Abstract
Objective
To assess and compare the attitudes and trust that African American and white parents have toward their children participating in research.
Design
Self-administered, cross-sectional survey of a convenience sample of parents.
Setting
Primary Care Center at Children’s Hospital of Pittsburgh from August 2004 through April 2005.
Participants
One hundred ninety parents (140 African American and 50 white parents).
Outcome Measure
Parental distrust of medical research as measured by a summative score of distrusting responses to 8 questions assessing trust in research.
Results
African American parents had significantly greater distrust than white parents (67% vs 50%, P=.04). Education was also associated with having significantly greater distrust (74% of those with <high school education vs 44% of college graduates, P=.03). However, African American race remained a predictor of distrust even when education was controlled for (odds ratio, 2.25; 95% confidence interval, 1.01–5.01).
Conclusions
The degree of parental distrust toward medical research was significantly greater among African American parents. Parental distrust may be a barrier to enrollment of African American children in clinical research. Strategies for overcoming the higher level of distrust in African American parents are warranted for ensuring adequate representation of African American children in clinical research.
Robust representation of ethnic minorities and children in research is critical for generalization of research findings, and the National Institutes of Health have mandated that researchers ensure such representation.1–3 Among minority groups, African Americans are frequently under-represented in clinical research. Poverty, lack of awareness and access to medical care, transportation and parking, and social and cultural norms combined with individual attitudes may contribute to reluctance to participate in clinical research.4–10 African Americans’ distrust of medical research has been suggested to be an important reason for their lack of participation. This distrust may be attributed both to a cultural memory of victimization and exploitation during clinical experiments, such as in the Tuskegee Syphilis Study,4,11–15 and to personal experiences with discrimination. Recent studies with adults suggest that the Tuskegee Syphilis Study is not a factor driving unwillingness to participate in research.16–18
Parents’ attitudes, prior experiences, and cultural beliefs determine whether or not their children are enrolled as research subjects. Although previous studies have investigated factors that influence parents’ decisions to enroll their children in clinical research,19–21 the literature does not specifically address the role of parental trust and racial differences. We conducted an exploratory study to assess the attitudes and prior experiences of African American and white parents who were attending a primary care clinic. Subjects were asked to complete a survey regarding participation of their children in research. We also examined differences in trust in medical research by race and investigated the relationship of distrust with prior participation and the effectiveness of incentives for participation in a hypothetical study.
A challenge of studying trust is those with high levels of distrust may not participate. Thus, the range of trust assessed may be more limited than in the overall population. In addition, in a clinic setting, a higher level of trust among patients is likely to be present. However, it is exactly this population that is often approached to participate in research, and their levels of trust are important and worth examining.
METHODS
From August 2004 through April 2005, a cross-sectional convenience sample of parents accompanying their child to the Primary Care Center at Children’s Hospital of Pittsburgh completed a self-administered questionnaire. Surveys were distributed and collected by 3 research assistants, all of whom were African American. All parents accompanying their child to the clinic were eligible. Parents were approached in the waiting room on different days of the week and at different times and were asked to complete the questionnaire while waiting for an appointment. A research assistant was available to answer any questions, and daily field notes were kept and regularly reviewed with the authors. Parents completed the surveys in private and returned them to the research assistant. Although no statistics on refusal to participate were kept, field notes from the research assistants indicated that the refusal rate was low and the primary reason given for refusal was lack of time before the appointment.
The Primary Care Center is a busy general pediatric clinic. There were 15 368 outpatient visits during the study period. The racial distribution of children seen in the clinic was 70% African American, 20% white, and 10% other. About 75% of children seen at the clinic were eligible for Medicaid, thus most parents were of lower socioeconomic status. A comparison of clinic demographics with the racial distribution and income level of the convenience sample indicates rough similarity. The University of Pittsburgh’s institutional review board approved the study.
