Abstract
The dorsal (A9) and ventral striatum (A10) of the midbrain mediate many of the effects of psychoactive drugs that alter emotion, cognition, and motor activity within the contexts of therapy or abuse. Although transgenic and knockout technologies have enabled development of genetic models to dissect contributions of specific dopamine (DA) receptor subtypes to psychoactive drug effects, few models exist that can distinguish contributions of A9 versus A10 circuits. Pitx3 is a transcription factor enriched in DA neurons. Aphakia (ak) mice deficient in Pitx3 show selective loss of nigrostriatal DA, while other DA pathways are relatively spared, and therefore could be a useful tool for investigating the role of this subclass of DA projections. We investigated the effects of stimulants amphetamine, apomorphine, and MK-801 and the antipsychotic drug haloperidol on behavior in ak mice. Whereas wild-type mice showed the characteristic locomotor hyperactivity in response to amphetamine (5mg/kg) and apomorphine (4mg/kg), these drugs caused a paradoxical suppression of locomotor hyperactivity in ak mice. MK-801 (0.2mg/kg) induced hyperactivity was maintained in both wt and ak mice. Additionally, mutant but not wild-type mice were insensitive to the cataleptic effects of haloperidol (1mg/kg). These studies indicate that the nigrostriatal DA circuit plays a critical role in maintaining normal responsiveness to psychotropic drugs that either stimulate or block DA neurotransmission. We propose that ak mice may represent a valuable genetic model not only to study Parkinson’s disease, but also to dissect the pathophysiologic and pharmacotherapuetic mechanisms of other DA-mediated disorders such as attention-deficit hyperactivity disorder, drug abuse and schizophrenia.
Keywords: Pitx3, amphetamine, dopamine, dorsal, ventral, striatum
Introduction
Midbrain dopamine (DA) neurons arising from the substantia nigra (A9) and ventral tegmental area (A10) form the nigrostriatal and mesolimbic DA circuits, respectively. Together with the mesocortical pathway, these circuits make up the core of the mesencephalic DA system (van Domburg and ten Donkelaar, 1991). Traditionally, these pathways have been ascribed distinct roles in the nervous system. The nigrostriatal pathway, terminating primarily in the dorsal striatum, is typically associated with the control of motor functions, and its degeneration results in Parkinson's disease. The mesolimbic circuit, terminating in the nucleus accumbens and olfactory tubercle, is typically associated with motivation and emotion, and is implicated in psychiatric disorders such as schizophrenia and drug addiction (Wise, 2004, Carlsson, 2006). These DA circuits have in common the synthesis, release, and reuptake of DA. Yet, they develop and are maintained via distinct molecular signaling pathways, which have only recently begun to be delineated. Among the signaling molecules implicated in DA neuron development is a bicoid-related homeobox transcription factor, Pitx3, which is restrictively expressed in DA-containing neurons and may differentially regulate the development of individual DA neuronal groups (Smidt et al., 2004).
Pitx3 and its rat homologue (Ptx3) were cloned independently by two groups, and are selectively expressed in the developing lens and midbrain regions (Semina et al., 1997, Smidt et al., 1997). The aphakia mouse, a naturally occurring mutant, has two genomic deletions in the Pitx3 gene; the first is a 765 base pair deletion at 2.5kb upstream of the transcription start site, and the second is a 1423-base-pair deletion that encompasses the upstream promoter region, the transcription start site, exon 1 and a portion of intron 1. As a consequence, Pitx3 expression is greatly reduced in homozygous ak mice. Previous studies of the ak mice suggest that Pitx3 is likely involved in eye development, as the homozygous ak mouse has small eyes that lack the lens (Semina et al., 1997). Consistent with this, mutations in the human homologue PITX3 gene were found in two unrelated families with congenital cataracts and anterior segment mesenchymal dysgenesis (Semina et al., 1998). Recently, our group and others have demonstrated that Pitx3 deficiency in ak mice causes selective loss of A9 DA neurons in the midbrain, while sparing A10 and other catecholaminergic neurons (Hwang et al., 2003, Nunes et al., 2003, van den Munckhof et al., 2003). This selective loss of neurons occurs during early brain development and is evident at birth.
