Abstract
We explored the time course of surround suppression and found clear evidence for two distinct mechanisms: one strong, transient, and largely monocular, the other weaker, sustained, and binocular. We measured detection thresholds for a Gabor target at 8 deg eccentricity surrounded by a large annulus of matching spatial frequency and orientation. At short stimulus durations surround suppression was very strong, but the suppression strength decreased precipitously for durations longer than ~100 msec. The strong transient component did not transfer between the eyes and occurred almost instantaneously (<1 frame delay, 12 msec) irrespective of the separation between target and surround. Both suppression components were tightly tuned to orientation, peaking at target orientation, but neither was tuned to target spatial phase. These results are in good agreement with surround suppression properties measured in macaque V1 neurons. The absence of interocular transfer, the strong orientation selectivity, and the high propagation speed incommensurate with slow horizontal connections in V1 suggest that the transient component of suppression originates between input layers and the subsequent layers in V1.
Keywords: surround suppression, time course, psychophysics, human subjects
Introduction
When a target viewed in the periphery is surrounded by a mask with similar properties, contrast sensitivity is suppressed, producing elevated thresholds (Andriessen & Bouma, 1976; Snowden & Hammett, 1998). This type of masking termed surround suppression (or surround masking) is distinct from the type of masking produced by masks transparently overlaid on the target. Surround suppression is a peripheral phenomenon and is tightly tuned to the target orientation and spatial frequency, while overlay suppression is equally strong in both the fovea and the periphery, and is broadly tuned to orientation and spatial frequency (Petrov, Carandini, & McKee, 2005).
The neurophysiological basis of surround suppression remains unknown. Stimulating the area outside the classical receptive field commonly produces strong suppression in LGN (Hubel & Wiesel, 1961), in V1 (Hubel & Wiesel, 1965, 1968), and beyond (Allman, Miezin, & McGuinness, 1985). In agreement with the psychophysically observed surround suppression, neuronal suppression is orientation-tuned in the areas beyond the LGN (Cavanaugh, Bair, & Movshon, 2002; DeAngelis, Freeman, & Ohzawa, 1994) and for cats possibly even in LGN (Murphy & Sillito, 1987; Naito, Sadakane, Okamoto, & Sato, 2007; Sillito, Cudeiro, & Murphy, 1993), but see (Bonin, Mante, & Carandini, 2005). It is possible that the suppression observed in the different visual areas is inherited from a single early source, or there might be separate suppressive mechanisms in many visual areas. In addition, there is evidence for a feedback input to suppression in LGN (Murphy & Sillito, 1987; Sillito et al., 1993; Solomon, White, & Martin, 2002; Webb et al., 2002).
Interocular masking is a straightforward test of an early site for surround suppression. In this condition mask and target are shown to opposite eyes. Because visual inputs from the two eyes converge beyond the V1 input layers, one can assume that interocular suppression originates downstream of these layers, while any purely monocular suppression originates either in these layers, or earlier. We will use the term ‘binocular’ to indicate the case where both monocular and interocular masking produce suppression.
DeAngelis et al. (1994) found that in cat V1 cells a surround mask in one eye suppressed the response to the target in the other eye, but not quite as strongly as when both stimuli were presented to the same eye. Macknik and Martinez-Conde (2004) compared interocular and monocular suppression in macaques (neurophysiology) and humans (psychophysics) using the “wave of invisibility illusion”. Their stimulus comprised a single target line flanked by two abutting mask lines. Neuronal responses in macaque LGN and V1 were almost completely suppressed, when the mask was shown to the same eye as the target. Interocular masking, on the other hand, was effective only for a small subpopulation of cells, even though most of the tested cells were binocular. These results seem to be in conflict with the DeAngelis et al. (1994) study. One possible explanation is that surround suppression in macaque remains monocular farther downstream than in cats. Tse, Martinez-Conde, Schlegel, and Macknik (2005) studied the neural correlates of the “wave of invisibility illusion” in human observers using fMRI methods and found signs of interocular masking only in the areas downstream of V2. Their stimuli were similar to those used in the Macknik and Martinez-Conde (2004) study.
It is not clear, however, to what extent the “wave of invisibility illusion” is relevant to surround suppression. As seen from the averaged neuronal response in the Macknik and Martinez-Conde (2004) study, the mask alone evoked strong response, which means that the mask was impinging on the classical receptive field of the studied cells. In this case the masking effect of the “wave of invisibility illusion” was mostly due to overlay suppression or some other local inhibitory effect rather than surround suppression.
Bair, Cavanaugh, and Movshon (2003) examined the time course of surround suppression in macaque V1 for indications of the source of the suppression. The target was a sine-grating disk and the mask was a sine-grating annulus. The mask inner diameter was carefully adjusted for an individual neuron's receptive field to preclude the mask encroaching on the classical receptive field. Their results demonstrated the existence of the two components to surround suppression: a strong transient component and a weak sustained component. The transient component was found to propagate swiftly across cortex, much faster than the estimated transmission speed of horizontal connections in V1. Bair et al. suggested a feedback from higher cortical areas as a likely alternative.
