/04 | 5,052 | 3,090 | 3,089 | NA | 1,294 | 86 | 5,473 | 3,853 | 3,848 | NA | 1,588 | 88 |
| 61.1 | 100.0 | 41.9 | 70.4 | 99.9 | 41.3 | |||||||
Excluding the 46 young people who were HIV positive at the first survey they attended (23 male, 23 female).
Throughout the study, attendance rates are higher for women than men, and higher in the remote rural villages (both sex attendance range 70% to 86%) than in the roadside villages (range 61% to 81%). The most common reason for non-attendance was temporary absence from home. Almost all those who completed the survey questionnaire also provided a blood sample for HIV testing. Change of residence out of the ward was the main reason for not attending more than one sero-survey: overall, 73% of those tested at sero1 tested again at some later date, but among those who remained resident in the ward throughout the entire period of the study, 97% tested again. Similarly, although only 41% of those who tested at sero4 had tested previously, the proportion testing previously among those who had been age eligible and resident in the study area throughout, was 98%.
Prevalence
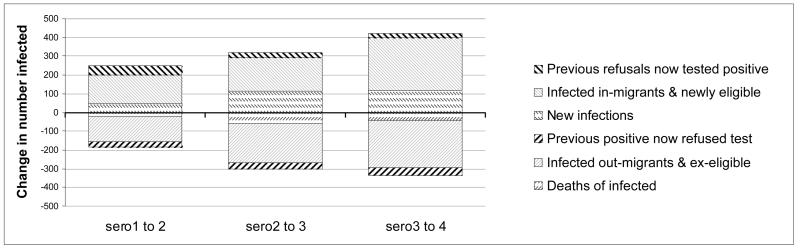
When comparing prevalence trend and incidence levels it should be noted that new infections are only one of many factors contributing to prevalence change. Prevalence is also affected by mortality of infected persons and by in-and out migration of infected and uninfected individuals and both prevalence and incidence estimates are affected by changing participation rates. Figure 1 illustrates the magnitude of the main influences that determine overall prevalence change. Migratory changes dwarf all the others, but in each of the intervals overall in-migration of HIV infected persons has been more or less balanced by out-migration of infected persons. However this is not always the case in narrower age bands, in which it is also necessary to account for aging in and out of the age group. This can lead to unexpected relationships between age-specific incidence and prevalence change in particular age groups.
Figure 1. Components of change in the HIV infected population aged 15-44 in each inter-survey interval.
In the 2003/04 survey, HIV prevalence among adults aged 15+ was 7.5% for men and 8.2% for women, compared to 6.9% for men and 7.8% for women in the previous survey. Data for the population aged 15-44, available for all four surveys, are shown in table 2.
Table 2. Crude prevalence and incidence estimates for population aged 15-44 years and the cumulated risk of acquiring HIV infection by the age 45, by sex, residence and survey / inter-survey interval.
| Roadside | Remote rural | Whole area | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Men | Women | Both sexes | Men | Women | Both sexes | Men | Women | Both sexes | |
| HIV Prevalence, percent, in sero-surveys (with range of adjustment for participation bias) | |||||||||
| Sero 1 | 9.2 (7.4-9.5) | 10.0 (9.3-11.0) | 9.6 (8.4-10.3) | 3.0 (2.7-3.3) | 4.3 (4.0-4.7) | 3.7 (3.4-3.9) | 5.1 (4.4 -5.5) | 6.9 (6.1 – 7.2) | 6.0 (5.3-6.4) |
| Sero 2 | 8.5 (8.3-9.1) | 12.4 (11.8-12.5) | 10.8 (10.3-10.9) | 3.3 (3.2-3.5) | 4.9 (4.7-5.0) | 4.2 (4.1-4.3) | 5.1 (4.9 – 5.5) | 8.0 (7.6 – 8.2) | 6.7 (6.4-6.9) |
