Abstract
The structure of neurons changes during development and in response to injury or alteration in sensory experience. Changes occur in the number, shape, and dimensions of dendritic spines together with their synapses. However, precise data on these changes in response to learning are sparse. Here, we show using quantitative transmission electron microscopy that a simple form of learning involving mystacial vibrissae results in ∼70% increase in the density of inhibitory synapses on spines of neurons located in layer IV barrels that represent the stimulated vibrissae. The spines contain one asymmetrical (excitatory) and one symmetrical (inhibitory) synapse (double-synapse spines), and their density increases threefold as a result of learning with no apparent change in the density of asymmetrical synapses. This effect seems to be specific for learning because pseudoconditioning (in which the conditioned and unconditioned stimuli are delivered at random) does not lead to the enhancement of symmetrical synapses but instead results in an upregulation of asymmetrical synapses on spines. Symmetrical synapses of cells located in barrels receiving the conditioned stimulus also show a greater concentration of GABA in their presynaptic terminals. These results indicate that the immediate effect of classical conditioning in the “conditioned” barrels is rapid, pronounced, and inhibitory.
Introduction
Neurons of the cerebral cortex are plastic, i.e., they are able to modify their structure and function. Plasticity occurs during development (LeVay et al., 1980; Stern et al., 2001; Desai et al., 2002), in response to injury (Darian-Smith and Gilbert, 1995; Kaas et al., 2008) and as a result of alterations of sensory experience (Wiesel and Hubel, 1965; Glazewski and Fox, 1996; Trachtenberg et al., 2002), including learning (Wilson and McNaughton, 1994; Kleim et al., 1996). Various reports show that the morphology of all major components of the neuron are malleable, i.e., the axons (Portera-Cailliau et al., 2005; De Paola et al., 2006), cell bodies (von der Ohe et al., 2006), and also the dendrites (Holtmaat et al., 2005; Knott and Holtmaat, 2008), together with their spines, in which the majority of brain synapses are located (Knott and Holtmaat, 2008). There is now unequivocal evidence that dendritic spines are at the center of the mechanisms underlying plasticity of the neuronal circuits (Harris and Woolsey, 1981; Mahajan and Desiraju, 1988; Engert and Bonhoeffer, 1999; Grutzendler et al., 2002; Trachtenberg et al., 2002). It has been shown that spine density, morphology, and motility are experience and activity dependent (Lendvai et al., 2000; Trachtenberg et al., 2002; Holtmaat et al., 2005). Although spines have the most capacity for change during early development, it seems that they also change in adulthood (Knott et al., 2002; Holtmaat et al., 2006).
It is commonly assumed that the mechanisms underlying learning and memory are based on changes in synaptic weights and connectivity between neurons, guided by neuronal activity and supported by molecular cues (Bailey and Kandel, 2008; Howland and Wang, 2008). Although several molecules are known to specifically interfere with memory, the detailed anatomical, physiological, and molecular mechanisms underlying memory in a mammalian brain are still not known (Costa-Mattioli and Sonenberg, 2008). A part of the somatosensory cortex of rodents that represents the mystacial vibrissae, the barrel cortex, offers an excellent model to study the mechanisms of learning and memory because of its clearly defined structure, connectivity, and the ease of inducing plasticity via a learning paradigm (Woolsey and Van der Loos, 1970; Siucinska and Kossut, 1996; Fox, 2002). The neurons of the barrel cortex have the ability to change their receptive field properties as a result of alterations in sensory experience (Armstrong-James and Fox, 1987). For example, sensory deprivation of selected vibrissae causes expansion of the cortical representations of the spared vibrissae (Glazewski and Fox, 1996). Similarly, stimulation of a row of vibrissae paired with a tail shock (classical conditioning) results in a short-lasting enlargement of the cortical representation of this row, mainly in layer IV (Siucinska and Kossut, 1996). The main aim of the present study was to investigate the effects of whisker conditioning on symmetrical and asymmetrical synapse number. Whisker conditioning resulted in selective increase in the density of inhibitory synapses on double-synapse spines in the “trained” barrels. Neither whisker stimulation alone nor pseudoconditioning produce a similar effect.
Materials and Methods
Animals.
The experiments were performed on Swiss Webster mice aged 6–7 weeks raised in standard conditions (Siucinska and Kossut, 1996). All experiments were compliant with the European Communities Council Directive of November 24, 1986 (86/609/EEC) and were approved by the Animal Care and Use Committees of the Polish Academy of Sciences and the Jagiellonian University.
Training protocols.
From 34 experimental mice, 24 were trained and 10 served as controls. From the trained mice, 10 were conditioned [conditioned stimulus–unconditioned stimulus (CS–UCS)], seven were pseudoconditioned, and seven had whiskers only stimulated (CS). Control animals were split into two groups from which one group was habituated in a homemade restrainer along with all mice awaiting the training, and the second group was left nonhabituated. During the habituation period, mice spent 10 min/d for 3 weeks in the restrainer. The restrainer holds the mouse's neck stationary, while leaving the rest of the body, including the head, free. After a period of habituation, mice were conditioned using a classical (Pavlovian) delay conditioning paradigm. A stroke of the selected vibrissae (B-row; CS) on one side of the snout was paired with a mild tail shock (UCS) (Siucinska and Kossut, 1996). The pairing procedure comprised three strokes lasting 3 s each, applied to the row of whiskers, repeated at a frequency of four times per minute for 10 min. This procedure was applied for 3 consecutive days. The UCS consisted of weak 0.5 mA electric current applied to the tail for 0.5 s at the end of the last stroke in the series. In the case of pseudoconditioned (random pairing of CS and UCS) and whisker-stimulated (only strokes to the selected row of vibrissae applied) animals, the number and frequency of stimuli applied were identical to those used during conditioning protocol.
Analysis of behavior during conditioning.
Animal behavior was assessed during training with the aim to provide evidence that animals actually alter their behavior (learn) in response to conditioning. The number of times the head turned toward the stimulating brush (CS) was counted during the first and last session of training of five CS–UCS, five CS, and five pseudoconditioned animals as an indication of learning.
Fixation, embedding protocol, and sectioning.
