Abstract
The protective immune response that develops following infection with many tissue-dwelling intestinal nematode parasites is characterized by elevations in IL-4 and IL-13 and increased numbers of CD4+ T cells, granulocytes and macrophages. These cells accumulate at the site of infection and in many cases can mediate resistance to these large multicellular pathogens. Recent studies suggest novel potential mechanisms mediated by these immune cell populations through their differential activation and ability to stimulate production of novel effector molecules. These newly discovered protective mechanisms may provide novel strategies to develop immunotherapies and vaccines against this group of pathogens. In this review, we will examine recent studies elucidating mechanisms of host protection against three widely-used experimental murine models of tissue-dwelling intestinal nematode parasites: Heligmosomoides polygyrus, Trichuris muris and Trichinella spiralis.
Keywords: Parasite, Inflammation, Immune, Intestine, Nematode, Th2
1. Introduction
Tissue-dwelling intestinal nematode parasites pose a major health problem throughout developing countries and a food safety concern worldwide. As well as contributing directly to morbidity and impaired childhood development, infection with these metazoan parasites may also impair effective immune responses against microbial pathogens, including Mycobacterium tuberculosis (MTb) and Human Immunodeficiency Virus (HIV), as well as those of veterinary importance (Urban et al., 2007). These helminth parasites range throughout the world and it is estimated that over 2 billion people are currently infected. They include such diverse species as: Trichuris (whipworms), Strongyloides (threadworms), Necator and Ancylostoma (hookworms), Ascaris, Anisakis, microfilaria and Trichinella. Each occupies a distinct micro-environment in the host, which is associated with an immune response that may contribute to the development of a protective response to the invading metazoan parasite. In this review, we will examine several murine models of tissue-dwelling intestinal nematode parasites, focusing on recent findings elucidating the host protective immune response elicited against each of them.
In most murine models, and to the extent to which this has been examined in human disease, tissue-dwelling nematodes generally trigger a T helper (Th) 2-type protective immune response identified by the characteristic production of Th2 cytokines, including IL-4, IL-5, IL-9 and IL-13. These cytokines may be produced by both Th and non-T cells including B cells, eosinophils, mast cells and basophils. Additional candidate Th2 cytokines include IL-21 (Wurster et al., 2002; Pesce et al., 2006; Frohlich et al., 2007), IL-31 (Perrigoue et al., 2007), IL-33 (Humphreys et al., 2008) and IL-25, the latter of which may be produced by a distinct Th25 cell (Owyang et al., 2006; Tato et al., 2006; Wang et al., 2007). With varying degrees of importance, all of these cytokines can contribute to the development of Th2-type responses, an important exception being IL-31, which has recently been shown to down modulateTh2-type responses (Perrigoue et al., 2007). Innate immune cells may initially produce several Th2 cytokines at early stages of the immune response, thereby contributing to an environment that supports Th2 cell differentiation from naïve T cells; however several studies now suggest that IL-2 and autocrine IL-4 are sufficient for Th2 cell differentiation (Noben-Trauth et al., 2002; Cote-Sierra et al., 2004; Liu et al., 2005), although this putative role of IL-2 may need to be re-examined given recent findings that anti-IL-2 antibody (S4B6) may enhance the agonistic effects of IL-2 rather than block IL-2 activity (Phelan et al., 2008). Innate cells producing Th2 cytokines may act in an autocrine manner and may also activate other innate cells including macrophages and dendritic cells. Generally Th2 cells, as a source of Th2 cytokines, are required to amplify and sustain the Th2-type response. Epithelial cells in the skin and mucosal tissues have recently been shown to promote the development of Th2 cells, in particular through their secretion of thymic stromal lymphopoietin (TSLP) which can condition dendritic cells or act directly on T cells to promote Th2 cell differentiation (Watanabe et al., 2004; Rimoldi et al., 2005; Allakhverdi et al., 2007; Holgate, 2007; Liu et al., 2007; Omori and Ziegler, 2007; Zaph et al., 2007).
Microbes, including many bacteria, protozoa and viruses, stimulate the development of a distinct Th1-type response where IFN-γ is produced and macrophages, CD8+ T cells, natural killer (NK) cells and neutrophils are primary players. In this milieu, macrophages are activated to up-regulate inducible nitric oxide synthase (iNOS) which generates nitric oxide and, together with neutrophils, are considered the first responders in the Th1-type response. Neither neutrophils nor macrophages were conventionally thought to be primary players in the Th2-type response. More recently, however, it has become clear that macrophages are indeed activated by IL-4, IL-13 and IL-21 (Mantovani et al., 2005; Pesce et al., 2006). These cytokines trigger signaling pathways in the macrophages which result in an alternative activation pathway with the production of molecules quite different from those produced following toll-like receptor (TLR)- or IFN-γ-mediated activation. Other results suggest that neutrophils are also activated during Th2-type responses and, under some experimental conditions, may mediate nematode parasite killing (Al-Qaoud et al., 2000; Saeftel et al., 2001; Saeftel et al., 2003; Galioto et al., 2006; Porthouse et al., 2006; Padigel et al., 2007). Although not as well studied, neutrophils may also undergo differential activation depending on the immune environment (Tsuda et al., 2004; Tsuda et al., 2008).
Thus, activated innate immune cell populations involved in clearance of helminth parasites are similar to those involved in immune responses to many bacteria, protozoa and viruses, but their differentiation and effector function in response to metazoan pathogens is different, as is the case for the adaptive T and B cell immune response. The ability of immune cells to alter their function depending on the type of invading pathogen is likely a considerable evolutionary advantage. In many cases, large multi-cellular parasites require a different set of immune effectors for their clearance compared with responses required to control bacteria, protozoa and viruses. For example, the phagocytosis and intracellular killing of microbes is different from the sequestration and killing of multi-cellular tissue-dwelling parasites. The array of control mechanisms may include: i) walling off the parasite from the surrounding tissue by creating an immune cell and/or connective tissue barrier; ii) impairing the ability of the parasite to migrate to or remain in its preferred tissue destination; iii) release of toxins or other factors that may directly damage or stress the parasite. It is notable that multi-cellular parasites may cause considerable tissue damage as they migrate through the host. Rapid induction of mechanisms of tissue healing and repair are required and, not surprisingly, recent studies have suggested that wound healing mechanisms are regulated by Th2-type immune responses (Gratchev et al., 2001; Wynn, 2004; Sakthianandeswaren et al., 2005).
The intestine is a unique immune environment where the resident mucosal immune system is subject to constant stimuli from luminal bacteria which may also invade the mucosal tissue during nematode parasite infection. Robust regulatory immune cell populations, including both macrophages and T regulatory (T reg) cells, have recently been identified that can control the development of Th1-type or Th17-type inflammation. Several recent reviews and papers have discussed these important regulators of potentially harmful gut inflammation (Belkaid, 2007; Denning et al., 2007). In this mucosal milieu, the Th2-type response and associated allergic inflammation mediated by Th2 cytokines may be similarly controlled. Alternatively, if these regulatory cell populations preferentially dampen Th1-type inflammation, the intestinal milieu may actually favor the development of potent and polarized Th2-type responses. More studies are needed to examine the effects of these regulatory populations on the development of the Th2-type immune response.
