Abstract
The hematopoietic cell transplantation comorbidity index (HCT-CI), a weighted index of 17 pretransplantation comorbidities, has been validated in nonmyeloablative and myeloablative allogeneic hematopoietic stem cell transplantation (HSCT) studies, but it has not been specifically tested in patients with non-Hodgkin lymphoma (NHL) receiving reduced-intensity conditioning (RIC). We performed a retrospective analysis to assess the impact of the HCT-CI on outcomes of NHL patients treated with HSCT relative to treatment-related mortality (TRM), disease-related mortality (DRM), with a specific emphasis on overall survival (OS). Individual pretransplantation and disease-related factors also were analyzed with HCT-CI relative to their impact on OS. All patients were uniformly treated with an identical pretransplantation induction regimen and an identical RIC regimen (cyclophosphamide [Cy]/fludarabine [Flu]), and received T cell–replete allografts from HLA-matched siblings. The analysis included 63 NHL patients with a median HCT-CI score of 2 (range, 0 to 11). The HCT-CI (0 to 2 comorbidities vs 3+ comorbidities) demonstrated a potential association with TRM, but not with DRM, at 100 days (4.5% vs 26.3%) and at 1 year (13.6% vs 36.8%) posttransplantation. The factor most strongly associated with OS was response to pretransplantation chemotherapy (P = .0001), based on a composite measure. In a Cox model, pretransplantation chemotherapy response remained the most important factor (P < .0001) relative to OS, and there was a trend (P = .056) toward HCT-CI adding predictive value for OS. Although HCT-CI may be useful for predicting TRM, our data further underscore the importance of response to chemotherapy before transplantation as a predictor of overall transplantation outcome in NHL patients being considered for RIC allogeneic HSCT.
Keywords: Comorbidity index, Non-Hodgkin lymphoma, Reduced intensity conditioning, Allogeneic
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) can result in sustained, disease-free survival (DFS) in patients with refractory and relapsed non-Hodgkin lymphoma (NHL) [1,2]. Previously, the option of allogeneic HSCT was relatively contraindicated for patients with NHL who were older, had comorbidities, or had previously received high-dose therapy and autologous HSCT [3]. The introduction of nonmyeloablative and reduced-intensity conditioning (RIC) regimens, which are associated with decreased acute morbidity and mortality, made the option of allogeneic HSCT available to many of these patients [4–7]. It has been demonstrated that nonmyeloablative and reduced-intensity allogeneic HSCT can result in sustained remission in patients with refractory and relapsed NHL [7,8]. These transplantations have been performed successfully in older patients, patients who had undergone previous autologous stem cell transplantations, and patients with preexisting comorbidities [6,9].
Despite evidence that nonmyeloablative and reduced-intensity allogeneic HSCT may be successful in patients with advanced NHL, determining whether or not certain patients are good candidates for transplantation can be difficult. Multiple patient-related factors, including age and performance status, are associated with transplantation outcomes [10,11]. In addition, disease-related factors (particularly chemotherapy sensitivity before transplantation) have been identified as prognostic factors for overall transplantation success in the nonmyeloablative and RIC settings [8,9,12]. The assessment of whether nonmyeloablative or RIC allogeneic HSCT is an appropriate option for patients with an NHL is even more difficult, because many of these patients have multiple adverse patient-and disease-related factors, as well as coexisting comorbidities. Because each of these factors has a unique impact on transplantation outcome, the ability to quantify the collective impact of these factors would be useful in better determining the applicability of nonmyeloablative or reduced-intensity allogeneic HSCT to this heterogeneous patient population.
Various comorbidity indices, including the Charlson comorbidity index and the Kaplan-Feinstein scale, have been developed in attempts to facilitate pretransplantation patient counseling and aid researchers comparing varied patient populations [13,14]. These assessment tools, particularly the Charlson comorbidity index, have been successful in predicting mortality risk in several medical conditions, including allogeneic HSCT. But these tools did not include several therapy- and disease-related factors that have been demonstrated to affect outcomes related to allogeneic HSCT. To address these inadequacies, a new assessment tool specific to HSCT was developed by investigators from the Fred Hutchinson Cancer Research Center [15]. This assessment tool, the hematopoietic cell transplantation comorbidity index (HCT-CI), is a weighted index calculated from 17 comorbidities and based on data from more than 1000 diverse patients who underwent HSCT. The HCT-CI has demonstrated its superiority in predicting outcomes in HSCT patients compared with the Charlson comorbidity index.