Twenty-four hours after each experiment, animals were deeply anesthetized with pentobarbitone and perfused through the heart with 20 ml of PBS containing 2.0% freshly dissolved paraformaldehyde and 0.2% of glutaraldehyde, followed by 100–150 ml of PBS of 2% paraformaldehyde and 2.5% glutaraldehyde. Immediately after perfusion, the brains were removed and left overnight in PBS of 2% paraformaldehyde and 2.5% glutaraldehyde at 4°C. Next day, slices of 60 μm of thickness were cut tangentially to the surface using a vibratome. Slices containing the barrel field (layer IV) were prepared using our standard procedure for transmission electron microscopy (TEM) (Kirov et al., 1999; Knott et al., 2002). Briefly, the slices were washed in 0.1 m sodium cacodylate buffer (three times for 5 min), postfixed at 4°C in 1% osmium tetroxide in 0.1 m sodium cacodylate buffer (two times for 1 h, the first change also containing 1.5% potassium ferrocyanide), washed in distilled water (three times for 5 min), incubated at 4°C in 70% alcohol containing 1% uranyl acetate, and dehydrated in increasing concentrations of ethanol (50, 70, 90, and 96%, 5 min each), 100% (three times for 5 min), and propylene oxide (two times for 10 min). Slices were next incubated in increasing concentrations of propylene oxide and embedded between silicon-coated glass slides in Epon (Polyscience). Embedded slices were photographed (5×) under Nikon Optiphot using a computer-assisted Nikon DXM 1200 F digital camera. The images were stacked together with use of the Adobe Photoshop CS (Adobe Systems), and the barrel field was reconstructed. The B2 barrel was identified from the pattern of barrels drawn from under the microscope together with the pattern of blood vessels characterizing its location within the barrel field. The embedded slices containing the B2 barrel were then trimmed with help of the drawings to blocks encompassing exclusively B1 and B2 or B2 and B3 barrels. Trimmed slices were cut in 30–50 series of ultrathin sections at 60–70 nm using an ultramicrotome (Ultracut; Reichert). The ultrathin sections were collected on Formvar-coated copper–palladium slots, counterstained with 1% lead citrate, and photographed at 7000–10,000× using a JEOL 100SX TEM aiming for the central part of the B2 barrel in which cell bodies are sparse.
Postembedding immunocytochemistry for GABA.
The tissue of three conditioned and three control animals was fixed and embedded as above with the omission of uranyl acetate and osmium/potassium ferrocyanide steps and replacing Epon with Durcupan resin. Ultrathin sections of the same region of B2 as before were cut and mounted onto 200 mesh nickel grids. Postembedding immunogold reaction with GABA antibody (anti-GABA 990; kindly provided by O.-P. Ottersen and J. Storm-Mathisen, Oslo, Norway) was used to identify inhibitory synapses according to the procedure of Ottersen (Ottersen, 1989; Mahendrasingam et al., 2004). Briefly, grids were successively incubated in drops of freshly made 1% aqueous sodium metaperiodate, Tris-buffered 1% human serum albumin (TBHSA), anti-GABA antibody diluted 1:1000 in TBHSA (primary antibody), washed, and then incubated in goat anti-rabbit secondary antibody conjugated to 10 nm gold particles (BioCell) diluted 1:100 in TBHSA. The anti-GABA antibody was preincubated with glutaraldehyde-conjugated β-alanine, glutamic acid, glycine, and taurine to eliminate unwanted immunoreactivity with other amino acids. Finally, grids were washed in TBHSA and then distilled water and counterstained with 2% aqueous uranyl acetate (Mahendrasingam et al., 2004). Grids were viewed in a JEOL 100CXII TEM, and images were taken at a magnification of 29,000–48,000× using an Olympus/SIS systems megaview III digital camera. Special care was taken to process the tissue from all experimental animals in the same way and to standardize staining conditions. The animals were perfused on the same day. Their brains were embedded together and sliced on the same day. All tissue samples were processed for immunochemistry in parallel and in identical conditions. The comparison between GABA content in the terminals of symmetrical and asymmetrical synapses was performed using the same set of sections. The only difference was that three animals have been trained and three were left untreated.
Quantitative analysis of synapses and dendritic spines.
The density of symmetrical and asymmetrical synapses located on dendritic shafts and single- and double-synapse spines together with the density of single- and double-synapse dendritic spines were estimated using the serial disector method (Weibel, 1979; Gundersen, 1986; Fiala and Harris, 2001). Axo-somatic synapses were then omitted from the analysis. Similarly, only synaptically connected spines have been included. The total density of synapses in this study indicates the number of synapses on dendritic spines and shafts counted in the defined area of the center of the B2 barrels. The centers of the barrels were chosen for counting synapses because the lower density of cell bodies minimizes obscuration of synapses on finer neuronal elements, in which most of them are located (White, 1976; Knott et al., 2002). Synapses and spines have been defined and counted according to Knott et al. (2002). Briefly, counting was done by placing a sample rectangle over the sequence of serial sections and counting each structure only once through the series; only structures fully within the rectangle or intersecting the left and the upper sides of the rectangle were included. The counting was done blind concerning the experimental group being counted.
Statistical analysis.
To compare the effects of learning on synapse density across the experimental groups, we counted the number of synapses in ∼100 μm3 volume of the B2 barrel hollow within each animal, averaged them across groups of treatment, and compared animal means using one-way ANOVA with post hoc Tukey's test after testing for normality and homogeneity. In the case of immunogold experiments, the data were compared using Kolmogorov–Smirnov and Mann–Whitney U statistics. In behavioral studies, the Mann–Whitney U test has been used. Both control groups (see above) appeared to be identical (Mann–Whitney U test) and were pooled together. The data were expressed as means ± SD throughout, unless stated otherwise.
Results
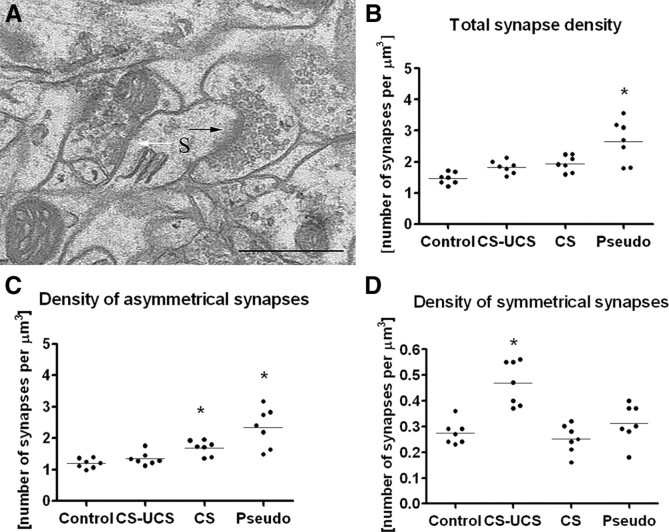
In this study, we compared the density of synapses in layer IV barrels of mice subjected to a brief learning paradigm involving mystacial vibrissae with that of untreated mice. Moreover, to test whether learning was the only cause of synaptic changes, we measured whether whisker stimulation alone or pseudoconditioning (in which the conditioned stimulus and the reinforcement are presented randomly) were enough to produce similar effects. With the aim of recognizing the neuronal elements in which synaptic changes take place, we counted synapses on dendritic shafts and spines independently, characterizing them as symmetrical (most likely inhibitory) or asymmetrical (most likely excitatory) (Fig. 1 A). During the course of the study, we noticed that the symmetrical synapses were the only pool of synapses that changed density specifically as a result of learning. Therefore, we also asked whether the level of GABA changed in the terminals lying presynaptic to the symmetrical synapses.