2. Intestinal nematode parasites
The predominant nematode genera of geohelminths, including Ascaris, Trichuris, Necator, and Ancylostoma, ultimately infect the human intestine. However, the different species occupy different micro-environmental niches including migration through specific tissue sites. In experimental murine models, the Th2-type response in the intestine is generally protective, in some cases resulting in effective expulsion of the parasite. Although most components of the Th2-type response exhibit stereotypical activation against the range of these metazoan pathogens, only certain effector functions are capable of mediating specific protective effects against a particular parasite. In this review, we will primarily examine the host protective response that occurs during the tissue-dwelling phase of three widely used experimental mouse models: Heligmosomoides polygyrus, Trichuris muris and Trichinella spiralis.
2.1. Heligmosomoides polygyrus
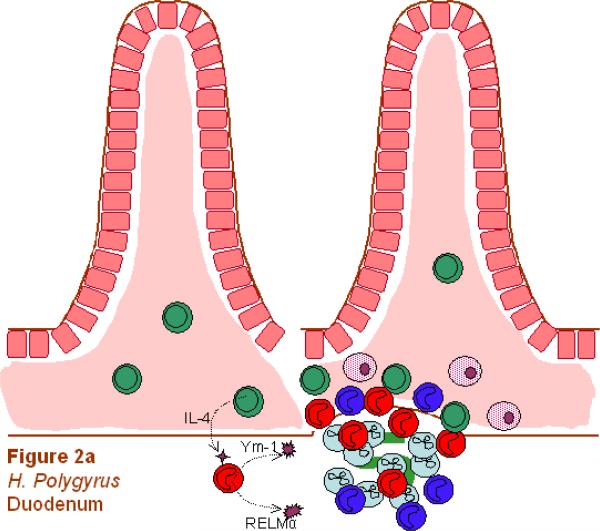
Heligmosomoides polygyrus is a natural murine intestinal trichostrongylid nematode parasite with a strictly enteral life cycle. The infective L3s are free living and orally ingested to initiate the infection (this exposure is experimentally mimicked by inoculation with a ball-tipped feeding tube). Primary inoculation results in chronic infection, but if the parasites are cleared with an anthelmintic drug treatment at 2 weeks after primary inoculation, a subsequent secondary challenge results in expulsion of the parasite by 2 weeks post-secondary inoculation. As such, this parasite is an excellent model for studying the memory Th2 response to nematodiasis. An interesting characteristic of the infection is that the parasitic L3 invades the mucosa of the duodenum and migrates to the sub-mucosa juxtaposed to the muscularis layer for a period of 8 days (Gause et al., 2003; Anthony et al., 2006). During this time the larva develops into an adult and subsequently migrates back to the lumen. In the initial 36-h period after challenge inoculation, L3s penetrate pits of the cardiac region of the stomach and by 12 h induce a pronounced infiltrate in the mucosa and sub-mucosa composed of eosinophils, mast cells and neutrophils (Liu et al., 1974). Whether this initial penetration affects subsequent L3 invasion of the duodenum is unclear.
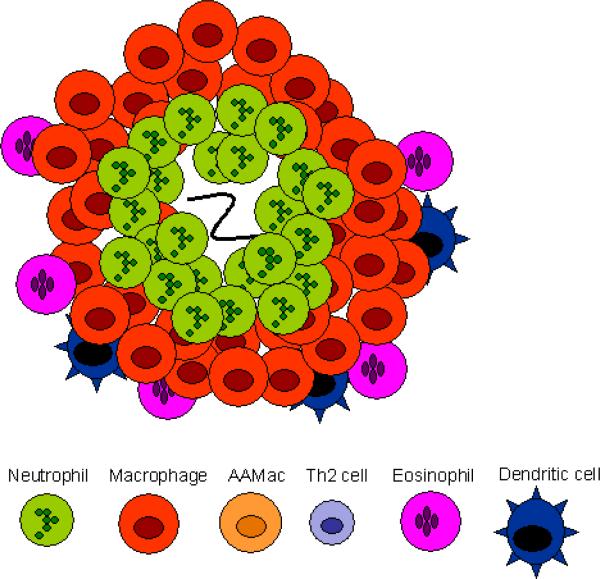
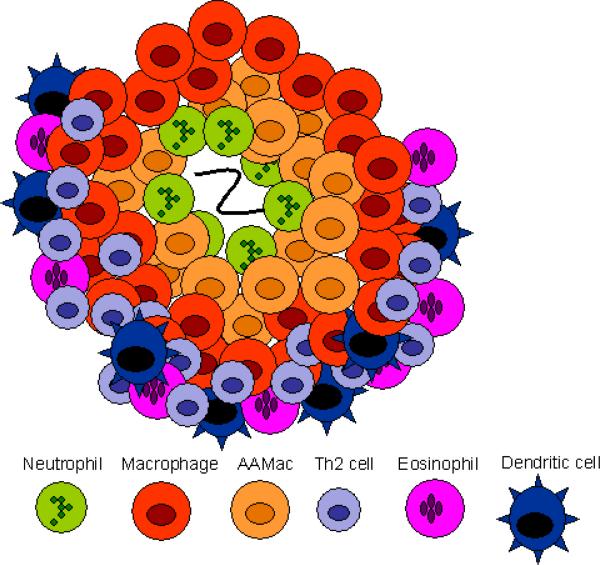
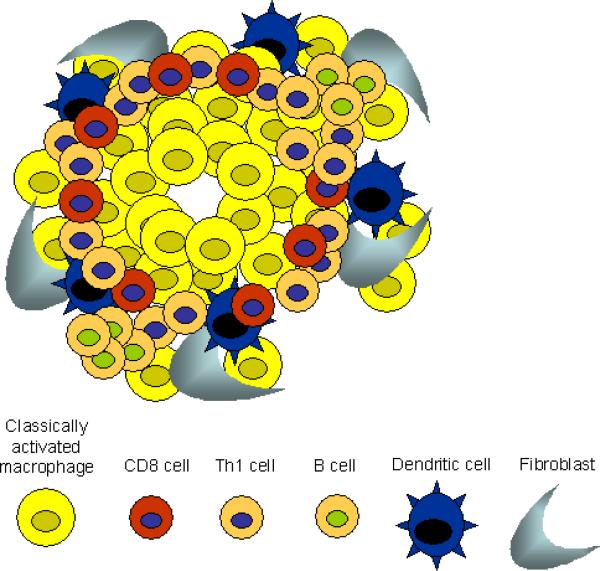
The memory response triggers a characteristic immune cell infiltrate that surrounds the parasite in the duodenum by 4 days after secondary inoculation. Thus, in the memory Th2-type response, as in the Th1-type response, immune cells are rapidly recruited to the host:parasite interface. The rapidly developing immune cell architecture includes neutrophils that accumulate immediately adjacent to the parasite (see Figs. 1 and 2) and alternatively activated macrophages (AAMacs) (CD206+, IL-4Rα+) that form a thick band around the neutrophils and parasite. Just beyond the AAMacs is another band of immune cells composed of dendritic cells, CD4+ T cells, eosinophils (Morimoto et al., 2004) and a population of apparently undifferentiated macrophages expressing macrophage markers F4/80 and CD11b but not the mannose receptor CD206 or the IL-4R (Kreider and Gause, unpublished data). This overall pattern is highly consistent. The rapid accumulation of AAMacs is CD4+ T cell-dependent and occurs primarily during the memory response, with only a few AAMacs detected during a comparable period after a primary response (Anthony et al., 2006). This infiltrate shares many features with the more conventional granulomas that typically develop during Th1-like responses as in Mycobacterium tuberculosis infection (Fig. 1). In both responses, macrophages are the dominant cell type and both neutrophils and macrophages are the first responders.