The HCT-CI has been extensively evaluated in patients receiving myeloablative and nonmyeloablative conditioning regimens [16,17], but it has not been specifically tested in a cohort of NHL patients receiving an RIC regimen before allogeneic HSCT. Because of the relative heterogeneity in the degree of myelosuppression, immunosuppression, and clinical outcomes with various nonmyeloablative and RIC regimens, exploring the utility of the HCT-CI with different regimens is of interest. Using a cohort of NHL patients who had been uniformly treated with RIC allogeneic HSCT, we performed a retrospective analysis comparing the HCT-CI with individual comorbidities and disease-related factors relative to their impact on transplantation outcomes, with a specific emphasis on overall survival (OS).
METHODS
Patient Selection
Patients with NHL who were enrolled in the sequential National Cancer Institute protocols 99-C-0143, 03-C-0077, and 04-C-0055, from January 2000 through November 2005, were included in this retrospective analysis. All 3 protocols required that the patient had disease with less than a complete response to primary treatment, relapsed and failed to respond to second-line therapies, or relapsed or progressed after autologous HSCT. A patient was not required to have chemotherapy-sensitive disease to participate in any of these protocols. All protocols had nearly identical eligibility criteria, which included an Eastern Cooperative Oncology Group (EGOG) performance status assessment of 0 to 2 (Karnofsky performance status > 60%), aged > 18 years, and consenting first-degree relative matched at HLA-A, -B, and -DR. Although severe cardiac, pulmonary, renal, or hepatic dysfunction was reason for exclusion from the study, mild to moderate organ dysfunction was permitted, including (1) cardiac left ventricular ejection fraction (LVEF) as low as 45%, (2) pulmonary diffusion capacity for carbon monoxide (DLCO) as low as 50% of the expected value when corrected for hemoglobin, (3) serum creatinine level up to 1.5 mg/dL and creatinine clearance as low as 50 mL/min/1.73 m2, and (4) serum total bilirubin as high as 2.5 mg/dL and serum alanine aminotransferase and aspartate aminotransferase values as high as 2.5 times the upper limit of normal. The National Cancer Institute’s Pathology Laboratory confirmed each patient’s diagnosis using the World Health Organization (WHO) classification system. The protocols in which the patients participated were approved by the National Cancer Institute Institutional Review Board, and informed written consent was obtained from each patient and his or her donor.
All patients were treated in a uniform manner. On enrollment onto their respective protocols, all patients received an induction chemotherapy regimen, EPOCH-F (consisting of etoposide, prednisone, vincristine, cyclophosphamide [Cy], doxorubicin, and fludarabine [Flu]), administered at conventional doses [10]. Patients with lymphoma expressing CD20 also received rituximab with the induction chemotherapy. The purpose of the induction chemotherapy was to further assess chemotherapy sensitivity, reduce circulating host T cells, and provide tumor control before proceeding to transplantation. All patients then received an identical reduced-intensity conditioning regimen consisting of Cy (4.8 g/m2) and Flu (120 mg/m2), both administered over 4 days, followed by a T cell–replete peripheral blood allograft collected from an HLA-matched siblings after mobilization with filgrastim. All patients received a cyclosporine (CsA)-based graft-versus-host disease (GVHD) prophylaxis regimen. The 3 protocols differed by the addition of donor T helper cells with type 2 cytokine profile (Th2) cells, methotrexate (MTX), and/or sirolimus for prevention of GVHD [18].
Variables
The following variables were analyzed for their potential association with OS: HCT-CI score, ECOG performance status, LVEF, DLCO, creatinine, creatinine clearance, total bilirubin, body mass index (BMI), CD34+ cell dose within the allograft, age, number of previous regimens (including the pretransplantation induction chemotherapy regimen), response to induction chemotherapy, cytomegalovirus (CMV) status, sex, history of previous autologous transplantation, GVHD prophylaxis, and histology. HCT-CI scores (0 to 2 vs 3+) were calculated using the method described by Sorror et al. [15], based on comorbidities present at study enrollment.