Figure 1.
Whisker conditioning induces a large increase in the density of symmetrical synapses (ANOVA, p < 0.001), whereas pseudoconditioning and whisker stimulation alone increased the density of asymmetrical synapses (ANOVA, p < 0.001 and p < 0.05, respectively). A, An electron micrograph taken from a B2 barrel hollow showing an asymmetrical synapse (black arrow) and a symmetrical synapse (white arrow), both located on the same dendritic spine (S). B, Total density of synapses for individual animals belonging to the following groups: control; CS–UCS, conditioned; CS, stimulated only; Pseudo, pseudoconditioned. C, Density of asymmetrical synapses for individual animals belonging to groups of treatment as in B. D, Density of symmetrical synapses for individual animals belonging to groups of treatment as in B. Scale bar, 0.5 μm. Horizontal line through the points in B–D indicates the mean.
Sampling
The synapses and dendritic spines were counted in the following volumes of the tissue: control group, 803.53 ± 73.05 μm3 (average per animal, 114.80 ± 35.10 μm3); conditioned group, 667.39 ± 60.67 μm3 (average per animal, 95.34 ± 46.3 μm3); whisker-stimulated group, 794.94 ± 72.27 μm3 (113.56 ± 24.18 μm3); and pseudoconditioned group, 675.52 ± 61.41 μm3 (average per animal, 96.50 ± 26.86 μm3). These sampling volumes were not significantly different across the treatment groups (ANOVA, p = 0.58, F = 0.67, total df = 27). The total number of spines and synapses counted in subsequent groups of treatment is summarized in Table 1, where the total number of ultrathin sections/photographs is also provided.
Table 1.
The total number of ultrathin sections/photographs, dendritic spines, and the synapses across groups of treatment
| Experimental treatment | Total number of ultrathin sections/photographs | Total number of spines | Total number of synapses |
|---|---|---|---|
| Control | 439 | 798 | 1165 |
| CS–UCS | 328 | 760 | 1206 |
| CS | 340 | 1161 | 1522 |
| Pseudo | 355 | 1360 | 1748 |
Control, Untreated; CS–UCS, conditioned; CS, whisker-stimulated only; Pseudo, pseudoconditioned group.
Quantitative analysis of synapse density
The total density of synapses
Conditioning did not change total synapse density in the hollow of the stimulated B2 barrel, but the density of synapses in the pseudoconditioned group (2.66 ± 0.69/μm3) was 82% higher than the density of synapses in the control group (1.46 ± 0.19/μm3; ANOVA, p < 0.001, F = 11.34, total df = 27), 46% higher than in the conditioned animal group (1.82 ± 0.21/μm3; ANOVA, p < 0.01), and 37% higher than in the stimulated group (1.94 ± 0.28/μm3; ANOVA, p < 0.05) (Fig. 1 B). These data indicate that only pseudoconditioning is capable of inducing changes in total synapse density. Our finding does not preclude the possibility that whisker stimulation and conditioning could evoke synaptic changes in specific subgroups of synapses that are less numerous and therefore obscured by the variance in total synapse density. We tested for this possibility by measuring symmetrical and asymmetrical synapse densities independently and also recognizing that some of them are located on dendritic shafts and some on dendritic spines.
The total density of asymmetrical synapses
The asymmetrical synapse density in both pseudoconditioned (2.34 ± 0.62/μm3) and stimulated (1.69 ± 0.24/μm3) animals was ∼97 and 42% higher than in controls, respectively (1.19 ± 0.15/μm3), which was statistically significant (ANOVA, p < 0.001 and p < 0.05, respectively, with F = 14.46 and total df = 27). The mean of the synaptic density of conditioned animals (1.34 ± 0.21/μm3) was found not to be different from controls (Fig. 1 C).
The total density of symmetrical synapses
The mean density of symmetrical synapses in the conditioned group (0.47 ± 0.09/μm3) was 74% higher than the mean of the control (0.27 ± 0.05/μm3; ANOVA, p < 0.001, F = 14.94, total df = 27), 88% larger than stimulated (0.25 ± 0.06/μm3; ANOVA, p < 0.001), and 52% larger than in the pseudoconditioned group (0.31 ± 0.08/μm3; ANOVA, p < 0.01) (Fig. 1 D).
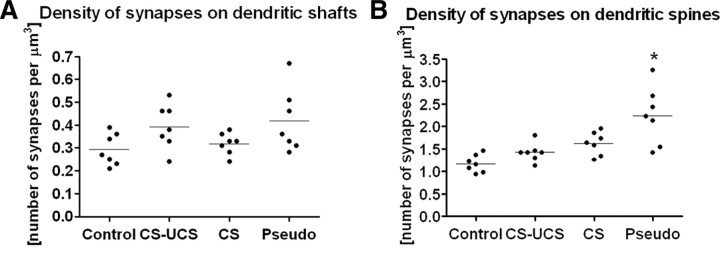
The total density of synapses on dendritic shafts and dendritic spines
In this study, only synapses located on the dendritic shafts and spines were counted in the hollows of the barrels. The density of synapses located on dendritic shafts was several-fold lower than the density of synapses found on dendritic spines, independent of treatment (synapses located on dendritic shafts: control, 0.29 ± 0.07/μm3; conditioned, 0.39 ± 0.10/μm3; stimulated, 0.32 ± 0.05/μm3; and pseudoconditioned, 0.42 ± 0.14/μm3) (Fig. 2 A). A twofold increase in the number of synapses on dendritic spines was found in the pseudoconditioned animals when compared with control animals (2.24 ± 0.64 and 1.17 ± 0.19/μm3, respectively; ANOVA, p < 0.001, F = 11.66, total df = 27), with no significant differences found between conditioned, stimulated, or control animals (Fig. 2 B). The large increase in asymmetrical synapse density attributable to pseudoconditioning was therefore localized to the synaptic spines.
Figure 2.
