Abstract
Objective
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by unpredictable flares of disease activity and irreversible damage to multiple organ systems. An earlier study showed that SLE patients carrying an interferon gene expression signature in blood have elevated serum levels of interferon (IFN)-regulated chemokines. These chemokines were associated with more severe and active disease and showed promise as SLE disease activity biomarkers. This study was designed to validate IFN regulated chemokines as biomarkers of SLE disease activity in 267 longitudinally-followed SLE patients.
Methods
To validate the potential utility of serum chemokine levels as biomarkers for disease activity, we measured serum chemokine levels – CXCL10 (IP-10), CCL2 (MCP-1), and CCL19 (MIP-3B) – in an independent cohort of 267 SLE patients followed longitudinally over one year (1166 total visits).
Results
Serum chemokine levels correlated with current visit lupus activity (p=2×10−10), rising at flare (p=1×10−3) and decreasing as disease remitted (p=1×10−3), and performed better than currently available laboratory tests. Chemokine levels measured at a single baseline visit in patients with SLEDAI ≤4 were predictive of lupus flare over the ensuing year (p=6×10−4).
Conclusion
Monitoring serum chemokine levels in SLE may improve assessment of current disease activity, the prediction of future flare, and overall clinical decision-making.
Systemic lupus erythematosus (SLE) is a chronic, inflammatory autoimmune disease defined by autoantibodies to nuclear components, immune complex deposition, and systemic vasculitis (1). Many organ systems are targeted, including the skin, joints, blood cells, kidneys, and nervous system. The disease affects 0.1 percent of the US population with a striking 9:1 female predominance. The factors contributing to the onset and progression of SLE are not well understood; however, genetic, environmental and hormonal factors are likely important.
SLE disease activity can be difficult to monitor, and flares are unpredictable in both frequency and severity. Certain clinical laboratory tests, including anti-double-stranded DNA antibodies (anti-dsDNA), complement factor levels, and the erythrocyte sedimentation rate (ESR) are often measured as potential indicators of disease activity. However, there is significant uncertainty regarding the utility of these tests in accurately assessing SLE activity, and several longitudinal studies have failed to establish these as reliable markers (2–8). Additional studies examining other potential markers of SLE activity have been inconclusive, and no biomarker for disease activity has been validated (3).
The type 1 interferon (IFN) pathway is dysregulated in SLE, and is a source of potential lupus biomarkers (9). We and others identified a group of type 1 IFN responsive genes (the ‘IFN gene signature’) that was upregulated in the peripheral blood cells of over 50% of adult lupus cases and the majority of pediatric SLE patients (10–12). The IFN gene signature correlates with current visit disease activity and severe complications including renal, central nervous system, and immunologic disease (10, 13, 14).
In a recent study of 30 SLE patients, we identified several IFN-regulated chemoattractant cytokines (chemokines) that were present at increased concentrations in lupus serum(15). Levels of these chemokines were significantly correlated with disease activity scores and clinical laboratory tests (ESR, low complement, anti-dsDNA, low leukocyte counts, etc.) and provided a more sensitive indicator of IFN pathway activation than the gene expression signature.(15) Other groups have similarly observed increased levels of these chemokines in SLE blood(16–18). In the current study, we utilized multiplexed sandwich-based immunoassays to measure the levels of three IFN-regulated chemokines, CCL2 (MCP-1), CCL19 (MIP-3B), and CXCL10 (IP-10), in serum samples from an independent group of 267 SLE patients followed longitudinally for approximately one year (total clinic visits=1166), to prospectively test the hypothesis that serum chemokine levels are biomarkers of SLE disease activity.
Materials and Methods
Research participants, clinical data, and sample collection
Consenting SLE patients from the Hopkins Lupus Cohort (19) were enrolled in the Autoimmune Biomarkers Collaborative Network (ABCoN) study (see Supplementary Information). All research protocols were approved by institutional review boards at the University of Minnesota, Johns Hopkins University, and The Feinstein Institute. The current study includes serum samples from 267 SLE patients followed longitudinally for one year (1166 total visits; average of ~4.5 visits per patient; Supplementary Figure S1). Samples were collected at regularly scheduled quarterly intervals, and also when patients were seen at interim visits due to flare or other complications. The patient group was 56% North Americans of European descent, 37% African Americans, and 7% other ethnicity, with an average age of 42 years (standard deviation = 12 years). Eighty-seven percent of the patients were female. All patients were examined by the same rheumatologist (MP) at each visit.
Clinical data included a comprehensive medical history, medication profile, clinical laboratory tests, and several validated disease activity measures, including a revision of the SLE Disease Activity Index (SLEDAI) (20) from the Safety of Exogenous Estrogens in Lupus Erythematosus National Assessment (SELENA) study (21) as well as the Physician’s Global Assessment (PGA) (see Supplementary Information). Current visit clinical data were available for 99% of visits (n=1152). Anti-double stranded DNA (anti-dsDNA) antibodies were measured by Crithidia luciliae immunoassay. Serum C3 and C4 levels were determined by the Johns Hopkins Hospital Diagnostic Immunology Laboratory (Baltimore, MD). The majority of patients received treatment for lupus during the observation period, including hydroxychloroquine (70%), immunosuppressive therapies (40%; including Cytoxan, CellCept, Immuran, Methotrexate, and Chlorambucil), and oral prednisone (63%).
Peripheral blood was collected by venipuncture and sera were isolated in serum-separator Vacutainer tubes (Becton-Dickinson). A protease inhibitor (aprotinin, 1 ug/ml) was added and aliquots were immediately frozen at −80°C.
