Abstract
Previous studies of patients with stable coronary artery disease (CAD) have demonstrated that decreases in left ventricular (LV) ejection fraction (EF) during acute mental stress are predictive of adverse clinical outcomes. The present study examined the prospective relationship of mental stress on clinical outcomes in a sample of 138 patients with stable CAD. Patients underwent mental stress testing and were followed for a median of 5.9 years to assess the occurrence of the combined endpoint of myocardial infarction or all-cause mortality. There were 32 events (17 nonfatal myocardial infarctions and 15 deaths) over the follow-up period. Of the 26 patients who exhibited myocardial ischemia during mental stress testing, 11 (42%) sustained a subsequent clinical event, compared to 21 (19%) of the 112 patients who showed no mental stress-induced ischemia. LVEF change during mental stress also was related to the clinical events in a graded, continuous fashion, with each 4 percentage point decrease from resting LVEF associated with an adjusted hazard ratio of = 1.7, 95% CI = 1.1, 2.6, p = .011. We conclude that reductions in LVEF during mental stress are prospectively associated with adverse clinical outcomes.
Keywords: Myocardial Ischemia, Mental Stress, Ejection Fraction, Coronary Artery Disease
Introduction
A decrease in left ventricular ejection fraction (LVEF) during mental stress (LVEF-MS) has been shown to identify patients at increased risk for adverse clinical events1-4 in patients with documented coronary artery disease (CAD). Previously, we have shown that reductions in LVEF in response to mental stress1 were associated with increased likelihood of subsequent clinical events, but these events were primarily revascularization procedures.1 Because revascularization procedures may not always reflect extra-clinical factors as well as disease progression,5 we reexamined this association using ‘hard’ endpoints of all-cause death and myocardial infarction in a sample of patients with stable CAD.
Methods
Data were available from baseline assessments of 138 of 144 patients with documented CAD who participated in a clinical trial of exercise and behavioral stress management.6 In order to participate in the trial patients had to have documented CAD (by prior myocardial infarction, coronary artery bypass graft surgery, coronary angioplasty, and/or ≥75% stenosis in at least 1 major coronary artery), and a positive treadmill exercise test within the prior year. Informed consent was obtained for all participants and the study protocol was approved by the Institutional Review Board at Duke University Medical Center.
Unless medically contraindicated, patients were briefly withdrawn from anti-ischemic medications (e.g., beta blockers, calcium channel blockers, and long-acting nitrates) at least 48 hours prior to testing; the medication washout period was at least 5 half-lives of the anti-ischemic medication. Twenty-eight patients were unable to be withdrawn from their medications and were tested on their usual dosage of anti-ischemic medications. After a 40-minute rest period, mental stress testing was performed in which patients were presented with two mental stress tasks, Public Speaking and Mirror Trace, in counterbalanced order. The Speaking stressor required participants to give a speech on a controversial current events topic after 1 minute of preparation. The Mirror Trace required participants to outline the shape of a star from its reflection in a mirror. Each task lasted for 5 minutes, with a 10 minute rest period between each stressor. These tasks have been used in prior work and have been found to elicit ischemia in vulnerable patients.7
To determine the presence of myocardial ischemia, R-wave-synchronized, gated equilibrium radionuclide ventriculography (RNV) with Paragon PBR software (Medasys Inc., Ann Arbor, MI) was performed prior to and during each stressor at 20 frames per cardiac cycle using a gamma camera (Siemens Gammasonics Inc., Des Plaines, IL) equipped with a sodium iodide crystal and an all-purpose collimator. Images were obtained following the labeling of autologous red blood cells with technetium 99m pertechnetate using the in vivo technique.8 Imaging was conducted during the last 2 minutes of the rest period, the first minute of speech preparation, at 2 min and 4 min for the Speaking and Mirror trace stressors with the camera in the left anterior oblique view. LVEF was obtained using the PBR software.