Selective loss of A9 DA neurons makes the ak mouse a potential animal model of Parkinson’s disease (PD). Indeed, we have demonstrated that it is a valid and useful model to investigate neurological and cognitive dysfunction as well as L-DOPA-induced dyskinesia (Hwang et al., 2005; Ding et al., 2007; Ardayfio et al., 2008). Furthermore, given the potential role of mesencephalic DA neurons in mediating the behavioral responses to DA-modulating drugs, we hypothesized that ak mice may demonstrate differential responses to psychomotor stimulants and antipsychotic drugs and could thus serve as a model to dissect anatomical substrates of DA modulating drugs of abuse and therapeutics. In particular psychostimulant induced locomotor activity is thought to be mediated largely by mesolimbic pathways (Creese and Iversen, 1975, Wise and Bozarth, 1987), while stereotypy and antipsychotic induced catalepsy are thought to be mediated by the nigrostriatal circuits. The present studies were designed to examine the effects of various classes of psychotropic drugs on locomotor activity in ak mice.
Materials and Methods
Animals
The Ak mice used in this study originated from the Jackson labs and homozygous ak/ak mice were generated in the Transgenic Core Facility at the University of Iowa. Several breeding pairs were transferred, expanded and maintained in the Animal Care Facility at McLean Hospital. Lights in the vivarium came on at 800 hours and off at 2000 hours. The use of animals was in accordance with McLean's Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines.
Drugs
D-amphetamine hydrochloride (5mg/kg,) and apomorphine (4mg/kg) were obtained from Sigma (St Louis MO) and dissolved in 0.9% saline. (+)- MK-801 maleate (0.2mg/kg) was obtained from Tocris Bioscience (St.Louis, MO). Haloperidol (1mg/kg, 1hr pre-treatment) was obtained from Cardinal Health (Dublin, OH), in a premixed solution (5mg/ml) made up in water with the following components: 0.38% lactic acid, 0.18 % methylparaben, and 0.02% propylparaben. Drugs were injected intraperitoneal at a volume of 10ml/kg.
Locomotor Activity
Locomotor activity was monitored individually for 90 min using an infrared photobeam activity monitoring system (San Diego Instruments, San Diego CA), which measured consecutive horizontal beam breaks. Testing was in a standardized novel environment (43X20X20 cm) transparent plastic cages with fresh bedding in a grid of 8 cm horizontal infrared beams), between 1000 and 1600 hours (day) or between 2100 and 2300 hours (night). Locomotor activity was defined as breaking of consecutive photobeams. Mice were given a one hour habituation session before being injected.
Catalepsy
Catalepsy was measured using the bar test (Boulay et al., 2000). One hour after injection of haloperidol mice were tested for catalepsy. The forepaws of each animal were placed on a metal pole 0.4mm in diameter and 3.5 cm above the surface of the bench. The latency to remove both paws or climb on top of the pole was recorded as the measure of catalepsy up to a maximum of 5 min.
Statistics
For the locomotor activity studies, analyses were conducted using either the Mann-Whitney U test, or a 2-way (treatment X time) analyses of variance (ANOVA) with repeated measures on time. Students t-test was used in the catalepsy experiments. Criteria for significance was p<0.05.