The following definitions were used for response to induction chemotherapy during the trial. Complete response (CR) was defined according to standard criteria available at the time the studies were performed, as regression of all lymph nodes to normal size (≤ 1.5 cm), resolution of soft tissue masses or palpable organomegaly resulting from lymphoma, and clearance of bone marrow infiltration if present previously [19]. As with standard criteria, partial response (PR) required a minimum 50% reduction in the sum of the products of the diameter of reference lesions without enlargement of other lesions, including liver and/or spleen. Progressive disease (PD) was defined by conventional criteria (appearance of any new lesion or a minimum 50% increase in sum of the products of the diameter of an existing lesion). Patients who did not meet criteria for PD, PR, or CR based on these definitions were categorized as having stable disease (SD). In addition, response to the composite response to pretransplantation chemotherapy (response to pre-enrollment chemotherapy + response to induction chemotherapy) was studied as CR + PR versus PD + SD as well as CR + PR + SD PD.
For histological analysis, patients were classified as diffuse large B cell lymphoma, follicular lymphoma (the 2 most common histologies in this analysis), or “other” (those without specific histological features of diffuse large B cell or follicular lymphoma, or with disease that transformed from one histological subtype to another). Because of the large number and heterogeneity of patients falling in the “other” category, disease was also classified and analyzed as either “indolent” or “aggressive,” as defined by the Physician Data Query Modification of the Revised European American Lymphoma (PDQ-REAL) classification of lymphoproliferative diseases (see http://www.cancer.gov/cancertopics/pdq/treatment/adult-non-Hodgkins/HealthProfessional/page3#Section_31). Treatment-related mortality (TRM) and disease-related mortality (DRM) were calculated from date of transplantation to date of death or to date of last follow-up.
Statistical Analysis
Survival was calculated from the date of stem cell infusion until date of death or last follow-up. Exploratory univariate analyses for survival were performed using Kaplan-Meier curves to report the probability of survival as a function of time. The significance of the difference between curves was determined using the log-rank test with 2-tailed P values. Because further evaluation of certain variables required dividing continuous parameters into quartiles and then using these preliminary results to determine where to choose the best of the 3 places to divide the patients for a more definitive evaluation, adjusted P values equal to triple the unadjusted value, along with the unadjusted value, are reported for those variables.
Individual prognostic factors with unadjusted P values < .10 were included for evaluation in an initial Cox model. A backward selection algorithm was used to select the final model. To test the gain to the model by including the HCT-CI, this variable was not included in the initial model; once the model was selected based on the more standard parameters, the HCT-CI was added to determine whether this parameter provided additional, statistically significant improvement to the model.
The HCT-CI also was analyzed using cumulative incidence curves of TRM, with DRM as the competing outcome, following the method described by Gooley et al. [20]. Likewise, curves for DRM were created using TRM as the competing outcome.
RESULTS
Patients
Table 1 summarizes characteristics of the 63 patients with NHL included in the analysis. The median patient age was 53 years, and the median ECOG performance status score was 1. The 2 most common histologies were diffuse large B cell lymphoma (n = 20) and follicular lymphomas (n = 12). The remaining histologies were quite diverse, including chronic lymphocytic leukemia, mantle cell lymphoma, marginal zone lymphoma, and T cell lymphomas, among others. Based on the PDQ-REAL classification system, 77% of patients were classified as having an “aggressive” histology. The median BMI was 25.2; only 4 patients had a BMI > 35. The median HCT-CI score was 2 (range, 0 to 11). Forty-four patients had a HCT-CI score of 0 to 2, and 19 patients had a score of 3 or greater.
Table 1.