We defined LVEF-MS as the change from resting levels in LVEF, averaged across the Speaking and Trace tasks. We also classified patients who had a reduction in LVEF of at least 5% (e.g., from 55% during rest to an average of 50% or lower during the tasks) exhibiting mental stress-induced myocardial ischemia. Participants were assessed for clinical events 4 months after testing and annually thereafter through a combination of telephone and mail contact with participants, examination of medical records, and public sources of vital statistics. The primary outcome was the combined endpoint of all-cause mortality or myocardial infarction.
We used multivariable Cox proportional hazards models9 to estimate the hazard associated with LVEF-MS, adjusting for age, gender, history of myocardial infarction, and LVEF at rest. LVEF-MS was modeled as a continuous variable and scaled such that the resulting hazard ratio represented the change in hazard for every 4% (the interquartile range of LVEF-MS) reduction in LVEF-MS. Resting LVEF and age also were modeled as continuous variables scaled to their interquartile range (14% and 15 years, respectively). We examined the association between the continuous LVEF-MS measure and the endpoint for possible nonlinearity using a flexible non-parametric curve-fitting algorithm.10,11 We also conducted a number of sensitivity analyses, adjusting for further potential confounders, including revascularization procedures that occurred during follow-up (modeled as a time-varying covariate). In addition, we evaluated whether the relation between LVEF-MS and the combined endpoint differed for patients who were tapered off their cardiac medication during the mental stress testing and those who were still on medication by testing an LVEF-MS by medication status (on versus off medication) interaction term in the Cox model. Finally, we re-estimated the primary Cox models for the separate endpoints of death and myocardial infarction.
Results
One-hundred thirty eight (138) participants (98%) had adequate RNV studies during mental stress testing. The average age of the sample was 62 years, with the majority being male and Caucasian. Twenty six patients (19%) of the sample exhibited mental-stress-induced ischemia. Patients with mental stress-induced ischemia were more likely to belong to an ethnic minority, to report a history of diabetes, and to have lower serum high density lipoprotein (HDL) levels at the time of testing compared to the non-ischemic patients (Table 1). The median follow-up time was 5.9 years (interquartile range = 4.8 – 7.2 years; range = 35 days – 8.8 years). There were 18 deaths and 17 non-fatal myocardial infarctions. Of the 18 deaths, 4 were known to be related to cardiac causes, 2 were known to be non-cardiac, and 12 were of unknown causes. In 3 cases death was preceded by myocardial infarction, so the initial myocardial infarction rather than death was used as the outcome. Thus, there were 32 unique events (15 deaths, 17 myocardial infarctions) available for analysis.
Table 1.