Only pseudoconditioning leads to the upregulation of synapse density and only on dendritic spines (mean ANOVA, p < 0.001). A, The density of synapses on dendritic shafts does not change despite treatment. B, Pseudoconditioning increases the number of synapses on dendritic spines. Labels as in Figure 1. Horizontal line through the points indicates the mean. Each dot represents data taken from one animal.
The density of symmetrical and asymmetrical synapses on dendriticshafts and dendritic spines
Asymmetrical synapses located on dendritic spines made up ∼73.3% of all synapses found in the area (1.07 ± 0.17/μm3), whereas 8.2% of them were located on the dendritic shafts (0.12 ± 0.06/μm3). Among the remaining synapses, 11.6% were recognized as symmetrical synapses connecting to the dendritic shafts (0.17 ± 0.03/μm3), and 6.9% were located on dendritic spines (0.10 ± 0.03/μm3).
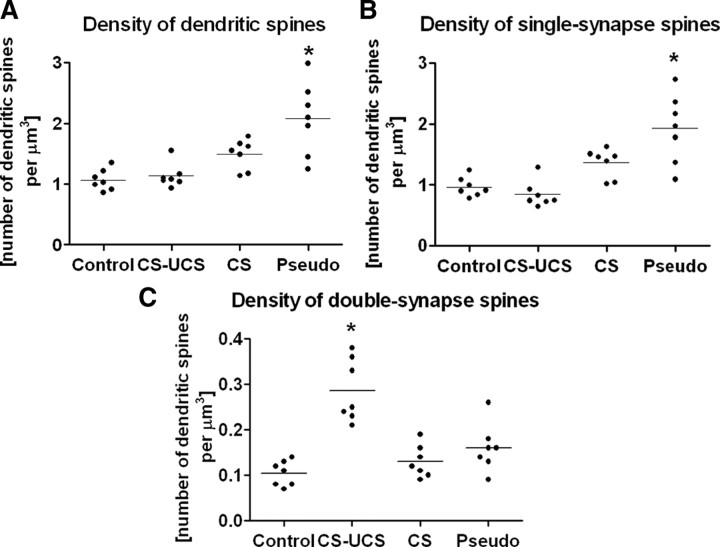
Quantitative analysis of dendritic spine density
The total density of dendritic spines
The density of dendritic spines increased approximately twofold after pseudoconditioning (control, 1.07 ± 0.17/μm3; pseudoconditioned, 2.08 ± 0.60/μm3; ANOVA, p < 0.001, F = 12.30, total df = 27) but not after conditioning (1.13 ± 0.20/μm3) or whisker stimulation (1.49 ± 0.25/μm3) (Fig. 3 A).
Figure 3.
Pseudoconditioning increases the density of single-synapse spines (ANOVA, p < 0.001), whereas whisker conditioning increases the density of double-synapse spines (ANOVA, p < 0.001). A, Total density of dendritic spines across treatment groups. B, Density of single-synapse spines across treatment groups. C, Density of double-synapse spines across treatment groups. Labels as in Figure 1. Horizontal line through the points indicates the mean. Each dot represents data taken from one animal.
The density of single-synapse and double-synapse spines
Most dendritic spines have only one synapse located on them. This is usually an asymmetrical synapse (single-synapse spines), but a small percentage of dendritic spines host two synapses (double-synapse spines); in the majority of cases, one is asymmetrical, located on the head of a dendritic spine, and, one is symmetrical, located on its neck (Fig. 1 A) (Knott et al., 2002; Jasinska et al., 2006; Kubota et al., 2007). In the present study, all synapses located on single-synapse spines were recognized as being asymmetrical, and on double-synapse spines, one was always symmetrical and the other asymmetrical. Spines with more than two synapses were not observed in this region of the barrel cortex. In the control animals, the density of single-synapse spines was calculated as 0.96 ± 0.16/μm3 and constituted ∼90% of the total number of dendritic spines in the area (1.07 ± 0.17/μm3). The density of double-synapse spines was estimated as 0.10 ± 0.03/μm3.
Pseudoconditioning increased the density of single-synapse spines by approximately twofold (from 0.96 ± 0.16/μm3 in control animals to 1.92 ± 0.57/μm3; ANOVA, p < 0.001, F = 14.80, total df = 27), whereas conditioning and stimulation alone did not induce significant increases (conditioned group, 0.84 ± 0.22/μm3; stimulated group, 1.36 ± 0.24/μm3) (Fig. 3 B). Thus, the large increase in the density of asymmetrical synapses attributable to pseudoconditioning (Fig. 1 C) could be interpreted as an increase in the density of asymmetrical synapses connecting to the single-synapse dendritic spines (Fig. 3 B).
Whisker conditioning increased the density of the double-synapse spines almost threefold, from 0.10 ± 0.03/μm3 in control animals to 0.29 ± 0.07/μm3 (ANOVA, p < 0.001, F = 18.95, total df = 27), with no change in the case of stimulated (0.13 ± 0.04/μm3) and pseudoconditioned (0.16 ± 0.05/μm3) animals (Fig. 3 C). Thus, the large increase in the density of conditioning-evoked symmetrical synapses is correlated with the increase in double-synapse spines (Figs. 1 D, 3 C).