Results
Locomotor Activity
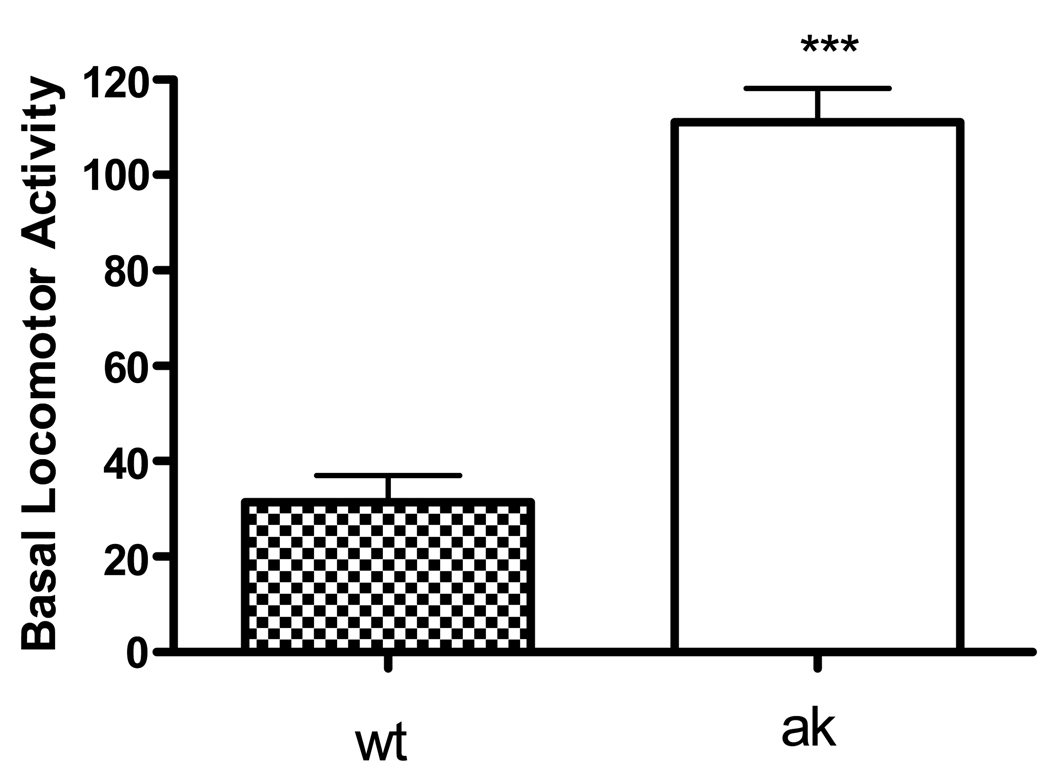
To determine whether d-amphetamine-induced hyperlocomotion is intact in ak mice, wild-type and ak mice were challenged with 5mg/kg d-amphetamine. Locomotor activity after saline injection was elevated (p<0.001) in ak mice relative to wt mice (Figure 1). This result is consistent with our previous studies demonstrating hyperactivity in ak mice during the light cycle (Hwang et al., 2005). During the light cycle, in wt mice, 5mg/kg of d-amphetamine caused a robust increase in locomotor activity compared with saline treatment. Two-way ANOVA demonstrated a main effect of time (F(8,171)= 13.6 p<0.001), drug (F(1,171)= 1296.96, p<0.0001) and a significant drug×time interaction (F(8,171)=13.12). In contrast to the increase in locomotor activity induced by amphetamine in wt mice, ak mice showed a marked suppression of locomotor activity in response to d-amphetamine in the 90 min following injection (Figure 2). It should be noted that there was a main effect of time (F(8,171) =21.09, p=0.001), drug (F(1,171) = 98.83, p=0.0001), and a drug × time interaction (F(8,171)=3.24, p=0.0018).
Figure 1.
Ak mice are hyperactive over a 90 min test session compared to wt mice after i.p. saline treatment in both groups. ***p<0.0001, Mann Whitney Test
Figure 2.
Amphetamine paradoxically suppresses locomotor activity in ak mice (n=10–11) animals per group). Data is horizontal beam breaks by wt (2a-day,2b-night), or ak (2c-day,2d-night) mice treated with amphetamine (5mg/kg, black squares) or saline (open circle) over 90 min in the day or night. Each point represents mean breaks over a 10 min interval. Repeated measures ANOVA shows main effect of treatment on locomotor activity. p<0.001
The amphetamine induced locomotion in wt mice was also maintained when the mice were tested in the dark cycle (Figure 2b). During the dark cycle, when we have previously shown ak mice to be hypoactive, there was no effect of amphetamine on locomotor activity in ak mice (Figure 2d). At high doses, psychostimulants such as amphetamine can elicit repetitive focused, stereotypic behaviors that interfere and compete with horizontal locomotor movements. We evaluated ak mice for amphetamine-induced stereotypic behaviors and found that ak mice did not differ from wildtype in amphetamine induced stereotyped behavior. There was no appreciable levels of stereotypy in either wt or ak mice treated with amphetamine at 5mg/kg (data not shown). This is consistent with the report that significant stereotypies typically occur at doses of 16mg/kg or higher of amphetamine in mice (Yates et al., 2007). Thus, the stimulant-induced reduction in locomotor activity appears not to be a confound of increased stereotypy in ak mice.