Characteristics of patients with and without mental stress-induce myocardial ischemia (n = 138)
| Variable | No Ischemia (N = 112) |
Ischemia (N = 26) |
All Participants (N = 138) |
P-value comparing Ischemia to No Ischemia groups a |
|---|---|---|---|---|
| Age (years) | 62.5 (55.8,71.2) | 60.0 (51.2,69.0) | 62.0 (55.0,70.0) | 0.471 |
| Men | 79 (71%) | 17 (65%) | 96 (70%) | 0.607 |
| Ethnicity | 0.030b | |||
| African America | 17 (15%) | 8 (31%) | 25 (18%) | |
| Caucasian | 91 (81%) | 15 (58%) | 106 (77%) | |
| Other Ethnicity | 4 (4%) | 3 (12%) | 7 (5%) | |
| Education | 0.391b | |||
| High School or Less | 32 (29%) | 7 (27%) | 39 (28%) | |
| Some college | 39 (35%) | 6 (23%) | 45 (33%) | |
| College or more | 41 (37%) | 13 (50%) | 54 (39%) | |
| Body Mass Index (kg/m2) | 28.4 (25.8,32.3) | 30.1 (25.5.,32.4) | 28.9 (25.7, 32.3) | 0.584 |
| Hypertension | 60 (54%) | 15 (58%) | 75 (54%) | 0.704 |
| Diabetes Mellitus | 21 (19%) | 10 (38%) | 31 (22%) | 0.030 |
| Current Smoker | 14 (12%) | 3 (12%) | 17 (12%) | 0.893 |
| Quit Smoking | 72 (64%) | 15 (58%) | 87 (63%) | 0.530 |
| Past Myocardial Reinfarction | 63 (56%) | 15 (58%) | 78 (57%) | 0.894 |
| Past Revascularization | 50 (45%) | 12 (46%) | 62 (45%) | 0.889 |
| Total Cholesterol (mg/dL) | 180 (160,205) | 183 (166,197) | 180 (161,201) | 0.819 |
| Low Density Lipoprotein (mg/dL) | 97 (87,124) | 114 (88,125) | 98 (87,125) | 0.539 |
| High Density Lipoprotein (mg/dL) | 45 (39,54) | 40 (35,47) | 44 (38,52) | 0.016 |
| Triglycerides (mg/dL) | 141 (102,211) | 138 (100,166) | 140 (102,210) | 0.888 |
| Beta Blockade | 81 (72%) | 18 (72%) | 99 (72%) | 0.974 |
| Nitrates | 34 (30%) | 8 (23%) | 42 (31%) | 0.872 |
| Calcium Channel Blockade | 25 (22%) | 5 (20%) | 30 (22%) | 0.800 |
| Anticoagulants | 102 (91%) | 22 (85%) | 124 (90%) | 0.326 |
| Statins | 87 (78%) | 20 (77%) | 107 (78%) | 0.934 |
| Left Ventricular Ejection Fraction at Rest (%) | 57.5 (51.0,66.2) | 56.2 (51.1,63.0) | 57.2 (51.0,64.9) | 0.729 |
| Reduction in Left Ventricular Ejection Fraction during Mental Stress (%) | -0.75 (-2.25,0.75) | -6.50 (-7.19, -5.56) | -1.50 (-3.50, 0.50) | < .001 |
| Medication During testing | 21 (19%) | 7 (27%) | 28 (20%) | 0.351 |
Data presented are median (interquartile range) or number (percent) of patients.
Wilcoxon Test used for continuous variables, Pearson chi-square for categorical variables
P-value is from global test of all categories.
Among the 26 patients who exhibited mental-stress induced ischemia, 42% (n = 11) sustained a clinical event during the follow-up period, compared to 19% (n = 21) of the 112 patients who showed no ischemia. Figure 1 shows the unadjusted Kaplan-Meier curves for patients with and without mental stress-induced ischemia. The log rank test comparing the two curves was statistically significant, p = .020. Turning to the Cox regression results, LVEF-MS was significantly associated with the time to the combined endpoint (See Table 2), adjusting for age, gender, prior MI, and resting LVEF. We found no evidence that the association between LVEF-MS and the combined endpoint was nonlinear (p = .485).
Figure 1.