Patient Characteristics
| Pretransplantation and disease-related factors | |
| Total number of patients | 63 |
| Median age, years | 53 (range, 32 to 74) |
| Serum creatinine, median, mg/dL | 0.8 (range, 0.5 to 1.7) |
| DLCO, median % predicted | 89.75% (range, 57.0% to 125.0%) |
| LVEF | 58% (range, 34% to 72%) |
| Sex, n (%) | |
| Donor | |
| Male | 33 (52%) |
| Female | 30 (48%) |
| Recipient | |
| Male | 37 (59%) |
| Female | 26 (41%) |
| CMV serology, n (%) | |
| Donor | |
| Positive | 35 (56%) |
| Negative | 27 (44%) |
| Recipient | |
| Positive | 39 (63%) |
| Negative | 23 (37%) |
| Previous chemotherapy regimens, median, n | 4 (range, 1 to 9) |
| Previous autologous HSCT, n (%) | |
| Yes | 14 (22%) |
| No | 49 (78%) |
| NHL histology, n (%) | |
| Diffuse large B cell lymphoma | 20 (32%) |
| Follicular lymphoma | 12 (19%) |
| T cell lymphomas | 11 (17%) |
| Mantle cell lymphoma | 7 (11%) |
| Transformed B cell lymphomas | 7 (11%) |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 4 (6%) |
| Marginal zone | 2 (3%) |
| PDQ-REAL disease classification, n (%) | |
| Indolent | 14 (22%) |
| Aggressive | 49 (78%) |
| Response to last therapy before study enrollment, n (%) | |
| Chemotherapy-sensitive | 23 (37%) |
| Chemotherapy-resistant | 33 (52%) |
| Untested | 7 (11%) |
| Response to pretransplantation induction chemotherapy, n (%) | |
| Complete response | 7 (11%) |
| Partial response | 17 (27%) |
| Stable disease | 26 (41%) |
| Progressive disease | 10 (16%) |
| Nonevaluable | 3 (5%) |
| Median ECOG performance status | 1 (range, 0 to 2) |
| Median CD34+ dose, × 106 cells/kg | 7.3 (range, 3.46 to 17.8) |
| HCT-CI | |
| Median HCT-CI score | 2 (range, 0 to 11) |
| Distribution of HCT-CI scores, n (%) | |
| 0 to 1 | 30 (48%) |
| 2 | 14 (22%) |
| 3 | 7 (11%) |
| ≥ 4 | 12 (19%) |
| Individual comorbidities as defined by the HCT-CI, n (%) | |
| Arrhythmia | 1 (2%) |
| Cardiac | 7 (11%) |
| Inflammatory bowel disease | 0 (0) |
| Diabetes | 1 (2%) |
| Cerebrovascular disease | 0 (0) |
| Psychiatric | 6 (9%) |
| Mild hepatic | 16 (25%) |
| Obesity | 5 (8%) |
| Infection | 19 (30%) |
| Rheumatologic | 1 (2%) |
| Peptic ulcer disease | 1 (2%) |
| Moderate/severe renal | 0 (0) |
| Moderate pulmonary | 14 (22%) |
| Previous solid tumor | 1 (2%) |
| Heart valve disease | 1 (2%) |
| Severe pulmonary | 7 (11%) |
| Moderate/severe hepatic | 2 (3%) |
DLCO indicates pulmonary diffusion capacity for carbon monoxide; LVEF, left ventricular ejection fraction; CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplantation; NHL, non-Hodgkin lymphoma; PDQ-REAL, Physician Data Query Modification of the Revised European American Lymphoma classification of lymphoproliferative diseases; HCT-CI, hematopoietic cell transplantation comorbidity index.
Patients received a median of 4 chemotherapy regimens before transplantation. Although all patients had relapsed or refractory disease at the time of study enrollment, 37% of evaluable patients were found to have chemotherapy-sensitive disease to salvage therapy before study enrollment. Seven patients had untreated relapse at time of study enrollment. Response to induction chemotherapy correlated well with preenrollment treatment response; 96% of patients were found to have maintained or improved on their previous response after treatment with EPOCH-F. Among patients with chemotherapy-resistant disease at enrollment, 27% actually had a response to EPOCH-F, and an additional 45% had SD. The overall composite response to pretransplantation chemotherapy was as follows: CR, 13%; PR, 43%; SD, 17%; and PD, 27%.
Transplantation Outcomes
TRM and DRM
For patients who were alive as of the date of analysis, the median time from transplantation to last follow- up was 35.5 months (range, 6 to 80.6 months). After transplantation, 16 patients died from treatment- related causes and 14 died from disease-related causes. The cumulative incidence of TRM, adjusting for the impact of the competing factor of death from progression, was 11.1% at 100 days posttransplantation and 20.7% at 12 months posttransplantation (Figure 1A). The cumulative incidence of DRM, adjusting for the impact of the competing effect of TRM, was 3.2% at 100 days posttransplantation and 16.2% at 12 months posttransplantation (Figure 1A).
Figure 1.
Transplantation outcomes in patients (n = 63) undergoing reduced-intensity allogeneic HSCT. (A) Cumulative incidences of TRM and DRM. (B) Probability of OS.
