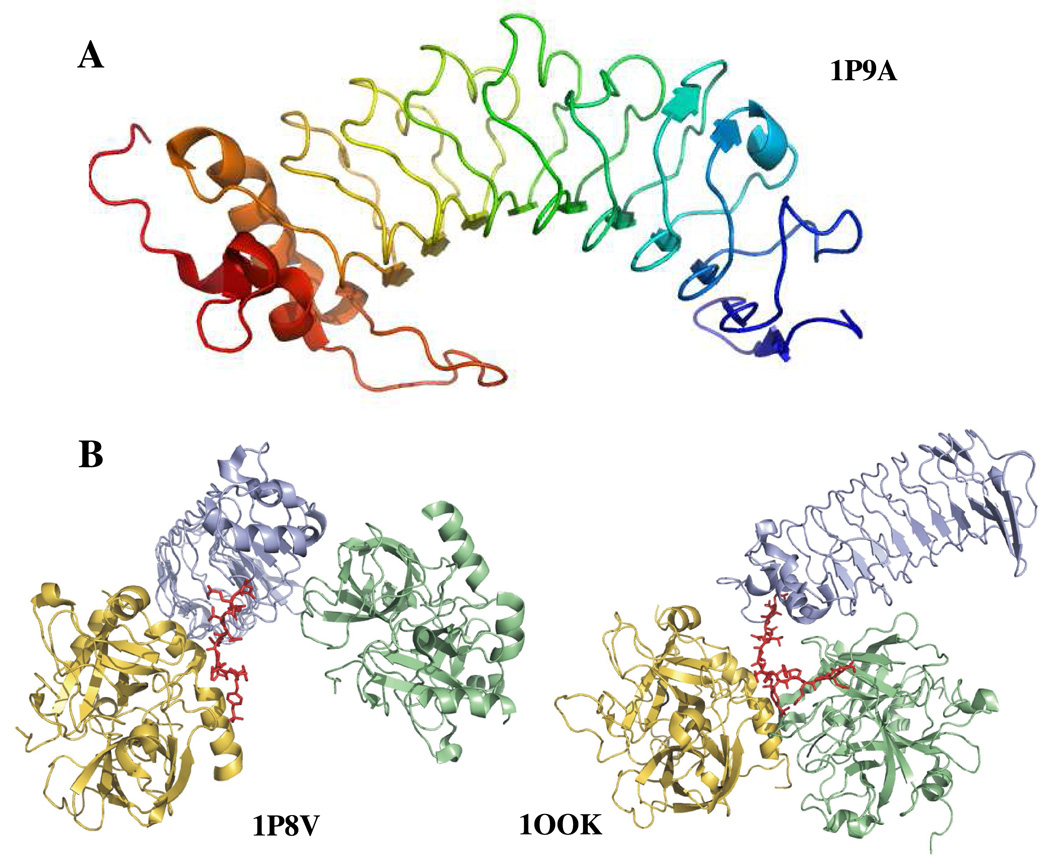
Figure 1.
A. Structure of glycoprotein Ibα (1–266) (1P9A) (25). The highly anionic C-terminal tail (269–286) is not modeled in this structure. The most prominent feature of the structure is the leucine rich repeat domain that forms the curvature of the protein. B. Two views of GpIbα binding to thrombin. The crystal structures depict similar ABE-II interactions but differ in the depiction of thrombin dimerization. In blue is GpIbα and the anionic C-terminal tail is in red. Thrombin in gold binds to the C-terminal tail, while thrombin in green binds at different interfaces. The structure on the left is 1P8V (26), the structure on the right 1OOK (27). These figures were created using PyMol (11).