Abstract
Survival of the human pathogen Streptococcus pneumoniae requires a functional mevalonate pathway, which produces isopentenyl diphosphate, the essential building block of isoprenoids. Flux through this pathway appears to be regulated at the mevalonate kinase (MK) step, which is strongly feedback-inhibited by diphosphomevalonate (DPM), the penultimate compound in the pathway. The human mevalonate pathway is not regulated by DPM, making the bacterial pathway an attractive antibiotic target. Since DPM has poor drug characteristics, being highly charged, we propose to use unphosphorylated, cell-permeable prodrugs based on mevalonate that will be phosphorylated in turn by MK and phosphomevalonate kinase (PMK) to generate the active compound in situ. To test the limits of this approach, we synthesized a series of C3-substituted mevalonate analogues to probe the steric and electronic requirements of the MK and PMK active sites. MK and PMK accepted substrates with up to two additional carbons, showing a preference for small substitutents. This result establishes the feasibility of using a prodrug strategy for DPM-based antibiotics in S. pneumoniae and identified several analogues to be tested as inhibitors of MK. Among the substrates accepted by both enzymes were cyclopropyl, vinyl, and ethynyl mevalonate analogues that, when diphosphorylated, might be mechanism-based inactivators of the next enzyme in the pathway, diphosphomevalonate decarboxylase.
Keywords: Isoprenoid pathway, mevalonic acid, phosphomevalonic acid, diphosphomevalonic acid, mevalonate kinase, phosphomevalonate kinase, diphosphomevalonate decarboxylase, prodrug
1. Introduction
Streptococcus pneumoniae kills over a million people a year, mostly children and the elderly, and is the primary cause of community-acquired pneumonia and bacterial meningitis.1, 2 Universal vaccination programs in the U.S. have led to a reduction in the targeted serotypes of S. pneumoniae; however, this effort has resulted in serotype replacement by nonvaccinated strains that show substantial antimicrobial resistance.3 Vancomycin, the antibiotic of last resort, has shown signs of weakness in some strains of S. pneumoniae,4, 5 and widespread resistance to beta-lactams and macrolides, including multiple-drug-resistance, makes treatment difficult and expensive.6–9 The need for new strategies to combat this deadly pathogen in the short term is unequivocal.
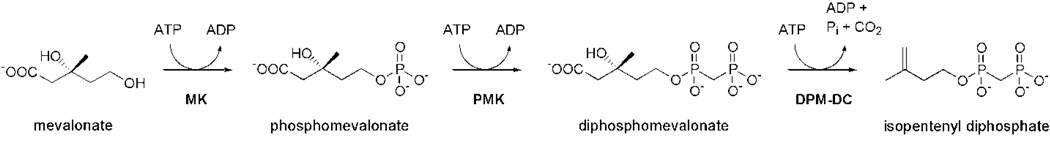
A presently unexploited antibiotic target in S. pneumoniae is the mevalonate pathway (Fig. 1), which is required for the organism to survive in lung and serum.10 This pathway converts mevalonate to isopentenyl diphosphate (IPP), the building block of isoprenoids, in three enzymatic steps, each carried out by a member of the GHMP kinase family. We recently discovered that the first enzyme, mevalonate kinase (MK), is feedback inhibited by diphosphomevalonate (DPM) through binding at a high-affinity allosteric site at concentrations (Ki = 0.5 µM) where the human homolog of MK is unaffected.11 The allosteric site is thought to lie at the MK dimer interface and appears to be absent from the MKs of mammals12 and some bacteria13, raising the possibility that the effects of DPM inhibition would be limited to few species.
Figure 1.
The mevalonate pathway. MK, mevalonate kinase; PMK, phosphomevalonate kinase; DPM-DC, diphosphomevalonate decarboxylase.
