Abstract
Biodegradable nanofibers simulate the fibril structure of natural extracellular matrix, and provide a cell-friendly microenvironment for tissue regeneration. However, the effects of nanofiber organization and immobilized biochemical factors on cell infiltration into three-dimensional scaffolds are not well understood. For example, cell infiltration into an electrospun nanofibrous matrix is often limited due to relatively small pore size between the fibers. Here we showed that biophysical and biochemical modification of nanofibrous scaffolds facilitated endothelial cell infiltration in three-dimensional scaffolds in vitro and in vivo. Aligned nanofibers significantly enhanced cell infiltration into the nanofibrous matrices in vitro. In a full-thickness dermal wound model, the nanofiber scaffolds enhanced epidermal skin cell migration across the wound when compared to a control group without scaffold. Aligned nanofibers promoted the infiltration of endothelial cells into the scaffolds. Furthermore, heparin-coated nanofibers also increased cell infiltration significantly. These results shed light on the importance of biophysical and biochemical properties of nanofibers in the regulation of cell infiltration into three-dimensional scaffolds and tissue remodeling.
Keywords: Nanotopography, Scaffold, Heparin, Electrospinning, Nanofibers, Tissue engineering, Wound healing, Endothelial cells, Polylactic acid
Introduction
Dermal wound healing is a complex process requiring coordination of several biological processes, including ingrowth of cells, organization of extracellular matrix (ECM), regulation of inflammation, and rapid wound coverage to prevent infection [1]. For many wounds however, the “repair” mechanism often dominates over a more desirable “regeneration” mechanism, resulting in the formation of disorganized scar tissue instead of well-ordered skin [2]. One potential solution to this problem is to create tissue engineered scaffolds with properties that can enhance the natural wound healing process, such as aligned, organized, or bioactive fibrils to replace lost/damaged ECM and to guide new cells directly into the wound area with improved speed and overall organization.
Native ECM contains biological fibrils with diameters ranging from tens of nanometers to micrometers in scale. The organized structure of these matrix fibrils guides tissue morphogenesis and remodeling. In addition, matrix fibrils serve as “depots” for the storage of bioactive factors for the regulation of cell migration, proliferation and differentiation. Synthetic polymers can be specifically engineered (both physically and chemically) to aid tissue regeneration, and it is known that the surface microstructure and chemistry of these engineered substrates can influence the ability of the cells and tissues to attach, grow and function [3]. Electrospinning technology has been used to fabricate nonwoven nanofibrous scaffolds from biological and/or synthetic polymers, and has tremendous potential for tissue engineering applications [4–7]. The diameter of the individual fibers can be specifically controlled down to the nanometer range, and the fibers can be patterned through a variety of methods [8–10]. Moreover, the electrospun nanofibers have large surface area to volume ratio, which allows for the direct attachment of ECM ligands, growth factors and other biomolecules onto fiber surfaces to locally modulate cell and tissue function and to guide and enhance regeneration. Although the patterning of the nanofibers has been shown to influence the alignment of cells and cellular processes [11–15], the effects of patterned and bioactive nanofibers on cellular migration and dermal wound healing have not been fully elucidated, especially with regard to the infiltration of cells into three-dimensional (3-D) scaffolds.
One limitation of electrospun nanofibrous scaffolds is the relatively small pore size (in comparison to the average diameter of most cells) and the resultant difficulty for cell infiltration into the 3-D structure, which retards matrix remodeling and tissue regeneration. Recently, salt-leaching methods have been used to increase the pore size of the electrospun scaffolds [16], but these methods still face the problems of collapse of fibrous structure after removing the salt. Our previous study showed excellent cell infiltration into vascular grafts with nanofibers aligned in the circumferential direction [11], but whether aligned fibers enhanced cell infiltration was not clear. In this study, we fabricated aligned nanofibrous scaffolds and studied their effect on cell infiltration in vitro and in vivo by using a dermal wound healing model. In addition, chemical modification of nanofibers could affect cell infiltration by directing cellular behavior and modifying physiological conditions. Since heparin can bind to many growth factors (e.g., basic fibroblast growth factor, epidermal growth factor, etc.) and matrix proteins (fibronectin) and can prevent clotting, we also investigated whether heparin modification of nanofibers can enhance dermal cell infiltration into 3-D scaffolds.