Serum chemokine measurement
SearchLight (Pierce, Woburn, MA) chemiluminescent sandwich-based immunoassays were used to quantitate the serum levels of CXCL10 (IP-10), CCL2 (MCP-1) and CCL19 (MIP-3B) at the University of Minnesota Cytokine Reference Laboratory. These chemokines are IFN-regulated, and exhibited the strongest correlations with disease activity in our initial report (15) (see also Supplementary Information and Supplementary Figure S2). Concentration values (pg/ml) were obtained using seven-point standard curves generated with recombinant protein. Each sample was run in duplicate. To permit normalization of slight differences in intensity readings between plates, two SLE samples were repeated on each plate (see Supplementary Information). For quality control, we also included samples from the 30 patients that were previously assayed on another platform (Supplementary Table S1) (15). Chemokine measurements were highly reproducible between the two platforms (r=0.88, p=0.0001; see Supplementary Information). All data analysis in the current study was performed by authors Bauer and Baechler. The distribution of chemokine values was non-normal, therefore non-parametric tests were utilized for analyses of chemokine measurements. Laboratory test values and disease activity measures were normal, allowing parametric tests to be used.
Chemokine score calculation
In order to use the information derived from the 3 chemokine measurements in a simplified manner, we calculated a normalized composite chemokine score. For each chemokine, concentrations above the 95th percentile value were assigned a value of 1.0. The remaining concentration values were then scaled to the 95th percentile value, and these scaled values for each chemokine were summed to derive the final score (possible range 0–3). The chemokine scores for all SLE patient visits were transformed to a 100 point scale. Patient visits were classified by chemokine score as either chemokine-low (bottom quartile; score ≤24), chemokine-high (top quartile; score ≥47), or chemokine-intermediate (middle two quartiles).
Comparison of chemokine levels in active vs. inactive SLE
We chose one visit from each patient with active SLE (SLEDAI ≥6; n=76) and one visit from each patient in an independent group of inactive cases (SLEDAI ≤2 and PGA=0; n=105). If patients had multiple active or inactive visits, the earliest available visit was selected. Nonparametric Mann-Whitney U-tests were used to compare chemokine levels and the chemokine score between active and inactive SLE. Categorical data were evaluated with Fisher’s exact test. Heat maps were generated using CLUSTER and visualized by Treeview (22).
Comparison of disease activity in chemokine-high vs. -low patients
The selection of chemokine-high and -low patients for this analysis is described in detail in the Supplementary Information. Briefly, from the full set of chemokine-high and -low visits, we selected only those visits that had current clinical data available. There were 286 visits in the chemokine-high group (127 total patients) and 302 visits in the chemokine-low group (116 total patients). Sixteen patients were excluded from the chemokine-low group because they had one or more visits that fell into the chemokine-high category. From these groups, we selected the earliest available visit from each patient. SLEDAI scores were compared between the chemokine-high (n=127) and chemokine-low (n=100) groups using Student’s unpaired t-test.
The presence of organ-specific manifestations was defined by Lupus Activity Index (LAI)(23) subscores ≥1. Student’s unpaired t-tests were used to compare continuous variables while Fisher’s exact tests were used for categorical data.
Longitudinal analysis of changes in disease activity
We selected paired, consecutive visits from patients who had either a flare in disease activity (6) (increase in SLEDAI ≥3; n=62 pairs; average time between visits, 88.3 days) or attenuation of activity (decrease in SLEDAI ≥3; n=73 pairs; average time between visits, 87.8 days). Paired Wilcoxon tests were used to compare chemokine levels at consecutive visits, either (1) before and during flare, or (2) before and during attenuation. Standard laboratory tests were analyzed by paired Student’s t-test.
Prediction of future disease activity
We selected all available patients who had a baseline visit with inactive or less active disease (SLEDAI ≤4) and ≥3 total visits (n=222 patients). These patients were then stratified by baseline chemokine score (chemokine-high, n=44; chemokine-intermediate, n=108; chemokine-low, n=72). We identified patients who flared (increase in SLEDAI ≥3) and recorded the number of days from baseline to flare. The same patient group was used to determine whether serum chemokine levels could specifically predict future renal activity, with renal flares defined as an increase in the renal SLEDAI subscore from 0 to 4+. In addition to the clinical data that was collected concurrently with serum sampling, we also had available longitudinal clinical data for 384 patient visits that lacked a matched blood sample for serum chemokine measurement. The clinical data from these visits was included in these analyses. The Physician’s Global Assessment (PGA) was also used for an alternate definition of flare (increase in PGA≥1).(24) Patients who did not flare were selected only if they completed at least 9 months of the study. Kaplan-Meier plots were used to compare the percentage of patients who remained flare-free in the three groups.
Using the same set of 222 patients, univariate and multivariate Cox regression analyses were used to identify variables with power to predict future flare. The tested variables included chemokine levels and laboratory tests measured at the baseline visit. Variables were kept in the model if p<0.1 using stepwise regression. For Cox regression analysis, all variables were continuous; the chemokine score was also tested as a binary variable (chemokine-high vs. - intermediate/low). To facilitate interpretation of hazard ratios, continuous variables were dichotomized; hazard ratios are relative to a 100 pg/ml change in chemokine level and 10 units for all clinical laboratory test results and the chemokine score.