OUTCOME MEASURES
The questionnaire was modified from a random-digit dialing telephone survey developed by Thomas and colleagues at the Centers for Disease Control and Prevention. These questions were designed to assess factors associated with adult participation in medical research.7 This adult survey was based on formative research exploring African American views on research and the Tuskegee Syphilis Study and was supplemented by questions used by the Presidential Advisory Committee on Human Radiation Experiments.7,12,22
In our study, modifications to the survey consisted of rewording and framing items to assess parents’ attitudes about their child’s participation and experiences. Additionally, we eliminated questions that were not related to children and added a few supplemental questions specifically about children. The final questionnaire consisted of 35 items. Nine assessed demographic characteristics; 8 assessed parents’ attitudes toward their child’s medical care; 14 assessed attitudes of medical research and participation in medical research; and 4 addressed the use of incentives to increase participation in a hypothetical study. All questions were close-ended and primarily had 4- and 5-point Likert scales or yes-or-no answers. The Flesch-Kincaid reading level of the questions was grade 8.3 according to Microsoft Word.
Trust was an independent variable. For the purpose of this study, trust and distrust should be viewed as 2 ends of a single continuum or as independently varying attitudes, each of which may affect participation in research. We chose to adopt the latter view and operationalize a measure of distrust in medical research because of the focus on African American distrust and the actual face validity of the questions. Our goal was to create a composite measure of distrust from the attitudinal questions, adding value beyond a simple examination of each question.
Using the original questionnaire by Thomas and colleagues, Corbie-Smith et al7 identified a 7- item distrust index. Because of questionnaire modifications and our study’s exploratory nature, the attitudinal questions were analyzed. Exploratory factor analyses (not reported) were conducted to investigate underlying dimensions. To measure distrust in medical research, a final set of 8 questions were selected based on face validity, measurement properties, conceptual similarity, internal consistency, and previous findings in the literature. Six of the 8 were modifications of questions used in the Corbie-Smith index.
Because the questions did not share a response set, they were dichotomized (indicating higher vs lower levels of distrust) to simplify construction of a measure. The response “Don’t know” was classified as indicating higher distrust. We justify this strategy with the fairly extreme wording of the items regarding trust; not knowing if one agrees or disagrees with these questions would indicate potential distrust. A composite distrust score was created by summing the number of distrusting responses. This score ranged from 0 to 8, with higher scores indicating higher levels of distrust. Cronbach α for the 8 questions was 0.66.
To assess the effects of higher and lower distrust, the score was dichotomized at the median value of 2.0. Low distrust was defined as a score of 1 or lower, high distrust as a score of 2 or higher. We chose this relatively low cutoff because any degree of distrust is significant in a study population recruited in a clinic setting. In this context, trust levels are likely to be higher compared with the general population.
As a form of construct validity, the association of the distrust score with missing information on the income question (which likely stems from a related form of distrust) was investigated. Parents with missing data on income had significantly higher levels of distrust compared with those who provided it (80% vs 20%, respectively, P=.001), lending some support to the validity of the measure.
STATISTICAL ANALYSIS
We used SPSS, version 15 (SPSS Inc, Chicago, Illinois), and Stata, version 9 (Stata Corp, College Station, Texas), to analyze the data. For bivariate analysis, χ2 and Fisher exact tests were used for categorical data and a t test was used for continuous data, with a significance level of P ≤ .05. Logistic and ordinary least-squares regressions were used to assess correlates of distrust controlling for covariates.
RESULTS
DEMOGRAPHIC CHARACTERISTICS
A total of 231 parents completed the survey. The racial distribution of the participating parents was similar to the clinic demography: 60% African American, 22% white, 9% other, and 9% refused. As the focus of this article is the difference between self-identified African American and white parents, we report only the data for these groups. Demographic characteristics of the parents are summarized in Table 1 by race. There were no significant differences between racial groups by sex, age, employment, or number of children. Statistically significant differences were noted for marital status, education, and income. African American parents were more likely to be unmarried (P = .001) and to have less education (P = .008) and lower incomes (P = .01). About 80% of African American and 85% of white participants had a high school education or higher; thus, literacy levels were not a major issue in questionnaire administration.
Table 1.