Webb, Dhruv, Solomon, Tailby, and Lennie (2005) studied spatiotemporal tuning of surround suppression for monocular and interocular surrounds using the same stimuli and techniques as Bair et al. (2003). They also found two components: a broadly tuned monocular component and a tightly tuned binocular component. The monocular component could originate in the input layers of V1 or earlier, while the binocular component could be feedback from extra-striate cortex. Because Bair et al. used a cross-oriented surround mask as the baseline (no-suppression) condition, the monocular component, which is broadly tuned to orientation was probably not measured in their study. It seems reasonable to identify both the transient and the sustained components in the Bair et al. (2003) study with the tightly tuned binocular component in the Webb et al. (2005) study.
A recent psychophysical study by Cai, Zhou, and Chen (2008) investigated the source of surround suppression by measuring the effect of binocular suppression of the surround mask on the surround suppression strength. The study used foveal targets and a contrast-matching paradigm. For these conditions iso-oriented and cross-oriented surround masks produce comparable suppression, (see also Xing & Heeger, 2001). Binocular suppression of the iso-oriented surround mask reduced (albeit very slightly) the surround suppression strength, but did not significantly affect surround suppression for the cross-oriented mask. This lends support to the Webb et al. (2005) results, because binocular suppression should not affect the early monocular component of surround suppression, which is only broadly tuned to orientation. It is worth pointing out that because neurophysiologists purposefully avoid the small receptive fields and the confluence of visual areas in the primate fovea it is not clear whether Cai et al. foveal results can be directly compared to the Webb et al. study. It is apparent, that surround suppression properties are quite different in the fovea and in the periphery (Petrov et al., 2005; Snowden & Hammett, 1998).
With the exception of the Cai et al. (2008) study prior psychophysical work on surround suppression did not explore the temporal aspects of suppression in a systematic fashion. Metacontrast studies, however, have frequently focused both on the binocularity and the time course of metacontrast masking. Unfortunately, it is hard to compare metacontrast studies with the recent electrophysiological work reviewed above, because of the very different stimuli traditionally used to study metacontrast. These included large abutting half-disks for mask and target (Baumgardt & Segal, 1942; Stigler, 1910), a large disk for target and an abutting ring for mask (Kolers & Rosner, 1960; Werner, 1940) small abutting bars (Alpern, 1953; Toch, 1956), small letter targets and large overlaying homogeneous/patterned/noisy large-field masks (Lindsley, 1961; Schiller, 1965, 1969; Schiller & Wiener, 1963; Turvey, 1973), Vernier target and Vernier masks (Duangudom, Francis, & Herzog, 2007), and a grating disk target with a grating disk annulus (Ishikawa, Shimegi, & Sato, 2006).
For the most part, the stimuli were presented centrally (e.g. Baumgardt & Segal, 1942; Duangudom et al., 2007; Lindsley, 1961; Schiller, 1965, 1969; Schiller & Wiener, 1963; Stigler, 1910; Toch, 1956; Turvey, 1973; Werner, 1940). Although in some cases the stimuli were large enough to extend into the periphery, the prevailing stimulation was foveal. This alone makes the relevance of these metacontrast studies to surround suppression rather tenuous. In a few studies stimuli were presented in the periphery (e.g. Alpern, 1953; Ishikawa et al., 2006; Kolers & Rosner, 1960), but the stimuli and the experimental tasks differed substantially from surround suppression studies, which makes it difficult to make comparisons.
Given the variety of metacontrast stimuli it is likely that the masking effects observed in the metacontrast studies result from a mixture of surround suppression, overlay suppression, crowding, and possibly other types of masking, e.g. attentional blink or visual masking due to motion integration. This would explain the wide variety of results in the metacontrast studies, where depending on the stimuli used, these various influences could be mixed in different proportions. Some metacontrast studies found interocular masking to be almost as strong as monocular masking (Baumgardt & Segal, 1942; Kolers & Rosner, 1960; Toch, 1956; Werner, 1940), while other studies did not find any sign of interocular masking (e.g. Alpern, 1953; Stigler, 1910; Turvey, 1973).
Based on the analysis carried out by Schiller (1965, 1969), Schiller and Wiener (1963), and Turvey (1973) and on further analysis of later metacontrast studies one can draw the tentative conclusion that interocular masking was observed for masks that were in some respect similar to the target. For example, the pattern of lines in the mask could be similar to the pattern of lines in the target (letter), or the inner boundary of the annulus mask was of the same radius as the outer boundary of the target disk, etc. The “wave of invisibility” illusion studied psychophysically by Macknik and Martinez-Conde (2004) and Tse et al. (2005) also falls into this category, because in both studies the mask and the target were very similar to each other and, accordingly, strong interocular masking was observed.
A parallel dichotomy describes the results pertaining to the time-course of the metacontrast masking. When the mask was similar to the target, the strongest effect was observed for a mask presented 50–150 msec after the target, which produced the classical U-shaped sensitivity pattern usually associated with metacontrast. When the mask and the target were dissimilar (e.g. a letter target and a full-field homogeneous mask), the masking effect was strongest for simultaneously presented target and mask (Duangudom et al., 2007; Schiller, 1965, 1969; Schiller & Wiener, 1963).
The aim of our study was to relate psychophysically observed suppression in humans to the electrophysiological observations of surround suppression in cats and monkeys. To this end we (wherever possible) modeled our stimuli on the stimuli used by Bair et al. (2003) and Webb et al. (2005). The differences were due to the nature of our psychophysical paradigm: we used relatively low-contrast stimuli presented as a brief flash instead of drifting/flashing high-contrast stimuli used in the electrophysiological studies. We compared monocular and interocular surround suppression, varied stimulus duration, mask onset with respect to the target onset, and the spatial separation between target and mask. Our results demonstrate that surround suppression in human observers has two distinct components: one strong, transient, and largely monocular, and the other weaker, sustained, and binocular.