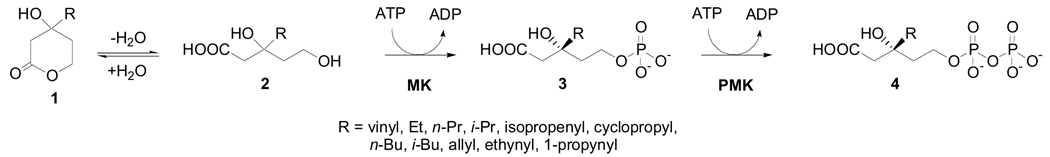
DPM is not expected to cross the cell membrane because of its high negative charge (4-); therefore, we have focused on a prodrug strategy in which mevalonate analogues are taken up by cells and phosphorylated by the mevalonate pathway enzymes to generate inhibitors in situ. An analogous strategy has been used to show that 6-fluoromevalonate inhibits isoprenoid biosynthesis in cultured human cells.14, 15 This result is consistent with the compound crossing the cell membrane and being converted in situ to the phosphorylated form; the diphosphorylated compound (6-fluoro-5-diphosphomevalonate) is a potent inhibitor of the human diphosphomevalonate decarboxylase (DPM-DC).16 The present study seeks to expand the repertoire of DPM prodrugs with a series of compounds elaborating the C3-methyl group with hydrocarbons of variable length, branching, and saturation (Scheme 1). Several mevalonate analogues were designed with an eye toward mechanism-based inhibition of DPM-DC, in addition to binding to the MK allosteric site, in a dual-acting antibiotic approach. In the present view of the DPM-DC transition state, substantial positive charge forms on C3 during turnover.17 To exploit this mechanism for inactivation, we synthesized DPM analogues (vinyl-, allyl-, ethynyl-, and cyclopropyl-substituted) that delocalize this positive charge through resonance, transiently forming a strong electrophile that could undergo attack by a protein nucleophile in the vicinity of the putative substrate-binding pocket of DPM-DC.18 An advantage of this strategy is that the potency of the antibiotic is enhanced because the inhibitor acts at two points in the same pathway. Because the human mevalonate pathway enzymes are closely related to those from S. pneumoniae, the specificity of antibiotics directed against DPM-DC is a concern. Whereas a functional mevalonate pathway is required for survival of the bacterium, the human pathway can be suppressed with minimal side-effects with the use of cholesterol-lowering drugs (statins, HMG-CoA reductase) and antiproliferative agents (bisphosphonates, farnesyl transferase).19, 20 Therapeutic benefits that arise by controlling flux through the human mevalonate pathway suggest that DPM-DC inhibitors may have additional clinical uses. In the present study, we assess the limitations on side chains for a prodrug strategy; binding of these analogues to the MK allosteric site and DPM-DC inactivation will be the subject of a future study.
Scheme 1.
2. Chemistry
The general synthetic route to the racemic vinyl, ethyl, n-propyl, n-butyl, i-butyl, 2-propenyl, allyl, ethynyl, and 1-propynyl mevalonate lactone analogues (1a–e, g, i–k) is shown in Scheme 2. Selective protection of the ketone functional group with ethylene glycol in the presence of BF3-OEt2, followed by LAH reduction provided diol 7 in a high yield. Next, selective protection of diol 7 using TBDPSCl gave mono-protected diol 8 in 86% yield. Then, the cyclic ketal group of 8 was readily cleaved using PPTS to give 9. The other hydroxyl group of 9 was protected as a MOM ether to give bis-protected diol 10 in a quantitative yield. Diol 10 was treated with Grignard reagents in the presence of CeCl3 21 to generate tertiary alcohols 11a–e, 11g, 11i–k in good yields (77–99%). Selective deprotection of MOM group in 11a–g, 11i–k, followed by an oxidation of the resulting primary alcohol using IBX and NaClO2 provided the corresponding carboxylic acids 13a–e, 13g, 13i–k. Finally, desilylation of the TBDPS group in 13a–e, 13g, 13i–k coincidently led to an intramolecular cyclization to give the desired lactones 1a–e, 1g, 1i–k.
Scheme 2.
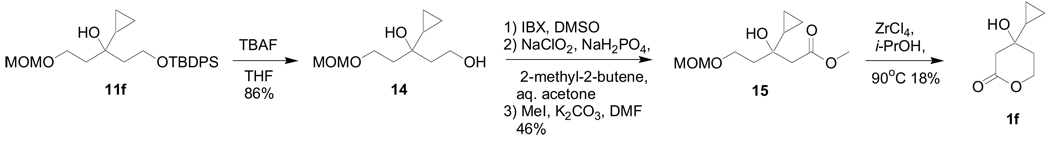
The cyclopropyl analogue (1f) had to be prepared by a slightly different route starting from the diprotected alcohol intermediate (11f) because of problems with the MOM ether deprotection step (Scheme 3).
Scheme 3.