GABA upregulates because of conditioning: immunogold studies
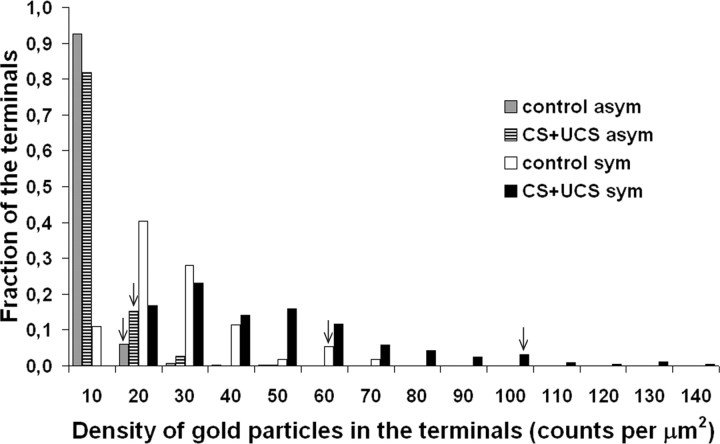
The conditioning paradigm led to an increase in the density of inhibitory synapses on double-synapse spines. To answer the question of whether this increase is accompanied by an increase in the concentration of GABA in the presynaptic terminals of inhibitory synapses, we immunolabeled ultrathin sections cut through layer IV of conditioned and untreated animals with anti-GABA antibodies, visualized with gold-conjugated secondary antibodies (Fig. 4). We counted the number of gold particles in the symmetrical and asymmetrical terminals in the same sections cut from the barrels that were involved in the process of conditioning (153 symmetrical and 308 asymmetrical terminals counted) and in matching controls (148 symmetrical and 309 asymmetrical terminals counted) and measured the areas of all terminals. Although the presence of gold particles in the asymmetrical synapse terminals is evident (Fig. 4), the presence of large amounts of GABA in them is unlikely (Martin and Rimvall, 1993), so we took the density of gold particles in the asymmetrical synapse terminals as the background. Having estimated the densities of gold particles in the individual synaptic terminals, we plotted the distributions of these densities for symmetrical and asymmetrical “trained” and “untrained” data pooled from all experimental animals as depicted in Fig. 5. Only the histogram representing the symmetrical synaptic terminals is shifted to the right because of the conditioning, suggesting an increase in the concentration of GABA. To compensate for the background, in each animal, we subtracted the 95th percentile value obtained from the distribution of density of gold labeling in the asymmetrical terminals from the density of gold particles measured in all symmetrical terminals individually and then averaged the results. From this average for each animal, we then calculated an average value of the three animals within the treatment groups, that is, control and conditioned, and compared them using the Mann–Whitney U test (mean ± SEM; control group, 12.31 ± 1.89 gold particles/μm2; conditioned group, 27.06 ± 1.06 gold particles/μm2; p < 0.0001). This analysis unequivocally shows that whisker conditioning increases the concentration of GABA in the terminals of the symmetrical synapses.
Figure 4.
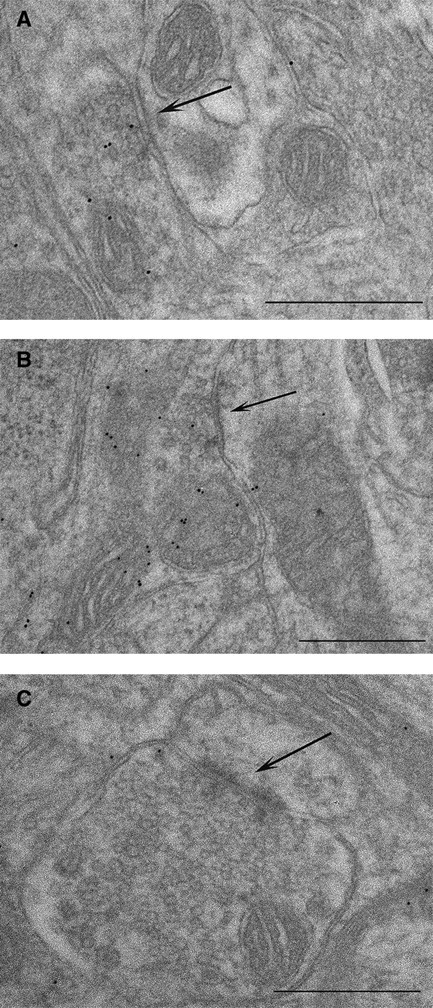
Immunogold labeling for GABA. Whisker conditioning upregulates GABA in symmetrical synapse terminals as indicated by an increase in the number of gold particles per terminal. Electron micrographs taken from B2 barrel hollow stained with anti-GABA antibodies showing the following: A, symmetrical synapse from the control animal; B, symmetrical synapse from the conditioned animal; C, asymmetrical synapse from the conditioned animal. Black arrows indicate the synapses. Scale bars, 0.5 μm.
Figure 5.
Quantification of immunogold labeling for GABA. Whisker conditioning upregulates GABA in the symmetrical synapse terminals. The histogram illustrates the distribution of gold particle densities in the symmetrical and asymmetrical synapse terminals (uncorrected for the background) of trained and untrained animals after pooling the data from all experimental animals. The arrowheads point to the 95th percentile of maximal density of all four density distributions to show that the substantial shift in the density of gold particles attributable to conditioning takes place only in the terminals of the symmetrical synapses (Kolmogorov–Smirnov, p < 0.001).
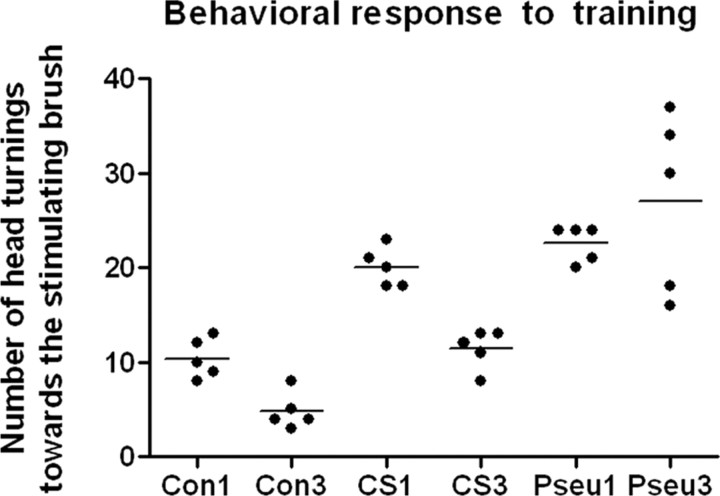
Behavioral effects of conditioning
During the initial trials of whisker conditioning (CS–UCS), the mice often reacted to vibrissal stimulation by turning the head toward the stimulus, but, during the subsequent trials, the frequency of this reaction decreased. The number of head turnings during CS application, which possibly indicates the amount of learning by the mouse, was counted from video recordings. In the course of CS–UCS, the number of head turnings decreased from 10.40 ± 0.93 in the first session to 4.80 ± 0.83 in the third session (p < 0.05); in the case of CS, the number decreased from 20.00 ± 0.95 in the first session to 11.40 ± 0.93 in the third session (p < 0.05), but the decrease has not been observed during pseudoconditioning (session 1, 22.60 ± 1.95; session 2, 27.00 ± 4.24) (Fig. 6). This shows that the animals learn not only during CS–UCS sessions but also during CS sessions, albeit in a different manner than in the CS–UCS case and far slower.
Figure 6.
The number of head turnings decreases during conditioning and whisker stimulation alone (Mann–Whitney U test, p < 0.05) but not during pseudoconditioning. Note that, in the case of conditioned animals, changes happen already during the first session. Con, Conditioned; CS, whisker-stimulated only; Pseu, pseudoconditioned. The numbers are session numbers.