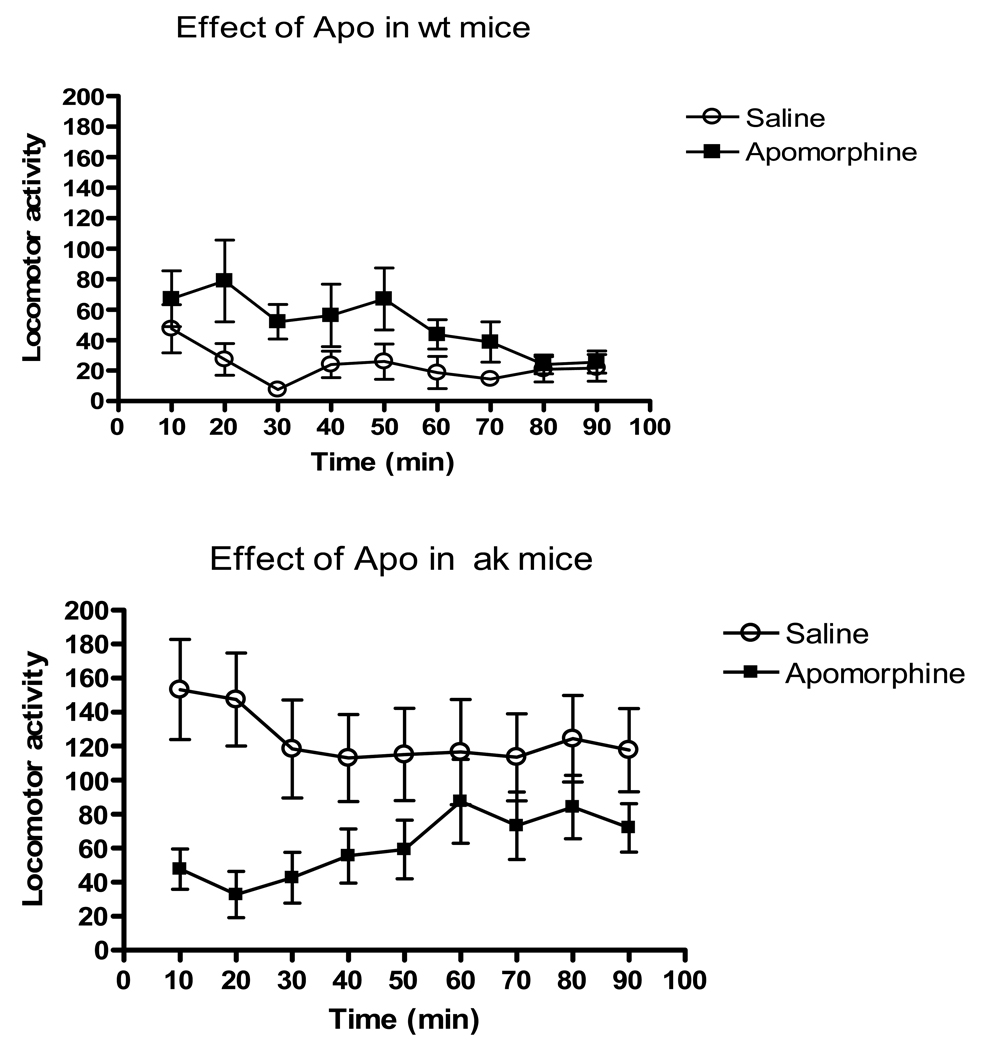
Because amphetamine increases extracellular DA primarily via presynaptic mechanisms, we examined the effects of the mixed D1/D2 receptor agonist, apomorphine, on locomotor activity in ak mice. As shown in Figure 3, apomorphine at 5mg/kg increased locomotor activity in wildtype mice compared to saline. There was a main effect of drug F(1,171) =17.72, p<0.0001. In contrast, apomorphine resulted in a suppression of locomotor activity in ak mice with a main effect of drug, F(1, 171)=35.45, p<0.0001.
Figure 3.
