Abstract
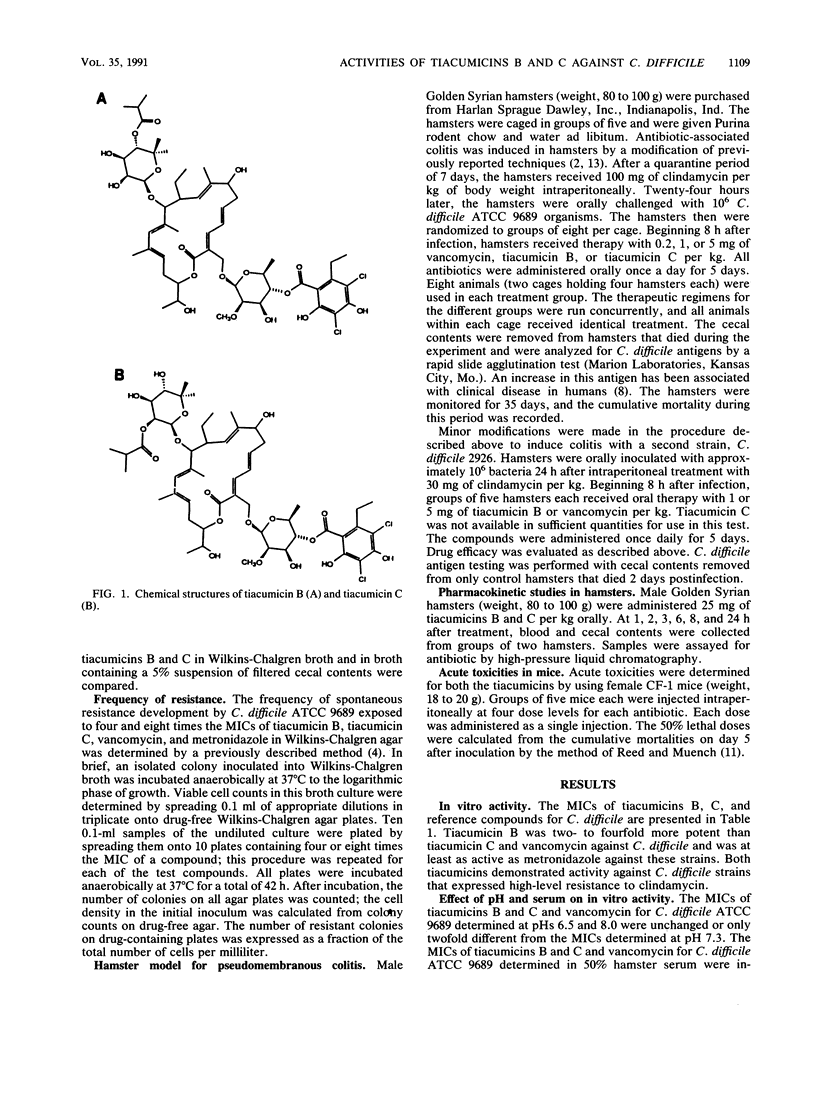
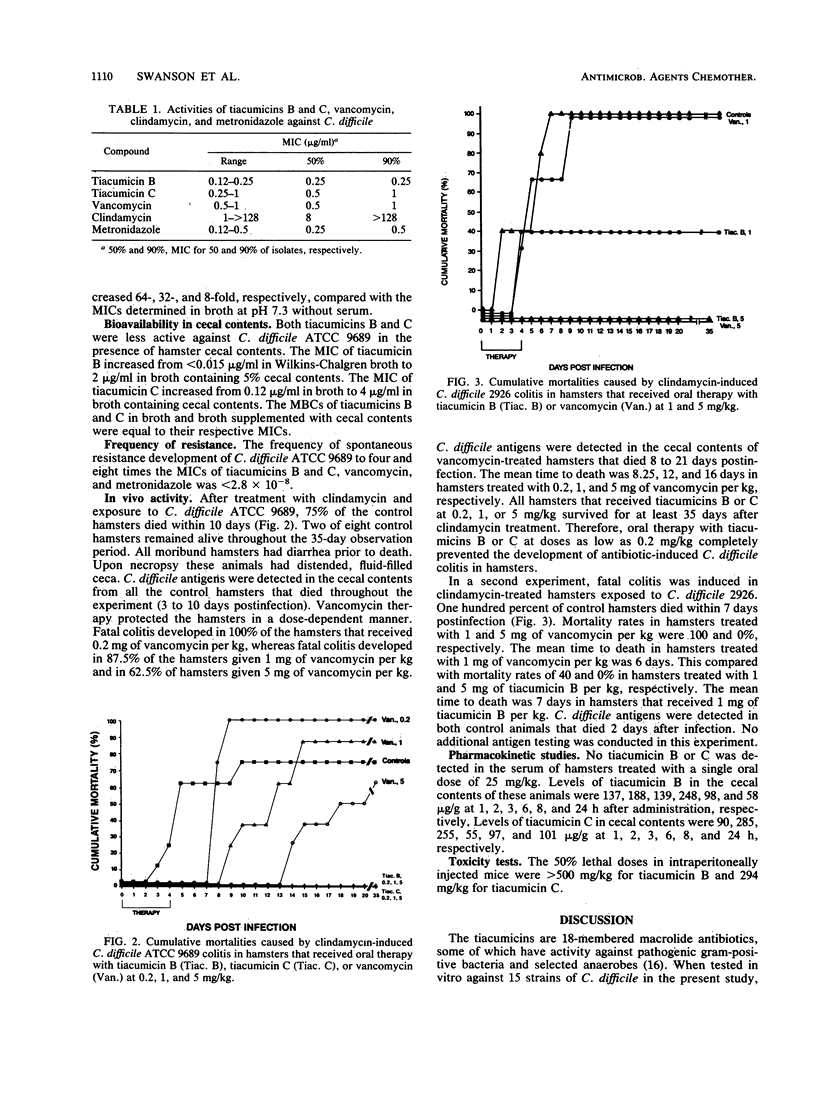
Tiacumicins B and C are members of a novel group of 18-membered macrolide antibiotics with in vitro activity against Clostridium difficile. The MICs against 15 strains of C. difficile were 0.12 to 0.25 microgram/ml for tiacumicin B, 0.25 to 1 microgram/ml for tiacumicin C, and 0.5 to 1 microgram/ml for vancomycin. The resistance frequency for both compounds against C. difficile was less than 2.8 x 10(-8) at four and eight times the MIC. The in vivo activities of the tiacumicins against two strains of C. difficile were compared with that of vancomycin in a hamster model of antibiotic-associated colitis. Oral therapy with 0.2, 1, or 5 mg of tiacumicin B or C per kg of body weight protected 100% of clindamycin-treated hamsters exposed to C. difficile ATCC 9689. Oral treatment with identical doses of vancomycin produced a prolonged, dose-dependent survival of hamsters, but it did not prevent the development of fatal colitis at doses of up to 5 mg/kg. When clindamycin-treated animals were exposed to another strain of C. difficile, both tiacumicin B and vancomycin were protective at 5 mg/kg, but not at lower doses. Tiacumicin C was not tested in vivo against the second strain of C. difficile. No tiacumicin B or C was detected in the sera of hamsters treated with single oral doses of 25 mg/kg, while antibiotic levels in the ceca of these hamsters reached 248 micrograms/ml and 285 mg/ml for tiacumicins B and C, respectively. The tiacumicins demonstrated in vitro and in vivo potencies against C. difficile and achieved high concentrations in the cecum, but not the serum, of hamsters after oral