| Sero 3 | 8.5 (7.5-9.1) | 13.5 (13.0-14.0) | 11.4 (10.7-11.8) | 6.0 (5.7-6.2) | 6.5 (6.4-6.5) | 6.2 (6.0-6.4) | 6.9 (6.3 – 8.0) | 9.3 (9.1 – 10.4) | 8.3 (7.8-9.3) |
| Sero 4 | 8.5 (7.6-9.2) | 10.6 (10.1-11.0) | 9.5 (9.0-10.2) | 6.8 (6.1-7.2) | 7.6 (7.2-8.1) | 7.2 (6.7-7.7) | 7.5 (6.7 – 8.2) | 8.8 (8.6 – 9.5) | 8.2 (7.7-8.8) |
|
| |||||||||
| Crude Incidence for survey interval: Infections per 1000 PYO (with uncertainty range due to interval censoring) | |||||||||
| Sero 1 - 2 | 10.1(7.8-12.4) | 12.2 (10.3-14.1) | 11.2 (8.2-14.2) | 6.4 (5.5-7.2) | 6.9 (6.0-7.8) | 6.6 (5.6-7.7) | 7.5 (6.6-8.4) | 8.8 (7.9-9.7) | 8.1(6.5-9.7) |
| Sero 2 - 3 | 18.4 (16.1-20.8) | 18.7(17.0-20.4) | 18.6 (16.5-20.7) | 9.7 (8.7-10.6) | 9.6 (8.7-10.5) | 9.6 (8.7-10.6) | 12.2 (11.2-13.2) | 12.5 (11.7-13.4) | 12.4 (11.4-13.4) |
| Sero 3 - 4 | 15.5 (13.1-17.9) | 9.8 (8.4-11.2) | 12.6 (6.4-18.9) | 9.7 (8.6-10.9) | 12.1(11.2-13.1) | 10.9 (8.2-13.7) | 11.5 (10.4-12.6) | 11.3 (10.5-12.1) | 11.4 (10.4-12.4) |
|
| |||||||||
| Cumulated risk of Infection by age 45, percent (with uncertainty range due to interval censoring) | |||||||||
| Sero 1 - 2 | 32 (26-38) | 30 (26-34) | 29 (26-33) | 18 (16–21) | 19 (17-21) | 19 (17-20) | 22 (21- 24) | 23 (20 - 25) | 22 (20-25) |
| Sero 2 - 3 | 44 (40-49) | 42 (39-45) | 42 (40-45) | 25 (23-28) | 23 (21-25) | 24 (22-25) | 30 (28 – 31) | 30 (28-32) | 30 (28-32) |
| Sero 3 - 4 | 41 (36-46) | 25 (22-29) | 31 (29-36) | 24 (22-27) | 31 (29-33) | 28 (26-30) | 29 (28- 31) | 29 (27-31) | 29 (27-31) |
| all intervals | 39 (38-41) | 32 (31-33) | 35 (34-36) | 23 (22-24) | 24 (23-25) | 24 (23-25) | 28 (28-29) | 28 (27-29) | 28 (27-29) |
In the area as a whole, prevalence appears to be rising still for men, but has declined slightly in recent years for women. Prevalence is lower in remote rural areas, but it has continued to rise in these areas for both sexes, whereas in the area closest to the road and trading centre it has levelled off for men and fallen sharply for women. Overall, prevalence is very little influenced by changes in the composition of the population between surveys: when standardised for age and residence, the standardised prevalence ratios (not shown) differed by less than 0.2% from the crude values.
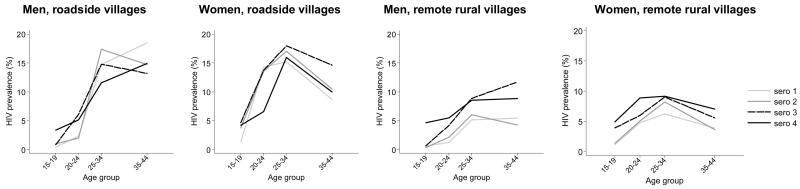
HIV prevalence varies by age group, and the pattern of age variation differs between the sexes and between roadside and remote rural areas; furthermore the age patterns change over time (see Figure 2). Prevalence is consistently higher across all ages in roadside residents compared to those living in more remote rural areas, but the gap has reduced over time. For females, prevalence peaks in the 25-34 age group, but for men it continues to be high in the 35-44 age group. It has increased over time amongst males and females under 20 in both residence categories – however, for men this increase has been recent (2000-03), whereas for young women the increase occurred mainly at the start of the study (1994-97). In older age groups, prevalence was fairly steady in roadside villages in the first three surveys but fell at the most recent survey.