Fig. 1.
Th1- and Th2-type granulomas have distinct cell types and phenotypes. (A) At day 4 after primary inoculation with Heligmosomoides polygyrus, the tissue-dwelling larva is surrounded by a neutrophilic infiltrate and macrophages. Th2 cells are not found at this early timepoint. (B) At day 4 after secondary challenge, the H. polygyrus larva provokes a Th2-type granuloma, characterized by Th2 cells, more eosinophils, and alternatively activated macrophages (AAMacs). (C) The response to Mycobacterium tuberculosis is typical of a Th1-type granuloma. Th1-derived IFN-γ results in classically activated macrophages, which use inducible nitric oxide synthase (iNOS) to generate microbicidal products that destroy phagocytosed bacteria.
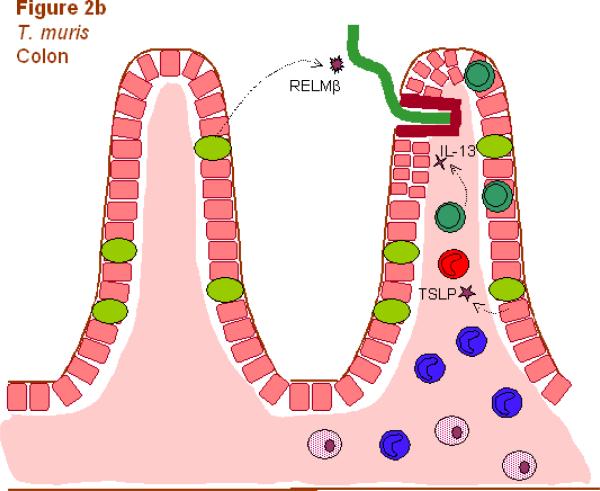
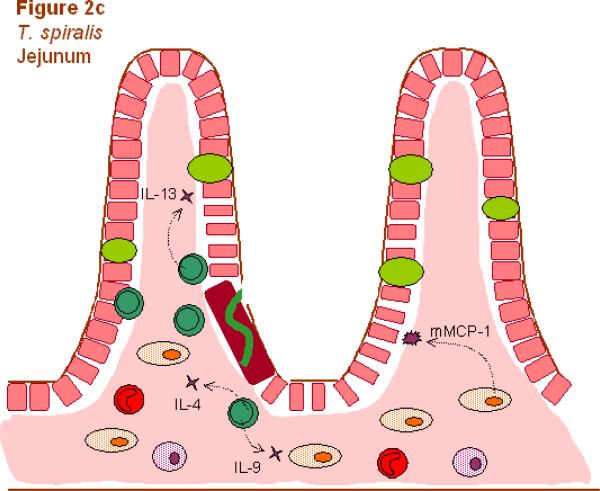
Fig. 2.
Different components of the Th2-type response are effective against different helminthic parasites. Responses involve Th2 cells (dark green), neutrophils (light blue), macrophages (dark blue), alternatively activated macrophages (AAMacs; red), eosinophils (purple), goblet cells (light green), epithelial cells (pink), epithelial syncytium (maroon), mast cells (orange) and secreted factors. (A) The localized memory response to the Heligmosomoides polygyrus tissue-dwelling larva is characterized by AAMacs. AAMac-mediated protection is arginase-dependent and includes secretion of the chitinase-like protein Ym-1 and resistin-like molecule α (RELMα). (B) An IL-13-dependent “epithelial escalator” uses increased cell turnover to displace the burrowing head of Trichuris muris. Epithelial cell thymic stromal lymphopoietin (TSLP) may stimulate Th2 responses and inhibit Th1-type inflammatory responses, and goblet cell resistin-like molecule β (RELMβ) may bind the worm. Eosinophils accumulate in the lamina propria but are not required for parasite expulsion. (C) Th2 cytokines induce mast cell protease 1 (mMCP-1) which disrupts epithelial cell tight junctions, and goblet cell hyperplasia, together creating a “leaky” gut environment that favors expulsion of Trichinella spiralis from the syncytium of epithelial cells where it rapidly matures and reproduces.
Analysis of the infiltrate using laser capture micro-dissection has shown high levels of Th2 cytokine gene expression in both regions where the neutrophils are located and in the outer band where the CD4+ T cells accumulate (Morimoto et al., 2004; Anthony et al., 2006). Immune histological staining demonstrates high IL-4R surface expression by macrophages that also express CD206 (the mannose receptor), a characteristic marker of AAMacs. Thus, the cellular composition of the infiltrate surrounding this parasite is similar to the granuloma formed during a Th1-like response to M. tuberculosis, but the differentiation state of the effector cells is characteristic of a Th2-type response. As such, this infiltrate is referred to as a Th2-type granuloma (Anthony et al., 2007). The ability of immune cells, including macrophages, to rapidly respond and accumulate at the site of parasite invasion in peripheral tissues is thus a major feature of both the Th1-type response and the Th2-type response. It also demonstrates the plasticity of the immune system, in which immune cells can rapidly differentiate into characteristic and quite different phenotypes depending on the cytokine milieu and the particular type of infectious agent. Besides H. polygyrus, experimental mouse models of filarial infection also show the development of distinct granulomas that contribute to parasite damage (Rajan et al., 2002; Chirgwin et al., 2003; Chirgwin et al., 2006; Rao and Klei, 2006).