Demographic Characteristics of Participants
| Participants, No. (%) |
||||
|---|---|---|---|---|
| Characteristic | African American (n=140) | White (n=50) | P Value | |
| Sex | ||||
| M | 14 (10.2) | 9 (18.0) | ||
| F | 123 (89.8) | 41 (82.0) | ||
| Age, y | ||||
| <20 | 13 (10.2) | 2 (4.1) | ||
| 20–29 | 69 (54.3) | 24 (49.0) | ||
| ≥30 | 45 (35.4) | 23 (46.9) | ||
| Education | ||||
| <High school | 27 (20.6) | 7 (14.6) | .008a | |
| High school graduate | 34 (26.0) | 16 (33.3) | ||
| Some college, 1–3 y | 59 (45.0) | 13 (27.1) | ||
| College graduate | 11 (8.4) | 12 (25.0) | ||
| Employed | ||||
| Yes | 72 (56.3) | 32 (68.1) | ||
| No | 56 (43.8) | 15 (31.9) | ||
| Income, $ | ||||
| <10 000 | 35 (39.3) | 6 (15.0) | .01a | |
| 10 000–19 999 | 22 (24.7) | 11 (27.5) | ||
| 20 000–29 999 | 19 (21.3) | 9 (22.5) | ||
| ≥30 000 | 13 (14.6) | 14 (35.0) | ||
| Marital status | ||||
| Not married | 93 (72.1) | 20 (42.6) | .001b | |
| Married or living with partner | 36 (27.9) | 27 (57.4) | ||
| No. of children in household | ||||
| 0–1 | 43 (32.6) | 19 (38.0) | ||
| 2 | 36 (27.3) | 18 (36.0) | ||
| 3 | 27 (20.5) | 10 (20.0) | ||
| ≥4 | 26 (19.7) | 3 (6.0) | ||
χ2 Test.
Fisher exact test.
DISTRUST IN MEDICAL RESEARCH AND TREATMENT
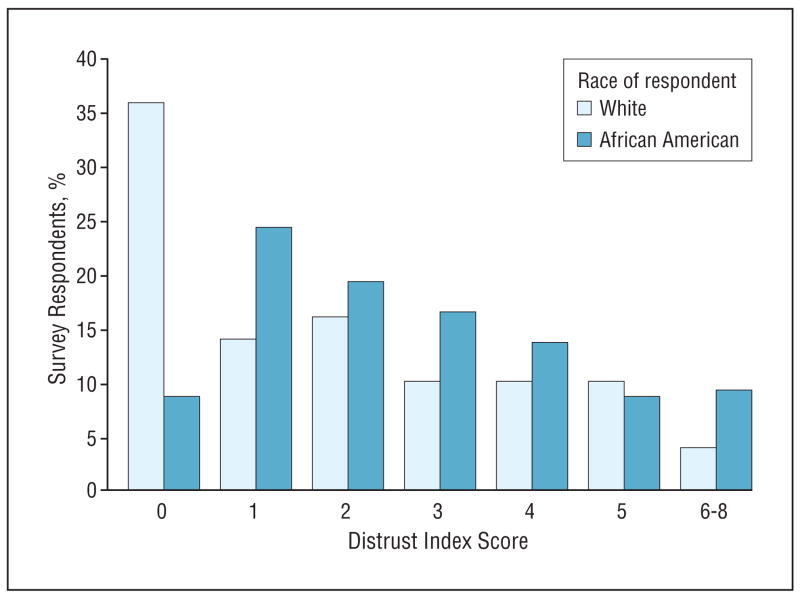
The percentage of respondents who gave a distrusting response are presented in Table 2 by race and survey question. African American parents were more likely than white parents to believe that medical research involves too much risk to the participant (46.8% vs 26.0%, P=.01), that physicians will not make full disclosure regarding their child’s participation (24.6% vs 10.0%, P=.04), and that participants in medical research will be favored and receive better medical care (48.6% vs 28.0%, P=.01). It should be noted that the overall level of trust toward research and medicine was relatively high among both groups. The Figure shows the distribution of distrust scores by race. A t test indicated that African American parents had a significantly higher mean ordinal distrust score compared with white parents (mean [standard deviation], 2.7 [1.9] vs 1.9 [2.0], P=.01).
Table 2.