Figure 2. HIV prevalence (%) among men and women resident in roadside and remote rural areas of Kisesa at each sero-survey, by age group.
Incidence
Incidence estimates are based on a total of 33,140 person-years of observation between ages 15 and 45 with 362 sero-conversions observed between these ages. This includes 3,690 person-years contributed by young residents in the interval before their first sero-survey attendance (31 sero-conversions), and 2,630 person-years contributed by those who skipped one or more sero-surveys (32 sero-conversions). The second panel of table 2 shows crude incidence rates by sex, residence and inter-survey interval. Incidence rose sharply between the first two inter-survey intervals in all areas, but roadside areas experienced a reversal of this trend in the last interval with a particularly dramatic fall among women, in contrast to remote rural areas where incidence has levelled out for men but continues to rise for women.
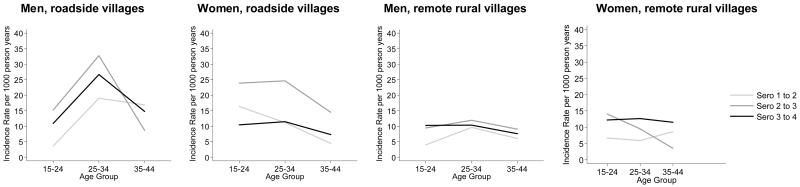
Figure 3 shows that women experience a younger incidence pattern than men, with higher rates than males in the 15-24 age group, and relatively little difference between incidence rates in the 15-24 and 25-34 age groups. This contrasts with the male age pattern which generally peaks in the 25-34 age group. The incidence age pattern has become less differentiated by age in rural areas, with little overall change in level, in urban areas the pattern has become older during the recent fall, and differences between the areas have decreased.
Figure 3. HIV incidence (per 1000 person years) among men and women resident in roadside and remote rural areas of Kisesa who attended at least two sero-surveys.
Incomplete coverage of the study population might affect incidence estimates if individuals who attend at least two sero-surveys are disproportionately concentrated in subgroups with unusually high or low incidence rates. The number of HIV negative persons who attended only one of the first three sero-surveys was 4,338 – of these 894 were eligible to attend at least one other sero-survey. They contributed a total of 5,008 person-years during the time that they were under observation according to the demographic surveillance. Had they experienced the sex- age- and residence specific incidence rates observed in their groups their crude incidence rate would have been 12.6 per thousand over the study period as a whole, compared to 10.9 observed in those who attended 2 or more sero-surveys. The difference arises because those who came to only one sero-survey are mainly found in the more mobile populations in the trading centre and roadside villages. The inclusion of these extra person-years with their expected incidence would raise the overall incidence to 11.1
The overall effect of differences in incidence patterns is clearly seen in the cumulated probability of infection shown in the last panel of Table 2. This life table measure is independent of the structure of the population at risk, and represents the probability that a person experiencing prevailing risks of infection between ages 15 and 45 would have become infected by age 45. The contrasting age patterns between the sexes generally cancel out by age 45, with cumulated infection being almost the same for men and women, except for the period between surveys 3 and 4, where women have an overall advantage over men in the roadside areas, whilst men are advantaged in rural areas. In recent times overall levels in rural and roadside villages have converged as a result of this contrasting trend between the sexes,
Discussion
HIV prevalence among adults in Kisesa ward increased from 6.5% to 8.3% between 1994 and 2000 and has remained at the same level until 2003/04. The incidence data do not show a similar time trend. The crude prevalence of HIV infection in Kisesa Ward in 2003/04 (7.5% for men, 8.8% for women) was reasonably close to the crude prevalence of HIV in Mwanza Region estimated in the 2003/4 national AIDS Impact Survey as 7.5% (4.1% - 10.8%) for men and 7.0% (3.6% - 10.4%) for women 13. Unlinked anonymous HIV testing carried out in Kisesa ante-natal clinics in 2000 produced an estimate of 4.6% in clinics serving remote rural areas and 11.3% in the roadside clinic 14. This is lower than the sero3 prevalence estimate for women in 2000 (5.7% remote, 12.4% roadside) because of lower fertility among HIV infected women.