MATERIALS AND METHODS
Electrospinning of Nanofibrous Polymer Scaffolds
We used biodegradable poly(L-lactide) (PLLA) (1.09 dL/g inherent viscosity) (Lactel Absorbable Polymers, Pelham, AL) to fabricate nanofibrous scaffolds by electrospinning, as described previously [11–13]. The PLLA (10% w/v) solution (dissolved in hexafluoroisopropanol (HFIP) solvent) was delivered by a programmable pump to an electrically charged needle, which formed a nanoscale polymer fiber at the needle tip. The electrostatically charged fiber was ejected toward a grounded collecting drum in the high electric field, resulting in a nonwoven nanofibrous membrane deposited on the drum. For each membrane produced, the alignment of the nanofibers was controlled during the electrospinning process by adjusting the rotational speed of the collecting drum. For randomly oriented nanofibers, a low speed of rotation was used (~20 rpm), whereas a high speed of rotation (~800 rpm) was used to generate nanofibers that were aligned in the direction of the rotation. Finalized nanofibrous scaffolds were approximately 200–400 μm in thickness, based on thickness gage (Mitutoyo America, Aurora, IL) measurements.
Chemical Functionalization and Characterization of Nanofibrous Scaffolds for Bioactivity
Heparin was conjugated to nanofibers by using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (sulfo-NHS) (Pierce Biotechnology, Rockford, IL) with or without di-amino-poly(ethylene glycol) as a linker [13]. The presence of heparin on nanofibers was verified by toluidine blue staining. Briefly, heparin-treated and non-heparin-treated membranes were incubated in toluidine blue solution (0.0005% in 0.001N hydrochloric acid with 0.02% (w/v) sodium chloride) on a shaker for 1 minute then promptly removed from solution. Purple coloring of the membranes indicated the presence of heparin.
Cell Culture
Bovine aortic endothelial cells (BAECs) were isolated from bovine aorta either with collagenase or by gently scraping with a rubber policeman as previously described [17]. All cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin antibiotic mix. Cell culture was maintained in a humidified incubator at 37°C and supplemented with 5% CO2.
In vitro Cell Infiltration Model
Random and aligned nanofibrous sheets of approximately 150–200 μm thickness were produced by electrospinning and were subsequently cut into 1-cm by 1-cm membranes. These membranes were sterilized in 70% ethanol while under germicidal ultraviolet light for 30 minutes and subsequently washed five times with sterile, deionized water. Afterwards, half of the sterilized random membranes and half of the sterilized aligned membranes were chemically functionalized via the conjugation of heparin, resulting in four groups of membranes: (1) Randomly oriented, heparin-modified nanofibers; (2) Aligned, heparin-modified nanofibers; (3) Randomly oriented nanofibers without heparin; and (4) Aligned nanofibers without heparin. A minimum of three nanofibrous membranes representing each of these four groups were then secured to non-tissue-culture-treated polystyrene dishes via sterile, double-sided tape.
BAECs were stained with DiI fluorescent cell tracker and seeded at 100% confluency onto the membranes. Seeding consistency among all four groups was confirmed by viewing the DiI fluorescent signal under a Zeiss Axioskop 2 microscope (Carl Zeiss MicroImaging Inc., Thornwood NY). The BAEC-seeded membranes were subsequently kept for seven days in a humidified incubator (37°C, 5% CO2). Sufficient media was used in each dish as to avoid the need to change the DMEM before the end of seven days.
At Day 7, each membrane was removed from the polystyrene dishes and cryopreserved in OCT compound (TissueTek, Elkhart, IN) on dry ice. Cross sections of 10-μm thickness were taken in the transverse plane in a −20°C cryosectioner. The DiI fluorescent signals from the cells within these cryosections were again viewed under a Zeiss Axioskop 2 microscope. Hematoxylin staining was subsequently performed to further visualize cell infiltration into the nanofiber membranes. A minimum of 3 cryosections were observed for each membrane to confirm consistency between sections.