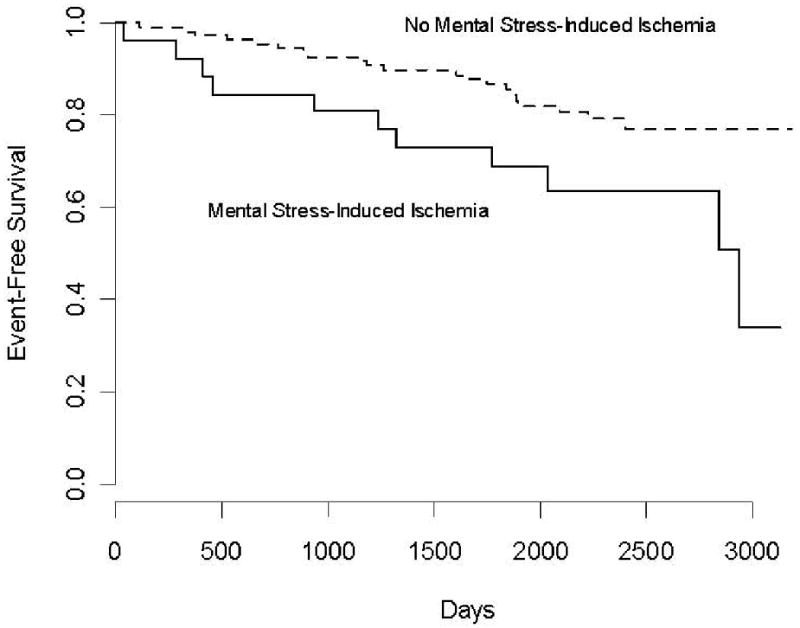
Kaplan-Meier curves comparing patients with myocardial ischemia (LVEF decrease ≥ 5%) and those without myocardial ischemia (LVEF decrease < 5%) during mental stress. The log-rank test was statistically significant, p = .012.
Table 2.
Cox regression results predicting time to combined endpoint of death (n = 15) or non-fatal myocardial infarction (n = 17)
| Factor | Scale Value for Predictor | Hazard Ratio | p-value | Lower 95% Confidence Interval | Upper 95% Confidence Interval |
|---|---|---|---|---|---|
| Age | 15 | 1.1 | .718 | 0.6 | 1.9 |
| Prior Myocardial Infarction | Yes vs. No | 1.1 | .824 | 0.5 | 2.4 |
| Left Ventricular Ejection fraction at Rest | 14 | 1.0 | .820 | 0.6 | 1.6 |
| Reduction in Left Ventricular | 4 | 1.7 | .011 | 1.1 | 2.6 |
| Ejection Fraction During Mental Stress |
Continuous predictor variables age, LVEF at rest, and LVEF reduction during mental stress are rescaled to their interquartile range. This preserves the continuous form of the predictor but generates a hazard ratio that represents a comparison of hazards across a meaningful distance on the range of the predictor. This scaling distance for each continuous variable is given in column 2. For example, the hazard ratio for age represents the increase in hazard for every 15 year increase in age. Gender was included in the model as a stratification variable.
Adjusting for revascularization procedures (as a time-varying covariate), diabetes, HDL, and ethnic minority status in the primary Cox model did not materially alter the estimate for LVEF-MS, HR = 1.8, 95% CI = 1.1, 2.9, p = .012. We also observed no evidence that the relation between LVEF-MS and the endpoint differed by medication status during the mental stress testing (p = .637). Considering only the 17 myocardial infarctions as the endpoint (censoring deceased patients at the time of death) the estimate for LVEF-MS remained similar to that in the primary analysis, HR = 1.8, 95% CI = 1.02, 3.2, p = .043. Using only the 18 deaths (including the 3 patients who had died after a myocardial infarction) as the endpoint, the HR for change in LVEF-MS was attenuated, HR = 1.6, but the confidence interval contained 1.0; 95% CI = 0.84, 2.9, p = .159. When we removed the 3 deceased cases with known MI from the analysis of deaths only, the HR was further attenuated and the confidence intervals became wider: HR = 1.5, 95% CI = 0.8-2.9, p = .221.
