Abstract
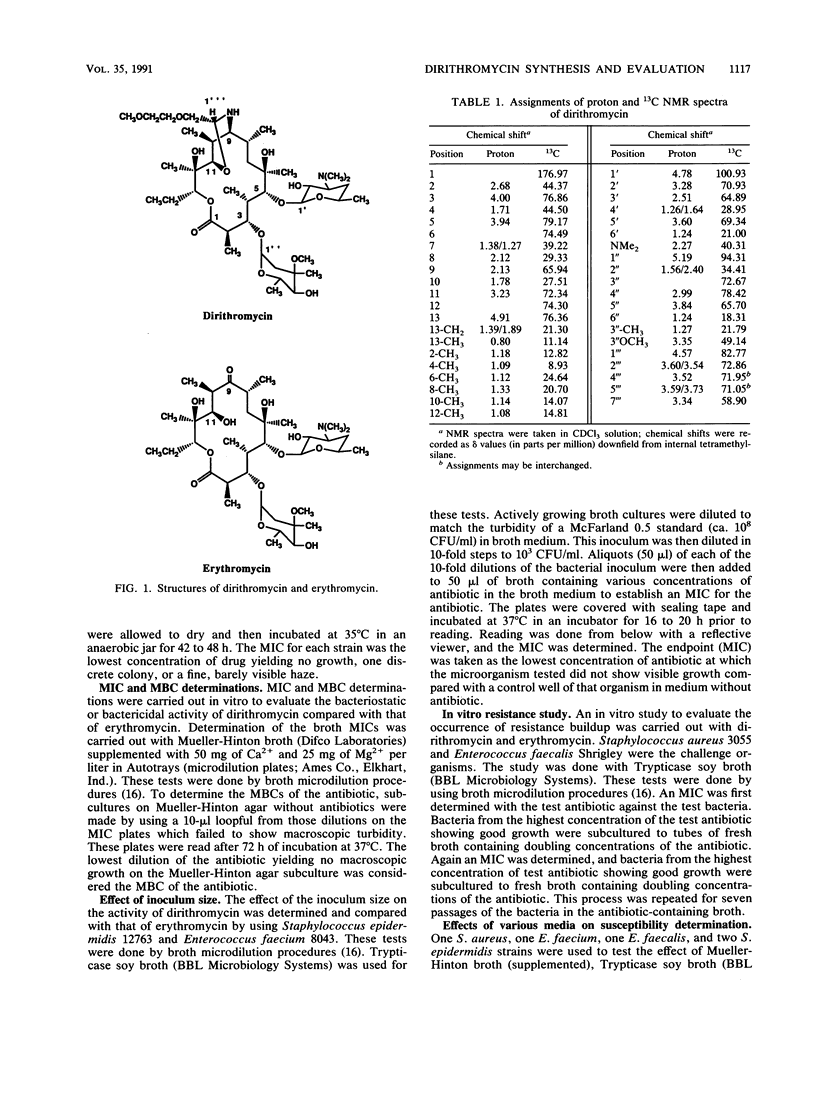
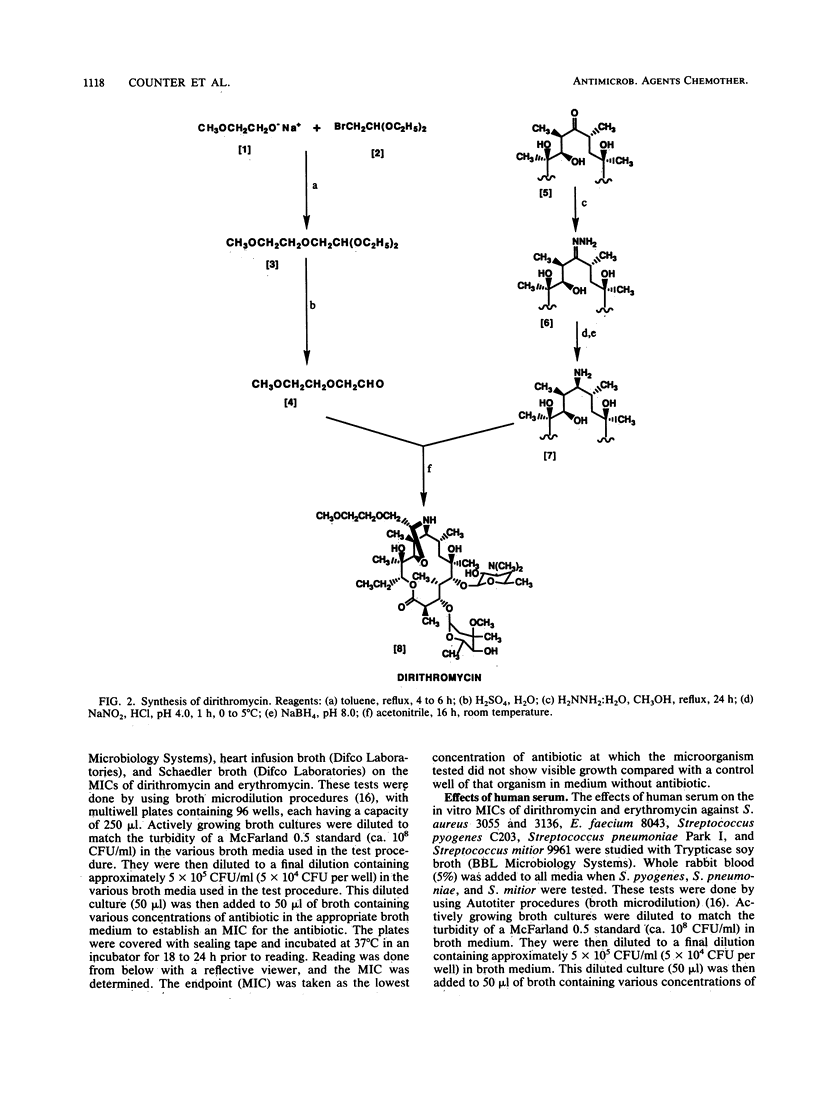
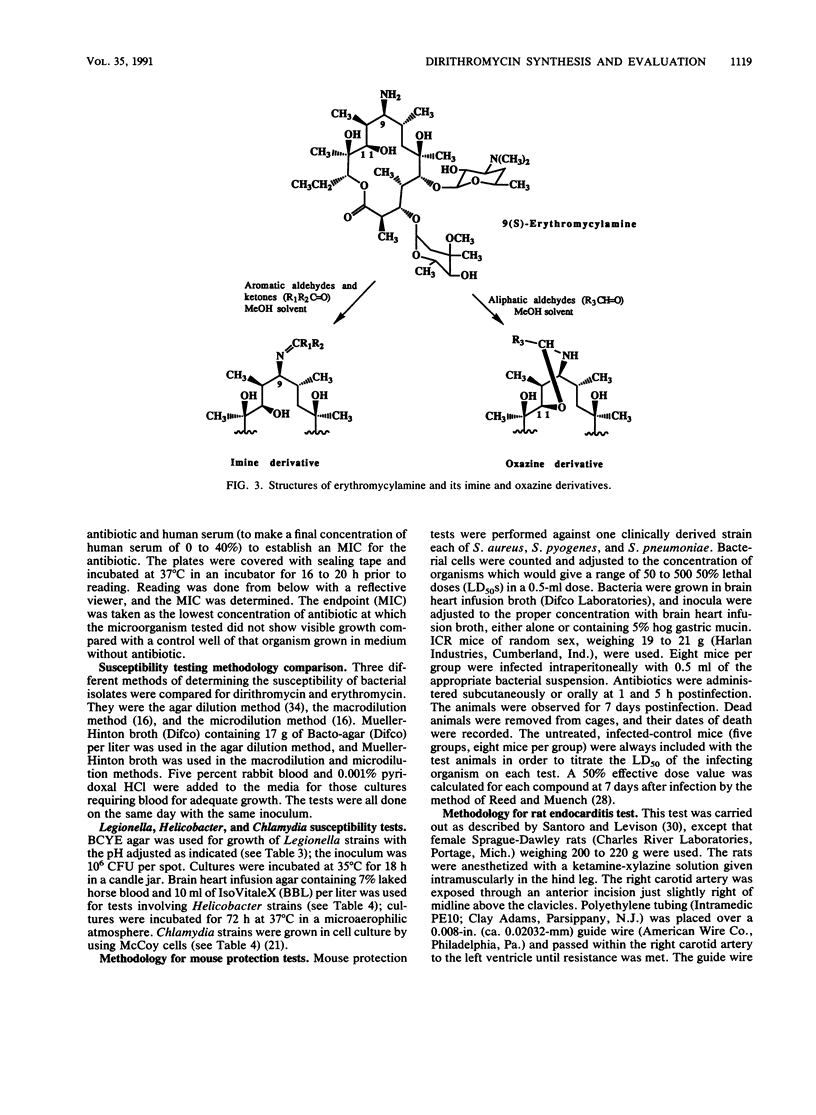
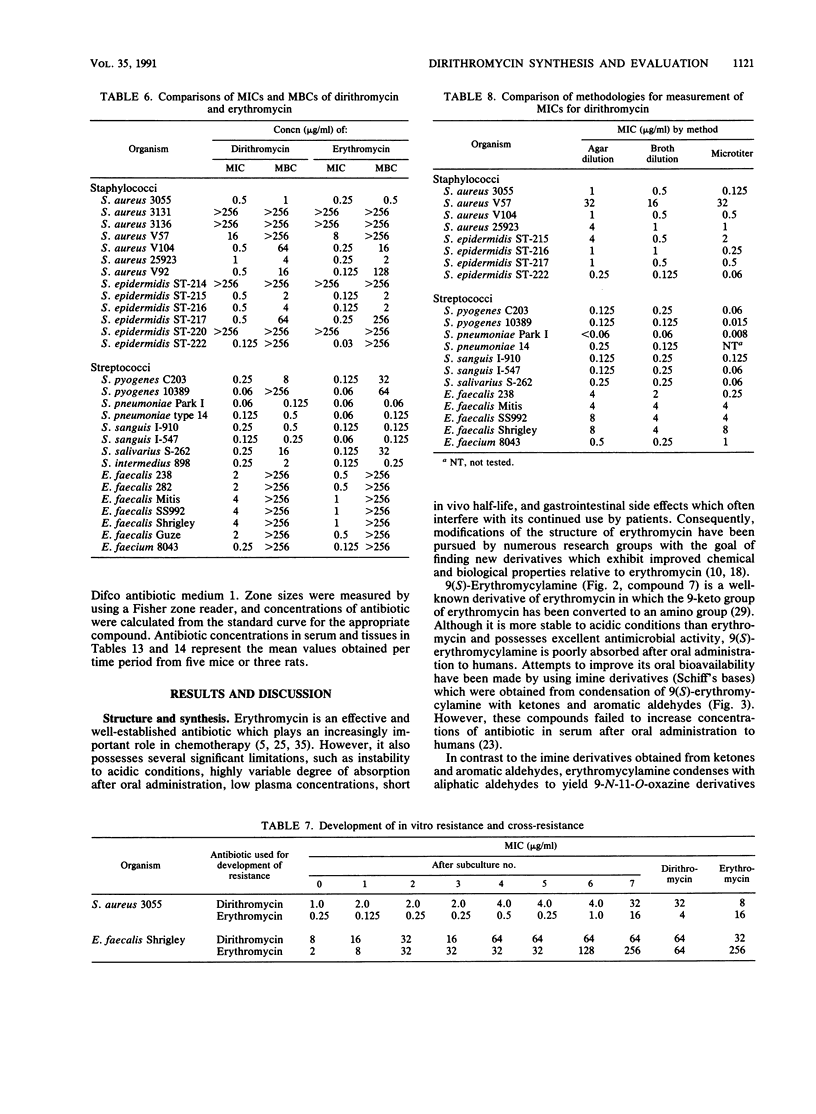
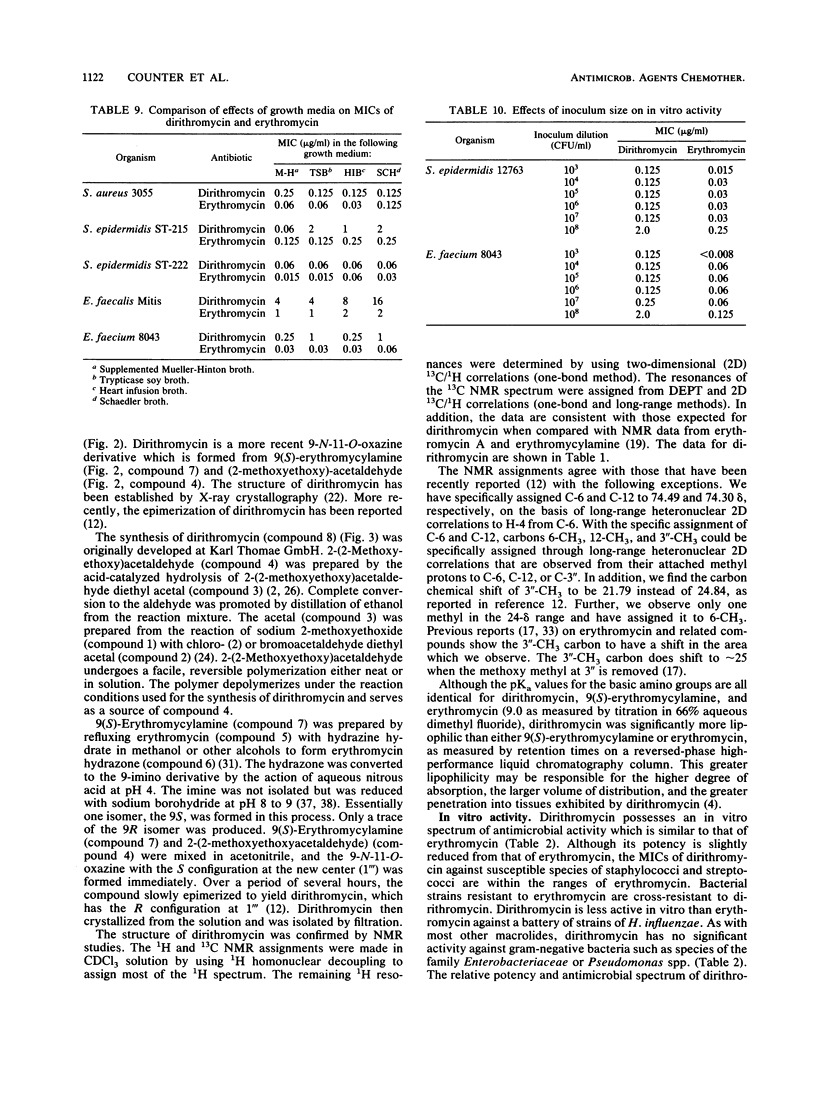
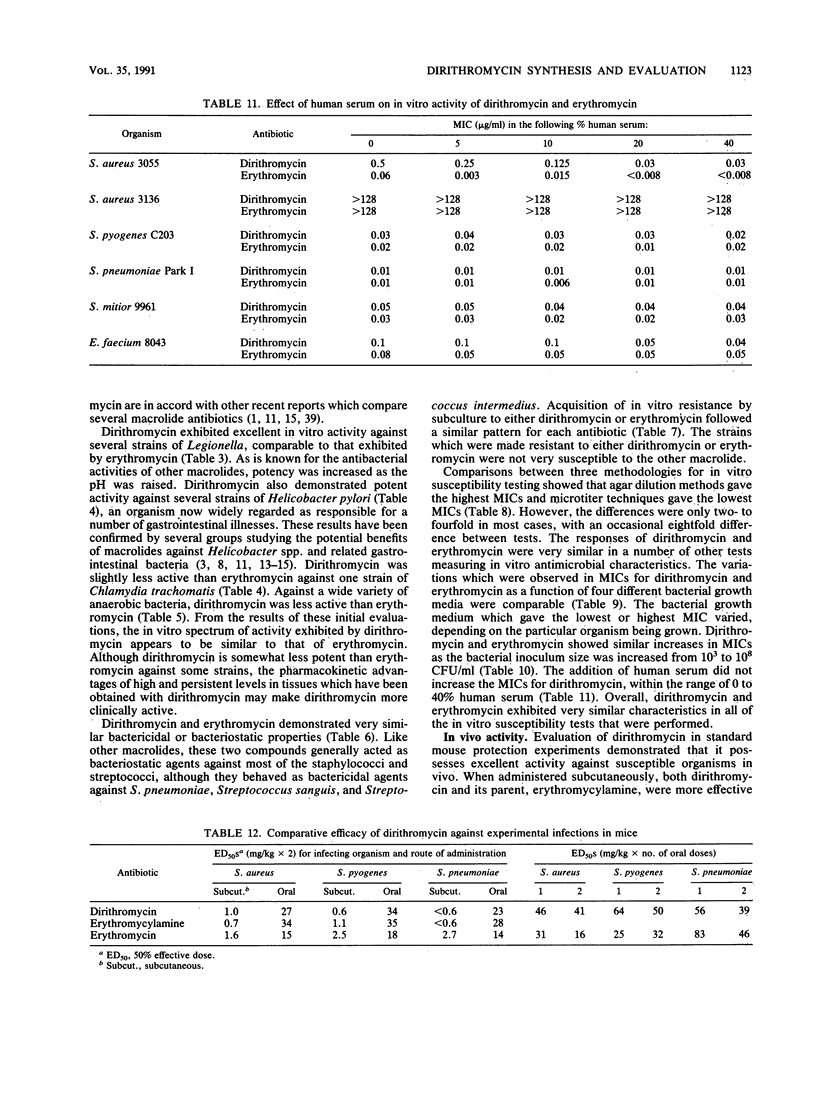
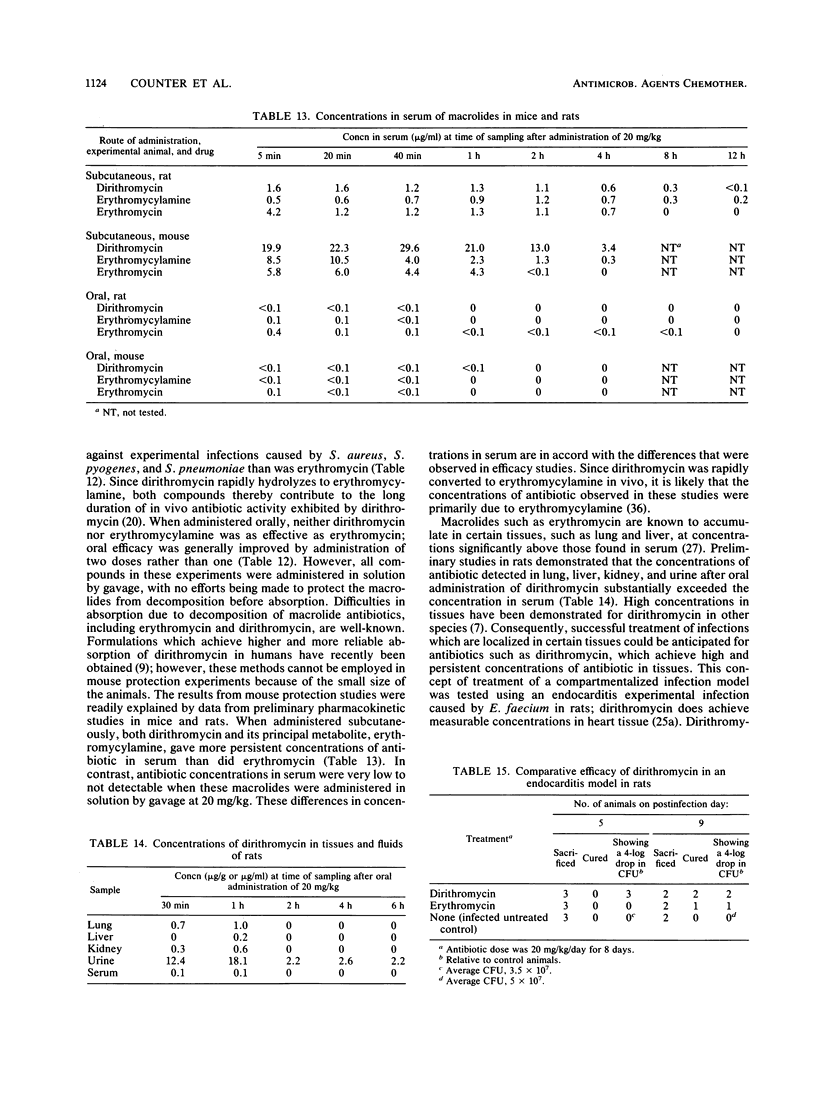
Dirithromycin is a 9-N-11-O-oxazine derivative which is formed by condensation of 9(S)-erythromycylamine with 2-(2-methoxyethoxy)acetaldehyde. Dirithromycin is hydrolyzed, either under acidic conditions or in vivo, to its major active metabolite, 9(S)-erythromycylamine. The antimicrobial spectrum of dirithromycin is similar to that of erythromycin; both antibiotics are active against gram-positive bacteria, Legionella spp., Helicobacter pylori, and Chlamydia trachomatis. Comparable results were obtained for each antibiotic