Rat Dermal Wound Healing Model
To investigate the effects of the bioactive nanofibrous meshes in a full-thickness dermal wound healing model in vivo, a rat animal model was chosen based on several factors, including ease of procedure and biological relevance. All animal experiments were carried out at the David Grant Medical Center at the Travis Air Force Base (Fairfield, CA).
Standard laboratory rats (Rattus norvegicus) were individually housed in wire bottom cages in temperature- and light-controlled rooms. All animals were allowed to acclimate to the housing facility for 5–7 days prior to intervention and had ad libitum access to food and water. All procedures were in accordance with the Institutional Animal Care and Use Committee (IACUC) at David Grant Medical Center. Rats were randomly assigned into one of four groups on the basis of nanofibrous mesh alignment (respective to the long axis of the wound) and heparin-modification of the mesh: (1) Control – no mesh implanted, (2) Random – mesh having randomly oriented fibers, (3) Aligned – mesh having aligned fibers oriented perpendicular to the long (3cm) axis of the wound. (4) Random+heparin – mesh with randomly aligned heparin-conjugated fibers. The animals were then taken to the operating room under general anesthesia and sterile conditions, where a rectangular 1 × 3cm full thickness skin flap was removed from the upper back and hemostasis was achieved. Interrupted 5-0 Monocryl (Ethicon, Inc., Somerville, NJ) mattress sutures were then placed circumferentially to secure the nanofiber mesh to the skin and the underlying muscle fascia (supplemental figure 1). The control group received similarly placed sutures, but with no nanofibrous mesh. For each group, the number of animals tested was n=4.
All rats were recovered from anesthesia and treated daily with buprenorphine for analgesia and bacitracin ointment for wound care. All animals were monitored daily by a veterinarian and there were no adverse events noted in any of the animals. The rats were returned to the operating room on day 7, where they were given general anesthesia and an overdose of euthanasia solution. Digital photographs were taken of all wounds, and each wound was then excised to include wound edges, underlying fascia, and mesh. Tissue samples were immediately placed in either formaldehyde or OCT (TissueTek, Elkhart, IN), and processed in paraffin or OCT blocks, respectively, for standard histologic examination.
Histology and Immunohistochemistry
Tissue cross sections (10μm in thickness) were generated from the embedded tissue samples. All sections were taken in the transverse plane with respect to the long (3cm) axis of the original wound. Routine hematoxylin and eosin (H&E) staining was performed to assess the tissue morphology. To investigate neovascularization and endothelial cell migration within the wounds, the sections were stained using an antibody against endothelial marker, CD31 (BD Pharmingen, San Diego, CA) and the 4plus detection system (Biocare Medical, Concord, CA) according to the manufacturer's instructions. Additionally, monocytes and macrophages were stained (to investigate inflammatory response) with an antibody against CD68 (Santa Cruz Biotechnology, Santa Cruz, CA) and the 4plus detection system. Images were taken using a Zeiss Axioskop as described above. At least 3 sections were observed for each sample to insure consistency between sections.
RESULTS
Electrospinning of nanofibrous scaffolds and heparin modification
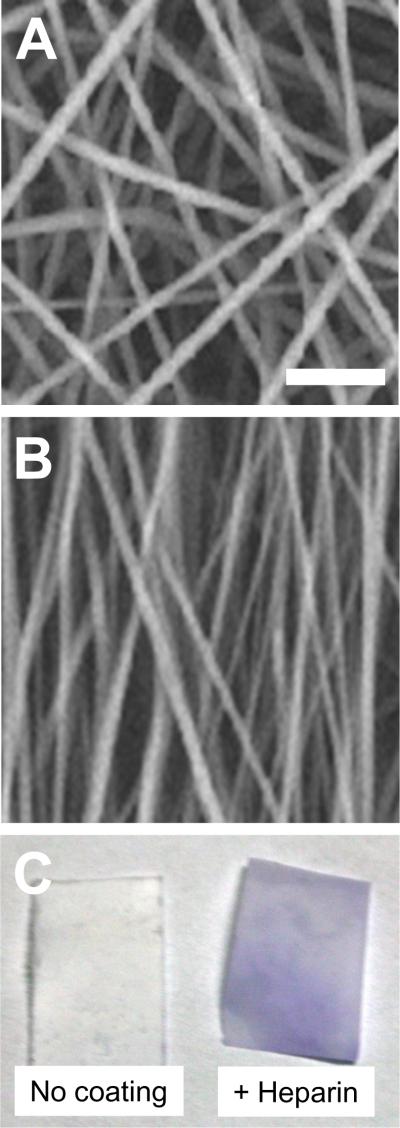
Before using the nanofibrous membranes for biological experimentation, they were characterized for desired fiber architecture and chemical functionalization. As shown in Figure 1A–B, we were able to obtain nanofibrous scaffolds with either random or aligned nanofibers by adjusting the rotation speed of the drum in electrospinning system, as described. Furthermore, toluidine blue staining verified the immobilization of heparin on the nanofibers (Figure 1C).