administration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coronelli C., White R. J., Lancini G. C., Parenti F. Lipiarmycin, a new antibiotic from Actinoplanes. II. Isolation, chemical, biological and biochemical characterization. J Antibiot (Tokyo) 1975 Apr;28(4):253–259. doi: 10.7164/antibiotics.28.253. [DOI] [PubMed] [Google Scholar]
- Dong M. Y., Chang T. W., Gorbach S. L. Treatment of Clostridium difficile colitis in hamsters with a lipopeptide antibiotic, LY146032. Antimicrob Agents Chemother. 1987 Jul;31(7):1135–1136. doi: 10.1128/aac.31.7.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Hanson C. W., Stamm J. M., Vojtko C., Shipkowitz N. L., St Martin E. The frequency of in-vitro resistance development to fluoroquinolones and the use of a murine pyelonephritis model to demonstrate selection of resistance in vivo. J Antimicrob Chemother. 1987 Apr;19(4):449–465. doi: 10.1093/jac/19.4.449. [DOI] [PubMed] [Google Scholar]
- Gross M. H. Management of antibiotic-associated pseudomembranous colitis. Clin Pharm. 1985 May-Jun;4(3):304–310. [PubMed] [Google Scholar]
- Hochlowski J. E., Swanson S. J., Ranfranz L. M., Whittern D. N., Buko A. M., McAlpine J. B. Tiacumicins, a novel complex of 18-membered macrolides. II. Isolation and structure determination. J Antibiot (Tokyo) 1987 May;40(5):575–588. doi: 10.7164/antibiotics.40.575. [DOI] [PubMed] [Google Scholar]
- Hächler H., Berger-Bächi B., Kayser F. H. Genetic characterization of a Clostridium difficile erythromycin-clindamycin resistance determinant that is transferable to Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Jul;31(7):1039–1045. doi: 10.1128/aac.31.