Methods
Apparatus
Stimuli were displayed on a gray background (42 cd/m2) and viewed through a Wheatstone stereoscope on a pair of linearized 17-in. Sony Trinitron G220 monitors. The monitor resolution was 1024 × 768 pixels; viewing distance was 65 cm. The video signal was rendered with (nominal) 8-bit precision, but an additional factor of 4 increase in precision was attained using 2 × 2 block pixel ordered dithering (analogous to the classical newspaper halftone technique). The resulting effective pixel subtended 3.4 arcmin, whereas the dithering artifacts (0.8% contrast modulation at 16 cpd) were approximately 20 times below the detection threshold. The effective luminance resolution of the screen at the background level (after γ-correction) was confirmed to be 0.2% (9 bits) by counting the number of gray levels in the stimulus screenshots and to be better than 0.3% with a Pritchard Photometer.
Subjects
Four observers with normal or corrected visual acuity were tested. Two of the observers were naive to the purpose of the study; all four were experienced psychophysical observers. Observers were trained for a short time (5–10 min) to get acquainted with the stimuli and the task.
Psychometric procedure
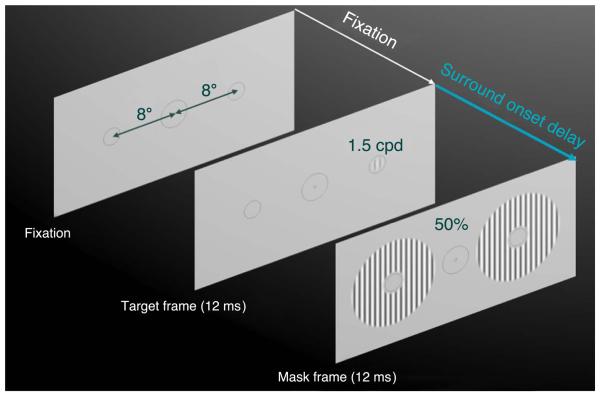
We used a two-alternative forced-choice procedure (2AFC), in which the test target appeared at one of two locations, 8° left and right of the fixation point (see Figure 1). A surround mask and a faint dark circle identifying each location were presented at both locations on all trials. The task was to indicate with a button press which location contained the test. Stimulus presentation interval was 11.8 ms (one video frame at 85 Hz screen refresh rate) in all of the experiments except Experiment 1, in which longer durations were also tested. A fixation pattern comprised of two low-contrast concentric circles and a pair of Nonius lines was displayed at the center of the screen at the beginning of each trial. The target location circles in the periphery and the fixation mark in the center were shown binocularly and were continuously visible throughout fixation and target presentation.
Figure 1.
An illustration of the experimental stimulus. The target and the mask durations varied in Experiment 1, the surround onset delay varied in Experiment 3. The surround mask orientation and eye-of-origin varied in Experiment 2.
We used a slightly modified version of the adaptive staircase algorithm, devised by Kontsevich and Tyler (1999), to estimate thresholds and steepness of psychometric functions. The modifications were merely technical (information maximization was replaced by variance minimization at each algorithm step) and were shown to produce slightly speedier estimates of both parameters of the psychometric function. Threshold contrast corresponded to 76% of the correct responses as estimated from the psychometric function. After several preliminary runs, the steepness (slope) parameter of the psychometric function was fixed at 1.5, which was found to be the typical value for all tested observers. The steepness parameter in the Kontsevich algorithm is similar but numerically different from the Weibull exponent β. Normally, observers carried out three blocks of 150 trials per block for each condition. Variability of the psycho-metric thresholds was taken as the maximum of these two estimates:
threshold variation calculated from the resulting probability distribution in the adaptive algorithm and
threshold variation among the three experimental blocks.
The test target was a standard (σ = λ/√2) cosine phase, vertically oriented Gabor in which ~1.5 periods (1 deg) of the sinusoidal pattern were visible, as shown in Figure 1. The Gabor spatial frequency was 1.5 cpd. Faint thin circles that were 15% in contrast, 1 pixel (1.2 arcmin) wide, and 2.5λ (1.67 deg) in diameter surrounded the 2AFC target regions at all times to reduce the observer's uncertainty about the target locations, which was particularly important for targets presented without the surround mask (Petrov, Verghese, & McKee, 2006).
The surround mask, shown in Figure 1, was a grating annulus centered on the target with an inner radius of 2λ (1.5 deg) and an outer radius of 8λ (6 deg). It contained a sinusoidal grating of the same orientation and spatial frequency as the target. The grating contrast was 50%. The phase of the grating was the same as that of the target, except in Experiment 2, where it was systematically varied. To ensure that no overlay masking was present, a blank region (at the background luminance) approximately 1 period wide (0.75 deg) separated the target from the mask. The mask orientation was varied in Experiment 2, and the separation was varied in Experiment 3.
Most of the parameters for this study were set to maximize the effect of surround suppression. Thus, we used a surround carrier that matched the target's spatial frequency and orientation to produce the maximum suppression for the detection task (Petrov et al., 2005).