Results
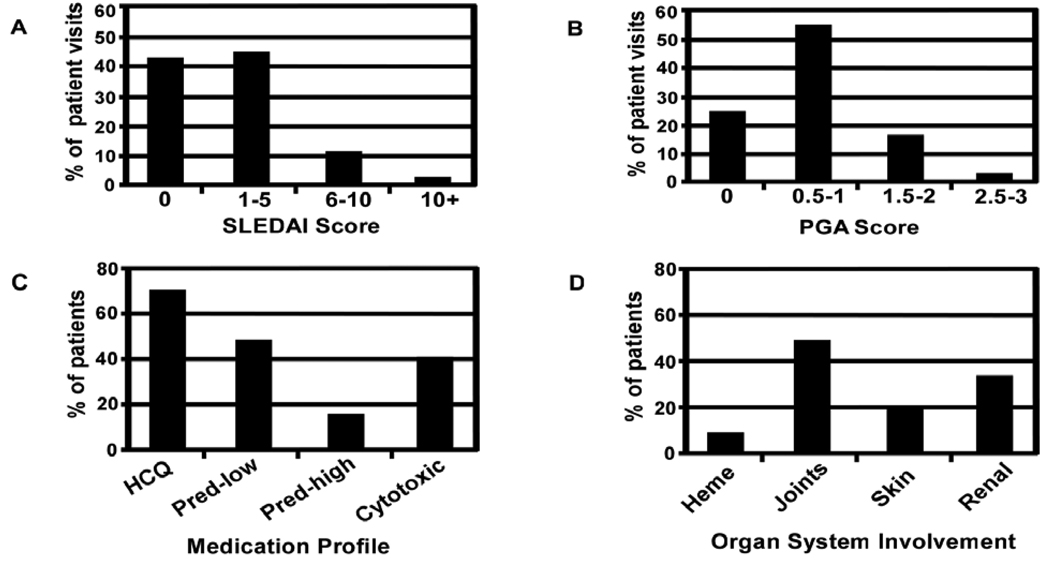
The current validation cohort was comprised of 267 SLE patients followed longitudinally for one year. The distribution of disease activity across all visits, as determined by the SLE Disease Activity Index (SLEDAI) or physician’s global assessment (PGA), is shown in Figure 1 (A,B). Most patients received treatment for lupus during their ABCoN observation period, including hydroxychloroquine (70%), immunosuppressive therapies (40%), and oral prednisone (63%) (Figure 1C). The most common clinical signs of SLE were arthritis (46%), followed by renal (35%), skin (19%) and hematologic (9%) manifestations (Figure 1D). The demographic and clinical features of these 267 patients were largely similar to those of the 30 patients from the previous study, although the patients in the earlier study had more active disease (Supplementary Table S1).
Figure 1. Clinical profile of ABCoN SLE patient visits.
The distribution of disease activity as measured by SLEDAI (panel A) and a physician’s global assessment (PGA, panel B) for 267 SLE patients followed longitudinally for one year (1152 total visits). The percentage of patients taking specific medications or exhibiting organ-specific manifestations at any time during the one year study are shown in panels C and D, respectively. HCQ - hydroxychloroquine, Pred - prednisone, mg/d - milligrams per day, Heme - hematologic, Cytotoxic − Imuran, Cytoxan, Chlorambucil, Methotrexate, and/or CellCept.
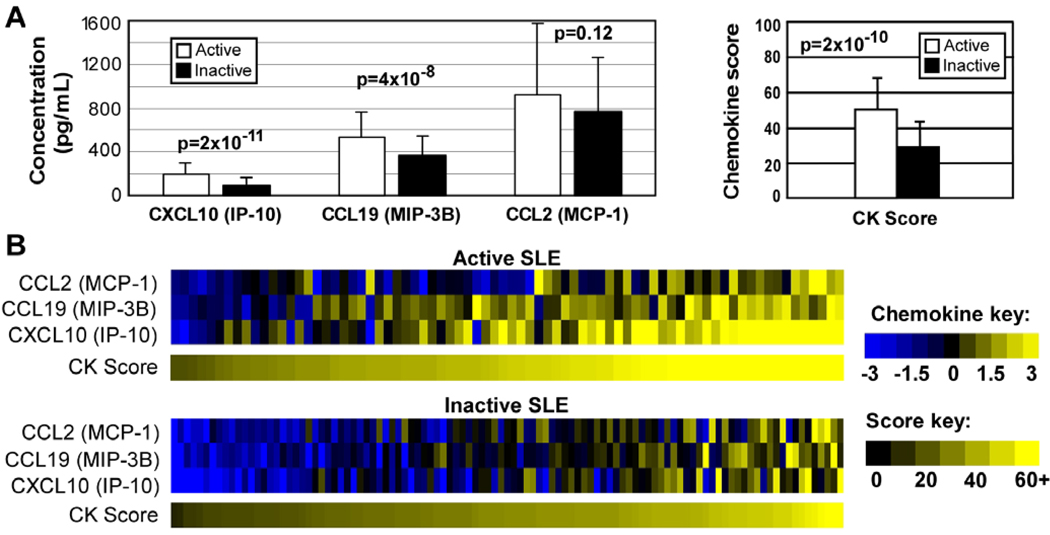
We first addressed the extent to which chemokine levels correlated with SLEDAI, a validated clinical index of current SLE disease activity. The levels of CXCL10 (IP-10) and CCL19 (MIP-3B), but not CCL2 (MCP-1), were significantly higher in patients with active SLE (defined as SLEDAI ≥6, n=76) compared to inactive SLE (SLEDAI ≤2 and PGA=0, n=105; Figure 2A and B). A composite chemokine score was significantly higher in active patients (mean ± SD chemokine score, 48 ± 18) than inactive patients (mean score 32 ± 12, p=2×10−10). In order to examine the pattern of chemokine expression across the full spectrum of disease activity, including patients with intermediate activity (those who did not meet the above criteria for either active or inactive SLE), we compared chemokine levels in the first visits of all patients. Chemokine scores were significantly elevated in active SLE patients when compared to patients with intermediate (p<0.0001) and low disease activity (p=0.0001). However, chemokine scores were not different between patients with intermediate and low disease activity (p=0.46; Supplementary Table S2).
Figure 2. Active SLE patients have elevated IFN-regulated chemokine levels.