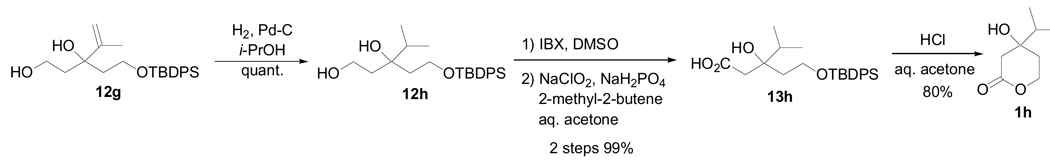
The bulky isopropyl group could not be installed using standard Grignard reaction conditions to make 11h, either with or without CeCl3. Consequently, the 2-propenyl intermediate (12g) was hydrogenated to the isopropyl analogue (12h), and the synthesis of 1h was completed as usual (Scheme 4).
Scheme 4.
To carry out the enzyme assays it was necessary to hydrolyze lactones 1a–k prior to incubation with the enzyme. Hydrolysis was carried out smoothly in aqueous 2.0 M LiOH at room temperature for 1 hour. It was found that re-lactonization occurred in water (t1/2 = 14 days), methanol (t1/2 = 7 days), or neat (t1/2 = 3 days), but was suppressed in 50 mM Hepes/K+ buffer containing 1 mM MgCl2 at pH 8.0 (stable at least one week), which is the buffer used to assay MK and phosphomevalonate kinase (PMK).
3. Results
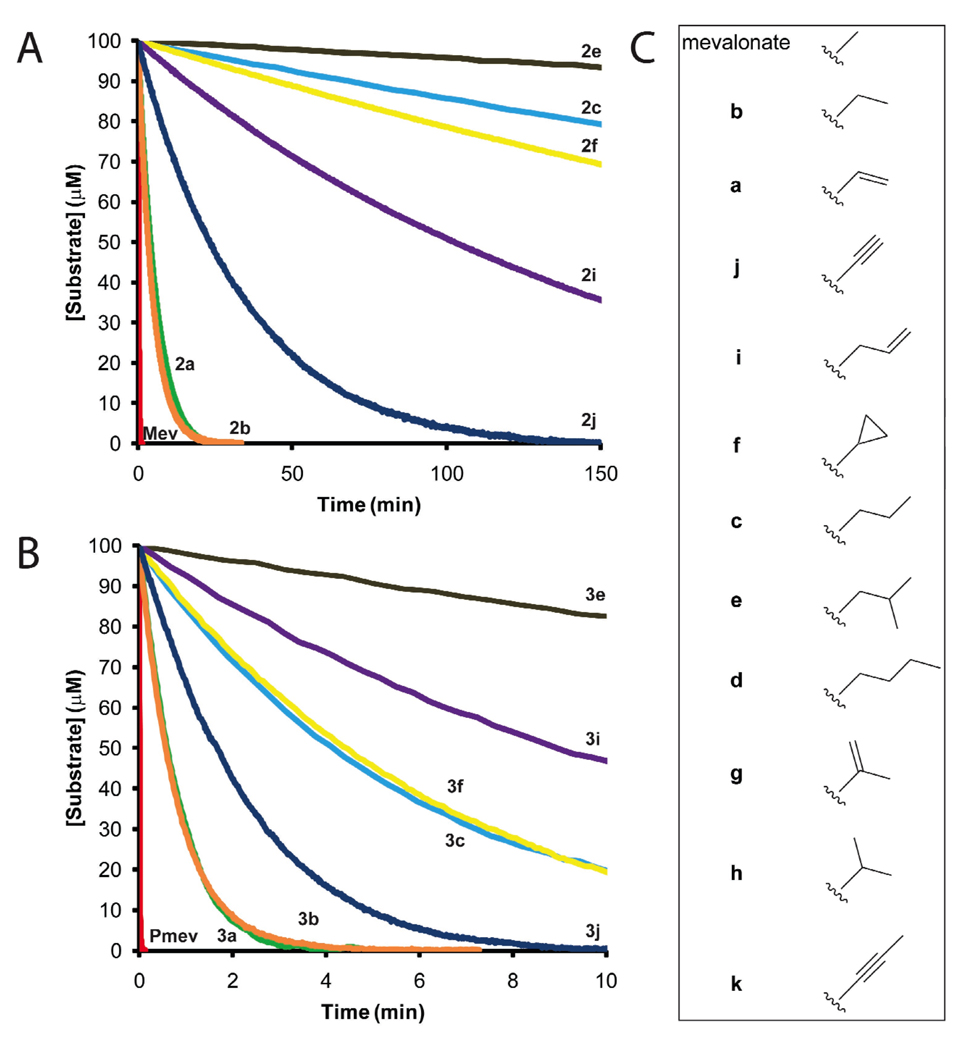
To assess their viability as substrates of the enzymes in the mevalonate pathway, each of the mevalonate analogues was tested as a substrate for both MK and PMK. These activities are imperative for in vivo conversion of the alcohols to the corresponding diphosphorylated analogues. Reaction progress was monitored optically at 386 nm using a well-established pyruvate kinase/lactate dehydrogenase coupled enzyme system in which the ADP produced by phosphorylation of the mevalonate analogue is linked stoichiometrically (1:1) to NADH oxidation.22 Reaction progress curves (Fig. 2) are the average of three assays done under identical conditions. The primary enzyme was varied from analogue to analogue to allow each progress curve to finish in a reasonable amount of time (see Fig. 2 Legend). To compare the activity of compounds, the data were normalized to a 1 µM enzyme concentration, since activity was linear with enzyme concentration (data not shown). MK reactions were initiated by addition of analogue (Fig. 2A). The MK reactions were allowed to reach completion before adding PMK, resulting in the progress curves shown in Fig. 2B. The endpoint of the MK reaction occurred at 50% of the initial concentration of the racemic compound, as would be expected for conversion of a single enantiomer.
Figure 2.
Mevalonate analogues as substrates for MK and PMK. Reaction progress curves for mevalonate kinase (A) and phosphomevalonate kinase (B). Color coding is identical in panels A and B. Reaction conditions: MK (Mev, 0.050 µM; 2a, 1 µM; 2b , 1 µM; 2c, 20 µM; 2e, 20 µM; 2f, 20 µM; 2i, 20 µM; 2j, 10 µM) or PMK (Pmev, 0.005 µM; 3a, 0.075 µM; 3b, 0.150 µM; 3c, 1.0 µM; 3e, 2.0 µM; 3f, 0.50 µM; 3i, 2 