The experimental data are shown in Figures 2-5. The threshold elevation between masked and unmasked conditions was used as a measure of the surround suppression strength in all figures except Figure 5, where the reduction of the perceived contrast was used instead. Thus, zero threshold elevation indicated no suppression.
Figure 2.
Results of Experiment 1. The surround mask appeared simultaneously with the target, the stimulus duration varied between 12 msec and 375 msec (shown on a logarithmic scale). Filled squares show detection threshold elevation for binocular viewing (target and mask in both eyes). Open circles show results for interocular viewing: target displayed to the left eye, surround mask displayed to the right eye. Hashed squares show results for the two control conditions: 95% contrast mask for the shortest stimulus duration, and 10% contrast mask for the longest stimulus duration.
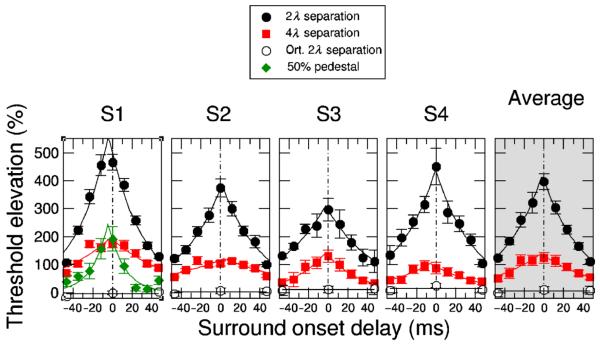
Figure 5.
Tuning of surround suppression to mask onset delay. The minus sign of the delay indicates that the mask appeared first, followed by the target. Target and mask were shown binocularly for 12 msec.
The unmasked thresholds (not shown) varied among subjects from 10–15% for the shortest stimulus duration to 1–3% for the longest duration.
Results and discussion
Experiment 1: Effect of stimulus duration
In the first experiment we varied the duration of the whole stimulus (target and surround mask) while keeping the mask onset delay with respect to the target at 0. Thus, the target and the mask appeared and disappeared simultaneously. We tested the two experimental conditions: binocular suppression, where the target and the mask were shown to both eyes, and interocular suppression, where the target was shown to the left eye and the mask was shown to the right eye. Figure 2 displays the resulting threshold elevations as a function of the stimulus duration for four observers. The rightmost panel displays the averaged data.
There were marked differences between the binocular and the interocular conditions. The interocular suppression, shown by open circles, was relatively weak (approximately a factor of 2) and was largely unaffected by the stimulus duration. In contrast, binocular suppression, shown by filled squares, greatly increased at short stimulus durations where it peaked at around 20 msec. This demonstrates the transient character of the monocular component: it declines to the interocular suppression level, when durations exceed 100 msec. This result is in agreement with Bair et al. (2003) study of surround suppression in macaque V1 neurons. This study also found a strong transient component and a weak sustained component of the suppression.
Because the effective mask contrast decreases at short stimulus durations, we wanted to see how the results were affected by higher mask contrasts. To this end we tried two control conditions. In the first condition the mask contrast was increased to 95% for the shortest duration (12 msec). In the second condition the mask contrast was decreased to 10% for the longest duration (375 msec). The results are shown by hashed squares in Figure 2. The effect of the mask contrast variation was insignificant in both cases.
Surround suppression appears to have a somewhat different effect on perceived contrast than on detection thresholds (see Petrov & McKee, 2006 for discussion), so we also investigated whether the difference between interocular and binocular forms of surround suppression affected the perceived contrast. In a contrast-matching version of the main experiment the perceived contrast of the Gabor target was measured by the method of constant stimuli. A 75% contrast Gabor target was shown on one side of fixation. The target was surrounded by the same annulus mask used in the main experiment, but at 100% contrast. Because contrast matching becomes unreliable for low-contrast targets and brief presentations, the high-contrast target was chosen to make the matching task easier. The mask contrast was maximized to maximize surround suppression. A reference unmasked Gabor was shown on the opposite side of fixation. The contrast of the reference Gabor randomly varied from trial to trial over 7 contrast values. The subject's task was to indicate whether the contrast of the unmasked Gabor appeared lower or higher than the contrast of the masked Gabor. The stimulus duration was 50 msec, short enough to produce strong monocular suppression, and long enough to make the perceptual judgment reliable. Both the test and the reference Gabor were shown to the left eye, while the surround mask was shown either to the left eye (monocular masking), or to the right eye (interocular masking). The test Gabor was shown either on the left or on the right of fixation in two separate experimental blocks.
Figure 3 shows the average of the two blocks, color-coded by subject. The dot-dashed line indicates the physical contrast of the target. When the mask was shown to the same eye as the target, the target's perceived contrast was approximately 40% lower than its physical contrast. The interocular mask produced no suppression, in fact it slightly increased the apparent contrast for all three observers.
Figure 3.
The effect of interocular and monocular surround mask on the Gabor target perceived contrast. The dot-dashed line shows the physical contrast of the target.
Experiment 2: Effect of mask orientation and spatial phase
Experiment 1 showed striking differences between monocular and interocular modes of surround suppression. Our second experiment explored these differences in more detail. We measured the orientation tuning of surround suppression separately for monocular and interocular surrounds. We chose a 17 msec stimulus presentation interval (one video frame at 60 Hz refresh rate) for this experiment, as the largest difference between the two conditions in Experiment 1 was observed around 20 msec duration. In the monocular condition both the target and the surround mask were simultaneously presented to the same eye. Both left and right eyes were tested in this experiment in separate blocks. In the interocular condition the target and the mask were simultaneously presented to opposite eyes: the target to left eye, the mask to right eye, or vice versa, also tested in separate blocks. Since there was no significant difference between the left and right-eye blocks, the results were averaged between the two blocks.