Figure 1.
Nanofiber organization and heparin modification. PLLA membranes were produced to be predominately composed of (A) randomly oriented nanofibers and (B) aligned nanofibers (with fibers aligned predominantly from top to bottom of image) by using low speed (~20 rpm) and high speed of rotation (~800 rpm), respectively. Scale bar=5 μm. (C) Heparin was conjugated to nanofibers, and stained by toluidine blue, showing purple color.
Effects of nanofiber alignment and heparin-modification on cell infiltration in vitro
To investigate the effects of nanofibrous scaffolds on cell infiltration in vitro, BAECs were seeded on the surface of nanofibrous scaffolds as described. After 7 days, a significant difference between BAEC infiltration into randomly oriented fiber meshes and aligned meshes was apparent (Figure 2). As evidenced by both the DiI cell tracking stain (Figure 2A) and hemotoxylin stain (Figure 2B), aligned nanofiber meshes enhanced cell infiltration into the thickness of the mesh when compared to randomly oriented nanofiber meshes, regardless of heparin-modification. Without nanofiber alignment, heparin-modification had no clear effect on the cells ability to penetrate into the membranes of randomly oriented nanofibers.
Figure 2.
BAEC infiltration into a nanofiber matrix in vitro after 7 days. (A) DiI staining of cells (in red) and (B) hemotoxylin staining of cells (dark purple).
Effects of nanofiber alignment and heparin-modification on would healing in vivo
Biological response to nanofibrous scaffolds was further characterized in an in vivo wound healing model as described. Digital photographs of wounds (Figure 3) were taken directly after graft implantation (day 0) and directly before harvesting (day 7). In all cases, nanofibrous PLLA grafts appeared to mildly improve eschar formation when compared to the control animal. However, no major differences in wound size were observed. Also, no major eschar differences were seen between the various PLLA grafts (eschar formation on all fiber orientations and chemical modifications appeared approximately equal in the wound photographs).
Figure 3.
Wound images with PLLA grafts. At day 0, grafts are implanted with appropriate nanofiber alignment and orientation with respect to the long axis of the wound. In this perspective, “aligned” means aligned nanofibers are oriented left to right in the plane of the image. At day 7, eschar formation is seen in all experimental groups.
Since no major differences in the wounds were observed at a macroscopic level, histological staining was employed to more closely investigate the microscopic effects of nanofibrous PLLA meshes on dermal wound healing in rats. H&E staining of healthy native skin tissue was performed in order to visualize the natural epidermis, dermis, and hypodermis (Supplemental Figure 2). These were used as reference for the H&E staining of wounds with the implanted PLLA grafts discussed above (Figure 4). When comparing wounds with grafts to that without (i.e. “control” wound), it was clearly evident that the epidermal layer grew/migrated out from the original wound edge toward the nanofibrous graft, whereas the epidermal layer showed no growth or migration from the wound edge when no graft was present. However, there was no obvious difference in epidermal growth/migration between the various implanted graft types. Furthermore, positive H&E staining within the grafts (Figure 4) illustrated that cells migrated into the scaffolds. Since H&E staining alone could not confirm which types of cells were within the scaffolds, immunohistochemical (IHC) staining was employed next.