Apomorphine paradoxically suppresses locomotor activity in ak mice. (n=10–11 per group). Data is horizontal beam breaks in wt (3a) or ak (3b) mice treated with apomorphine (4mg/kg, black squares) or saline (open circles) over 90 min. Each point represents mean breaks over a 10 min interval. Repeated measures ANOVA demonstrates main effect of treatment on locomotor activity. p<0.001
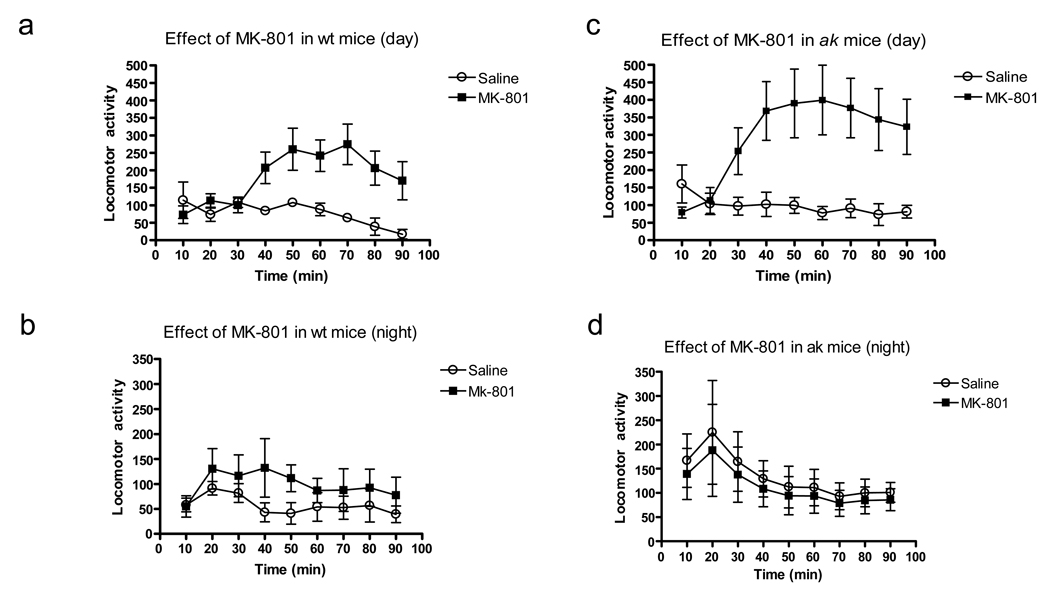
To explore whether Pitx3 deficiency in ak mice would affect nondopaminergic stimulants, we tested the effect of the non-competitive NMDA receptor antagonist MK-801 on locomotor activity in wt and ak mice. During the day, MK-801 (0.2mg/kg) increased locomotor activity in wt mice, (F(1,64)=10.93, p=0.018, Figure 4a). At night however, this effect was not observed (Figure 4b). ak mice also showed an increase in locomotor activity in response to MK-801, (F(1,80)=7.86, p=0.0187, Figure 4c),which was absent at night (Figure 4d).
Figure 4.
MK-801 induced hyperlocomotion is maintained in ak mice. Data is horizontal beam breaks in wt (4a,4b) and ak mice (4c,4d) after treatment with saline (open circles) or MK-801 (0.2 mg/kg, black squares) in the day or night over 90 min.
Catalepsy
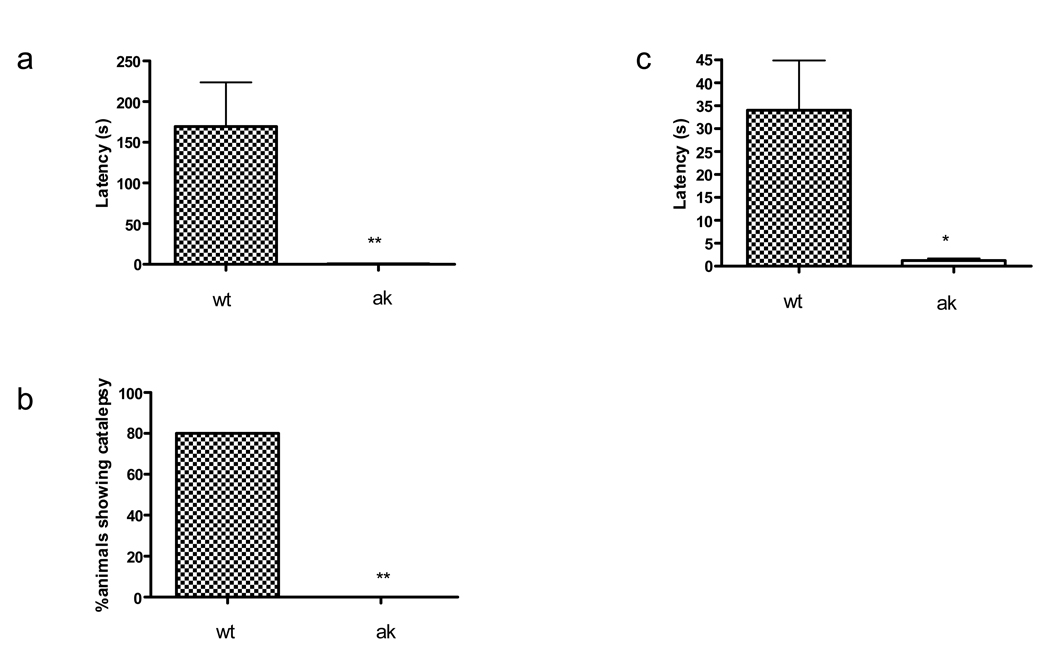
To determine whether ak mice also differed in their response to a DA antagonist, the catalepsy response to haloperidol was examined in wt and ak mice. As shown in Figure 5a, 1mg/kg haloperidol induced marked catalepsy in wt mice with a mean latency of 169.2 ± 54.58. The same treatment had virtually no effect in ak mice 0.5 ± 0.5 (p<0.01). The number of mice showing catalepsy, defined as greater 10s latency to removal of forepaws from bar, was significantly (p<0.01) greater in wt (5 out of 6) mice compared to ak (1 out of 6) mice (Figure 5b). A similar effect was seen when wt and ak mice were tested at night (Figure 5c).
Figure 5.
Haloperidol (1mg/kg) induced catalepsy is absent in ak mice. (n=6 per group) (5a) mean and SEM of latency (in seconds) to remove both forepaws from bar in wt (checkered bar) and ak (clear bar) mice during the day. (5b) percentage of wt or ak mice showing catelpsy defined as >10s on bar) after haloperidol treatment. ***p<0.01, students t-test. **p<0.01, Fisher’s exact test. The above effects were maintained when wt and ak mice were tested at night(5c).
Discussion