Figure 4 presents the results for monocular and interocular conditions; the effect of the iso-oriented surround is shown in black, the effect of the cross-oriented surround is shown in red. In the iso-oriented surround condition the mask orientation was vertical, the same as the target Gabor. In the cross-oriented condition the mask orientation was horizontal.
Figure 4.
Effect of the surround mask orientation for monocular and interocular masking. Black bars show the effect of the surround mask of the same orientation as the target, red bars show the effect of the cross-oriented mask.
The results clearly demonstrate that surround suppression was orientation-tuned for both interocular and monocular conditions: When the mask was iso-oriented, the suppression was strong in the monocular condition and much weaker, but still significant, in the interocular condition. Note that the interocular masking for the iso-oriented mask is comparable to the level of masking in Figure 2 for all stimulus durations. When the mask was cross-oriented, there was no suppression in either condition. This result is again in agreement with the Bair et al. (2003) results on surround suppression in macaque V1. Figure 3b in their paper shows that transient suppression rises, when the surround orientation is abruptly changed from cross-oriented to iso-oriented. Their Figure 3d shows how the sustained component disappears, when the surround is changed back to cross-oriented. Altogether, the two figures indicate that both components are orientation-tuned. On the other hand, our result is at odds with the Webb et al. (2005) study, where the monocular component of the surround suppression in macaque V1 was found to be only slightly weaker for the cross-oriented surround.
For a typical stimulus the target and the mask spatial phases were matched. We measured the effect of spatial phase on surround suppression by repeating the experiment with 90 deg and 180 deg phase shift between the center and the surround. The experiment showed that there was no significant effect of the phase shift on surround suppression. In our previous study, where longer (150 msec) stimuli were used, we also did not find any effect of surround phase (Petrov & McKee, 2006). Thus, neither the transient component of the surround suppression measured in the present study, nor the sustained component measured in the earlier study was affected by the surround phase. This agrees with the neurophysiological observations of DeAngelis et al. (1994) in cat and Webb et al. (2005) in macaque.
Experiment 3: Effect of mask onset delay
The first two experiments demonstrate that there is a strong monocular component to surround suppression, which indicates an early visual area (or areas) as the likely origin of this component. As discussed in the Introduction, the study of surround suppression in primate V1 by Bair et al. (2003) found that the onset of suppression in this area was very fast, with delays of arrival of suppression ranging from 0 to 30 msec. The suppression delay is important, because it constrains possible mechanisms of surround suppression. We wanted to see how this compares with the suppression delays in our study. To measure the delays psychophysically we used a stimulus with the shortest possible duration (12 msec) and varied the mask onset with respect to the target frame, as illustrated in Figure 1. Because suppression at such short durations was found to be mostly transient, we expected to see a pronounced effect of mask delay on the suppression strength.
Figure 5 shows the suppression strength plotted as a function the onset delay between the mask and the target tested in separate trial blocks. Negative delays correspond to the blocks in which the mask appeared prior to the target. To test whether the orientation tuning of surround suppression depends on the mask delay we tried cross-oriented and iso-oriented masks.
The cross-oriented mask produced zero suppression irrespective of the mask delay, which is shown by the open circles (and horizontal line at the bottom of graphs). The iso-oriented mask produced strong suppression, which peaked around zero mask delay. The suppression was symmetrical about the peak position. The decay of suppression on both sides of the peak was well fitted by an exponential function with 28 msec half-life time for the averaged data. The fit is shown by solid curves in Figure 5. The precise suppression peak position varied from −1 to 5 msec, as estimated from the exponential fit for each observer.
Assuming that neural responses to the mask and to the target have a similar time course, the peak values should indicate delays of the arrival of suppression. Our results demonstrate that suppression onset delays were very short. It might be argued that the assumption of the similar time course for the target and the surround mask was an oversimplification given the differences in the contrast of target and surround. At 50%, the contrast of the surround mask was about 3 times higher than the average target contrast needed for thresholds. It is well known that neurons in V1 respond faster when higher contrast stimuli are used (e.g. Cavanaugh et al., 2002). Therefore, the mask's higher contrast could shorten the arrival time of surround suppression in our study. To control for this possibility we modified the experiment. The contrast detection task was replaced by a contrast discrimination task, in which a contrast increment was added to one of two pedestals (50% contrast) presented at both target locations. Thus, in the control experiment the target contrast was always higher than the mask contrast. Unfortunately, surround suppression is very weak in this case; only one observer showed strong enough suppression to obtain data on this condition. The results for this observer are shown by green symbols in Figure 5. Besides the obvious reduction of the suppression strength no other change could be observed. In particular, the position of the suppression peak did not change, which demonstrates that the higher contrast of the mask did not affect the arrival of suppression delay.
In the second part of the experiment we increased the target-mask separation from 2λ to 4λ to study how the suppression delay depended on the target-mask separation. The results are shown by red symbols in Figure 5. While the suppression strength decreased, the timing of the suppression peak did not change significantly.