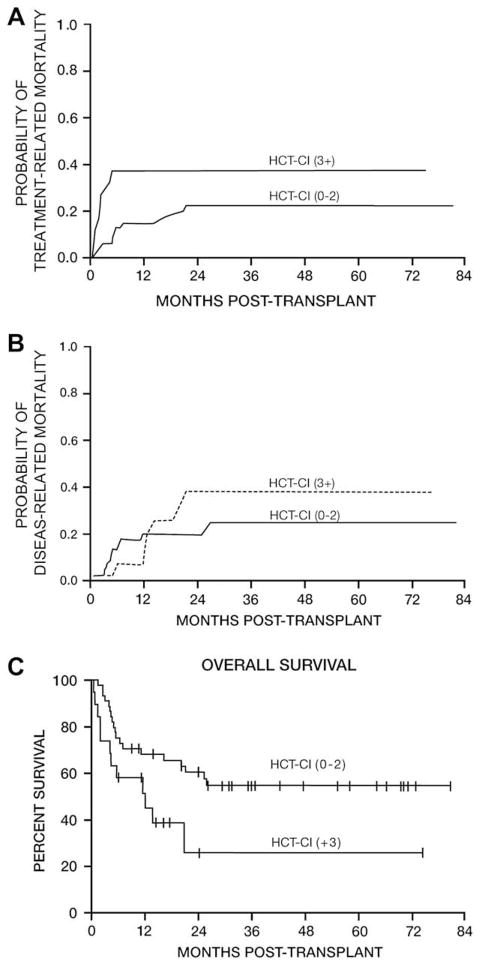
The cumulative incidence curves for TRM and DRM based on HCT-CI score are summarized in Table 2 and depicted in Figure 2. The HCT-CI (0 to 2 comorbidities vs 3+ comorbidities) demonstrated a potential association with TRM at 100 days (4.5% vs 26.3%) and 1 year (13.6% vs 36.8%) posttransplantation. The HCT-CI (0 to 2 vs 3+) was not statistically associated with DRM at 100 days (4.5%vs 0) or 1 year (18.3% vs 11.7%) posttransplantation.
Table 2.
Estimated Probabilities of Mortality Based on HCTCI Score
| 100 Days |
1 Year |
|||
|---|---|---|---|---|
| 0 to 2 | ≥ 3 | 0 to 2 | ≥ 3 | |
| All causes of mortality | 9.1% | 26.3% | 32% | 48.5% |
| Cumulative incidence of TRM | 4.5% | 26.3% | 13.6% | 36.8% |
| Cumulative incidence of DRM | 4.5% | 0 | 18.3% | 11.7% |
HCT-CI indicates hematopoietic cell transplantation comorbidity index; TRM, treatment-related mortality; DRM, disease-related mortality.
Figure 2.
Impact of HCT-CI on transplantation outcome: (A) TRM; (B) DRM; (C) OS.
OS
The median OS for all 63 patients was 24.1 months (Figure 1B). The 12- and 24-month probabilities of OS were 63.1% and 51.6%, respectively. The overall probability of death from all causes at 100 days was estimated to be 9.1% for patients with HCT-CI scores of 0 to 2 and 26.3% for those with scores of 3+. Mortality from all causes at 1 year was estimated to be 32% for patients with an HCT-CI score of 0 to 2 and 48.5% for those with a score of 3+ (Figure 2C).
Univariate Analysis and Resulting Cox Model for OS
In univariate analysis, various factors were identified as having a potential association with OS, with unadjusted P values < .10 (Table 3), and thus were selected for inclusion in an initial Cox model. After a backward elimination of variables, the composite pretransplantation response (either PD vs SD + PR + CR or PD + SD vs PR + CR) along with GVHD prophylaxis (CsA + Th2 vs all other forms), CMV donor status and creatinine clearance were found to be jointly statistically significant for an association with survival; response to EPOCH-F (SD + PR + CR vs PD) also was important in some models (Table 4A). The HCT-CI parameter (0–2 vs 3+) was marginally statistically significant when adjusted for the parameters included in the first model presented in the bottom part of Table 4 (P = .056). This parameter did not retain its level of significance in a related model including both the pretransplantation response and EPOCH-F response (P = .14; data not shown).
Table 3.