Discussion
These results demonstrate that brief classical conditioning, in which stimulation of whiskers is paired with tail shock, leads to a substantial upregulation of the density of GABAergic synapses in the hollow of “trained” barrels. The supernumerary GABAergic synapses were found on double-synapse spines, which showed an increased number after conditioning. Moreover, GABA content increased in the presynaptic terminals of symmetrical synapses (not only in double-spine synapses) in the “trained” barrel hollows. Pseudoconditioning led to a specific, high variance, increase in the density of the asymmetrical synapses on the single-synapse spines, whereas whisker stimulation alone increased the density of excitatory synapses in an unspecified pool.
Specificity of anatomical changes after learning
One interpretation of the data would be that whisker stimulation in the absence of a meaningful context (i.e., pseudoconditioning or whisker stimulation alone) results in an increased density of excitatory synapses, and whisker stimulation paired with reinforcement (i.e., conditioning) leads to an increase in the density of GABAergic synapses. However, at first glance, this reasoning is at odds with the results of other experiments in which whisker stimulation alone (Knott et al., 2002) led to an increase in the density of GABAergic synapses or whisker deprivation (Micheva and Beaulieu, 1995) led to a decrease, specifically on double-synapse spines. This would suggest that the length, intensity, frequency, or pattern of stimulation and not the behavioral paradigm is the differentiating factor leading to a specific change in synapse density. If so, the reinforcement in our experiments would eventually only modulate the demand for an appropriate stimulation needed for a particular synaptic change. Alternatively, the aforementioned “stimulation/deprivation” experimental paradigms may contain elements of learning.
Coherence of anatomical and physiological changes after learning
Whisker conditioning produces an upregulation of GABAergic synapses that is consistent with an elevation of several GABAergic markers in the barrel hollows of trained vibrissae, like the density of small GABA-immunoreactive, non-parvalbumin-containing neurons (Siucinska et al., 1999; Siucinska and Kossut, 2006), GAD-67 mRNA (Gierdalski et al., 2001), GABAergic puncta (Siucinska, 2006), and GAD-67 protein level (Gierdalski et al., 2001). Moreover, in the excitatory neurons of the barrels representing vibrissae involved in training, a selective increase in frequency, but not amplitude, of spontaneous IPSCs, but not EPSCs, was observed (Tokarski et al., 2007). Also, the amplitude of field potentials evoked by the stimulation of layer VI and recorded in layer IV was significantly reduced (Tokarski et al., 2007).
Because the increase in GABAergic synapse density appears on double-synapse spines with no apparent change in the density on single-synapse spines, a concomitant increase in excitatory synapse markers would be expected, and these indeed have been found again exclusively in barrels involved in learning (Jablonska et al., 1996; Skibinska et al., 2001, 2005).
Although synapses were counted only in “conditioned” barrels, there is evidence that their density in neighboring barrels either does not change or the change does not have an impact on neuronal transmission. First, the 2-DG response to stimulation of the principal whisker of the barrel immediately neighboring the “conditioned” barrel is indistinguishable from the control (Kossut and Siucinska, 1998). Second, neuronal transmission is affected by the learning procedure only in the output from, not input to, the “conditioned” barrel (Urban-Ciecko et al., 2005). The changes in GABAergic markers, GABAergic synapse density, and in vitro physiology appear to be well orchestrated. A similar coherence between physiological, histological, and anatomical response was observed in the barrel cortex in response to sustained whisker stimulation (Welker et al., 1989, 1992; Knott et al., 2002; Quairiaux et al., 2007). Together, these data unequivocally show that the response of the neurons of the barrels to vibrissae conditioning is net inhibitory in nature, at least at the time when measurements have been taken, and confined to “conditioned” barrels.
Conversely, the trained whisker-evoked 2-DG uptake spreads well beyond the borders of the “trained” barrels (Siucinska and Kossut, 1996). This result is intuitively at odds with an inhibitory response to learning. It should be expected that rising inhibition inside the barrel diminishes the uptake, intensity, and spread of the 2-DG response (Fox et al., 2003; Tokarski et al., 2007). With regard to the 2-DG uptake inside the barrel, the unchanged response could be a consequence of both the additive nature of the 2-DG signal, in which inhibition and excitation have the same metabolic meaning (Sokoloff et al., 1977), and high metabolic activity of inhibitory neurons (McCasland and Hibbard, 1997). In such circumstances, a slight increase in inhibition leading to a diminished excitatory response could be metabolically more pronounced than a loss of label attributable to decreased excitation (Melzer et al., 1985). 2-DG spread could be similarly explained as an effect of a learning-induced shift in lateral excitation and inhibition. The main substrates for such changes, that is, excitatory and inhibitory connections between barrels (Aroniadou-Anderjaska and Keller, 1996; Salin and Prince, 1996; Fox et al., 2003; Schubert et al., 2003), including the inhibitory network (Gibson et al., 1999; Sun et al., 2006; Cruikshank et al., 2007), are known, but details of the mechanism are missing. Alternatively, because our data were obtained 1 d after the cessation of training, we may be observing upregulation of the GABAergic system in response to training-enhanced excitation. We do not know at present how long the changes in the inhibitory system last after cessation of the conditioning procedure. The metabolic change fades within 5 d of the last training session (Siucinska and Kossut, 1996), which corresponds well with the time course of the disappearance of electrophysiological changes attributable to passive whisker stimulation, the procedure that may contain elements of learning (Knott et al., 2002).
The sources of input to double-synapse spinesand their location
The source of excitatory and inhibitory terminals engaged in synaptic contacts on double-synapse spines in the barrel cortex is not precisely known. The excitatory terminals could originate from the thalamus, as was recently shown in the rat frontal cortex (Kubota et al., 2007), or be intracortical, most likely belonging to excitatory cells of the same barrel (Feldmeyer et al., 1999; Lübke et al., 2000). The inhibitory terminals are intracortical (Fox, 2008), but it is not known which cells they belong to from the variety of dendrite-targeting inhibitory cell types (Markram et al., 2004). Initially, double bouquet cells were thought to be likely candidates because they are involved in the cat visual cortex (Tamás et al., 1997; Knott et al., 2002), but later it appeared that they are missing in rodent brains (Yáñez et al., 2005). Because non-parvalbumin GABAergic cells were found to be upregulated by conditioning (Siucinska and Kossut, 2006), the “donors” of GABAergic innervation to double-synapse spines possibly belong to this class of neurons with the Martinotti cells as possible and testable candidates (Markram et al., 2004). Double-synapse spines are most likely located on the dendrites of stellate and/or star pyramidal cells of the same barrels from which their afferentation originates (Woolsey et al., 1975; White and Peters, 1993; Aroniadou-Anderjaska and Keller, 1996; Salin and Prince, 1996; Lübke et al., 2000; Fox et al., 2003; Schubert et al., 2003). Spiny inhibitory interneurons are another possible, but less likely, target (Kawaguchi et al., 2006; Sun et al., 2006).