In Figure 6 we overlaid the suppression delays observed in this experiment (shown by red circles) in Figure 7a from Bair et al. (2003) study. To make the comparison we converted the target-mask separation for our experimental stimulus to the corresponding horizontal distance on human V1 cortex, using the cortical magnification formula by Rovamo and Virsu (1979). This comparison showed a close agreement between our results and the suppression delays measured in macaque V1 neurons. The slope of the red line in Figure 6 shows the estimated average conduction speeds for long-range horizontal connections in V1 (Bringuier, Chavane, Glaeser, & Frégnac, 1999; Girard, Hupé, & Bullier, 2001; Grinvald, Lieke, Frostig, & Hildesheim, 1994; Slovin, Arieli, Hildesheim, & Grinvald, 2002). In agreement with Bair et al. (2003) study our results indicate that surround suppression propagates much faster than is consistent with the horizontal connections in V1.
Figure 6.
Comparison of suppression delays measured in Experiment 3 (red circles) with suppression delays in macaque V1 neurons (Bair et al., 2003). The red line shows estimated conduction speed for horizontal connections in V1.
General discussion
This study demonstrated that surround suppression in human observers has two distinct components. The monocular component, which affects both contrast detection thresholds and perceived contrast, dominates for short stimulus durations (<100 msec) but quickly disappears for longer durations. This transient suppression component propagates across the cortex at speeds that significantly exceed the estimated propagation speed for horizontal connections in V1. The binocular component, which is about three times weaker than the monocular component is largely unaffected by the stimulus duration and thus dominates suppression for long stimulus durations (>150 msec). Both components of suppression are orientation tuned, yet are insensitive to the surround spatial phase.
Altogether, these results are in agreement with the properties of surround suppression observed in macaque V1 neurons (Bair et al., 2003; Webb et al., 2005) with the only exception: according to Webb et al. the average suppression in macaque V1 neurons was only slightly smaller for cross-oriented masks. Yet, Cavanaugh et al. (2002) found orientation tuning in macaque V1 neurons to be quite significant: for 50% contrast targets suppression from iso-oriented surround was 3 times stronger than from the cross-oriented surround on average. However, both studies along with earlier research (Levitt & Lund, 2002; Polat, Mizobe, Pettet, Kasamatsu, & Norcia, 1998) indicate that the cross-oriented surround becomes more suppressive for low target contrasts. We did not observe any cross-oriented suppression for low-contrast targets in this study or in our previous studies of surround suppression (Petrov et al., 2005; Petrov & McKee, 2006). It is quite possible, that for the detection task used in these studies human observers relied primarily on those V1 cells, which escaped suppression by the cross-oriented surround. Indeed, Figures 2a, 2c, 4b, and 6 in the Webb et al. (2005) study demonstrate that such neurons are not uncommon in V1.
The Webb et al. results correlate well with the recent psychophysical study of surround suppression (termed metacontrast masking) by Ishikawa et al. (2006). The results of this study indicated two components to surround suppression. The component arising at relatively high surround contrasts was tightly tuned to orientation and spatial frequency of the target; the other component arising at lower surround contrasts was broadly tuned to the target orientation and spatial frequency. The latter component was observed for masks presented 30–60 msec after the target. Based on the mask delay with respect to the target Ishikawa et al. characterize the tuned component as slow, and the less-tuned component as fast.
Unlike Ishikawa et al. (2006) we did not find any suppression from the cross-oriented surround up to the largest tested mask delay (48 msec), where the iso-oriented surround mask was still producing significant suppression. This is surprising, because the stimuli used in the two studies were quite similar. We believe that the cross-oriented component of surround suppression observed by Ishikawa et al. could be an artifact of the “yes-no” paradigm used in this study. Observers had to indicate whether a fixed 30% contrast target was present or absent. A mask was presented on all trials: iso-oriented or cross-oriented conditions were interleaved in random order. In half of the trials the target was absent. Given that for the optimally delayed iso-oriented mask observers almost invariably reported “no target”, 75% of the trials for these mask delays were “no” trials. Because there was no feedback, this response pattern could produce a strong (uncorrected) bias to report “no target” for the cross-oriented mask as well as for the iso-oriented mask, when the “right” mask delay was perceived. Indeed all “no target” responses for the cross-oriented mask were observed for those delays, which also produced the strongest iso-oriented suppression.
Webb et al. (2005) suggested that the results of their study were best explained by two suppression components: one that is prominent for high-contrast stimuli, is orientation selective, has relatively sharp spatiotemporal tuning, and is binocularly driven; the other is relatively more prominent for low-contrast stimuli, has very broad spatiotemporal tuning, and is monocularly driven. Based on these properties, the suggested origins of the two components were respectively input layers of V1 or LGN, and visual areas downstream of V1. Ishikawa et al. (2006) proposed instead that the two components indicated by their results arise within the magnocellular and parvocellular pathways. Because all stimuli were presented binocularly, the effect of the mask's eye-of-origin was not analyzed in their study.
The results reported here better support the Webb et al. scheme. We note, however, that the strong orientation tuning of surround suppression in humans makes its origin in LGN very unlikely. Because V1 is the only other likely site for the monocular component, and because the speed of surround suppression rules out V1 horizontal connections, we surmise that the monocular suppression could arise in a feed-forward fashion between the input layers and the subsequent layers within V1.