Percentage of Participants Who Gave Distrusting Responses to Survey Questions
| Participants With Distrusting Responses, % |
|||
|---|---|---|---|
| Survey Item | African American (n=140) | White (n=50) | P Valuea |
| “With regard to treatments your child has received, how often do you feel that your child has been exposed to unnecessary risks?” (answered “very often,” “fairly often,” or “don’t know”) | 12.4 | 14.0 | |
| “How often have you felt that doctors had given your child any treatment that was experimental in nature without asking your permission?” (answered “very often,” “fairly often,” or “don’t know”) | 7.1 | 10.0 | |
| “Do you believe that doctors prescribe medication as a way of experimenting on people without their knowledge or permission?” (answered “definitely believe,” “probably believe,” or “don’t know”) | 40.0 | 28.0 | |
| “If a doctor wanted your child to participate in medical research, how much do you trust he or she would fully explain it to you?” (answered “not trust at all” or “don’t know”) | 24.6 | 10.0 | .04 |
| “How often do you believe that a doctor would ask you to allow your child to participate in medical research if he or she thought it would harm your child?” (answered “frequently,” “occasionally,” or “don’t know”) | 39.6 | 30.0 | |
| “How often do you think that medical research involves too much risk to the people who participate in it?” (answered “always,” “very frequently,” or “don’t know”) | 46.8 | 26.0 | .01 |
| “How often do you think patients in medical research get better health care than other patients?” (answered “always,” “most of the time,” or “don’t know”) | 48.6 | 28.0 | .01 |
| “How often do you think the doctors running the medical research care more about the research than the people they study?” (“always,” “very frequently,” or “don’t know”) | 51.8 | 46.0 | |
Fisher exact test.
Figure.
Association between distrust index score and race. Higher scores indicate higher levels of distrust of medical research.
Table 3 shows the association between the demographic variables and higher levels of distrust (defined as a distrust score of ≥2). Overall, 62.6% of respondents had a high distrust score. We observed significant differences in distrust by race, education, and number of children. African American parents were 2 times more likely than white parents to have a high distrust score (95% confidence interval [CI], 1.06–3.94). In addition, education was significantly inversely associated with high distrust scores; college graduates were less likely to have a high distrust score compared with respondents with less than a high school education (odds ratio [OR], 0.28; 95% CI, 0.09–0.85). Finally, respondents living in households with 4 or more children had higher distrust scores than those living with 1 or no children (OR, 3.16; 95% CI, 1.13–8.83).
Table 3.
Likelihood of Having a High Distrust Score by Demographic Characteristics
| Predictor | Respondents, No. (%)(n=190) | Odds Ratio (95% Confidence Interval) |
|---|---|---|
| Overall | 119 (62.6) | |
| Race | ||
| White | 25 (50.0) | 1 [Reference] |
| African American | 94 (67.1) | 2.04 (1.06–3.94)a |
| Sex | ||
| M | 16 (69.6) | 1 [Reference] |
| F | 101 (61.6) | 0.70 (0.27–1.80) |
| Age, y | ||
| <20 | 10 (66.7) | 1 [Reference] |
| 20–29 | 55 (59.1) | 0.72 (0.23–2.29) |
| ≥30 | 42 (61.8) | 0.81 (0.25–2.63) |
| Education | ||
| <High school | 25 (73.5) | 1 [Reference] |
| High school graduate | 32 (64.0) | 0.64 (0.25–1.67) |
| Some college, 1–3 y | 43 (59.7) | 0.53 (0.22–1.31) |
| College graduate | 10 (43.5) | 0.28 (0.09–0.85)a |
| Employed | ||
| Yes | 65 (62.5) | 1 [Reference] |
| No | 42 (59.2) | 0.87 (0.47–1.61) |
| Income, $ | ||
| <10 000 | 23 (56.1) | 1 [Reference] |
| 10 000–19 999 | 20 (60.6) | 1.20 (0.47–3.06) |
| 20 000–29 999 | 17 (60.7) | 1.21 (0.46–3.22) |
| ≥30 000 | 11 (40.7) | 0.54 (0.20–1.44) |
| Marital status | ||
| Not married | 72 (63.7) | 1 [Reference] |
| Married or living with partner | 37 (58.7) | 0.81 (0.43–1.52) |
| No. of children in household | ||
| 0–1 | 34 (54.8) | 1 [Reference] |
| 2 | 33 (61.1) | 1.29 (0.62–2.72) |
| 3 | 23 (62.2) | 1.35 (0.59–3.11) |
| ≥4 | 23 (79.3) | 3.16 (1.13–8.83)a |
P <. 05.