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S., Imamura N., Oiwa R., Kuga H., Iwata R., Masuma R., Iwai Y. Clostomicins, new antibiotics produced by Micromonospora echinospora subsp. armeniaca subsp. nov. I. Production, isolation, and physico-chemical and biological properties. J Antibiot (Tokyo) 1986 Oct;39(10):1407–1412. doi: 10.7164/antibiotics.39.1407. [DOI] [PubMed] [Google Scholar]
- Parenti F., Pagani H., Beretta G. Lipiarmycin, a new antibiotic from Actinoplanes. I. Description of the producer strain and fermentation studies. J Antibiot (Tokyo) 1975 Apr;28(4):247–252. doi: 10.7164/antibiotics.28.247. [DOI] [PubMed] [Google Scholar]
- Sergio S., Pirali G., White R., Parenti F. Lipiarmycin, a new antibiotic from Actinoplanes III. Mechanism of action. J Antibiot (Tokyo) 1975 Jul;28(7):543–549. doi: 10.7164/antibiotics.28.543. [DOI] [PubMed] [Google Scholar]
- Swanson R. N., Hardy D. J., Shipkowitz N. L., Hanson C. W., Ramer N. R., Coen L. J., Fernandes P. B. Phenelfamycins, a novel complex of elfamycin-type antibiotics. III. Activity in vitro and in a hamster colitis model. J Antibiot (Tokyo) 1989 Jan;42(1):94–101. doi: 10.7164/antibiotics.42.94. [DOI] [PubMed] [Google Scholar]
- Teasley D. G., Gerding D. N., Olson M. M., Peterson L. R., Gebhard R. L., Schwartz M. J., Lee J. T., Jr Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983 Nov 5;2(8358):1043–1046. doi: 10.1016/s0140-6736(83)91036-x. [DOI] [PubMed] [Google Scholar]
- Theriault R. J., Karwowski J. P., Jackson M., Girolami R. L., Sunga G. N., Vojtko C. M., Coen L. J. Tiacumicins, a novel complex of 18-membered macrolide antibiotics. I. Taxonomy, fermentation and antibacterial activity. J Antibiot (Tokyo) 1987 May;40(5):567–574. doi: 10.7164/antibiotics.40.567. [DOI] [PubMed] [Google Scholar]