Conclusions
The time course of surround suppression in human observers revealed a strong monocular component existing for about 100 msec after the surround onset. A weaker binocular component was observed for longer durations. The fast onset of the transient monocular component was incommensurate with slow propagation speed estimated for horizontal connections in V1. These results are in good agreement with properties of surround suppression in macaque V1.
Acknowledgments
We would like to thank Dr. Matteo Carandini for helpful discussions. This work was supported by National Institutes of Health National Eye Institute Grants RO1 EY017071 and RO1EY01728 and by the Smith-Kettlewell Eye Research Institute.
Footnotes
Commercial relationships: none.
Contributor Information
Yury Petrov, Department of Psychology, Northeastern University, Boston, MA, USA.
Suzanne P. McKee, Smith-Kettlewell Eye Research Institute, San Francisco, CA, USA
References
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: Neurophysiological mechanisms for local-global comparisons in visual neurons. Annual Review of Neuroscience. 1985;8:407–430. doi: 10.1146/annurev.ne.08.030185.002203. PubMed. [DOI] [PubMed] [Google Scholar]
- Alpern M. Metacontrast. Journal of the Optical Society of America. 1953;43:648–657. doi: 10.1364/josa.43.000648. PubMed. [DOI] [PubMed] [Google Scholar]
- Andriessen JJ, Bouma H. Eccentric vision: Adverse interactions between line segments. Vision Research. 1976;16:71–78. doi: 10.1016/0042-6989(76)90078-x. PubMed. [DOI] [PubMed] [Google Scholar]
- Bair W, Cavanaugh JR, Movshon JA. Time course and time-distance relationships for surround suppression in macaque V1 neurons. Journal of Neuroscience. 2003;23:7690–7701. doi: 10.1523/JNEUROSCI.23-20-07690.2003. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgardt E, Segal J. Facilitation et inhibition parametres de la fonction visuelle. Annee Psychologique. 1942;43:43–44. [Google Scholar]
- Bonin V, Mante V, Carandini M. The suppressive field of neurons in lateral geniculate nucleus. Journal of Neuroscience. 2005;25:10844–10856. doi: 10.1523/JNEUROSCI.3562-05.2005. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringuier V, Chavane F, Glaeser L, Frégnac Y. Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science. 1999;283:695–699. doi: 10.1126/science.283.5402.695. PubMed. [DOI] [PubMed] [Google Scholar]
- Cai Y, Zhou T, Chen L. Effects of binocular suppression on surround suppression. Journal of Vision. 2008;8(9):9, 1–10. doi: 10.1167/8.9.9. http://journalofvision.org/8/9/9/, doi:10.1167/8.9.9. PubMed. Article. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Selectivity and spatial distribution of signals from the receptive field surround in macaque V1 neurons. Journal of Neurophysiology. 2002;88:2547–2556. doi: 10.1152/jn.00693.2001. PubMed. Article. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat's primary visual cortex. Journal of Neurophysiology. 1994;71:347–374. doi: 10.1152/jn.1994.71.1.347. PubMed. [DOI] [PubMed] [Google Scholar]
- Duangudom V, Francis G, Herzog MH. What is the strength of a mask in visual metacontrast masking? Journal of Vision. 2007;7(1):7, 1–10. doi: 10.1167/7.1.7. http:// journalofvision.org/7/1/7/, doi:10.1167/7.1.7. PubMed. Article. [DOI] [PubMed] [Google Scholar]
- Girard P, Hupé JM, Bullier J. Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities. Journal of Neurophysiology. 2001;85:1328–1331. doi: 10.1152/jn.2001.85.3.1328. PubMed. Article. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke EE, Frostig RD, Hildesheim R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. Journal of Neuroscience. 1994;14:2545–2568. doi: 10.1523/JNEUROSCI.14-05-02545.1994. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Integrative action in the cat's lateral geniculate body. The Journal of Physiology. 1961;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. Journal of Neuro-physiology. 1965;28:229–289. doi: 10.1152/jn.1965.28.2.229. PubMed. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. The Journal of Physiology. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Shimegi S, Sato H. Metacontrast masking suggests interaction between visual pathways with different spatial and temporal properties. Vision Research. 2006;46:2130–2138. doi: 10.1016/j.visres.2005.12.013. PubMed. [DOI] [PubMed] [Google Scholar]
- Kolers PA, Rosner BS. On visual masking (metacontrast): Dichoptic observation. American Journal of Psychology. 1960;73:2–21. PubMed. [PubMed] [Google Scholar]
- Kontsevich LL, Tyler CW. Bayesian adaptive estimation of psychometric slope and threshold. Vision Research. 1999;39:2729–2737. doi: 10.1016/s0042-6989(98)00285-5. PubMed. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Lund JS. The spatial extent over which neurons in macaque striate cortex pool visual signals. Visual Neuroscience. 2002;19:439–452. doi: 10.1017/s0952523802194065. PubMed. [DOI] [PubMed] [Google Scholar]
- Lindsley D. Electrophysiology of the visual system and its relation to perceptual phenomena. Brain and Behavior. 1961;1:359–392. [Google Scholar]