The assessment of national trends in HIV prevalence in Tanzania is hampered by lack of data from a sufficient number of clinics providing data continuously over a prolonged period of time. A general assessment of the trend based on the antenatal clinic-based HIV surveillance system suggested that the epidemic reached a peak at 8.1% HIV prevalence among adults 15-49 years in 1995 and had decreased to 6.5% in 2004 15. Our analysis suggests that trends in different parts of the country may have followed divergent paths.
The main factor influencing the accuracy with which our prevalence estimates reflect true prevalence in Kisesa ward is attendance at sero-surveys. If survey participation is in any way associated with HIV status this could bias our results. However, our attempts to predict HIV status of non attenders, using past or future known HIV status, and predictions based on age, sex and residence for those who never attended, have produced a range of crude prevalence estimates (shown in table 2) that in every case encompass the prevalence measured among those who did attend. Our predictions for non-attenders were based solely on demographic characteristics, available from the regular household censuses, not on behavioural data, since the latter were collected at the same time as sero-status was determined. Overall, the observed prevalence lies slightly closer to the lower bound of the predicted range (average deviation 0.5) than the upper bound (average deviation 0.6, but there is no indication of a strong bias in either direction.
We found that failure to attend a follow-up sero-survey by eligible persons was more common in persons resident in the roadside and trading centre area, who might be expected to have higher than average incidence. However, the person-years of missed follow-up for eligible persons who did not attend a follow-up survey (5,008) compared to the person-years observed for those who did attend a subsequent survey (33,140) is relatively small, so that the downward bias in our incidence estimate is probably only of the order of 0.2 per thousand person years.
Multiple imputation of sero-conversion dates does not suffer from the same bias as mid-point estimation, but introduces random error since each person is unlikely to have been allocated the true date of sero-conversion. This random error will not have any effect on the number of sero-conversions, and only a negligible effect on overall exposure time, but it could affect the classification by age group at sero-conversion. The uncertainty range due to interval censoring (which indicates the effect of a particular sero-conversion falling outside the age-range of interest, 15-44; or taking place in a later or earlier inter-survey period in the case of those who became infected in an interval that included a missed survey round) is narrow, suggesting that imputation has not had an appreciable effect on these results. A comparison of the different methods for estimating sero-conversion dates found that the method we used performed less well than conditional mean imputation16, however that method needs additional assumptions about the distribution of sero-conversion times by calendar year.
Mid-point imputation of incidence rates produced some extreme and improbable estimates for certain age and sex groupings and which further justifies the choice of an alternative imputation method.
Our analysis highlights the problems of relying on prevalence data to gauge the general trend of the epidemic. For example, in the most recent interval prevalence rose for men and fell for women (by +0.6 and −0.5 percentage points respectively) whereas crude incidence rates and cumulated infection risk indicators were virtually identical for both sexes. Prevalence data will become even less reliable as an indicator of epidemic spread if ART roll-out is successful, hence the importance of continuing to collect high quality incidence data.
Our data show that the gap in incidence level (as measured by the lifetime risk of infection indicator) between rural and roadside communities is narrowing, mainly because women in rural areas have recently experienced higher infection risks. This information should prompt further research to try to discover whether HIV prevention messages are reaching these women, whether they are engaging in risky behaviour, or whether this trend could be due to their increasingly becoming the sexual partners of choice of men from the roadside villages, of whom a relatively large proportion are infected.
The fact that incidence appears to be falling in roadside areas is an encouraging sign, but the continued gradual rise in incidence in “remote” rural areas is worrying, especially as the majority (2/3) of the Kisesa population lives in these areas. There is an urgent need to promote and expand access to the existing HIV prevention efforts such as behavioural change including abstinence and condom use, VCT services and early diagnosis and treatment of STIs, and to ensure that these services reach the most remote rural parts of the country. The roll out of ART affords an opportunity to strengthen prevention messages in a de-stigmatising context of providing treatment – it will be equally important to ensure that these treatment services also reach rural areas.