A. Chemokine (CK) levels were compared between a group of single visits from active SLE patients (SLEDAI ≥6; n=76) and an independent group of inactive SLE patients (PGA=0 and SLEDAI ≤2; n=100). Chemokine scores and individual chemokine levels were compared using Mann-Whitney U-tests. Bars represent mean ± standard deviation. B. Individual chemokine levels were normalized to the average of the inactive patients and log2-transformed. Chemokine scores were visualized on 100 point scale.
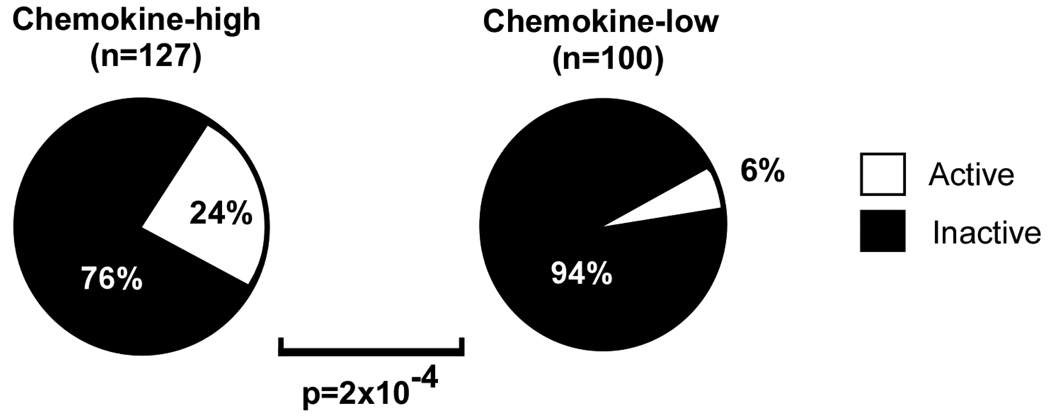
To further explore the relationship between chemokine levels and disease activity, we examined single visits from patients who were either chemokine-high (n=127) or chemokine-low (n=100). The chemokine-high group had a significantly higher fraction of active SLE cases (SLEDAI ≥6) as compared to the chemokine-low group (p=2×10−4; Figure 3). Chemokine-high patients also had higher absolute levels of disease activity as measured by the SLEDAI compared to chemokine-low patients (p=8×10−8, Supplementary Table S3). Furthermore, the chemokine-high group showed higher ESR (p=8×10−5), higher anti dsDNA antibody titers (p=2×10−4), lower complement C3 levels (p=0.03), and lower leukocyte counts (p=6×10−4) than the chemokine-low group (Supplementary Table S3). In order to compare disease activity across the full range of chemokine scores, including patients with intermediate chemokine levels, we examined activity scores in the first visits of all patients. SLEDAI scores were significantly elevated in chemokine-high patients as compared to chemokine-intermediate (p=3.8×10−5) and chemokine-low patients (p=1.0×10−7), but SLEDAI scores were similar between chemokine-intermediate and chemokine-low patients (p=0.06; Supplementary Table S4).
Figure 3. Patients with high chemokine levels are more likely to have elevated SLE disease activity.
The percentage of patients with active SLE (SLEDAI ≥6) were compared between patients with high levels of chemokines (chemokine score ≥47, n=127) and an independent group of patients with low levels of chemokines (chemokine score ≤24, n=100). P-values were determined by Fisher’s exact test.
To determine whether the relationship between the chemokine score and disease activity extended beyond these indicators of serologic activity, we performed a similar analysis using a modified SLEDAI (mSLEDAI) which lacks the anti-dsDNA and complement components (20, 23). The chemokine-high group had significantly higher mSLEDAI scores compared to the chemokine-low group (p=1×10−3, Supplementary Table S3). Furthermore, the chemokine score was significantly correlated with mSLEDAI (p<0.0001), as were anti-dsDNA, complement components, and lymphocyte count (p<0.0001), ESR (p=0.0004), and WBC count (p=0.003; Supplementary Table S5). The only organ-specific manifestation that was significantly enriched in the chemokine-high group was skin involvement (p=0.02, Supplementary Table S6). Based on these data, we conclude that serum IFN-regulated chemokine levels serve as biomarkers of current SLE disease activity.
We next examined whether chemokine levels were altered in patients treated with high dose prednisone (10+ mg/day) or immunosuppressive drugs (see Methods). In order to control for disease activity, we compared chemokine levels separately in active and inactive SLE patients. In patients with active disease, chemokine levels were not significantly different between patients receiving high dose prednisone and those who were not taking prednisone (p=0.16; Supplementary Table S7). Furthermore, active patients treated with immunosuppressive drugs (cytotoxic +) had similar chemokine levels as patients who were not being treated with immunosuppressants (cytotoxic −) (p=0.84). In patients with inactive disease, chemokine levels were not significantly different between cytotoxic + and cytotoxic − patients (p=0.49). However, inactive patients receiving high dose prednisone had significantly lower levels of chemokines as compared to inactive patients who were not taking prednisone (p=0.0002; Supplementary Table S7). Further prospective studies are needed to fully elucidate the effects of medication usage on serum chemokine levels.