Mechanism of synaptic changes after learning
The increase in density of symmetrical synapses occurs on double-synapse spines and may be obtained in two ways that are not mutually exclusive. The first possibility relies on the addition of symmetrical synapses to the preexisting single-asymmetrical synapse spines with concomitant replenishment of their own density. The second possibility is that the double-synapse spines are constructed de novo. New synapses could be formed using an existing pattern of neuronal connectivity or, alternatively, orchestrated with changes in the density of neuropil inside the “trained” barrel. Retractions and additions of small axonal elements over a timescale of days and the distances comparable with the dimensions of barrel hollows have recently been observed during development of the barrel cortex (De Paola et al., 2006). The dendrites, excluding dendritic spines, appear to be more stable, at least over a relatively short timescale (Trachtenberg et al., 2002).
Possible meaning of the changes
We showed that animals reduce head movements in response to whisker stimulation coupled with conditioning, signaling an inescapable fearful stimulus, significantly more than mice that received only whisker stimulation. This possibly indicates that the animal is undergoing learning (Siucinska and Kossut, 1996; Cybulska-Klosowicz et al., 2009). Additionally, the animals clearly remember the conditioning for at least 24 h, as is indicated in Figure 6. The mechanism underlying this may reside in the barrels. Such a mechanism should include a specific memory trace but also regulated activity environment necessary for its formation. In our case, the increased inhibitory interactions in layer IV, triggered by sensory training, could decrease the effects of learning-associated release of neuromodulators and in consequence general arousal to maintain activity homeostasis (Froemke et al., 2007). With time, conditioning-induced changes observed in barrels could propagate elsewhere (Berry et al., 2008).
Footnotes
This work was supported by the Wellcome Trust (S.G., M.K.), the Royal Society and the Physiological Society (S.G.), and Erasmus, SBMiK, and Jagiellonian University Grant BW/IZ/72/2007 (M.J.). We thank Drs. Alison Barth, Kevin Fox, Egbert Welker, and Gerald Finnerty for their advice on the manuscript, Drs. Graham Knott, Egbert Welker, and Karen Walker for the training of M.J., and Drs. Polwart and Preater for advice on the statistical analysis.
References
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol. 1987;263:265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Keller A. Intrinsic inhibitory pathways in mouse barrel cortex. Neuroreport. 1996;7:2363–2368. doi: 10.1097/00001756-199610020-00017. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia . Prog Brain Res. 2008;169:179–198. doi: 10.1016/S0079-6123(07)00010-6. [DOI] [PubMed] [Google Scholar]
- Berry J, Krause WC, Davis RL. Olfactory memory traces in Drosophila . Prog Brain Res. 2008;169:293–304. doi: 10.1016/S0079-6123(07)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sonenberg N. Translational control of gene expression: a molecular switch for memory storage. Prog Brain Res. 2008;169:81–95. doi: 10.1016/S0079-6123(07)00005-2. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Cybulska-Klosowicz A, Zakrzewska R, Kossut M. Brain activation patterns during classical conditioning with appetitive or aversive UCS. Behav Brain Res. 2009;204:102–111. doi: 10.1016/j.bbr.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Egger V, Lubke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single “barrel” of developing rat somatosensory cortex. J Physiol. 1999;521:169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Harris KM. Extending unbiased stereology of brain ultrastructure to three-dimensional volumes. J Am Med Inform Assoc. 2001;8:1–16. doi: 10.1136/jamia.2001.0080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Fox K. Barrel cortex. Cambridge UP: 2008. [Google Scholar]
- Fox K, Wright N, Wallace H, Glazewski S. The origin of cortical surround receptive fields studied in the barrel cortex. J Neurosci. 2003;23:8380–8391. doi: 10.1523/JNEUROSCI.23-23-08380.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gierdalski M, Jablonska B, Siucinska E, Lech M, Skibinska A, Kossut M. Rapid regulation of GAD67 mRNA and protein level in cortical neurons after sensory learning. Cereb Cortex. 2001;11:806–815. doi: 10.1093/cercor/11.9.806. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996;75:1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Harris RM, Woolsey TA. Dendritic plasticity in mouse barrel cortex following postnatal vibrissa follicle damage. J Comp Neurol. 1981;196:357–376. doi: 10.1002/cne.901960302. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- Jablońska B, Kossut M, Skangiel-Kramska J. Transient increase of AMPA and NMDA receptor binding in the barrel cortex of mice after tactile stimulation. Neurobiol Learn Mem. 1996;66:36–43. doi: 10.1006/nlme.1996.0041. [DOI] [PubMed] [Google Scholar]
- Jasinska M, Siucinska E, Glazewski S, Pyza E, Kossut M. Characterization and plasticity of the double synapse spines in the barrel cortex of the mouse. Acta Neurobiol Exp. 2006;66:99–104. doi: 10.55782/ane-2006-1595. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Karube F, Kubota Y. Dendritic branch typing and spine expression patterns in cortical nonpyramidal cells. Cereb Cortex. 2006;16:696–711. doi: 10.1093/cercor/bhj015. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Sorra KE, Harris KM. Slices have more synapses than perfusion-fixed hippocampus from both young mature rats. J Neurosci. 1999;19:2876–2886. doi: 10.1523/JNEUROSCI.19-08-02876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery EA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott G, Holtmaat A. Dendritic spine plasticity-Current understanding from in vivo studies. Brain Res Rev. 2008;58:282–289. doi: 10.1016/j.brainresrev.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Kossut M, Siucinska E. Learning-induced expansion of cortical maps: what happens to adjacent cortical representations? Neuroreport. 1998;9:4025–4028. doi: 10.1097/00001756-199812210-00007. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hatada S, Kondo S, Karube F, Kawaguchi Y. Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J Neurosci. 2007;27:1139–1150. doi: 10.1523/JNEUROSCI.3846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Lübke J, Egger V, Sakmann B, Feldmeyer D. Columnar organization of dendrites and axons of single and synaptically coupled excitatory spiny neurons in layer 4 of the rat barrel cortex. J Neurosci. 2000;20:5300–5311. doi: 10.1523/JNEUROSCI.20-14-05300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan DS, Desiraju T. Alterations of dendritic branching and spine densities of hippocampal CA3 pyramidal neurons induced by operant conditioning in the phase of brain growth spurt. Exp Neurol. 1988;100:1–15. doi: 10.1016/0014-4886(88)90196-3. [DOI] [PubMed] [Google Scholar]