Figure 4.
H&E staining of wounds with PLLA grafts 7 days after wounding and implantation. Sections are transverse to the long axis of the wound, and are taken near the midpoint of the long axis. Images are taken at edge of wound to show interface between remaining healthy tissue and PLLA graft (no graft in “control” sample). Red circle indicates epidermal layer migration from skin/wound edge. The grafts are labeled with “G”, and the boundaries of the grafts are marked by dashed lines (no graft in “control” group).
Effects of nanofiber alignment and heparin-modification on cell infiltration in vivo
Although advanced nanofiber architecture and chemical modification had no apparent effect on epidermal outgrowth from the wound edge toward the nanofibrous graft, these characteristics did have an effect on endothelial cell infiltration into the grafts (Figure 5). CD31+ staining for endothelial cells showed that in a control wound with no implanted graft, endothelial cells were distributed approximately evenly and were organized randomly with no apparent formation of microvasculature. Similarly, when a nanofibrous graft with randomly aligned fibers was implanted in the wound, endothelial cells were still organized relatively randomly beneath the graft, and no CD31+ cells were seen within the graft. However, when the fibers were aligned, CD31+ cells could be seen infiltrating into the graft from the underside. Furthermore, CD31+ cell infiltration was greatest in the random heparin-conjugated nanofibrous mesh, as seen by many CD31+ endothelial cells in the center of the graft.
Figure 5.
CD31 IHC images of wounds with PLLA grafts 7 days after wounding and implantation. Sections are transverse to the long axis of the wound, and are taken near the midpoint of the long axis. Brown staining indicates CD31+ endothelial cells. The grafts are labeled with “G”, and the boundaries of the grafts are marked by dashed lines (no graft in “control” group).
Additionally, staining for CD68+ macrophages (Figure 6) showed enhanced infiltration of cells into the scaffolds with aligned nanofibers and the most cell infiltration into the heparin-modified scaffolds.
Figure 6.
CD68 IHC images of wounds with PLLA grafts 7 days after wounding and implantation. Sections are transverse to the long axis of the wound, and are taken near the midpoint of the long axis. Brown staining indicates CD68+ macrophages. The grafts are labeled with “G”, and the boundaries of the grafts are marked by dashed lines (no graft in “control” group).
DISCUSSION
Nanofibrous materials created by electrospinning have enormous potential for tissue engineering since they can mimic the structure and function of native ECM. These nano-scale fibers are similar in dimension to natural collagen fibers found throughout the ECM, and can be fabricated with varying alignment and orientation. In terms of wound healing, nanofibers have been characterized both in vitro [18–20] and in preliminary studies with rats [21] and guinea pigs [22], but these were limited to randomly oriented fiber meshes without chemical modification. This study demonstrates that nanofiber alignment plays a major role in cell infiltration into a nanofibrous scaffold, and that chemical modification of nanofibers with heparin can have additional effects.
A major finding in this study is that aligned nanofibers can promote cell infiltration into 3-D scaffolds. We speculate that the alignment of cells with nanofibers and the resulting cell morphology makes it easier for the cells to penetrate the space between aligned nanofibers. In addition, the aligned nanofibers may provide greater spacing or openness and change the spatial organization of “pores” between the fibers for more efficient cell migration.
With regard to wound healing on the surface of the nanofibrous matrices, our previous studies have shown that aligned nanofibers promote cell migration, wound healing and axon growth on their 2-D surfaces of the scaffolds [13]. These changes in cell behavior can be explained by contact guidance of cytoskeletal filaments via aligned nanofibers. However, in this 1-week dermal wound healing model, nanofibrous scaffolds enhance dermal tissue growth across the surface of the wound regardless of nanofiber orientation. It is possible that the different effects of random and aligned nanofibers could be detected at an earlier time point of the experiments.