in MIC and MBC determinations and in the potential development of resistance in vitro. The effects of human serum, bacterial growth media, test methodology, and inoculum size on MICs were similar for each antibiotic. In standard mouse protection studies, dirithromycin was more efficacious than erythromycin against experimental infections after subcutaneous administration of antibiotic. These results were consistent with pharmacokinetic studies in rodents, which showed that dirithromycin gave more persistent concentrations of antibiotic in serum and tissues than were achieved with erythromycin. These studies indicate that dirithromycin possesses antimicrobial activity comparable to that of erythromycin in vitro but is more active than erythromycin in vivo, which may be attributable to the persistence of antimicrobial activity in the tissue(s) of the test animals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brittain D. C. Erythromycin. Med Clin North Am. 1987 Nov;71(6):1147–1154. doi: 10.1016/s0025-7125(16)30802-1. [DOI] [PubMed] [Google Scholar]
- Elharrif Z., Mégraud F., Marchand A. M. Susceptibility of Campylobacter jejuni and Campylobacter coli to macrolides and related compounds. Antimicrob Agents Chemother. 1985 Nov;28(5):695–697. doi: 10.1128/aac.28.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Hardy D. J. Comparative in vitro potencies of nine new macrolides. Drugs Exp Clin Res. 1988;14(7):445–451. [PubMed] [Google Scholar]
- Firl J., Prox A., Luger P., Maier R., Woitun E., Daneck K. Epimerization of erythromycin derivatives. J Antibiot (Tokyo) 1990 Oct;43(10):1271–1277. doi: 10.7164/antibiotics.43.1271. [DOI] [PubMed] [Google Scholar]