References
- 1.García-Calleja JM, EGa, PDG National population based HIV prevalence surveys in sub-Saharan Africa: results and implications for HIV and AIDS estimates. Sexually Transmitted Infections. 2006;82(suppl_3):iii64–iii70. doi: 10.1136/sti.2006.019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National AIDS Control Programme . Surveillance of HIV and Syphilis Among Antenatal Clinic Enrollees 2003 - 2004. The United Republic of Tanzania Ministry of Health Tanzania Mainland; Dar es Salaam: 2004. [Google Scholar]
- 3.Wawer MJS, David Gray, Ronald H, Sewankambo Nelson, Li Chuanjun, Nalugoda F, Lutalo T, Konde-Lule JK. Trends in HIV-1 prevalence may not reflect trends in incidence in mature epidemics: data from the Rakai population-based cohort, Uganda. AIDS. 1997;11(8):1023–1030. doi: 10.1097/00002030-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Mwaluko G, Urassa M, Isingo R, Zaba B, Boerma JT. Trends in HIV and sexual behaviour in a longitudinal study in a rural population in Tanzania, 1994-2000. Aids. 2003;17(18):2645–2651. doi: 10.1097/00002030-200312050-00012. [DOI] [PubMed] [Google Scholar]
- 5.Boerma J, Urassa M, Senkoro K, Klokke A, Ngweshemi J. Spread of HIV infection in a rural area of Tanzania. AIDS. 1999;13(10):1233–1240. doi: 10.1097/00002030-199907090-00013. [DOI] [PubMed] [Google Scholar]
- 6.Urassa M, Boerma J, Isingo R, Ngalula J, Ng'weshemi J, Mwaluko G, et al. The impact of HIV/AIDS on mortality and household mobility in rural Tanzania. AIDS. 2001;15(15):2017–2023. doi: 10.1097/00002030-200110190-00015. [DOI] [PubMed] [Google Scholar]
- 7.Bloom S, Urassa M, Isingo R, Ng'weshemi J, Boerma J. Community effects on the risk of HIV infection in rural Tanzania. Sexually Transmitted Infections. 2002;78(4):261–266. doi: 10.1136/sti.78.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vironostika HIV-MIXT B. Organon,. The Netherlands: [Google Scholar]
- 9.Enzygnost HIV1/HIV2 DBMG. Emil-von-Behring- Str. 76 D-35041, Marburg. Germany: [Google Scholar]
- 10.Uniform 2 BbB. 5281RM, Boxtel. The Netherlands: [Google Scholar]
- 11.Law CG, Brookmeyer R. Effect of mid-point imputation on the analysis of double censored data. Statistics in Medicine. 1992;11:1569–1578. doi: 10.1002/sim.4780111204. [DOI] [PubMed] [Google Scholar]
- 12.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Second edition John Wiley and Sons; New Jersey: 2002. [Google Scholar]
- 13.Tanzania Commission for AIDS (TACAIDS) National Bureau of Statistics (NBS) ORC Macro . Tanzania HIV/AIDS Indicator Survey 2003-04. TACAIDS, NBS, and ORC Macro; Calverton, Maryland, USA: 2005. [Google Scholar]
- 14.Urassa M, Kumogola Y, Isingo R, Mwaluko G, Makelemo B, Mugeye K, et al. HIV prevalence and sexual behaviour changes measured in an Ante-Natal Clinic setting in northern Tanzania. STI. 2006;82:301–306. doi: 10.1136/sti.2005.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somi GRMM, Swai RO, Lyamuya EF, Killewo J, Kwesigabo G, Tulli T, Kabalimu TK, Ng'ang'a L, Isingo R, Ndayongeje J. Estimating and projecting HIV prevalence and AIDS deaths in Tanzania using antenatal surveillance data. BMC Public Health. 2006;6(120) doi: 10.1186/1471-2458-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geskus RB. Methods for estimating the AIDS incubation time distribution when date of seroconversion is censored. Statistics in Medicine. 2001;20:795–812. doi: 10.1002/sim.700. [DOI] [PubMed] [Google Scholar]