It has long been recognized that individual DA neuronal groups have distinct functions and characteristics, and are differentially susceptible to trauma or neurodegeneration (White and Wang, 1983). The homeodomain protein Pitx3 is the first known transcription factor to regulate differentially the development of individual DA neuronal groups. Pitx3-deficient ak mice show selective neuronal loss within A9 circuits while A10 and other catecholamine pathways are largely spared (Hwang et al., 2003).
In this study, we report that ak mice are hyperactive as demonstrated by an enhanced baseline locomotor activity compared to wt, and show a paradoxical suppression of locomotor activity in response to the indirect DA agonist amphetamine and nonselective DA receptor agonist apomorphine. The psychomotor stimulant response to the non-competitive NMDA receptor antagonist MK-801 was intact in ak mice. Additionally, the cataleptic response to the D2-like receptor antagonist haloperidol is absent in ak mice. These studies suggest a role for Pitx3 and A9 DA neurons in mediating basal locomotor activity as well as the behavioral response to psychostimulant and antipsychotic drugs.
Knock-out and transgenic technologies have enabled the development of mouse models with alterations in various aspects of the DA system. Knock-down and over-expression of DA receptor subtypes, transporters, and synthesizing and degrading enzymes have greatly facilitated understanding of the role of DA in normal and abnormal behavior and the response to drugs (Son and Joh, 1997, Pich and Epping-Jordan, 1998). Although there are numerous genetic models to investigate many aspects of the DA system, there are currently few animal models available to dissect the contribution of individual DA neuronal groups to behavior. Currently available mice with naturally occurring genetic mutations which show interesting region-specific dopamine phenotypes include the coloboma (Wilson, 2000, Jones et al., 2001), reeler (Nishikawa et al., 2003, Lalonde and Strazielle, 2007) and weaver mice (Schmidt et al., 1982, Maharajan et al., 2001). Our group and others have demonstrated severe and gross abnormalities of the A9 DA circuits relative to A10 circuits in ak mice: this includes selective loss of A9 DA content as measured by HPLC, A9 cell loss measured by nissl staining, loss of the DA markers AADC, TH, and DAT, loss of retrograde labeling from the striatum, as well as nigrostriatal-mediated behavioral deficits (Hwang et al 2005). Altogether this suggests that these animals may also be a useful tool for this purpose.