The association between race and distrust was further investigated using logistic regression (not reported) examining correlates of a distrust score of 2 or higher while adjusting for all demographic covariates except income. Income was excluded because of the large amount of missing data (67% of respondents provided income data). Both race and education remained significant predictors of high distrust in the regression, while number of children in the household was no longer significant. African American parents were 2 times more likely to be distrusting of medical research than white parents after controlling for education level (OR, 2.25; 95% CI, 1.01–5.01). Similar ordinary least-squares regressions using the ordinal measure of distrust as the outcome produced similar results (not reported).
ASSOCIATION OF DISTRUST WITH ATTITUDES TOWARD AND PARTICIPATION IN MEDICAL RESEARCH
Table 4 shows the association of distrust with respondents’ attitudes toward and experiences with medical research by race. The overall attitude toward medical research was quite favorable in both racial groups. However, African American parents with high distrust were significantly less likely to have a favorable attitude than those with low distrust (OR, 0.39; 95% CI, 0.16–0.97). There was a similar trend in white parents, though the association was not significant owing to smaller sample size.
Table 4.
Likelihood of Participants Having an Attitude or Experience Related to Medical Research by Distrust Score and Race
| African American (n=140) |
White (n=50) |
|||||
|---|---|---|---|---|---|---|
| Participants, No. (%) |
Participants, No. (%) |
|||||
| Attitude or Experience | Low Distrust | High Distrust | OR (95% CI) | Low Distrust | High Distrust | OR (95% CI) |
| General favorable attitude toward medical research | 39 (84.4) | 63 (67.7) | 0.39 (0.16–0.97)a | 20 (80.0) | 15 (60.0) | 0.38 (0.11–1.33) |
| Ever participated in medical research | 20 (43.5) | 30 (32.3) | 0.62 (0.30–1.28) | 11 (45.8) | 10 (40.0) | 0.79 (0.25–2.45) |
| Child ever participated in medical research | 15 (33.3) | 18 (19.4) | 0.48 (0.22–1.07)b | 10 (40.0) | 5 (24.0) | 0.47 (0.14–1.60) |
| More likely to enroll their child “in a medical research study that required blood draw and x-ray” if given incentive | ||||||
| Free medical care | 17 (37.0) | 13 (14.1) | 0.28 (0.12–0.65)c | 6 (24.0) | 5 (20.0) | 0.79 (0.21–3.03) |
| Free transportation | 16 (35.6) | 19 (20.4) | 0.47 (0.21–1.03)b | 6 (24.0) | 3 (12.0) | 0.43 (0.10–1.97) |
| $100 | 16 (34.8) | 19 (20.7) | 0.49 (0.22–1.08)b | 8 (32.0) | 7 (28.0) | 0.83 (0.25–2.78) |
| Free medicine | 9 (20.5) | 15 (16.3) | 0.76 (0.30–1.90) | 6 (24.0) | 5 (20.0) | 0.79 (0.21–3.03) |
| Any of the incentives | 25 (54.3) | 30 (31.9) | 0.39 (0.19–0.81)c | 9 (36.0) | 9 (36.0) | 1.00 (0.32–3.17) |
Abbreviations: CI, confidence interval; OR, odds ratio.
P ≤ .05.
P ≤ .10.
P ≤ .01.
A fairly high percentage of both parents and children had previously participated in medical research. Distrust was not significantly associated with parents’ previous research participation. A significantly higher percentage of children of parents with low levels of distrust had participated in medical research compared with children of parents with high levels of distrust (36% vs 20%, P = .02). When stratified by race (Table 4), the association of high distrust with parents’ and children’s previous participation in medical research, though not statistically significant, was stronger (as suggested by the narrower CI) in African American parents than in white parents owing to smaller sample size, especially with regard to children’s previous participation (African American children: OR, 0.48; 95% CI, 0.22–1.07). Distrust was not significantly associated with previous participation for white parents.