Although the CD4+ T cell-dependent Th2-type memory response is required for host protection against this parasite, the components that actually mediate worm expulsion remain unclear. It is possible that multiple immune mechanisms are involved at different stages of parasite development that together contribute to an effective response. In the tissue-dwelling phase, recent studies suggest that AAMacs play an important role in contributing to larval stress and ultimately to adult worm expulsion (Anthony et al., 2006). AAMacs may mediate protective effects through a number of potential mechanisms. AAMacs express certain chitinase and found in inflammatory zone (FIZZ) family member proteins (ChaFFs). This intriguing family of molecules includes chitinases, chitinase-like proteins that have lost their enzymatic activity e.g., Ym-1, and resistin-like molecules including RELMα and RELMβ. Both Ym-1 and RELMα are expressed at high levels in AAMacs during H. polygyrus infection. Although some studies have suggested that Ym-1 may have eosinophil chemotactic ability (Owhashi et al., 1998; Owhashi et al., 2000; Boot et al., 2005), its actual in vivo function remains uncertain. Ym-1 is the most highly expressed gene in AAMacs, suggesting high production levels of this molecule may be important in the function of these cells. It also binds heparin, indicating that it may be important in mediating interactions between cells and the extracellular matrix (Hung et al., 2002). We have recently found that Ym-1 is highly expressed on H. polygyrus developing larvae (Kreider and Gause, unpublished data), and whether this may affect parasite activity is under examination. FIZZ1/RELMα is also expressed by AAMacs and together with Ym-1 is considered a key marker for AAMac differentiation (Raes et al., 2002; Nair et al., 2003). Its function is also unclear, although some studies suggest it may contribute to fibrosis as it can induce myofibroblast differentiation in vitro including increased Type I collagen production (Blagoev et al., 2002; Rajala et al., 2003; Liu et al., 2004). Recent studies suggest that the H. polygyrus protective immune response against tissue-dwelling larvae is also arginase-dependent (Anthony et al., 2006). Thus it will be important in future studies to determine which molecules associated with AAMac activation are arginase-dependent since these may contribute to parasite vulnerability during the tissue-dwelling phase. Arginase metabolism can result in increased polyamine production, a known down-regulator of Th1-type inflammation (Hasko et al., 2000; Cordeiro-da-Silva et al., 2004). It is thus possible that polyamines produced by AAMacs may help support the localized Th2-type response by controlling Th1-type cytokines, in this way promoting through a regulatory mechanism host protection against tissue-dwelling nematode parasites (Zhang et al., 1997; ter Steege et al., 1999). In addition, increased production of proline from arginase activity may contribute to fibrosis (Hesse et al., 2001) and this and other wound-healing functions of AAMacs may help to wall off the invading parasite, impairing its ability to obtain nutrients. It should be noted that these effector mechanisms may be most important during secondary infections, such as the memory response to H. polygyrus and other memory T cell-independent mechanisms may be more important in mediating resistance during primary infections.
2.2. Trichuris muris
This parasite is also a natural murine parasite but with a different life history and ecological niche compared to H. polygyrus. Mice ingest (experimentally inoculated) environmentally-resistant eggs that hatch into L1 in the ileum of the small intestine. The larvae then migrate to the cecum and proximal colon where they invade the mucosal epithelial cells at the crest of the crypt. Here they live in tunnels, previously thought to be composed of live cells (Lee and Wright, 1978), but which now appear to be a wall of dead cells kept structurally intact by actin associated with the apical brush border that invaginates as the parasite penetrates the epithelium (Tilney et al., 2005). Within this tunnel of actin-rich brush border epithelium, the parasites moves and feeds, ultimately reaching an adult length up to 2 cm. The corresponding immune response is mouse and parasite strain-dependent, with some mouse strains supporting a Th2-type immune response that leads to parasite expulsion, and others initiating a Th1-type response associated with susceptibility. In BALB/c mice, the Th2-type response can be deviated to a Th1-type response by blockade of costimulatory molecules (Urban et al., 2000), similar to the switch between Th2 to Th1-type response that follows B7 blockade during Leishmania major infection (Corry et al., 1994). An essential difference is that in L. major infection the Th1-type response results in resistance while in T. muris infection the Th2-type response is protective. In other studies, specific T. muris isolates can differentially affect Th responses, indicating that the specific parasite variant, as well as the mouse strain, can influence whether a protective Th2-type response is elicited (Bellaby et al., 1996).
Thus, both H. polygyrus and T. muris dwell in mucosal intestinal tissues but they occupy distinct ecological niches (see Fig. 2). In the case of T. muris, the epithelial cell micro-environment and the adjacent lamina propria are probably of particular importance. In fact, changes in immune cell number and constitution in these tissues can vary in resistant and susceptible strains. In particular, CD4+ Th cells preferentially accumulate in the epithelium of resistant mice around the time of worm expulsion while macrophages appear to markedly increase in the lamina propria (Little et al., 2005). Although further studies are required to examine whether macrophages mediate resistance to T. muris, as they do in the immune response to H. polygyrus, it is clear that Th cells polarized in resistant mice are a major source of the Th2 cytokines required for worm expulsion. Their accumulation in the epithelium and lamina propria of the large intestine at the time of worm expulsion may provide the levels of localized Th2 cytokines required to trigger effector functions that mediate resistance. This may include novel Th2 cytokines such as amphiregulin, a member of the epidermal growth factor (EGF) family, that directly induces epithelial cell proliferation and parasite clearance via EGF receptors on epithelial cells (Zaiss et al., 2006). Recent studies have also suggested that intestinal epithelial cells may support the development of this localized Th2-type response. Blockade of IκB kinase (IKK)-β, the catalytic subunit of the IKK complex that mediates NF-κB activation, in epithelial cells inhibits TSLP production and the development of the Th2-type response leading to elevations in IFN-γ and IL-17, and severe intestinal inflammation (Zaph et al., 2007). Similar results were obtained with mice deficient in TSLPR (Tpte2−/−mice), suggesting that TSLP produced by intestinal epithelial cells (IECs) may be a critical NF-κB-dependent cytokine required for the development of the protective Th2-type response against T. muris (Zaph et al., 2007). Another cytokine recently implicated in protection against T. muris is IL-33, which can increase TSLP when administered to infected SCID mice and induce a protective Th2-type response in normally susceptible AKR mice (Humphreys et al., 2008).
Several mechanisms may be involved in the ultimate expulsion of T. muris. In resistant strains developing a Th2-type response, IL-13 promotes increased migration and turnover of IECs. This “epithelial escalator” propels IECs up the crypt column dislodging T. muris from its ecological niche into the lumen (Cliffe et al., 2005). The IL-13-induced rapid IEC turnover is blocked in susceptible strains by IFN-γ-induced CXCL10 (IP10), resulting in the accumulation of proliferating epithelial cells in the crypt column and thickening of the IEC layer. This function of the Th2-type immune response to accelerate epithelial cell turnover is particularly well-suited to expel T. muris, which dwells in the epithelial cell layer. In fact, Cliffe et al. (2005) provided evidence suggesting that this protective mechanism alone is sufficient for T. muris expulsion as blockade of CXCL10 triggered increased epithelial cell turnover and worm expulsion in severe combined immunodeficient (SCID) mice lacking B and T cells. The Th1-type response both suppresses the “epithelial cell escalator” and promotes thickening of the IEC layer, both of which are beneficial for T. muris persistence in the host. As such, it is not surprising that T. muris may have evolved ways of promoting a Th1-type response (Grencis and Entwistle, 1997) and subsequent epithelial cell proliferation (Artis et al., 1999) during infection.
Another potential host protective mechanism against T. muris may involve RELMβ. In the intestine, RELMβ is specifically expressed by goblet cells and in resistant strains its expression is markedly up-regulated following T. muris infection. Both IL-4 and IL-13 up regulate RELMβ expression by goblet cells, indicating that expression of this molecule by differentiated epithelial cells is yet another component of the Th2-type enteric immune response. Intriguingly, RELMβ binds directly to pores or pore-like structures associated with chemosensory function of Strongyloides stercoralis (Artis et al., 2004). In vitro studies further showed that rRELMβ impaired chemoattraction of S. stercoralis suggesting that binding of RELMβ to nematode parasites in vivo may interfere with environmental cues that support parasitism (Artis et al., 2004). In future studies, it will be of interest to examine whether RELMβ bound to T. muris in vivo may prevent niche localization and movement between epithelial cells impairing the ability of the parasite to reside in its micro-environment.