Our initial experiments demonstrate a paradoxical suppression of locomotor hyperactivity after amphetamine treatment in ak mice. Many studies have attempted to elucidate both the neurochemical and neuroanatomical locus of amphetamine effects on behavior. Lesion studies designed to identify the relative role of A9 verse A10 DA regions in mediating amphetamine behavioral effects, although conflicting, suggest that amphetamine-induced hyperlocomotion is more dependent upon mesolimbic structures while stereotypy is mediated by the striatum (Iversen et al., 1975). Since we have previously shown that A10 regions are relatively intact in ak mice, our results suggest that Pitx3 and A9 regions are required for normal basal and stimulant-induced locomotor activity. Interestingly, our observation of stimulant-induced reduction in locomotor hyperactivity in ak mice resembles that observed in juvenile rats lesioned with intracisternal 6-hydroxydopamine as neonates (Zhang et al 2001, 2002) and in young patients diagnosed with ADHD and treated with stimulants (Biederman and Faraone 2005). Interestingly intra-nigral MPTP also has been reported to cause a transient hyperactivity in rats (Ferro et al., 2005). Therefore, the ak mouse may be a useful tool to elucidate the mechanism and neuroanatomical regions involved in stimulant induced reduction of locomotor hyperactivity.
It is of great interest that the locomotor hyperactivity induced by the NMDA receptor antagonist MK-801 remained intact in ak mice when administered during the day. This finding lends further support to evidence that dopamine is not required for the locomotor effects of NMDA receptor antagonists as PCP and MK-801 induced hyperactivity is intact in dopamine deficient mice (Chartoff et al., 2005). Of interest to the present studies, intra-accumbens administration of amphetamine or MK-801 induced locomotor hyperactivity in rats. This effect was blocked by 6-OHDA lesions of the nucleus accumbens in the case of amphetamine, but not MK-801 (Mele et al., 1998). These studies further support that NMDA receptor antagonists induced hyperlocomotion can occur independent of nigrostriatal DA circuits.
All classical antipsychotic agents, in addition to their antipsychotic properties, can induce akinetic motor deficits or extrapyramidal side effects (EPS) in humans. In animals, it is generally assumed that antipsychotic-induced catalepsy reflects the ability of these agents to produce EPS in man (Hoffman and Donovan, 1995). Haloperidol, the prototypical antipsychotic, elicits a characteristic catalepsy response in rodents that is thought to be more dependent upon striatal DA than mesolimbic DA circuits. We found that ak mice were insensitive to the cataleptic effects of haloperidol. Altogether, these studies indicate that an intact striatum and Pitx3 are required for a normal behavioral response to stimulant and antipsychotic drugs. Accordingly, we propose that the ak mouse is a useful model for exploring the mechanisms and neuroanatomical sites of action of therapeutics used for treating DA-related disorders such as ADHD and schizophrenia, as well as drugs of abuse.
Acknowledgements
This work was supported by NIH grants MH48866 and MH087903, and an International Grant from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Oblin A, Sanger DJ, Schoemaker H, Perrault G. Haloperidol-induced catalepsy is absent in dopamine D(2), but maintained in dopamine D(3) receptor knock-out mice. European journal of pharmacology. 2000;391:63–73. doi: 10.1016/s0014-2999(99)00916-4. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;(39 Suppl 1):S10–S14. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30:1324–1333. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain research. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- Ferro MM, Bellissimo MI, Anselmo-Franci JA, Angellucci ME, Canteras NS, Da Cunha C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson's disease: histological, neurochemical, motor and memory alterations. Journal of neuroscience methods. 2005;148:78–87. doi: 10.1016/j.jneumeth.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hoffman DC, Donovan H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology. 1995;120:128–133. doi: 10.1007/BF02246184. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res. 2003;114:123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Fleming SM, Ardayfio P, Moran-Gates T, Kim H, Tarazi FI, Chesselet MF, Kim KS. 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson's disease. J Neurosci. 2005;25:2132–2137. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Kelly PH, Miller RJ, Seviour P. Proceedings: Amphetamine and apomorphine responses in the rat after lesion of mesolimbic or striatal dopamine neurones. British journal of pharmacology. 1975;54 [PMC free article] [PubMed] [Google Scholar]