- Mahendrasingam S, Wallam CA, Polwart A, Hackney CM. An immunogold investigation of the distribution of GABA and glycine in nerve terminals on the somata of spherical bushy cells in the anteroventral cochlear nucleus of guinea pig. Eur J Neurosci. 2004;19:993–1004. doi: 10.1111/j.1460-9568.2004.03193.x. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem. 1993;60:395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- McCasland JS, Hibbard LS. GABAergic neurons in barrel cortex show strong, whisker-dependent metabolic activation during normal behavior. J Neurosci. 1997;17:5509–5527. doi: 10.1523/JNEUROSCI.17-14-05509.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer P, Van der Loos H, Dörfl J, Welker E, Robert P, Emery D, Berrini JC. A magnetic device to stimulate selected whiskers of freely moving or restrained small rodents: its application in a deoxyglucose study. Brain Res. 1985;348:229–240. doi: 10.1016/0006-8993(85)90441-x. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. An anatomical substrate for experience-dependent plasticity of the rat barrel field cortex. Proc Natl Acad Sci U S A. 1995;92:11834–11838. doi: 10.1073/pnas.92.25.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Quantitative electron microscopic immunocytochemistry of neuroactive amino acids. Anat Embryol (Berl) 1989;180:1–15. doi: 10.1007/BF00321895. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Weimer RM, De Paola V, Caroni P, Svoboda K. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol. 2005;3:e272. doi: 10.1371/journal.pbio.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quairiaux C, Armstrong-James M, Welker E. Modified sensory processing in the barrel cortex of the adult mouse after chronic whisker stimulation. J Neurophysiol. 2007;97:2130–2147. doi: 10.1152/jn.00338.2006. [DOI] [PubMed] [Google Scholar]
- Salin PA, Prince DA. Electrophysiological mapping of GABAA receptor-mediated inhibition in adult rat somatosensory cortex. J Neurophysiol. 1996;75:1589–1600. doi: 10.1152/jn.1996.75.4.1589. [DOI] [PubMed] [Google Scholar]
- Schubert D, Kötter R, Zilles K, Luhmann HJ, Staiger JF. Cell type-specific circuits of cortical layer IV spiny neurons. J Neurosci. 2003;23:2961–2970. doi: 10.1523/JNEUROSCI.23-07-02961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siucinska E. GAD67-positive puncta: contributors to learning-dependent plasticity in the barrel cortex of adult mice. Brain Res. 2006;1106:52–62. doi: 10.1016/j.brainres.2006.05.061. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. Short-lasting classical conditioning induces reversible changes of representational maps of vibrissae in mouse SI cortex-a 2DG study. Cereb Cortex. 1996;6:506–513. doi: 10.1093/cercor/6.3.506. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. Short-term sensory learning does not alter parvalbumin neurons in the barrel cortex of adult mice: a double-labeling study. Neuroscience. 2006;138:715–724. doi: 10.1016/j.neuroscience.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M, Stewart MG. GABA immunoreactivity in mouse barrel field after aversive and appetitive classical conditioning training involving facial vibrissae. Brain Res. 1999;843:62–70. doi: 10.1016/s0006-8993(99)01881-8. [DOI] [PubMed] [Google Scholar]
- Skibinska A, Lech M, Kossut M. PSD95 protein level rises in murine somatosensory cortex after sensory training. Neuroreport. 2001;12:2907–2910. doi: 10.1097/00001756-200109170-00030. [DOI] [PubMed] [Google Scholar]
- Skibinska A, Lech M, Kossut M. Differential regulation of cortical NMDA receptor subunits by sensory learning. Brain Res. 2005;1065:26–36. doi: 10.1016/j.brainres.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Svoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci. 2006;26:1219–1230. doi: 10.1523/JNEUROSCI.4727-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás G, Buhl EH, Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol. 1997;500:715–738. doi: 10.1113/jphysiol.1997.sp022054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski K, Urban-Ciecko J, Kossut M, Hess G. Sensory learning-induced enhancement of inhibitory synaptic transmission in the barrel cortex of the mouse. Eur J Neurosci. 2007;26:134–141. doi: 10.1111/j.1460-9568.2007.05629.x. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Urban-Ciecko J, Kossut M, Hess G. Effects of sensory learning on intracortical synaptic transmission in the barrel cortex of mice. Acta Neurobiol Exp. 2005;65:195–200. doi: 10.55782/ane-2005-1555. [DOI] [PubMed] [Google Scholar]
- von der Ohe CG, Darian-Smith C, Garner CC, Heller HC. Ubiquitous and temperature-dependent neural plasticity in hibernators. J Neurosci. 2006;26:10590–10598. doi: 10.1523/JNEUROSCI.2874-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER. Morphometry of the human lung: the state of the art after two decades. Bull Eur Physiopathol Respir. 1979;15:999–1013. [PubMed] [Google Scholar]
- Welker E, Soriano E, Dörfl J, Van der Loos H. Plasticity in the barrel cortex of the adult mouse: transient increase of GAD-immunoreactivity following sensory stimulation. Exp Brain Res. 1989;78:659–664. doi: 10.1007/BF00230256. [DOI] [PubMed] [Google Scholar]
- Welker E, Rao SB, Dörfl J, Melzer P, van der Loos H. Plasticity in the barrel cortex of the adult mouse: effects of chronic stimulation upon deoxyglucose uptake in the behaving animal. J Neurosci. 1992;12:153–170. doi: 10.1523/JNEUROSCI.12-01-00153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EL. Ultrastructure and synaptic contacts in barrels of mouse SI cortex. Brain Res. 1976;105:229–251. doi: 10.1016/0006-8993(76)90423-6. [DOI] [PubMed] [Google Scholar]
- White EL, Peters A. Cortical modules in the posteromedial barrel subfield (Sml) of the mouse. J Comp Neurol. 1993;334:86–96. doi: 10.1002/cne.903340107. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic cells. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Welker C, Schwartz RH. Comparative anatomical studies of the SmL face cortex with special reference to the occurrence of “barrels” in layer IV. J Comp Neurol. 1975;164:79–94. doi: 10.1002/cne.901640107. [DOI] [PubMed] [Google Scholar]
- Yáñez IB, Muñoz A, Contreras J, Gonzalez J, Rodriguez-Veiga E, DeFelipe J. Double bouquet cell in the human cerebral cortex and a comparison with other mammals. J Comp Neurol. 2005;486:344–360. doi: 10.1002/cne.20533. [DOI] [PubMed] [Google Scholar]