µM; 3j, 0.10 µM), pyruvate kinase (10.0 units/ml), lactate dehydrogenase (20.0 units/ml), ATP (4.0 mM), MgCl2 (5.0 mM), NADH (0.82 mM), phosphoenolpyruvate (1.0 mM), KCl (50 mM), β-mercaptoethanol (20.0 mM), compounds 2a–2k (200 µM, racemic mixture) or 3a–c, e, f, i, j (100 µM), Hepes/K+ (50 mM, pH 8.0), T = 25 ± 2 °C. The activities of compounds 2d, 2g, 2h and 2k with MK were indistinguishable from background. (C) Mevalonate analogues (R-groups) listed in the order of their reaction rate with MK.
4. Discussion
4.1 Synthetic Approach
The yields of all of the steps in the synthetic route were excellent (see Scheme 2–Scheme 4 for yields). The main complications came in the selection of protecting groups for the terminal alcohols. Initially, p-methoxybenzyl was selected as one of the alcohol protecting groups, but it caused problems later in the synthesis when it was removed; the methoxymethyl (MOM) protecting group was much more suitable. Steric hindrance seems to be the main cause for failure of the Grignard reaction; neither the isopropyl nor the tert-butyl analogue could be made directly by this route. However, the isopropyl analogue was prepared by catalytic hydrogenation of the 2-propenyl analogue.
4.2 Validation of the Prodrug Strategy
To evaluate their potential as prodrugs, eleven mevalonate analogues were tested as substrates for MK and PMK. Seven of the analogues (a, b, c, e, f, i, and j) proved to be substrates for both enzymes, and are, therefore, viable prodrug candidates. The remaining four analogues (d, g, h, and k) were inactive with MK (< 0.0025% of the activity of mevalonate).
The progress-curve based comparison of the reactivity of each active analogue reveals an inverse relationship between reaction rate and the size of the R-group (Figure 2). For example, increasing the length of the mevalonate C3 methyl group by a single methylene (b) has only a small effect on the reaction rate with MK and PMK. However, a two-carbon increase (c) significantly reduces reactivity, and a three-carbon increase (e) profoundly decreases the reactivity. The geometry of the R-group also has a dramatic effect on reactivity; branched compounds are excluded (cf., 2g vs. 2i, or 2h vs. 2f). The similar trend in reactivity for both enzymes is not surprising given their similar GHMP-kinase active-site architectures.23,24 The changes in reaction rate may be due to alterations in Vmax and/or Km, and a full kinetic analysis of seven compounds with the three mevalonate pathway enzymes (as substrates, inhibitors, and allosteric regulators) will be reported in due course.