- Jones MD, Williams ME, Hess EJ. Expression of catecholaminergic mRNAs in the hyperactive mouse mutant coloboma. Brain Res Mol Brain Res. 2001;96:114–121. doi: 10.1016/s0169-328x(01)00281-9. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Spontaneous and induced mouse mutations with cerebellar dysfunctions: behavior and neurochemistry. Brain research. 2007;1140:51–74. doi: 10.1016/j.brainres.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Maharajan P, Maharajan V, Ravagnan G, Paino G. The weaver mutant mouse: a model to study the ontogeny of dopamine transmission systems and their role in drug addiction. Progress in neurobiology. 2001;64:269–276. doi: 10.1016/s0301-0082(00)00061-7. [DOI] [PubMed] [Google Scholar]
- Mele A, Thomas DN, Pert A. Different neural mechanisms underlie dizocilpine maleateand dopamine agonist-induced locomotor activity. Neuroscience. 1998;82:43–58. doi: 10.1016/s0306-4522(97)00277-7. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Goto S, Yamada K, Hamasaki T, Ushio Y. Lack of Reelin causes malpositioning of nigral dopaminergic neurons: evidence from comparison of normal and Reln(rl) mutant mice. The Journal of comparative neurology. 2003;461:166–173. doi: 10.1002/cne.10610. [DOI] [PubMed] [Google Scholar]
- Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A. 2003;100:4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Epping-Jordan MP. Transgenic mice in drug dependence research. Annals of medicine. 1998;30:390–396. doi: 10.3109/07853899809029939. [DOI] [PubMed] [Google Scholar]
- Schmidt MJ, Sawyer BD, Perry KW, Fuller RW, Foreman MM, Ghetti B. Dopamine deficiency in the weaver mutant mouse. J Neurosci. 1982;2:376–380. doi: 10.1523/JNEUROSCI.02-03-00376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkhauser C, Daack-Hirsch S, Murray JC. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet. 1998;19:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter RS, Murray JC. Isolation of a new homeobox gene belonging to the Pitx/Rieg family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet. 1997;6:2109–2116. doi: 10.1093/hmg/6.12.2109. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Smits SM, Burbach JP. Homeobox gene Pitx3 and its role in the development of dopamine neurons of the substantia nigra. Cell Tissue Res. 2004;318:35–43. doi: 10.1007/s00441-004-0943-1. [DOI] [PubMed] [Google Scholar]
- Smidt MP, van Schaic HS, Lanctot C, Tremblay JJ, Cox JJ, van der Kleij AA, Wolterink G, Drouin J, Burbach JP. A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci U S A. 1997;94:13305–13310. doi: 10.1073/pnas.94.24.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Joh TH. Genetically engineered neural transmission. Molecular psychiatry. 1997;2:26–31. doi: 10.1038/sj.mp.4000181. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130:2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- van Domburg PH, ten Donkelaar HJ. The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases. Adv Anat Embryol Cell Biol. 1991;121:1–132. [PubMed] [Google Scholar]
- White FJ, Wang RY. Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science (New York, NY. 1983;221:1054–1057. doi: 10.1126/science.6136093. [DOI] [PubMed] [Google Scholar]
- Wilson MC. Coloboma mouse mutant as an animal model of hyperkinesis and attention deficit hyperactivity disorder. Neuroscience and biobehavioral reviews. 2000;24:51–57. doi: 10.1016/s0149-7634(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological review. 1987;94:469–492. [PubMed] [Google Scholar]
- Yates JW, Meij JT, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neuroscience letters. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Tarazi FI, Baldessarini RJ. Role of dopamine D(4) receptors in motor hyperactivity induced by neonatal 6-hydroxydopamine lesions in rats. Neuropsychopharmacology. 2001;25:624–632. doi: 10.1016/S0893-133X(01)00262-7. [DOI] [PubMed] [Google Scholar]
- Zhang K, Tarazi FI, Davids E, Baldessarini RJ. Plasticity of dopamine D4 receptors in rat forebrain: temporal association with motor hyperactivity following neonatal 6-hydroxydopamine lesioning. Neuropsychopharmacology. 2002;26:625–633. doi: 10.1016/S0893-133X(01)00404-3. [DOI] [PubMed] [Google Scholar]