Discussion
Our finding of an association between clinical events and LVEF change during mental stress is consistent with several prior reports using a variety of methodologies to evaluate mental stress-induced changes in left ventricular function,1-4 supporting the robustness of the association across a relatively broad population of patients with stable CAD. In the present sample, the hazard ratio for every 4 percentage point decrease in LVEF during mental stress was 1.7, which is consistent with our prior study, in which the adjusted hazard ratio was about 1.5 for every 4 percentage point reduction in LVEF during mental stress.1 We also found that the hazard estimate for LVEF-MS remained relatively unchanged after adjustment for a number of additional covariates, and also when examining myocardial infarction and all-cause mortality as a separate endpoint. The association of LVEF-MS with all-cause mortality alone was somewhat weaker, especially after the three overlapping MI cases were removed from among the deaths. This latter finding suggests that the inclusion of non-cardiac related death may have attenuated the association in the primary analysis of the combined endpoint. Sensitivity analyses also indicated that the present finding is unlikely explained by confounding baseline differences between patients with and without ischemic responses. We also found that although the conventional binary definition of ischemia during mental stress was related to the risk of an event, the continuous measures of LVEF change also was associated with clinical events. Indeed, given the relatively few patients (19%) with responses that met the definition of mental stress-induced ischemia, the association between LVEF change and prognosis was driven to a large extent by patients who did not have a fall in LVEF of at least 5%. We observed a similar continuous linear association in our previous study.1 Because LVEF changes with stress may reflect hemodynamic responses that are not due to myocardial ischemia, other mechanisms may contribute to the relationship between LVEF change and subsequent cardiovascular events.
Despite the important diagnostic and prognostic utility of mental stress ischemia, the mechanisms underlying its occurrence are not well understood. Because mental stress typically elicits marked hemodynamic adjustments, including increased blood pressure and heart rate, as well as cardiac contractility, it results in increased myocardial oxygen demand.12 Unlike normal coronary arteries, atherosclerotic vessels are prone to constrict rather than dilate, and constriction of the coronary arteries leads to reduced myocardial oxygen supply.13 Myocardial ischemia is understood to be the manifestation of a myocardial O2 supply/demand imbalance.14 On the demand side, we have previously shown that mental stress-induced ischemia was associated with increases in systolic blood pressure and rate pressure-product, but not with increased heart rate.7 We also observed that mental stress-induced ischemia was associated with elevated diastolic blood pressure and suggested that ischemia may result from reduced myocardial oxygen supply.7 Indeed, increased systemic vascular resistance (SVR) during mental stress has been linked to myocardial ischemia in the PIMI study15 and increased SVR may be considered to be a manifestation of arterial vasoconstriction and represent a marker of coronary vasoconstriction. Our previous work has shown that mental stress-induced increases in SVR are associated with vascular endothelial dysfunction, as indexed by low flow-mediated dilation (FMD).16 Indeed, the occurrence of mental stress-induced myocardial ischemia has been found to be associated with impaired FMD in postmenopausal women with angina.17 Acute mental stress may also result in unfavorable transient alterations in endothelial function. Ghiadoni and colleagues18 found that exposure to a laboratory-based, simulated public speaking stressor, resulted in transiently impaired FMD (falling from 5% at rest to 2.8% post-stress) in 10 healthy middle-aged men. Gottdiener et al.19 also documented that mental stress, associated with laboratory anger recall and mental arithmetic stressors, impaired FMD in a study sample comprised of 38 men and women. Findings of mental-stress related impairment of FMD have been replicated in several additional studies, which have utilized a variety of laboratory-based mental stressors, including mental arithmetic,20 psychomotor reaction time tasks,21 and cold pressor.22,23 Therefore, mental stress ischemia may be a manifestation of increased myocardial demand, combined with impaired vascular regulation that may transiently compromise myocardial oxygen supply and increase vulnerability to adverse cardiac events.