Variables Considered for Potential Incorporation into an Initial Cox Model of Survival
| Variable | P value (unadjusted; adjusted if appropriate) |
|---|---|
| HCT-CI | .026; .052* |
| ECOG performance status (0 vs ≥ 1) | .094 |
| LVEF (< 63% vs ≥ 63%) | .035; .11 |
| DLCO (# 100 vs ≥ 100.1) | .013; .038 |
| Creatinine (< 0.8 vs ≥ 0.9 mg/dL) | .075; .23 |
| Creatinine clearance (< 87 vs ≥ 87 mL/min) | .036; .23 |
| Total bilirubin (< 0.7 vs ≥ 0.7) | .046; .14 |
| BMI | † |
| Previous regimens (< 5 vs ≥ 5) | .045; .13 |
| EPOCH-F response (PR, CR, and PD vs SD) | .0025 |
| CD34+ cell dose | † |
| Donor CMV (positive vs negative) | .094 |
| Recipient CMV (positive vs negative) | .33 |
| Donor or recipient CMV positive (yes vs no) | .61 |
| Donor (male vs female) | .45 |
| Recipient (male vs female) | .72 |
| Sex (match vs mismatch) | .28 |
| Age, years (30 to 50 vs ≥ 51) | .11; .33 |
| Histology (indolent vs aggressive)‡ | .32 |
| Previous autologous HSCT (yes vs no) | .96 |
| GVHD prophylaxis | |
| CsA alone versus CsA + Th2 | .25 |
| CsA alone versus CsA + MTX | .61 |
| CsA alone versus CsA + rapamicin (alone or with Th2) | .29. |
| CsA + Th2 versus CsA + MTX | .033 |
| CsA + Th2 versus CsA + rapamicin (alone or with Th2) | .077 |
| CsA + MTX versus CsA+ rapamicin (alone or with Th2) | .61 |
| CsA + Th2 versus all others | .018; .054 |
HCT-CI indicates hematopoietic cell transplantation comorbidity index; DLCO, pulmonary diffusion capacity for carbonmonoxide; PR, partial response; CR, complete response; PD, progressive disease; SD, stable disease; CMV, cytomegalovirus; CsA, cyclosporine A; MTX, methotrexate.
Adjusted P value is double the unadjusted value, because there were 2 possible groupings. Variables with unadjusted P values < .10 were incorporated into a Cox model of survival.
Inconsistent ordering in association of categories for this parameter relative to response; thus, the parameter would not be included in a Cox model.
Based on the PDQ-REAL classification of lymphoproliferative diseases.
Table 4.
Cox Model Results Based on the Backward Selection Model, with and without Adding in the Effect of HCT-CI
| Variable | Parameter Estimate | P Value | Hazard Ratio (95% Confidence Interval) |
|---|---|---|---|
| Without adding in the effect of HCT-CI | |||
| Pretransplantation composite response (CR + PR + SD vs PD) | 2.411 | < .0001 | 11.147 (4.660 to 26.662) |
| GVHD prophylaxis (CsA + Th2 vs other) | −1.836 | < .0001 | 0.159 (0.064 to 0.396) |
| CMV donor (positive vs negative) | −1.479 | .0007 | 0.228 (0.096 to 0.538) |
| Creatinine clearance (< 87 vs ≥ 87 mL/min) | −1.441 | .0013 | 0.228 (0.098 to 0.570) |
| Adding in the effect of HCT-CI | |||
| HCT-CI (0 to 2 vs 3+) | 0.773 | .056 | 2.166 (0.981 to 4.784) |
| Pretransplantation composite response (CR + PR + SD vs PD) | 2.396 | <.0001 | 10.983 (4.472 to 26.976) |
| GVHD prophylaxis (CsA + Th2 vs other) | −2.003 | <.0001 | 0.135 (0.053 to 0.344) |
| CMV donor (positive vs negative) | −1.527 | 0.0005 | 0.217 (0.092 to 0.515) |
| Creatinine clearance (< 87 vs ≥ 87 mL/min) | −1.558 | 0.0007 | 0.211 (0.086 to 0.518) |
HCT-CI indicates hematopoietic cell transplantation comorbidity index; CR, complete response; PR, partial response, SD, stable disease; PD, progressive disease; CsA, cyclosporine A; GVHD, graft-versus-host disease; CMV, cytomegalovirus.
DISCUSSION
As the use of nonmyeloablative and RIC regimens opens up the option of allogeneic HSCT to patients with multiple comorbidities, it can be difficult to determine when risks outweigh the benefits of this potentially life-prolonging therapy. Our data demonstrate the potential validity of the HCT-CI as an instrument for estimating survival in a group of patients with relapsed and refractory NHL undergoing RIC allogeneic HSCT from HLA-matched siblings. HCT-CI scores of 3+ were statistically associated with decreased OS in univariate analysis, and there was a trend toward decreased OS in multivariate analysis. Also, specific individual transplant and tumor characteristics were predictive of OS, particularly response to pretransplantation chemotherapy. Interestingly, NHL histology, as defined by the PDQ-REAL classification system, did not affect survival after allogeneic HSCT; this result differs from those obtained in previous studies [21,22].