Parents were also asked whether incentives ($100 and free medical care, transportation, and medicine) would affect their decision about allowing their child to participate in a hypothetical research study that would require a blood draw and radiography. Overall, incentives were moderately effective in increasing the likelihood of participation (38% reported that ≥1 of the incentives would encourage them to participate). When stratified by race (Table 4), there was no significant association between distrust and participation with incentives for white parents. For African American parents, however, higher distrust was significantly negatively associated with the effectiveness of free medical care as an incentive (OR, 0.28; 95% CI, 0.12–0.65) and was marginally negatively associated with the effectiveness of free transportation (OR, 0.47; 95% CI, 0.21–1.03) and $100 (OR, 0.49; 95% CI, 0.22–1.08).
COMMENT
The results of this study demonstrate that parents’ distrust in medicine and research is an important determinant of enrolling their children in research. Although a few studies have looked at the characteristics of consenting parents, our study is the first to explore the effect of race on participation.19–21 We found that African American parents have greater distrust toward medical research than white parents. African American parents were significantly more likely than white parents to believe that medical research involved too much risk to the participant; that physicians will not make full disclosures regarding their child’s participation; and that participants in medical research will be favored and will receive better medical care.
Less education and more children in the household were also significantly associated with greater parental distrust. Adjusting for covariates, we found that race and education alone remained independent correlates of greater distrust. Thus, the greater degree of distrust in African American parents in this sample was not explained by their demographic characteristics.
In our sample, parental education was negatively associated with distrust, with distrust highest in those who had not graduated from high school and lowest among college graduates. However, Harth and Thong21 reported that volunteering parents in Australia, when compared with nonvolunteering parents, who were approached for enrollment of their children into a clinical trial had lower levels of education, lower levels of employment, greater health-seeking behavior, and a greater degree of trust. Also contradictory to our findings regarding the relationship between education and trust are the findings of Moseley et al,23 who, in a cross-sectional survey of parents’ trust in their child’s physician, observed lower levels of trust in those who had more than a high school education and private insurance. These findings suggest that well-educated parents from higher socioeconomic strata are likely to be more knowledgeable and may have higher expectations from their child’s physician and could have lower levels of trust if their expectations are not met. We believe the relationship between parental education, trust, and willingness to enroll one’s child in research is complex and multifactorial and requires further study.
Additionally, it seems likely that other aspects of socioeconomic status may be important determinants of parental distrust of medical research. When the subset of respondents who reported their income was further analyzed, the association between race and distrust was attenuated somewhat, suggesting that part of this association may be because of both education and income. Additional studies are warranted to examine the relationship between parental distrust, race, income, education, and other indicators of socioeconomic status. Future studies should attempt to measure wealth as a more complex income variable.
We were reassured that the overall attitude toward medical research was favorable among both African American and white parents. However, African American parents with less distrust had a significantly more favorable attitude toward medical research than those with greater distrust, and a similar nonsignificant trend was observed in the white parents. Clearly, gaining the trust of parents may help increase the participation of their children in clinical research.
Among African American parents, there was a marginally significant association between lower levels of distrust and a history of their child participating in research. Although the causal direction of this relationship is unknown, it is likely that positive experiences in research participation may lead to greater levels of trust and perhaps to greater willingness to participate in the future. Thus, greater personal knowledge about medical research is one way to dispel some of the distrust and fears about research. The use of culturally appropriate recruitment materials as well as using research assistants with similar racial and cultural backgrounds as the subject population can help provide accurate information and quell parental distrust toward clinical research. Establishment of community research advisory boards, which provide feedback at all stages of a research study, as has been done in Pittsburgh, is another means to ensure that minority community members participate and to disseminate information about studies while protecting the interests of research subjects and potentially lessening distrust.24
Incentives have always been perceived as an essential tool for research enrollment. This study suggests that their effectiveness may be attenuated by a greater level of distrust, more so among African American parents than white parents. Thus, incentives to participate are less likely to be effective among African Americans with high distrust compared with those with low distrust. In a similar finding among pediatric asthma research participants and their parents, financial compensation was not a significant factor influencing their decision to enroll.25 Incentives are important but may be insufficient to overcome cultural barriers among African Americans. The relationship between research compensation and trust may be more complex than usually thought and deserves further exploration.