Acknowledgments
Supported by grants from the National Institutes of Health HL59672 and M01-RR-30.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, Frid DJ, McNulty S, Morris JJ, O'Connor CM, Blumenthal JA. Mental stress--induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 2.Jain D, Burg M, Soufer R, Zaret BL. Prognostic implications of mental stress-induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76:31–35. doi: 10.1016/s0002-9149(99)80796-6. [DOI] [PubMed] [Google Scholar]
- 3.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, Kaufmann PG. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the psychophysiological investigations of myocardial ischemia study. Circulation. 2002;105:1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 4.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–1297. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 5.Pilote L, Miller DP, Califf RM, Rao JS, Weaver WD, Topol EJ. Determinants of the use of coronary angiography and revascularization after thrombolysis for acute myocardial infarction. NEJM. 1996;335:1198–205. doi: 10.1056/NEJM199610173351606. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Waugh R, Georgiades A, Bacon SL, Hayano J, Coleman RE, Hinderliter A. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: A randomized controlled trial. JAMA. 2005;293:1626–1634. doi: 10.1001/jama.293.13.1626. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal JA, Jiang W, Waugh RA, Frid DJ, Morris JJ, Coleman RE, Hanson M, Babyak M, Thyrum ET, Krantz DS. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation. 1995;92:2102–2108. doi: 10.1161/01.cir.92.8.2102. [DOI] [PubMed] [Google Scholar]
- 8.Metler FA, Guiberteau MJ. In: Cardiovascular system. Metler FA, Guiberteau MJ, editors. Essentials of Nuclear Imaging; New York: 1986. pp. 151–152. [Google Scholar]
- 9.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 10.Harrell FE. Regression Modeling Strategies: With applications to linear modeling, logistic regression, and survival analysis. New York: Springer; 2001. p. 568. [Google Scholar]
- 11.Stone CJ, Koo CY. Additive Splines in Statistics Proceedings of the Statistical Computing Section. American Statistical Association; Washington, D.C.: 1985. [Google Scholar]
- 12.Sherwood A, Turner JR. Hemodynamic responses during psychological stress: implications for studying disease processes. Int J Behav Med. 1995;2:193–218. doi: 10.1207/s15327558ijbm0203_1. [DOI] [PubMed] [Google Scholar]
- 13.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO., III Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76:125–130. doi: 10.1016/s0002-9149(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 14.Vetrovec GW. Changing concepts in the pathophysiology of myocardial ischemia. Am J Cardiol. 1989;64:3F–9F. doi: 10.1016/0002-9149(89)90739-x. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, Taylor H, Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress: experience from the psychophysiological investigations of myocardial ischemia study (PIMI) Circulation. 1996;94:2402–2409. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 16.Sherwood A, Johnson K, Blumenthal JA, Hinderliter AL. Endothelial function and hemodynamic responses during mental stress. Psychosom Med. 1999;61:365–370. doi: 10.1097/00006842-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Peix A, Trapaga A, Asen L, Ponce F, Infante O, Valiente J, Tornes F, Cabrera LO, Guerrero I, Garcia EJ, Carrillo R, Garcia-Barreto D. Mental stress-induced myocardial ischemia in women with angina and normal coronary angiograms. J Nucl Cardiol. 2006;13:507–513. doi: 10.1016/j.nuclcard.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 19.Gottdiener JS, Kop WJ, Hausner E, McCeney MK, Herrington D, Krantz DS. Effects of mental stress on flow-mediated brachial arterial dilation and influence of behavioral factors and hypercholesterolemia in subjects without cardiovascular disease. Am J Cardiol. 2003;92:687–691. doi: 10.1016/s0002-9149(03)00823-3. [DOI] [PubMed] [Google Scholar]
- 20.Jambrik Z, Sebastiani L, Picano E, Ghelarducci B, Santarcangelo EL. Hypnotic modulation of flow-mediated endothelial response to mental stress. Int J Psychophysiol. 2005;55:221–227. doi: 10.1016/j.ijpsycho.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Luscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105:2817–2820. doi: 10.1161/01.cir.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 22.Jambrik Z, Santarcangelo EL, Rudisch T, Varga A, Forster T, Carli G. Modulation of pain-induced endothelial dysfunction by hypnotisability. Pain. 2005;116:181–186. doi: 10.1016/j.pain.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 23.Lind L, Johansson K, Hall J. The effects of mental stress and the cold pressure test on flow-mediated vasodilation. Blood Press. 2002;11:22–27. doi: 10.1080/080370502753543927. [DOI] [PubMed] [Google Scholar]