Another novel finding is that immobilized heparin improved cell infiltration into the scaffolds with either random or aligned nanofibers. This could be explained by the anti-clotting effects of heparin, as evidenced in Figure 3 (more bleeding on the scaffolds with heparin). The prevention of clot formation around the scaffolds will allow more cells (either from blood or surrounding tissue) to penetrate the scaffolds. In addition, the cells in the scaffolds could release cytokines and growth factors to further increase the recruitment of cells. This also explains why heparin did not show a significant effect on cell infiltration in vitro because the anti-clotting mechanism is missing in the cell culture model. Moreover, the in vitro system may lack the complex multiple cell-type signaling of an in vivo environment. These apparent discrepancies underscore the difference between the in vitro and in vivo systems. On the other hand, heparin can bind to multiple ECM proteins and growth factors in the blood and interstitial fluid, which may facilitate cell proliferation and migration into the scaffolds. These results suggest that chemical modification of nanofibers can be as critical as the physical nanofiber architecture for enhancing cell infiltration.
CONCLUSIONS
Aligned nanofibers can enhance cell infiltration into 3-D scaffolds in vitro and in vivo. Heparin modification of nanofibers promotes cell infiltration into 3-D scaffolds in vivo, possibly due to the anti-clotting property and biomolecule-binding capabilities of heparin. These results shed light on the importance of biophysical and biochemical properties of nanofibers in the regulation of cell infiltration into 3-D scaffold and tissue remodeling, and emphasize that complex 3-D nanofibrous matrices can be specifically tailored to promote desired cell and tissue responses.
Supplementary Material
ACKNOWLEGEMENT
This work was supported in part by a grant from NIH (HL083900, to S.L.), a grant from the Office of the Surgeon General of the Air Force (grant number FDG200712A, to J.S.) and a NIGMS-IMSD Training Grant (GM56847, to R.R.R.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359(1445):777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metcalfe AD, Ferguson MW. Harnessing wound healing and regeneration for tissue engineering. Biochem Soc Trans. 2005;33(Pt 2):413–417. doi: 10.1042/BST0330413. [DOI] [PubMed] [Google Scholar]
- 3.Cima LG, Vacanti JP, Vacanti C, Ingber D, Mooney D, Langer R. Tissue engineering by cell transplantation using degradable polymer substrates. J Biomech Eng. 1991;113(2):143–151. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- 4.Doshi J, Reneker DH. Electrospinning process and applications of electrospun fibers. J Electrostatics. 1995;35:151–160. [Google Scholar]
- 5.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11(1–2):101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 6.Liang D, Hsiao BS, Chu B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv Drug Deliv Rev. 2007;59(14):1392–1412. doi: 10.1016/j.addr.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 2004;9:1422–1432. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Wang Y, Xia Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003;3(8):1167–1171. [Google Scholar]
- 9.Zong XH, Ran SF, Fang DF, Hsiao BS, Chu B. Control of structure, morphology and property in electrospun poly (glycolide-co-lactide) non-woven membranes via post-draw treatments. Polymer. 2003;44(17):4959–4967. [Google Scholar]
- 10.Theron A, Zussman E, Yarin AL. Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology. 2001;12(3):384–390. [Google Scholar]
- 11.Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104(29):11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang NF, Patel S, Thakar RG, Wu J, Hsiao BS, Chu B, et al. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006;6(3):537–542. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- 13.Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo MM, et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7(7):2122–2128. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 14.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25(5):877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Nam J, Huang Y, Agarwal S, Lannutti J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007;13(9):2249–2257. doi: 10.1089/ten.2006.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Kim M, Hu YL, Jalali S, Schlaepfer DD, Hunter T, et al. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem. 1997;272(48):30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- 18.Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res B Appl Biomater. 2004;70(2):286–296. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 19.Noh HK, Lee SW, Kim JM, Oh JE, Kim KH, Chung CP, et al. Electrospinning of chitin nanofibers: degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials. 2006;27(21):3934–3944. doi: 10.1016/j.biomaterials.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Venugopal J, Ramakrishna S. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 2005;11(5–6):847–854. doi: 10.1089/ten.2005.11.847. [DOI] [PubMed] [Google Scholar]
- 21.Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, et al. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27(8):1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res B Appl Biomater. 2003;67(2):675–679. doi: 10.1002/jbm.b.10058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.