- Macknik SL, Martinez-Conde S. Dichoptic visual masking reveals that early binocular neurons exhibit weak interocular suppression: Implications for binocular vision and visual awareness. Journal of Cognitive Neuroscience. 2004;16:1049–1059. doi: 10.1162/0898929041502788. PubMed. [DOI] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature. 1987;329:727–729. doi: 10.1038/329727a0. PubMed. [DOI] [PubMed] [Google Scholar]
- Naito T, Sadakane O, Okamoto M, Sato H. Orientation tuning of surround suppression in lateral geniculate nucleus and primary visual cortex of cat. Neuroscience. 2007;149:962–975. doi: 10.1016/j.neuroscience.2007.08.001. PubMed. [DOI] [PubMed] [Google Scholar]
- Petrov Y, Carandini M, McKee S. Two distinct mechanisms of suppression in human vision. Journal of Neuroscience. 2005;25:8704–8707. doi: 10.1523/JNEUROSCI.2871-05.2005. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov Y, McKee SP. The effect of spatial configuration on surround suppression of contrast sensitivity. Journal of Vision. 2006;6(3):4, 224–238. doi: 10.1167/6.3.4. http://journalofvision.org/6/3/4/, doi:10.1167/6.3.4. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov Y, Verghese P, McKee SP. Collinear facilitation is largely uncertainty reduction. Journal of Vision. 2006;6(2):8, 170–178. doi: 10.1167/6.2.8. http://journalofvision.org/6/2/8/, doi:10.1167/6.2.8. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Mizobe K, Pettet MW, Kasamatsu T, Norcia AM. Collinear stimuli regulate visual responses depending on cell's contrast threshold. Nature. 1998;391:580–584. doi: 10.1038/35372. PubMed. [DOI] [PubMed] [Google Scholar]
- Rovamo J, Virsu V. An estimation and application of the human cortical magnification factor. Experimental Brain Research. 1979;37:495–510. doi: 10.1007/BF00236819. PubMed. [DOI] [PubMed] [Google Scholar]
- Schiller PH. Monoptic and dichoptic visual masking by patterns and flashes. Journal of Experimental Psychology. 1965;69:193–199. doi: 10.1037/h0021574. PubMed. [DOI] [PubMed] [Google Scholar]
- Schiller P. Behavioral and electrophysiological studies of visual masking. In: Leibovic N, editor. Information processing in the nervous system. Springer-Verlag; New York: 1969. pp. 141–165. K. [Google Scholar]
- Schiller PH, Wiener M. Monoptic and dichoptic visual masking. Journal of Experimental Psychology. 1963;66:386–393. doi: 10.1037/h0041390. PubMed. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Cudeiro J, Murphy PC. Orientation sensitive elements in the corticofugal influence on centre-surround interactions in the dorsal lateral geniculate nucleus. Experimental Brain Research. 1993;93:6–16. doi: 10.1007/BF00227775. PubMed. [DOI] [PubMed] [Google Scholar]
- Slovin H, Arieli A, Hildesheim R, Grinvald A. Long-term voltage-sensitive dye imaging reveals cortical dynamics in behaving monkeys. Journal of Neurophysiology. 2002;88:3421–3438. doi: 10.1152/jn.00194.2002. PubMed. Article. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Hammett ST. The effects of surround contrast on contrast thresholds, perceived contrast and contrast discrimination. Vision Research. 1998;38:1935–1945. doi: 10.1016/s0042-6989(97)00379-9. PubMed. [DOI] [PubMed] [Google Scholar]
- Solomon SG, White AJ, Martin PR. Extraclassical receptive field properties of parvocellular, magnocellular, and koniocellular cells in the primate lateral geniculate nucleus. Journal of Neuro-science. 2002;22:338–349. doi: 10.1523/JNEUROSCI.22-01-00338.2002. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigler R. Chronophotische studien uber den umgebungskontrast. Pflugers Archiv European Journal of Physiology. 1910;134:365–435. [Google Scholar]
- Toch HH. The perceptual elaboration of stroboscopic presentations. American Journal of Psychology. 1956;69:345–358. PubMed. [PubMed] [Google Scholar]
- Tse PU, Martinez-Conde S, Schlegel AA, Macknik SL. Visibility, visual awareness, and visual masking of simple unattended targets are confined to areas in the occipital cortex beyond human V1/V2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17178–17183. doi: 10.1073/pnas.0508010102. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey MT. On peripheral and central processes in vision: Inferences from an information-processing analysis of masking with patterned stimuli. Psychological Review. 1973;80:1–52. doi: 10.1037/h0033872. PubMed. [DOI] [PubMed] [Google Scholar]
- Webb BS, Dhruv NT, Solomon SG, Tailby C, Lennie P. Early and late mechanisms of surround suppression in striate cortex of macaque. Journal of Neuroscience. 2005;25:11666–11675. doi: 10.1523/JNEUROSCI.3414-05.2005. PubMed. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BS, Tinsley CJ, Barraclough NE, Easton A, Parker A, Derrington AM. Feedback from V1 and inhibition from beyond the classical receptive field modulates the responses of neurons in the primate lateral geniculate nucleus. Visual Neuro-science. 2002;19:583–592. doi: 10.1017/s0952523802195046. PubMed. [DOI] [PubMed] [Google Scholar]
- Werner H. Studies on contour: Strobostereoscopic phenomena. American Journal of Psychology. 1940;53:418–422. [Google Scholar]
- Xing J, Heeger DJ. Measurement and modeling of center-surround suppression and enhancement. Vision Research. 2001;41:571–583. doi: 10.1016/s0042-6989(00)00270-4. PubMed. [DOI] [PubMed] [Google Scholar]