There are several limitations to our study. The data are based on the opinions of a convenience sample of parents at a single institution and thus have limited generalizability. The research is descriptive and intended to suggest avenues for future exploration. In addition, the opinions regarding incentives and research participation are hypothetical. Furthermore, it is likely that the degree of distrust that participating parents expressed is underestimated, as they were willing to take the survey despite any potential underlying distrust.
Despite these limitations, we found that African American parents had higher levels of distrust of medical research than white parents and that this may present a barrier to enrollment of their children in clinical research. Even if their rates of enrollment are not affected, the issue of higher levels of distrust among minority parents must be addressed because it could affect adherence to protocol and retention.26 To be altruistic, the decision to enroll one’s child in clinical research has to be value-based, not coerced, with a clear understanding of the risks and benefits of the study and a sense of empowerment to dissent, without distrust or fear of losing medical services.27
CONCLUSIONS
Although the overall attitude toward medicine and research was positive in both African American and white parents, the degree of distrust was significantly greater among African American parents. Our data suggest that African American parents with higher levels of distrust are less likely to enroll their children in clinical research. Additionally, traditional incentives (financial compensation and free medicine, transportation, and medical care) did not overcome the barrier of high distrust. Strategies for overcoming the distrust in medicine and research among African American parents are warranted to ensure adequate representation of African American children in clinical research.
Acknowledgments
Funding/Support: This study was supported in part by grant P60 MD000207 from the National Center on Minority Health Disparities, National Institutes of Health, and grant K23HD052550 from the National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Author Contributions: Dr Rajakumar had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Rajakumar and Thomas. Acquisition of data: Rajakumar and Thomas. Analysis and interpretation of data: Musa, Almario, and Garza. Drafting of the manuscript: Rajakumar, Thomas, Almario, and Garza. Critical revision of the manuscript for important intellectual content: Rajakumar, Thomas, Musa, Almario, and Garza. Statistical analysis: Musa and Almario. Obtained funding: Thomas. Administrative, technical, and material support: Rajakumar, Thomas, Almario, and Garza. Study supervision: Rajakumar.
Financial Disclosure: None reported.
Additional Contributions: Eugenia Mosby, Ina A. Ramos, MPH, and Mario C. Browne, MPH, CHES, from the Center for Minority Health, University of Pittsburgh, assisted in data collection for this project.
References
- 1.US Department of Health and Human Services. NIH guidelines on the inclusion of women and minorities as subjects in clinical research. Notice Fed Reg. 1994;59:14508–14513. [Google Scholar]
- 2.US Department of Health and Human Services. [Accessed November 4, 2007.];NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research: amended. 2001 October; http://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm.
- 3.US Department of Health and Human Services. [Accessed November 4, 2007.];NIH policy and guidelines on the inclusion of children as participants in research involving human subjects. http://grants.nih.gov/grants/guide/notice-files/not98-024.html Published March 6, 1998.