The fact that in the Cox model analysis, the HCTCI exhibited a trend toward a statistically significant association with overall transplantation outcome highlights the importance of disease-related factors in NHL patients being considered for RIC allogeneic HSCT. Chemotherapy resistance has been identified as important prognostic factor in previous analyses of both nonmyeloablative and RIC studies in NHL patients, and chemotherapy-sensitive disease is an eligibility requirement in many trials, including those being conducted by the Blood and Marrow Transplant Clinical Trials Network in the United States [4,6,8,9]. Our analysis, which did not exclude patients with either SD or PD before transplantation, supports and further explicates these results based on specific responses to pretransplantation chemotherapy. Something that remains unclear from our analysis is whether the chemotherapy sensitivity of the disease or the disease remission status before transplantation is of greater clinical importance—both of these factors have been previously observed to potentially influence posttransplantation outcomes [23,24]. This analysis could be interpreted as suggesting that patients who have achieved a clinically significant remission, and thus have a lower disease burden, are more likely to have a successful outcome after transplantation. This would be consistent with the theory that low volumes of malignant disease are most vulnerable to the graft-versus-malignancy effect [25]. The other possibilities are that resistance to chemotherapy is associated with a lack of response to cellular therapy (ie, the “graft-versus-lymphoma effect”), or that response to the effects of cytotoxic chemotherapy is essential for successful outcomes with allogeneic HSCT [26]. Alternatively, or in addition, it is possible that patients with chemotherapy-resistant NHL fare more poorly after transplantation because of in part the toxicity from posttransplantation interventions that are necessitated by the progression of malignancy, including early discontinuation of GVHD chemoprophylaxis, administration of donor lymphocyte infusions, and administration of chemotherapy posttransplantation.
In light of this information, it is possible that the option of allogeneic HSCT may be optimally performed relatively early in the disease course before exposure to regimens that may potentially generate chemotherapy resistance and/or result in excessive toxicity. Patients with good responses to previous chemotherapy who have an HCT-CI score < 3 are most likely to do well after RIC allogeneic HSCT. Our results indicate that patients with very poor response (ie, progressive disease) to chemotherapy are unlikely to benefit from RIC allogeneic HSCT, but patients with stable disease appear to derive potential benefits. If such patients are considered for transplantation, they are most likely to do well if they have a low HCT-CI score (< 3). Patients who have had poor response to chemotherapy and also have multiple comorbidities, as reflected in high HCT-CI scores (3+), are unlikely to do well after transplantation. This group of patients has increased incidences of relapse, death related to relapse, and nonrelapse mortality (NRM); they should be considered for therapeutic options other than RIC allogeneic HSCT. Unfortunately, for this group, nontransplantation options also have a relatively limited success rate. Our analysis confirms that the HCT-CI may be of potential clinical utility in patients with NHL undergoing RIC allogeneic HSCT. However, in light of the retrospective nature of the analysis, which was limited to patients with NHL undergoing RIC allogeneic HSCT, there results need to be verified in larger patient groups. If our findings are substantiated, we would expect them to be useful to physicians in attempting to evaluate whether patients are appropriate candidates for this procedure as well as in patient counseling regarding the potential risks and benefits of the procedure.
Acknowledgments
Financial disclosure: This work was supported by the Center for Cancer Research, National Cancer Institute, Intramural Research Program.