2.3. Trichinella spiralis
The infective stage of T. spiralis is part of a nurse cell-larva complex found in striated muscle of prey eaten by carnivores. Digestive enzymes in the stomach release the larva from the muscle tissue and the parasitic L1s migrate to small intestinal sites at the base of villi where they reside in a syncytium of epithelial cells (see Fig. 2). The larvae rapidly develop over a period of 30 h into adults with female worms measuring about 3 mm in length. Newborn larvae are produced from female worms within 5 days after mating and larva production continues until host protective immunity expels the adult worms. The larvae, produced in the intestine, migrate to striated muscle where they induce differentiation of the muscle cell into a nurse cell approximately 17 days after inoculation. The resistance of this infective stage of the parasite in muscle tissue is partially dependent on CD4+ T cell-regulated levels of IL-10 and TGF-β that normally control the level of inflammation surrounding the parasite-modified nurse cell (Beiting et al., 2007).
As with host protective immunity to both T. muris and H. polygyrus, worm expulsion is CD4+ T cell-dependent and requires Th2 cytokines. In particular, both IL-4 and IL-13 are important in mediating protection against T. spiralis and only when the effects of both these cytokines are inhibited is worm survival prolonged (Finkelman et al., 2004). Goblet cell hyperplasia, eosinophilia and mucosal mastocytosis are characteristic of this Th2-type response and, interestingly, although elevated, other Th2 cytokines are not required. Blocking IL-5 does not impair host protection (Grencis et al., 1991; Herndon and Kayes, 1992; Dixon et al., 2006), suggesting eosinophils are not essential, although eosinophils can kill larvae in vitro (Grove et al., 1977; Gurish et al., 2002). Similarly, eosinophilia does not appear to play a major protective role in the responses to either H. polygyrus (Urban et al., 1991) or T. muris (Betts and Else, 1999). Blocking IL-9 also does not impair T. spiralis expulsion (Khan et al., 2003), but IL-9 can under some circumstances act as an adjuvant to promote protective immunity (Leech and Grencis, 2006). Expulsion of T. muris is impaired, however, following IL-9 blockade (Richard et al., 2000). Studies with B cell deficient (μMt) mice indicate that neither B cells nor a specific antibody are required for T. spiralis expulsion, although under these conditions mast cell degranulation (as detected by serum mast cell protease levels) is reduced by as much as 50–60% (Finkelman et al., 2004). Mast cells, however, as well as CD4+ T cells, appear to play an essential role leading to worm expulsion (Knight et al., 2002; Brown et al., 2003; McDermott et al., 2003), and there is some support for a role for parasite-specific IgE in both the intestinal response to adult T. spiralis and L1 in the tissue (Gurish et al., 2004).
Mucins are highly branched glycoproteins that form the mucus layer covering epithelial cells and may contribute to protection during the lumen-dwelling stages of T. spiralis (Knight et al., 2008). Mice infected with T. spiralis (Shekels et al., 2001) and rats infected with Nippostrongylus brasiliensis (Kawai et al., 2007) both show increased expression of Muc2 and Muc3, but expression remains high in infected animals deficient in T cells or cytokine signaling necessary for clearance of the parasite. Increased expression of enzymes involved in mucin glycosylation is T cell-dependent in these rats, suggesting that the quality as well as the quantity of mucins may affect protection (Kawai et al., 2007). Studies of helminth infection in mucin-deficient mice have not yet been reported, so the protective role of these glycoproteins remains speculative.
Other possible effectors against T. spiralis are intelectins, a family of galactofuranose-binding lectins found primarily in goblet cells and Paneth cells of the intestine. Resistant BALB/c mice, but not susceptible C57BL/10 mice, show increased expression of intelectin-2 early during T. spiralis infection, suggesting a role in parasite recognition or expulsion (Pemberton et al., 2004). Similar results are found for resistant BALB/c versus susceptible AKR mice infected with T. muris (Datta et al., 2005); furthermore, intelectin-2 is induced by STAT6 in both lung and intestine of BALB/c mice infected with N. brasiliensis (Voehringer et al., 2007). However, transgenic mice over-expressing intelectins show no change in the clearance of N. brasiliensis or M. tuberculosis (Voehringer et al., 2007), leaving a protective role for these molecules still undefined.
During infection with T. spiralis, the jejunum becomes edematous and inflamed around the time of expulsion. It has been hypothesized that the increased intestinal permeability associated with this Th2-type inflammation is an important host protective mechanism (the leak-lesion hypothesis; (Murray et al., 1971). Indeed, IL-4/IL-13 signaling during infection with T. spiralis can trigger increased epithelial cell resistance and Na+-linked glucose absorption leading to increased luminal fluids (Madden et al., 2004) similar to what has been described for resistance to adult H. polygyrus (Shea-Donohue et al., 2001). Recent studies with another intestinal parasite that resides in the lumen, N. brasiliensis, suggests that AAMacs may also affect intestinal physiology by promoting smooth muscle contractility (Zhao et al., 2008). Whether their ability to impair N. brasiliensis egg production (Zhao et al., 2008) results from changes in intestinal physiology and/or macrophages damaging developing tissue-dwelling larvae in the lung prior to their migration to the intestinal lumen needs to be examined. Depletion of mast cells, with anti-c-kit antibody or by using mast cell deficient mice (Ha et al., 1983; Alizadeh and Murrell, 1984; Grencis et al., 1993; Donaldson et al., 1996; Finkelman et al., 2004), indicates that an absence of mast cells inhibits worm expulsion and blocks intestinal permeability during T. spiralis infection, consistent with the hypothesis that mast cells are instrumental in promoting IEC permeability. Mast cells secrete β-chymases (chymotrypsin-like serine proteases) which have been proposed to increase gut permeability. Recent studies suggest that the β-chymase, mouse mast cell protease 1 (mMCP-1), is essential as T. spiralis-infected mMCP-1 deficient mice did not show increased epithelial cell permeability or effective worm expulsion (Knight et al., 2000; Lawrence et al., 2004; Knight et al., 2008). The tight junctions that form between epithelial cells regulate the flow of fluids and solutes through the paracellular channels. Previous studies have indicated that serine proteases, such as mMCP-1, may degrade proteins, such as occludin, which are required for tight junction formation (McDermott et al., 2003). mMCP-1 may thus function by disrupting tight junction structure, thereby promoting paracellular permeability.
Th2 cytokines, including IL-4 and IL-9, promote mastocytosis (Finkelman et al., 2004). For example, IL-9 transgenic mice have pronounced mast cell hyperplasia and also exhibit increased intestinal permeability (Faulkner et al., 1997; Temann et al., 2002; Temann et al., 2007). At the initial stages of the immune response i.e., within the first few hours, mast cells and basophils may be important sources of Th2 cytokines. IgE cross-linking may be an important factor in enhancing mast cell/basophil activation in previously primed mice. As the response progresses, however, Th2 cytokine production by CD4+ T cells is of increasing importance. Recent findings also raise the possibility that exposure of non-bone marrow-derived cells, including IECs, to even low levels of IL-4 and IL-13 may increase their sensitivity to mast cell mediators, including mMCP-1 (Strait et al., 2003; Finkelman et al., 2004). Thus, Th2 cytokines may enhance the mast cell response by promoting mast cell activation and hyperplasia and by increasing the sensitivity of target cells to mast cell mediators.