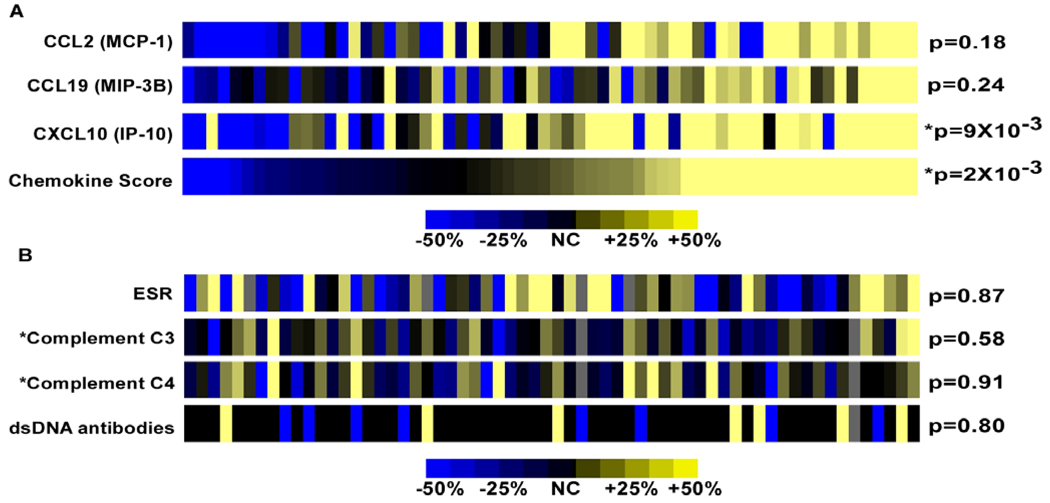
We next leveraged the longitudinal aspect of this collection to ask whether chemokine levels rise during flares of disease activity. All pairs of consecutive visits from patients who had a lupus flare (increase in SLEDAI ≥3; n=62 pairs) were identified to assess the ability of these chemokines to serve as dynamic biomarkers reflecting changes in activity. Strikingly, chemokine levels generally increased in patients between their pre-flare and flare visits (chemokine score, p=2×10−3; Figure 4A). Flares marked by a corresponding increase in chemokine levels affected a variety of organ systems, but were most commonly associated with increased renal (n=16), musculoskeletal (n=13), and skin (n=12) SLEDAI subscores. Importantly, standard clinical laboratory tests did not exhibit consistent changes concurrent with flare (range p=0.58 to p=0.91; Figure 4B).
Figure 4. IFN-regulated chemokines as biomarkers for disease flares.
Chemokine levels and standard laboratory tests were measured in 62 paired pre-flare and flare visits. Flares were defined as an increase in SLEDAI ≥3. Heat maps depict the magnitude and direction of the change in chemokine levels (A) or laboratory results (B) from the pre-flare to the flare visit. Nonparametric paired Wilcoxon tests were used to generate p-values for chemokine levels while paired Student’s t-tests were used for classic laboratory tests. *Changes in complement levels (C3 and C4) are reported inversely, so that a decrease in complement components appears yellow on the heat map. Chemokine levels generally increased with flare, although about 10% of flares were associated with ≥ 25% decrease in chemokine levels.
We also examined paired, consecutive visits from patients who exhibited a reduction in disease activity (decrease in SLEDAI ≥3; n=73 pairs). Chemokine levels generally dropped at the time of disease activity reduction (chemokine score, p=1×10−3; Supplementary Figure S3). Standard clinical laboratory tests did not perform as well as the chemokine levels, with complement C4 showing the only significant result (p=0.03; Supplementary Figure S3). Together, these results provide evidence supporting the utility of IFN-regulated chemokines as biomarkers of lupus activity.
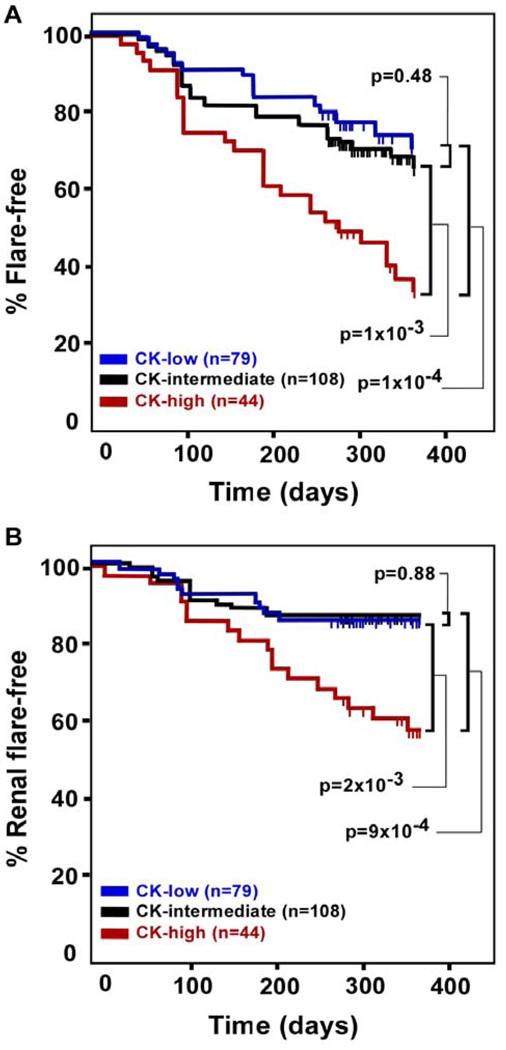
We next examined the ability of serum chemokine levels to predict future SLE disease activity. We first selected all patients with low disease activity at their baseline visit (SLEDAI ≤4), and separated them based on chemokine score (chemokine-high, n=44; chemokine-intermediate, n=108; chemokine-low, n=79). We then used Kaplan-Meier plots to visualize time to flare (increase in SLEDAI ≥3) for each group (Figure 5A). Interestingly, patients with high baseline chemokine levels were at increased risk for future SLE flares (36% of chemokine-high patients flare-free at 1 year vs. 72% of chemokine-low patients; p=0.0001). Patients in the chemokine-intermediate group were at similar risk compared to the chemokine-low group (p=0.48), but were significantly less likely to flare than the chemokine-high group (p=0.001). Strikingly, the difference in flare rates between chemokine-high and chemokine-low patients becomes significant as early as 100 days after the baseline visit (p=0.02), with an increase in significance at 200 days post-baseline (p=0.005). When the PGA was used as an alternative disease activity instrument for identifying flares, the chemokine-high group was again most likely to flare, although this trend was not significant (Supplemental Figure S4). These results suggest that clinically inactive patients with high serum chemokine levels are at increased risk for future lupus flares.