References
- 1.Ratanatharathorn V, Uberti J, Karanes C, et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin’s lymphoma. Blood. 1994;84:1050–1055. [PubMed] [Google Scholar]
- 2.Bierman PJ, Sweetenham JW, Loberiza FR, Jr, et al. for the Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin’s lymphoma: a comparison with allogeneic and autologous transplantation. J Clin Oncol. 2003;21:3744–3753. doi: 10.1200/JCO.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 3.Freytes CO, Loberiza FR, Rizzo JD, et al. for Lymphoma Working Committee of the International Bone Marrow Transplant Registry. Myeloablative allogeneic hematopoietic stem cell transplantation in patients who experience relapse after autologous stem cell transplantation for lymphoma: a report of the International Bone Marrow Transplant Registry. Blood. 2004;104:3797–3803. doi: 10.1182/blood-2004-01-0231. [DOI] [PubMed] [Google Scholar]
- 4.Khouri IF, Keating M, Körbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 5.Georges GE, Maris M, Sandmaier BM, et al. Related and unrelated nonmyeloablative hematopoietic stem cell transplantation for malignant diseases. Int J Hematol. 2002;76(Suppl 1):184–189. doi: 10.1007/BF03165242. [DOI] [PubMed] [Google Scholar]
- 6.Branson K, Chopra R, Kottaridis PD, et al. Role of nonmyeloablative allogeneic stem-cell transplantation after failure of autologous transplantation in patients with lymphoproliferative malignancies. J Clin Oncol. 2002;20:4022–4031. doi: 10.1200/JCO.2002.11.088. [DOI] [PubMed] [Google Scholar]
- 7.Rezvani AR, Storer B, Maris M, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 8.Robinson SP, Goldstone AH, Mackinnon S, et al. for Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 9.Escalón MP, Champlin RE, Saliba RM, et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin’s lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol. 2004;22:2419–2423. doi: 10.1200/JCO.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 10.Ringdén O, Horowitz MM, Gale RP, et al. Outcome after allogeneic bone marrow transplant for leukemia in older adults. JAMA. 1993;270:57–60. doi: 10.1001/jama.1993.03510010063030. [DOI] [PubMed] [Google Scholar]
- 11.Wallen H, Gooley TA, Deeg HJ, et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23:3439–3446. doi: 10.1200/JCO.2005.05.694. [DOI] [PubMed] [Google Scholar]
- 12.Dean RM, Fowler DH, Wilson WH, et al. Efficacy of reduced-intensity allogeneic stem cell transplantation in chemotherapy- refractory non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:593–599. doi: 10.1016/j.bbmt.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Alamo J, Shahjahan M, Lazarus HM, et al. Comorbidity indices in hematopoietic stem cell transplantation: a new report card. Bone Marrow Transplant. 2005;36:475–479. doi: 10.1038/sj.bmt.1705041. [DOI] [PubMed] [Google Scholar]
- 14.Artz AS, Pollyea DA, Kocherginsky M, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:954–964. doi: 10.1016/j.bbmt.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)–specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorror ML, Storer BE, Maloney DG, et al. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–452. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 18.Fowler DH, Odom J, Steinberg SM, et al. Phase I clinical trial of costimulated, IL-4 polarized donor CD4+ T cells as augmentation of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:1150–1160. doi: 10.1016/j.bbmt.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI-Sponsored International Working Group. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. erratum: 2000;18;2351–2352. [DOI] [PubMed] [Google Scholar]
- 20.Gooley TA, Leisinring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Faulkner RD, Craddock C, Byrne JL, et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood. 2004;103:428–434. doi: 10.1182/blood-2003-05-1406. [DOI] [PubMed] [Google Scholar]
- 22.van Besien KW, Mehra RC, Giralt SA, et al. Allogeneic bone marrow transplantation for poor-prognosis lymphoma: response, toxicity and survival depend on disease histology. Am J Med. 1996;100:299–307. doi: 10.1016/S0002-9343(97)89488-0. [DOI] [PubMed] [Google Scholar]
- 23.Michallet M, Bilger K, Garban F, et al. Allogeneic hematopoietic stem-cell transplantation after nonmyeloablative preparative regimens: impact of pretransplantation and posttransplantation factors on outcome. J Clin Oncol. 2001;19:3340–3349. doi: 10.1200/JCO.2001.19.14.3340. [DOI] [PubMed] [Google Scholar]
- 24.Baron F, Storb R, Storer BE, et al. Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopoietic cell transplantation. J Clin Oncol. 2006;24:4150–4157. doi: 10.1200/JCO.2006.06.9914. [DOI] [PubMed] [Google Scholar]
- 25.Bishop MR. The graft-versus-lymphoma effect: fact, fiction, or opportunity? J Clin Oncol. 2003;21:3713–3715. doi: 10.1200/JCO.2003.05.984. [DOI] [PubMed] [Google Scholar]
- 26.Grigg A, Ritchie D. Graft-versus-lymphoma effects: clinical review, policy proposals, and immunobiology. Biol Blood Marrow Transplant. 2004;10:579–590. doi: 10.1016/j.bbmt.2004.05.008. [DOI] [PubMed] [Google Scholar]