3. Conclusions and discussion
The components of the Th2-type response that are most effective against a tissue-dwelling intestinal nematode parasite are greatly influenced by the life cycle. This includes the specific micro-environment of the parasite and characteristics such as parasite behavior and tissue sites. It is also possible that different groups of parasites have physical and metabolic characteristics that make them differentially susceptible to specific effector cell functions. Unlike T. muris and T. spiralis, H. polygyrus migrates past the epithelial cell boundary to reside in the sub-mucosa where it remains until it develops into an adult that emerges into the lumen. A pronounced memory Th2-type granuloma develops within 4 days after inoculation, while a Th2-type granuloma of similar intensity follows about 8 days after a primary infection: too late to have much effect on the adult worm that has already migrated back to the intestinal lumen. Once in the lumen, adult worms are likely affected by other components of the Th2-type response, including changes in gut physiology induced by IL-4 and IL-13, such as increased mucous secretion into the gut, increased luminal fluid flow and increased smooth muscle contractility, which contribute to a generally inhospitable environment for adult worms (Shea-Donohue and Urban, 2004).
As both Trichuris and Trichinella reside in the intestinal epithelium, one might initially expect those to have similar protective immune mechanisms. In fact, the niches they occupy are quite different as Trichuris dwells in the colon while Trichinella inhabits the small intestine. Trichinella larvae need to migrate from the intestine to muscle tissue, whereas Trichuris worms remain in the lumen laying eggs to be passed in the host's feces. Also, Trichinella is much smaller and more mobile than Trichuris. All these factors may influence the effector mechanisms involved in protection (Richard Grencis, personal communication). For example, increased fluid flow may create an inhospitable environment for the smaller Trichinella, while the “epithelial escalator” may be more effective against the larger Trichuris encased in its tunnel of dead cells. It should also be noted that the mast cell response is reduced in Trichuris compared to Trichinella, perhaps because the colon does not support mastocytosis, mast cell effects on colonic smooth muscle and epithelial secretion are muted, or Trichuris has direct suppressive effects on the immune response. Future studies are needed to further characterize differences in the effective properties of the host Th2-type response against these two intestinal epithelial parasites. An additional area of particular importance includes examination of how the immune response differs in the colon and small intestine.
There are also a number of common features of the Th2-type response that seem to mediate protection against all three tissue-dwelling parasites. In particular, IL-4 and IL-13 are essential for worm expulsion as are Th2 cells. However, other Th2 cytokines, for example IL-5 and associated eosinophilia, are not essential. It is certainly possible that eosinophils play a more important role in resistance to other tissue-dwelling parasites and also the associated blood eosinophilia may be an important effector mechanism when parasites are present in the circulation. The pleiotropic effects of IL-4 and IL-13 are broad however, and IL-4/IL-13-dependent effector mechanisms may vary greatly in importance with the particular tissue-dwelling intestinal helminth. In the case of Trichinella, IL-4/IL-13-mediated increases in sensitivity to mast cell mediators may be of considerable importance, while IL-4/IL-13-dependent epithelial cell turnover may be dominant in the response to Trichuris, and IL-4/IL-13-mediated Th2 granuloma formation and alternative macrophage activation may be more important in the response to H. polygyrus. Many of these components of the Th2-type response, such as eosinophilia, AAMacs and neutrophils, are present during infection with these different intestinal nematode parasites whether or not they play an important role in worm expulsion. This suggests that the host immune response is not so fine-tuned that it distinguishes between these different metazoan pathogens. Rather, it appears that a generalized Th2-type response is elicited, with only a subset of the activated components actually mediating protection against the specific infecting nematode parasite. The variability in the immune response that is observed between these different tissue-dwelling parasitic nematodes may be more a result of the specific region and micro-environment of the gut that is infected than any differences in specific responses to the type of parasite.
The recent identification of ChaFFs expressed at high levels during intestinal nematode infection raises the possibility that new effector mechanisms dependent on these intriguing molecules may be characterized in vivo. It may be broadly significant if RELMβ or other ChaFFs generally impair migration of tissue-dwelling parasites in vivo. In the case of H. polygyrus, rapid up-regulation of IL-4 and IL-13 in the memory response may stimulate RELMβ release from goblet cells when H. polygyrus larvae are initially penetrating the epithelial barrier and migrating to the sub-mucosa, thereby blocking their ability to home to their preferred site. This mechanism may have some precedence in parasite binding by passive maternal antibodies, which can protect neonates against invasion of parasitic H. polygyrus L3 (Harris et al., 2006). Alternatively, RELMβ may impair adult feeding or fecundity in the lumen. Similarly, RELMβ or other ChaFFs may also contribute to expulsion of Trichuris or Trichinella, impairing their homing or feeding abilities at particular stages of their life cycles. In vivo studies with antagonists or mice deficient in one or more of these potential inhibitors of helminth chemosensory capability are required to further explore this potentially important area. Other molecules recently found to be elevated during helminth infections such as intelectins may play an essential role in protective immunity against these parasites (Artis, 2006; Knight et al., 2008).
Major advances have been made in the past several years towards understanding the immune mechanisms that contribute to host protection against intestinal nematode parasites. These insights may prove useful in the development of new immunologically-based treatments including vaccines that enhance resistance to intestinal nematode parasites.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Qaoud KM, Pearlman E, Hartung T, Klukowski J, Fleischer B, Hoerauf A. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int Immunol. 2000;12:899–908. doi: 10.1093/intimm/12.6.899. [DOI] [PubMed] [Google Scholar]
- Alizadeh H, Murrell KD. The intestinal mast cell response to Trichinella spiralis infection in mast cell-deficient w/wv mice. J Parasitol. 1984;70:767–773. [PubMed] [Google Scholar]
- Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Urban JF, Jr., Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D, Potten CS, Else KJ, Finkelman FD, Grencis RK. Trichuris muris: host intestinal epithelial cell hyperproliferation during chronic infection is regulated by interferon-gamma. Exp Parasitol. 1999;92:144–153. doi: 10.1006/expr.1999.4407. [DOI] [PubMed] [Google Scholar]
- Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D. New weapons in the war on worms: identification of putative mechanisms of immune-mediated expulsion of gastrointestinal nematodes. Int J Parasitol. 2006;36:723–733. doi: 10.1016/j.ijpara.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- Bellaby T, Robinson K, Wakelin D. Induction of differential T-helper-cell responses in mice infected with variants of the parasitic nematode Trichuris muris. Infect Immun. 1996;64:791–795. doi: 10.1128/iai.64.3.791-795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts CJ, Else KJ. Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunol. 1999;21:45–52. doi: 10.1046/j.1365-3024.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- Blagoev B, Kratchmarova I, Nielsen MM, Fernandez MM, Voldby J, Andersen JS, Kristiansen K, Pandey A, Mann M. Inhibition of adipocyte differentiation by resistin-like molecule alpha. Biochemical characterization of its oligomeric nature. J Biol Chem. 2002;277:42011–42016. doi: 10.1074/jbc.M206975200. [DOI] [PubMed] [Google Scholar]
- Boot RG, Bussink AP, Aerts JM. Human acidic mammalian chitinase erroneously known as eosinophil chemotactic cytokine is not the ortholog of mouse YM1. J Immunol. 2005;175:2041–2042. doi: 10.4049/jimmunol.175.4.2041-a. [DOI] [PubMed] [Google Scholar]
- Brown JK, Donaldson DS, Wright SH, Miller HR. Mucosal mast cells and nematode infection: strain-specific differences in mast cell precursor frequency revisited. J Helminthol. 2003;77:155–161. doi: 10.1079/JOH2002160. [DOI] [PubMed] [Google Scholar]
- Chirgwin SR, Nowling JM, Coleman SU, Klei TR. Effect of immunostimulatory oligodeoxynucleotides on host responses and the establishment of Brugia pahangi in Mongolian gerbils (Meriones unguiculatus) J Parasitol. 2003;89:483–489. doi: 10.1645/GE-3088. [DOI] [PubMed] [Google Scholar]
- Chirgwin SR, Coleman SU, Porthouse KH, Klei TR. Tissue migration capability of larval and adult Brugia pahangi. J Parasitol. 2006;92:46–51. doi: 10.1645/GE-599R.1. [DOI] [PubMed] [Google Scholar]
- Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- Corry DB, Reiner SL, Linsley PS, Locksley RM. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Dixon H, Blanchard C, Deschoolmeester ML, Yuill NC, Christie JW, Rothenberg ME, Else KJ. The role of Th2 cytokines, chemokines and parasite products in eosinophil recruitment to the gastrointestinal mucosa during helminth infection. Eur J Immunol. 2006;36:1753–1763. doi: 10.1002/eji.200535492. [DOI] [PubMed] [Google Scholar]
- Donaldson LE, Schmitt E, Huntley JF, Newlands GF, Grencis RK. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol. 1996;8:559–567. doi: 10.1093/intimm/8.4.559. [DOI] [PubMed] [Google Scholar]
- Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- Frohlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, Harris NL, Kopf M. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, Abraham D. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval strongyloides stercoralis in mice. Infect Immun. 2006;74:5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause WC, Urban JF, Jr., Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, Goerdt S. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand J Immunol. 2001;53:386–392. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- Grencis RK, Hultner L, Else KJ. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology. 1991;74:329–332. [PMC free article] [PubMed] [Google Scholar]
- Grencis RK, Else KJ, Huntley JF, Nishikawa SI. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 1993;15:55–59. doi: 10.1111/j.1365-3024.1993.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Grencis RK, Entwistle GM. Production of an interferon-gamma homologue by an intestinal nematode: functionally significant or interesting artefact? Parasitology. 1997;115(Suppl):S101–106. doi: 10.1017/s0031182097002114. [DOI] [PubMed] [Google Scholar]
- Grove DI, Mahmoud AA, Warren KS. Eosinophils and resistance to Trichinella spiralis. J Exp Med. 1977;145:755–759. doi: 10.1084/jem.145.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurish MF, Humbles A, Tao H, Finkelstein S, Boyce JA, Gerard C, Friend DS, Austen KF. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J Immunol. 2002;168:5730–5736. doi: 10.4049/jimmunol.168.11.5730. [DOI] [PubMed] [Google Scholar]
- Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139–1145. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun. 1983;41:445–447. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J, Urban JF, Jr., Lamarre A, Burki K, Odermatt B, Zinkernagel RM, Macpherson AJ. Mechanisms of neonatal mucosal antibody protection. J Immunol. 2006;177:6256–6262. doi: 10.4049/jimmunol.177.9.6256. [DOI] [PubMed] [Google Scholar]
- Herndon FJ, Kayes SG. Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J Immunol. 1992;149:3642–3647. [PubMed] [Google Scholar]
- Holgate ST. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 2007;28:248–251. doi: 10.1016/j.it.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- Hung SI, Chang AC, Kato I, Chang NC. Transient expression of Ym1, a heparin-binding lectin, during developmental hematopoiesis and inflammation. J Leukoc Biol. 2002;72:72–82. [PubMed] [Google Scholar]
- Kawai Y, Yamauchi J, Soga K, Yamada M, Uchikawa R, Tegoshi T, Arizono N. T cell-dependent and -independent expression of intestinal epithelial cell-related molecules in rats infected with the nematode Nippostrongylus brasiliensis. APMIS. 2007;115:210–217. doi: 10.1111/j.1600-0463.2007.apm_510.x. [DOI] [PubMed] [Google Scholar]
- Khan WI, Richard M, Akiho H, Blennerhasset PA, Humphreys NE, Grencis RK, Van Snick J, Collins SM. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun. 2003;71:2430–2438. doi: 10.1128/IAI.71.5.2430-2438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight PA, Wright SH, Brown JK, Huang X, Sheppard D, Miller HR. Enteric expression of the integrin alpha(v)beta(6) is essential for nematode-induced mucosal mast cell hyperplasia and expression of the granule chymase, mouse mast cell protease-1. Am J Pathol. 2002;161:771–779. doi: 10.1016/s0002-9440(10)64236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight PA, Brown JK, Pemberton AD. Innate immune response mechanisms in the intestinal epithelium: potential roles for mast cells and goblet cells in the expulsion of adult Trichinella spiralis. Parasitology. 2008;135:655–670. doi: 10.1017/S0031182008004319. [DOI] [PubMed] [Google Scholar]
- Lee TD, Wright KA. The morphology of the attachment and probable feeding site of the nematode Trichuris muris (Schrank, 1788) Hall, 1916. Can J Zool. 1978;56:1889–1905. doi: 10.1139/z78-258. [DOI] [PubMed] [Google Scholar]
- Leech MD, Grencis RK. Induction of enhanced immunity to intestinal nematodes using IL-9-producing dendritic cells. J Immunol. 2006;176:2505–2511. doi: 10.4049/jimmunol.176.4.2505. [DOI] [PubMed] [Google Scholar]
- Little MC, Bell LV, Cliffe LJ, Else KJ. The characterization of intraepithelial lymphocytes, lamina propria leukocytes, and isolated lymphoid follicles in the large intestine of mice infected with the intestinal nematode parasite Trichuris muris. J Immunol. 2005;175:6713–6722. doi: 10.4049/jimmunol.175.10.6713. [DOI] [PubMed] [Google Scholar]
- Liu SK, Cypess RH, van Zandt P. Gastritis caused by multiple Nematospiroides dubius infections. J Parasitol. 1974;60:790–793. [PubMed] [Google Scholar]
- Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164:1315–1326. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- Liu Z, Liu Q, Hamed H, Anthony RM, Foster A, Finkelman FD, Urban JF, Jr., Gause WC. IL-2 and autocrine IL-4 drive the in vivo development of antigen-specific Th2 T cells elicited by nematode parasites. J Immunol. 2005;174:2242–2249. doi: 10.4049/jimmunol.174.4.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF, Jr., Shea-Donohue T. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol. 2004;172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M, Whitmire J, Xiao S, Anthony RM, Mirakami H, Star RA, Urban JF, Jr., Gause WC. Peripheral CD4 T cells rapidly accumulate at the host: parasite interface during an inflammatory Th2 memory response. J Immunol. 2004;172:2424–2430. doi: 10.4049/jimmunol.172.4.2424. [DOI] [PubMed] [Google Scholar]
- Murray M, Jarrett WF, Jennings FW. Mast cells and macromolecular leak in intestinal immunological reactions. The influence of sex of rats infected with Nippostrongylus brasiliensis. Immunology. 1971;21:17–31. [PMC free article] [PubMed] [Google Scholar]
- Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85:173–180. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth N, Hu-Li J, Paul WE. IL-4 secreted from individual naive CD4+ T cells acts in an autocrine manner to induce Th2 differentiation. Eur J Immunol. 2002;32:1428–1433. doi: 10.1002/1521-4141(200205)32:5<1428::AID-IMMU1428>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- Owhashi M, Arita H, Niwa A. Production of eosinophil chemotactic factor by CD8+ T-cells in Toxocara canis-infected mice. Parasitol Res. 1998;84:136–138. doi: 10.1007/s004360050370. [DOI] [PubMed] [Google Scholar]
- Owhashi M, Arita H, Hayai N. Identification of a novel eosinophil chemotactic cytokine (ECF-L) as a chitinase family protein. J Biol Chem. 2000;275:1279–1286. doi: 10.1074/jbc.275.2.1279. [DOI] [PubMed] [Google Scholar]
- Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padigel UM, Stein L, Redding K, Lee JJ, Nolan TJ, Schad GA, Birnbaumer L, Abraham D. Signaling through Galphai2 protein is required for recruitment of neutrophils for antibody-mediated elimination of larval Strongyloides stercoralis in mice. J Leukoc Biol. 2007;81:1120–1126. doi: 10.1189/jlb.1106695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrigoue JG, Li J, Zaph C, Goldschmidt M, Scott P, de Sauvage FJ, Pearce EJ, Ghilardi N, Artis D. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J Exp Med. 2007;204:481–487. doi: 10.1084/jem.20061791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr., Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JD, Orekov T, Finkelman FD. Cutting edge: mechanism of enhancement of in vivo cytokine effects by anti-cytokine monoclonal antibodies. J Immunol. 2008;180:44–48. doi: 10.4049/jimmunol.180.1.44. [DOI] [PubMed] [Google Scholar]
- Porthouse KH, Chirgwin SR, Coleman SU, Taylor HW, Klei TR. Inflammatory responses to migrating Brugia pahangi third-stage larvae. Infect Immun. 2006;74:2366–2372. doi: 10.1128/IAI.74.4.2366-2372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest. 2003;111:225–230. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan TV, Ganley L, Paciorkowski N, Spencer L, Klei TR, Shultz LD. Brugian infections in the peritoneal cavities of laboratory mice: kinetics of infection and cellular responses. Exp Parasitol. 2002;100:235–247. doi: 10.1016/s0014-4894(02)00015-2. [DOI] [PubMed] [Google Scholar]
- Rao RU, Klei TR. Cytokine profiles of filarial granulomas in jirds infected with Brugia pahangi. Filaria J. 2006;5:3. doi: 10.1186/1475-2883-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc Natl Acad Sci U S A. 2000;97:767–772. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- Saeftel M, Volkmann L, Korten S, Brattig N, Al-Qaoud K, Fleischer B, Hoerauf A. Lack of interferon-gamma confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect. 2001;3:203–213. doi: 10.1016/s1286-4579(01)01372-7. [DOI] [PubMed] [Google Scholar]
- Saeftel M, Arndt M, Specht S, Volkmann L, Hoerauf A. Synergism of gamma interferon and interleukin-5 in the control of murine filariasis. Infect Immun. 2003;71:6978–6985. doi: 10.1128/IAI.71.12.6978-6985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthianandeswaren A, Elso CM, Simpson K, Curtis JM, Kumar B, Speed TP, Handman E, Foote SJ. The wound repair response controls outcome to cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 2005;102:15551–15556. doi: 10.1073/pnas.0505630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea-Donohue T, Urban JF., Jr. Gastrointestinal parasite and host interactions. Curr Opin Gastroenterol. 2004;20:3–9. doi: 10.1097/00001574-200401000-00003. [DOI] [PubMed] [Google Scholar]
- Shekels LL, Anway RE, Lin J, Kennedy MW, Garside P, Lawrence CE, Ho SB. Coordinated Muc2 and Muc3 mucin gene expression in Trichinella spiralis infection in wild-type and cytokine-deficient mice. Dig Dis Sci. 2001;46:1757–1764. doi: 10.1023/a:1010622125040. [DOI] [PubMed] [Google Scholar]
- Strait RT, Morris SC, Smiley K, Urban JF, Jr., Finkelman FD. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–3842. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- Tato CM, Laurence A, O'Shea JJ. Helper T cell differentiation enters a new era: le roi est mort; vive le roi! J Exp Med. 2006;203:809–812. doi: 10.1084/jem.20060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temann UA, Ray P, Flavell RA. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J Clin Invest. 2002;109:29–39. doi: 10.1172/JCI13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. Int Immunol. 2007;19:1–10. doi: 10.1093/intimm/dxl117. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Connelly PS, Guild GM, Vranich KA, Artis D. Adaptation of a nematode parasite to living within the mammalian epithelium. J Exp Zoolog A Comp Exp Biol. 2005;303:927–945. doi: 10.1002/jez.a.214. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Shigematsu K, Kobayashi M, Herndon DN, Suzuki F. Role of polymorphonuclear neutrophils on infectious complications stemming from Enterococcus faecalis oral infection in thermally injured mice. J Immunol. 2008;180:4133–4138. doi: 10.4049/jimmunol.180.6.4133. [DOI] [PubMed] [Google Scholar]
- Urban J, Fang H, Liu Q, Ekkens MJ, Chen SJ, Nguyen D, Mitro V, Donaldson DD, Byrd C, Peach R, Morris SC, Finkelman FD, Schopf L, Gause WC. IL-13-mediated worm expulsion is B7 independent and IFN-gamma sensitive. J Immunol. 2000;164:4250–4256. doi: 10.4049/jimmunol.164.8.4250. [DOI] [PubMed] [Google Scholar]
- Urban JF, Jr., Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci U S A. 1991;88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JF, Jr., Steenhard NR, Solano-Aguilar GI, Dawson HD, Iweala OI, Nagler CR, Noland GS, Kumar N, Anthony RM, Shea-Donohue T, Weinstock J, Gause WC. Infection with parasitic nematodes confounds vaccination efficacy. Vet Parasitol. 2007;148:14–20. doi: 10.1016/j.vetpar.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, Stanley SA, Cox JS, Completo GC, Lowary TL, Locksley RM. Nippostrongylus brasiliensis: identification of intelectin-1 and -2 as Stat6-dependent genes expressed in lung and intestine during infection. Exp Parasitol. 2007;116:458–466. doi: 10.1016/j.exppara.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Hanabuchi S, Soumelis V, Yuan W, Ho S, de Waal Malefyt R, Liu YJ. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5:426–434. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosmann TR. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314:1746. doi: 10.1126/science.1133715. [DOI] [PubMed] [Google Scholar]
- Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- Zhao A, Urban JF, Jr., Anthony RM, Sun R, Stiltz J, Rooijen NV, Wynn TA, Gause WC, Shea-Donohue T. Th2 Cytokine-Induced Alterations in Intestinal Smooth Muscle Function Depend on Alternatively Activated Macrophages. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]