- 4.Clay C, Ellis MA, Amodeo M, Fassler I, Griffin ML. Recruiting a community sample of African American subjects: the nuts and bolts of a successful effort. J Con-temp Soc Serv. 2003;84:396–404. [Google Scholar]
- 5.Ballard EL, Nash F, Raiford K, et al. Recruitment of black elderly for clinical research studies of dementia: the CERAD experience. Gerontologist. 1993;33 (4):561–565. doi: 10.1093/geront/33.4.561. [DOI] [PubMed] [Google Scholar]
- 6.DeNavas-Walt C, Proctor BD, Smith JUS. Census Bureau, Current Population Reports, P60-233, Income, Poverty, and Health Insurance Coverage in the United States: 2006. Washington, DC: US Government Printing Office; 2007. [Google Scholar]
- 7.Corbie-Smith G, Thomas SB, Marie DM, et al. Distrust, race, and research. Arch Intern Med. 2002;162(21):2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 8.Corbie-Smith GM. Minority recruitment and participation in health research. N C Med J. 2004;65(6):385–387. [PubMed] [Google Scholar]
- 9.Ferrari A, Montello M, Budd T, et al. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer. 2008;50(5 suppl):1101–1104. doi: 10.1002/pbc.21459. [DOI] [PubMed] [Google Scholar]
- 10.Lara PN, Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282–9289. doi: 10.1200/JCO.2005.02.6245. [DOI] [PubMed] [Google Scholar]
- 11.Napoles-Springer AM, Grumbach K, Alexander M, et al. Clinical research with older African Americans and Latinos. Res Aging. 2000;22(6):668–691. [Google Scholar]
- 12.Freimuth VS, Quinn SC, Thomas SB, et al. African American’s views on research and the Tuskegee Syphilis study. Soc Sci Med. 2001;52(5):797–808. doi: 10.1016/s0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 13.Corbie-Smith G, Thomas SB, Williams MV, et al. Attitudes and beliefs of African Americans towards participation in medical research. J Gen Intern Med. 1999;14(9):537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones J. Bad Blood: The Tuskegee Syphilis Experiment. New York, NY: Macmillan Publishing Co Inc; 1993. [Google Scholar]
- 15.Boulware LE, Cooper LA, Ratner LE, et al. Race and trust in the health care system. Public Health Rep. 2003;118(4):358–365. doi: 10.1016/S0033-3549(04)50262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandon DT, Issac LA, LaVeist TA. The legacy of Tuskegee and trust in medical care: is Tuskegee responsible for race differences in mistrust of medical care? J Natl Med Assoc. 2005;97(7):951–956. [PMC free article] [PubMed] [Google Scholar]
- 17.Katz RV, Kegeles SS, Kressin NR, et al. The Tuskegee legacy project: willingness of minorities to participate in biomedical research. J Health Care Poor Underserved. 2006;17(4):698–715. doi: 10.1353/hpu.2006.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz RV, Kegeles SS, Kressin NR, et al. Awareness of the Tuskegee syphilis study and the US Presidential apology and their influence on minority participation in biomedical research. Am J Public Health. 2008;98(6):1137–1142. doi: 10.2105/AJPH.2006.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tait AR, Voepel-Lewis T, Malviya S. Participation of children in clinical research: factors that influence a parent’s decision to consent. Anesthesiology. 2003;99(4):819–825. doi: 10.1097/00000542-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Tait AR, Voepel-Lewis T, Siewert M, et al. Factors that influence parent’s decision to consent to their child’s participation in clinical anesthesia research. Anesth Analg. 1998;86(1):50–53. doi: 10.1097/00000539-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Harth SC, Thong YH. Sociodemographic and motivational characteristics of parents who volunteer their children for clinical research: a controlled study. BMJ. 1990;300(6736):1372–1375. doi: 10.1136/bmj.300.6736.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Advisory Committee on Human Radiation Experiments. The Human Radiation Experiments. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 23.Moseley KL, Clark SJ, Gebremariam A, et al. Parents’ trust in child’s physician: using an adapted trust in physician scale. Ambul Pediatr. 2006;6(1):58–61. doi: 10.1016/j.ambp.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Quinn SC. Protecting human subjects: the role of community advisory boards. Am J Public Health. 2004;94(6):918–922. doi: 10.2105/ajph.94.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherer DG, Brody JL, Annett RD, et al. Financial compensation to adolescents for participation in biomedical research: adolescent and parent perspectives in seven studies. J Pediatr. 2005;146(4):552–558. doi: 10.1016/j.jpeds.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Walsh C, Ross LF. Are minority children under- or overrepresented in pediatric research? Pediatrics. 2003;112(4):890–895. doi: 10.1542/peds.112.4.890. [DOI] [PubMed] [Google Scholar]
- 27.Volunteering for research. Lancet. 1992;340(8823):823–824. [PubMed] [Google Scholar]