Figure 5. High baseline chemokine levels identify patients with elevated risk for future SLE flares.
SLE patients with low baseline disease activity (SLEDAI <4) were followed for one year. A. Patients who had a flare in any organ system (increase in SLEDAI ≥3) were recorded. Kaplan-Meier plots show the percentage of patients who remained free of flare in any organ system. B. Renal flares were defined as an increase in the renal SLEDAI subscore from 0 to 4+. Plots show the percentage of patients in each group who remained free of renal flares. Vertical tick marks along each curve represent patients who remained flare-free but did not have a full year of clinical follow-up (censored data).
Of the 79 chemokine-low patients depicted in Figure 5A, 19 patients (24%) had a flare within one year despite having low serum chemokine levels at their baseline visit. We next asked whether chemokine levels in these 19 patients rose over time as they approached flare by examining their chemokine levels at interim visits (between the baseline visit and the flare) and at the flare visit. On average, these patients had a 42% increase in chemokine scores at an interim visit (p-value = 0.009 by paired Wilcoxon test for the comparison of baseline chemokine score vs. interim chemokine score). Furthermore, these patients had an average increase in chemokine levels of 53% at the flare visit (p = 0.0002, baseline visit vs. flare visit). As another approach to this question, we determined the number of patients from this group who transitioned from their baseline chemokine-low state to a chemokine-high or - intermediate state either before or during the flare. Of these 19 patients, 13 individuals transitioned to chemokine-high or - intermediate status at either an interim visit (n=10) or at the flare visit (n=3). These data suggest that most of the patients who were chemokine-low at their baseline visit, but still flared within one year, had increasing levels of serum chemokines as they approached the flare event.
Since flares of lupus nephritis (LN) are among the most feared complications of lupus, we next tested the ability of chemokine levels to specifically predict renal flare. We selected patients who had no evidence for active LN at their baseline visit (renal SLEDAI subscore = 0) and compared LN flare rates (defined as a renal SLEDAI subscore ≥4 at the renal flare) among chemokine-high (n=45), chemokine-intermediate (n=100), and chemokine-low patients (n=70). Chemokine-high patients were at significantly increased risk for renal flares as compared to both chemokine-low (p=2×10−3) and chemokine-intermediate patients (p=9×10−4) (Figure 5B). Renal flare rates were not significantly different between chemokine-low and chemokine-intermediate patients (p=0.88). These data suggest that patients with elevated serum chemokine levels are at increased risk for lupus flares affecting the kidneys.
Since chemokine levels appear to distinguish patients likely to flare, we next tested whether standard laboratory measures could improve the prediction of lupus flares. We first performed univariate Cox proportional hazards regression to identify variables with significant ability to predict flare (increase in SLEDAI ≥3; Table 1). As expected, the chemokine score was significantly associated with flare, both as a binary variable (chemokine-high vs. chemokine-intermediate/low; p=0.0001), and as a continuous variable (p=0.0002). The individual chemokine levels for CXCL10 (IP-10) and CCL19 (Mip-3B) were also significant (p=0.0001 and p=0.03, respectively). Of interest, none of the common laboratory test results (ESR, complements, dsDNA abs) were significant predictors of flare, although ESR showed a positive trend (p=0.07).
Table 1.
Univariate and multivariate Cox proportional analysis to identify variables with power to predict flares in 222 SLE patients (77 with flare, 145 without). Bold type indicates significant variables (p<0.05). Chemokine score was tested both as a continuous variable and as a binary variable (chemokine-high vs. -low/intermeidiate).
|
Univariate | |||
| Variable | p-value | Hazard ratio | 95% CI |
| CXCL10 (IP-10) | 0.0001 | 1.16 | 1.08 to 1.25 |
| CCL2 (MCP-1) | 0.2973 | 1.02 | 0.99 to 1.05 |
| CCL19 (MIP-3b) | 0.032 | 1.08 | 1.00 to 1.17 |
| CK score (continuous) | 0.0002 | 1.25 | 1.11 to 1.41 |
| CK score (binary) | 0.0001 | 2.57 | 1.61 to 4.10 |
| ESR | 0.071 | 1.08 | 1.00 to 1.18 |
| C3 | 0.8106 | 1.00 | 0.94 to 1.07 |
| C4 | 0.5475 | 0.95 | 0.74 to 1.23 |
| DNA | 0.4509 | 1.01 | 0.99 to 1.03 |
| Multivariate | |||
| p-value | Hazard ratio | 95% CI | |
| CK score (binary) | 0.0001 | 2.52 | 1.63 to 4.09 |
Next, we used multivariate Cox proportional hazards regression to determine whether laboratory test results could further enhance the predictive ability of the chemokine score. Using a stepwise approach, the only variable that was included in the model was the chemokine score (p=0.0001; hazard ratio=2.5). These data suggest that monitoring serum chemokine levels could greatly improve the identification of patients at risk for flare and would provide a significant advancement over laboratory tests currently used to guide SLE management decisions.