- García-Rodríguez J. A., García Sánchez J. E., García García M. I., García Sánchez E., Muñoz Bellido J. L. In vitro activities of new oral beta-lactams and macrolides against Campylobacter pylori. Antimicrob Agents Chemother. 1989 Sep;33(9):1650–1651. doi: 10.1128/aac.33.9.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Hanson C. W., Hensey D. M., Beyer J. M., Fernandes P. B. Susceptibility of Campylobacter pylori to macrolides and fluoroquinolones. J Antimicrob Chemother. 1988 Nov;22(5):631–636. doi: 10.1093/jac/22.5.631. [DOI] [PubMed] [Google Scholar]
- Hardy D. J., Hensey D. M., Beyer J. M., Vojtko C., McDonald E. J., Fernandes P. B. Comparative in vitro activities of new 14-, 15-, and 16-membered macrolides. Antimicrob Agents Chemother. 1988 Nov;32(11):1710–1719. doi: 10.1128/aac.32.11.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibwage I. O., Janssen G., Busson R., Hoogmartens J., Vanderhaeghe H., Verbist L. Identification of novel erythromycin derivatives in mother liquor concentrates of Streptomyces erythraeus. J Antibiot (Tokyo) 1987 Jan;40(1):1–6. doi: 10.7164/antibiotics.40.1. [DOI] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: pharmacokinetics and clinical efficacy. Antimicrob Agents Chemother. 1989 Sep;33(9):1419–1422. doi: 10.1128/aac.33.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst H. A., Wind J. A., Leeds J. P., Willard K. E., Debono M., Bonjouklian R., Greene J. M., Sullivan K. A., Paschal J. W., Deeter J. B. Synthesis and structure-activity relationships of new 9-N-alkyl derivatives of 9(S)-erythromycylamine. J Med Chem. 1990 Nov;33(11):3086–3094. doi: 10.1021/jm00173a028. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Bowie W. R., Alexander E. R. In vitro assays of the efficacy of antimicrobial agents in controlling Chlamydia trachomatis propagation. Antimicrob Agents Chemother. 1978 Mar;13(3):441–445. doi: 10.1128/aac.13.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey E. H., Kitchell B. S., Martin L. D., Gerzon K. Antibacterial activity of 9(S)-erythromycylamine-aldehyde condensation products. J Med Chem. 1974 Jan;17(1):105–107. doi: 10.1021/jm00247a018. [DOI] [PubMed] [Google Scholar]
- Nelson J. D. The evolving role of erythromycin in medicine. Pediatr Infect Dis. 1986 Jan-Feb;5(1):118–119. [PubMed] [Google Scholar]
- Periti P., Mazzei T., Mini E., Novelli A. Clinical pharmacokinetic properties of the macrolide antibiotics. Effects of age and various pathophysiological states (Part I). Clin Pharmacokinet. 1989 Apr;16(4):193–214. doi: 10.2165/00003088-198916040-00001. [DOI] [PubMed] [Google Scholar]
- Santoro J., Levison M. E. Rat model of experimental endocarditis. Infect Immun. 1978 Mar;19(3):915–918. doi: 10.1128/iai.19.3.915-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Wilson W. R. Erythromycin: a microbial and clinical perspective after 30 years of clinical use (2). Mayo Clin Proc. 1985 Apr;60(4):271–278. doi: 10.1016/s0025-6196(12)60322-x. [DOI] [PubMed] [Google Scholar]
- Yu K. W., Neu H. C. In vitro activity of dirithromycin (LY 237216) compared with activities of other macrolide antibiotics. Antimicrob Agents Chemother. 1990 Sep;34(9):1839–1842. doi: 10.1128/aac.34.9.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]