Discussion
Here we provide data that validate serum IFN-regulated chemokine levels as biomarkers for disease activity in SLE. Serum chemokine levels correlated with SLE disease activity as measured by the SLEDAI, and changes in SLEDAI were accompanied by significant changes in chemokine levels. IFN-regulated chemokines also appear to serve as predictive markers of future lupus flare. Importantly, although chemokine levels and clinical laboratory tests (e.g. anti-dsDNA, serum complements, and ESR) demonstrated similar correlations with disease activity in some cross-sectional analyses, the levels of these chemokines significantly outperformed the clinical laboratory tests in longitudinal analyses. Serum chemokine levels may thus be clinically useful as an objective indicator of activity, and have the potential to improve disease management and therapeutic decision-making. Development of new SLE therapies has proven difficult, in part due to the lack of clearly defined, validated outcome measures for use in clinical trials (25). Our data suggest that measurement of IFN-regulated chemokines could potentially serve as a surrogate endpoint for SLE clinical trials of novel therapeutics.
Evaluating the risk of future flare is a major clinical challenge in SLE. Our data show that quiescent patients with high serum chemokine levels are much more likely to flare within one year compared to patients with low chemokine levels. Interestingly, flare rates in patients with intermediate chemokine levels were similar to the chemokine-low group, suggesting that increased risk of future flare may be largely restricted to patients with marked elevation of serum chemokines. Although some patients who were chemokine-low at baseline still flared within one year, most of these patients exhibited increasing chemokine levels as they approached the flare event, suggesting that frequent monitoring of chemokine levels may be useful in identifying patients who are progressing towards flare. In addition, the separation between flare rates for chemokine-high and chemokine-low individuals becomes significant at 100 days after the baseline visit, suggesting that chemokine levels may be useful for predicting flare over an even shorter time window. Our data indicate that commonly-used laboratory test results are not significant predictors of SLE flare, and these tests do not further enhance the predictive ability of serum chemokines in multivariate analyses. These data suggest that monitoring chemokine levels in SLE could give physicians an important tool to estimate the likelihood of future flares in patients who are clinically inactive. At a minimum, patients with elevated chemokine levels could be monitored more frequently.
Many studies have implicated type I IFN pathway activation in human lupus,(10–12, 26) and the IFN regulatory factor IRF5 was recently identified as a major risk gene for SLE (27, 28). Type I IFNs can stimulate B-cell proliferation and differentiation into antibody-secreting plasma cells, and differentiation of immature monocytes into antigen presenting dendritic cells. These dendritic cells can activate autoreactive lymphocytes and promote autoantibody production (29). These functions of type I IFN, coupled with impaired clearance of apoptotic debris in SLE patients, promote formation of immune complexes, which are potent inducers of type I IFN (30). Inappropriate IFN production and/or an inability to dampen IFN responses thus may initiate a positive feedback loop, resulting in perpetuation of the autoimmune response.
Another important link between IFN pathway activation and lupus may involve the upregulation of chemokines. Chemokines direct the migration of leukocytes throughout the body and thus orchestrate the inflammatory response. Type 1 IFN-dependent upregulation of chemokines in peripheral tissues may result in the inappropriate recruitment of autoreactive lymphocytes to sites of inflammation (15, 31, 32). Alternatively, systemic elevation of chemokines may desensitize chemokine receptors expressed on activated leukocytes, resulting in loss of normal homing mechanisms and consequently global inflammatory responses. Although the precise mechanism is unknown, IFN-regulated chemokines may be markers for the relevant underlying pathophysiology in SLE (discussed in (15)).
Progress in the discovery and validation of novel biomarkers for SLE has been hampered by the lack of suitable biorepositories. A significant strength of the current study is that samples comprising the ABCoN biorepository were collected by the same clinical laboratory and processed using a strict protocol to minimize variability and sample degradation. Additional strengths of the current study include the fact that several ethnic groups are represented, and all patients were examined and treated by the same rheumatologist, eliminating the confounding effect of inter-observer variability in assessment of disease activity. Furthermore, the large size of the ABCoN repository allowed us to conduct this prospective validation study based on an hypothesis generated in an earlier pilot study.
Here, we measured the three chemokines – CXCL10 (IP-10), CCL2 (MCP-1), and CCL19 (MIP-3B) – that showed the strongest correlations with disease activity in the pilot study. A potential limitation of the current study is that other proteins dysregulated in lupus serum, but not tested here, may improve the predictive ability of the composite chemokine biomarker. In addition, since cases in the current study were selected from a single cohort, it will now be important to determine whether the findings of this study translate to additional SLE cohorts. Clearly, attention to sample collection protocols and assay development will be important for these future studies. It should also be noted that CXCL10 (IP-10) was consistently the most strongly associated chemokine with current and future disease activity, raising the possibility that serum CXCL10 (IP-10) levels may be a stand-alone biomarker of SLE activity. Further studies are required to determine exactly which combination of markers will have the greatest clinical utility.
In summary, the data suggest that longitudinal measurement of IFN-regulated serum chemokines in SLE may prove useful in the clinical management of SLE, providing a tool to evaluate current disease activity and identify patients at risk for future flares. Furthermore, these results provide additional evidence implicating type I IFN in SLE pathogenesis and suggest that therapies targeting the IFN pathway may prove effective in modulating disease activity in lupus.
Supplementary Material
Acknowledgements
We would like to thank Ryan Fremming for his technical assistance with the Luminex and SearchLight assays at the Cytokine Reference Laboratory (University of Minnesota). This study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (N01-AR-1-2256), the National Institutes of Health (NIH AR 43727), and the Lupus Research Institute. The Johns Hopkins General Clinical Research Center is supported by the National Institutes of Health (M01-RR-00052).
Footnotes
Disclosures
Dr. Behrens reports being an employee of Genentech and having an equity interest in the company; Dr. Gregersen reports serving on the Abbott Scholar Award Advisory Committee and receiving honoraria from Biogen Idec, Genentech, and Roche Pharmaceuticals. No other potential conflict of interest relevant to this article was reported.
References
- 1.Wallace DJ, Hahn BH, editors. 7th ed. Baltimore: Williams & Wilkins; 2007. [Google Scholar]
- 2.Esdaile JM, Abrahamowicz M, Joseph L, MacKenzie T, Li Y, Danoff D. Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail. Arthritis Rheum. 1996;39(3):370–378. doi: 10.1002/art.1780390304. [DOI] [PubMed] [Google Scholar]
- 3.Illei GG, Tackey E, Lapteva L, Lipsky PE. Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis Rheum. 2004;50(7):2048–2065. doi: 10.1002/art.20345. [DOI] [PubMed] [Google Scholar]
- 4.Kavanaugh A. The utility of immunologic laboratory tests in patients with rheumatic diseases. Arthritis Rheum. 2001;44(10):2221–2223. doi: 10.1002/1529-0131(200110)44:10<2221::aid-art383>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Liu CC, Manzi S, Ahearn JM. Biomarkers for systemic lupus erythematosus: a review and perspective. Curr Opin Rheumatol. 2005;17(5):543–549. doi: 10.1097/01.bor.0000174182.70159.22. [DOI] [PubMed] [Google Scholar]
- 6.Ho A, Magder LS, Barr SG, Petri M. Decreases in anti-double-stranded DNA levels are associated with concurrent flares in patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2342–2349. doi: 10.1002/1529-0131(200110)44:10<2342::aid-art397>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Swaak AJ, Groenwold J, Bronsveld W. Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann Rheum Dis. 1986;45(5):359–366. doi: 10.1136/ard.45.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gladman DD, Hirani N, Ibanez D, Urowitz MB. Clinically active serologically quiescent systemic lupus erythematosus. J Rheumatol. 2003;30(9):1960–1962. [PubMed] [Google Scholar]
- 9.Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol. 2004;16(6):801–807. doi: 10.1016/j.coi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, et al. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50(12):3958–3967. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 13.Dall'era MC, Cardarelli PM, Preston BT, Witte A, Davis JC., Jr Type I interferon correlates with serological and clinical manifestations of SLE. Ann Rheum Dis. 2005;64(12):1692–1697. doi: 10.1136/ard.2004.033753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 15.Bauer JW, Baechler EC, Petri M, Batliwalla FM, Crawford D, Ortmann WA, et al. Elevated Serum Levels of Interferon-Regulated Chemokines Are Biomarkers for Active Human Systemic Lupus Erythematosus. PLoS Med. 2006;3(12):e491. doi: 10.1371/journal.pmed.0030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lit LC, Wong CK, Tam LS, Li EK, Lam CW. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:209–215. doi: 10.1136/ard.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narumi S, Takeuchi T, Kobayashi Y, Konishi K. Serum levels of IFN-inducible protein-10 relating to the activity of systemic lupus erythematosus. Cytokine. 2000;12(10):1561–1565. doi: 10.1006/cyto.2000.0757. [DOI] [PubMed] [Google Scholar]
- 18.Vila LM, Molina MJ, Mayor AM, Cruz JJ, Rios-Olivares E, Rios Z. Association of serum MIP-1alpha, MIP-1beta, and RANTES with clinical manifestations, disease activity, and damage accrual in systemic lupus erythematosus. Clin Rheumatol. 2007;26(5):718–722. doi: 10.1007/s10067-006-0387-y. [DOI] [PubMed] [Google Scholar]
- 19.Petri M. Hopkins Lupus Cohort. 1999 update. Rheum Dis Clin North Am. 2000;26(2):199–213. doi: 10.1016/s0889-857x(05)70135-6. [DOI] [PubMed] [Google Scholar]
- 20.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 21.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142(12 Pt 1):953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 22.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petri M, Hellmann D, Hochberg M. Validity and reliability of lupus activity measures in the routine clinic setting. J Rheumatol. 1992;19(1):53–59. [PubMed] [Google Scholar]
- 24.Petri M, Genovese M, Engle E, Hochberg M. Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study. Arthritis Rheum. 1991;34(8):937–944. doi: 10.1002/art.1780340802. [DOI] [PubMed] [Google Scholar]
- 25.Schiffenbauer J, Hahn B, Weisman MH, Simon LS. Biomarkers, surrogate markers, and design of clinical trials of new therapies for systemic lupus erythematosus. Arthritis Rheum. 2004;50(8):2415–2422. doi: 10.1002/art.20353. [DOI] [PubMed] [Google Scholar]
- 26.Ronnblom L, Alm GV. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J Exp Med. 2001;194(12):F59–F63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham RR, Kozyrev SV, Baechler EC, Reddy MV, Plenge RM, Bauer JW, et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006;38(5):550–555. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- 28.Graham RR, Kyogoku C, Sigurdsson S, Vlasova IA, Davies LR, Baechler EC, et al. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc Natl Acad Sci U S A. 2007;104(16):6758–6763. doi: 10.1073/pnas.0701266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 30.Vallin H, Blomberg S, Alm GV, Cederblad B, Ronnblom L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-alpha) production acting on leucocytes resembling immature dendritic cells. Clin Exp Immunol. 1999;115(1):196–202. doi: 10.1046/j.1365-2249.1999.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meller S, Winterberg F, Gilliet M, Muller A, Lauceviciute I, Rieker J, et al. Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: An amplification cycle triggering cutaneous lupus erythematosus. Arthritis Rheum. 2005;52(5):1504–1516. doi: 10.1002/art.21034. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel J, Worenkamper E, Freutel S, Henze S, Haller O, Bieber T, et al. Enhanced type I interferon signalling promotes Th1-biased inflammation in cutaneous lupus erythematosus. J Pathol. 2005;205(4):435–442. doi: 10.1002/path.1721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.