5. Conclusions
We have demonstrated the feasibility of using C3-substituted mevalonates as prodrugs to generate DPM analogues in situ. By knowing which analogues are suitable substrates for these enzymes, we can synthesize the appropriate DPM analogues for testing as allosteric inhibitors of MK as well as potential inhibitors and mechanism-based inactivators of DPM-DC, the next enzyme in the isoprenoid biosynthetic pathway.
6. Experimental procedures
4.1 General Methods
Flash chromatography was performed with 230–400 mesh silica gel. 1H-NMR, 13C-NMR and 31P-NMR spectra were obtained using Varian 300, 400 and 500 MHz spectrometers. NMR spectra were recorded in ppm (δ) relative to tetramethylsilane (δ = 0.00) as an internal standard unless stated otherwise and are reported as follows; chemical shift, multiplicity (br = broad, s = singlet, t = triplet, q = quartet, m = multiplet), coupling constant, and integration. Solvents and liquid reagents were transferred using hypodermic syringes. All other reagents and solvents used were reagent grade. All glassware was dried in an oven at 150 °C prior to use. Tetrahydrofuran (THF) and diethyl ether (Et2O) were distilled from sodium/benzophenone. Methylene chloride and triethylamine were dried over calcium hydride prior to use. Reactions were monitored by thin layer chromatography (TLC) with 0.25 mm E. Merck pre-coated silica gel plate (60 F254). Small and medium scale purifications were performed by flash chromatography. Pyruvate kinase and lactate dehydrogenase were obtained from Roche Applied Science (Indianapolis, IN). Kinetic measurements of enzymatic reactions were made using a Cary 100 or 400 UV/Vis spectrophotometer (Varian Inc., Palo Alto, CA).
6.2. 1,5-Dihydroxy-3-pentanone-ethylideneacetal (7)
A mixture of diethyl acetone-1,3-dicarboxylate (5, 4.04 g, 20.0 mmol), ethylene glycol (4.46 mL, 80.0 mmol), and BF3 · Et2O (3.80 mL, 30.0 mmol) in CH2Cl2 (40 mL) was stirred at 0 °C for 90 min and room temperature for 46 h. H2O was added to the mixture at 0 °C, and the mixture was stirred at 0 °C for 30 min and extracted with AcOEt. The organic layer was washed with H2O, brine, dried with Na2SO4, and evaporated. The residue was treated again as mentioned above and worked up the same way. A mixture of the crude product and LiAlH4 (1.0 M in THF, 40 mL, 40 mmol) in THF (150 mL, HPLC grade) was stirred at 0 °C for 1 h and room temperature for 15 h, and aqueous 2.0 M NaOH was added to the mixture at 0 °C. The mixture was stirred at 0 °C for 30 min, and the resulting gel was filtered off with Celite and washed with acetone. The combined filtrate and washings were evaporated, and the residue was co-evaporated with i-PrOH (× 2) and purified by silica gel column chromatography (5% MeOH in AcOEt) to give 7 (3.13 g, 96%) as a colorless oil: 1H-NMR (300 MHz, CDCl3) δ 4.06 (s, 4H, acetal-CH2 × 2), 3.70–3.77 (m, 4H, CH2OH × 2), 2.56 (br-s, 2H, OH × 2), 1.97–2.05 (m, 4H, CH2CH2OH × 2) 13C-NMR (125 MHz, CDCl3) δ 112.27, 64.78, 58.67, 38.32. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C7H14NaO4: 185.07843. Found: 185.07876.
6.3. 1-O-(tert-Butyldiphenylsilyl)-1,5-dihydroxy-3-pentanone-ethylideneacetal (8)
A mixture of 7 (2.61 g, 16.1 mmol) and NaH (964 mg, 24.1 mmol) in THF (150 mL, HPLC grade) was stirred at 0 °C for 1 h, and TBDPSCl (6.27 mL, 24.1 mmol) was added at 0 °C. The mixture was stirred at 0 °C for 30 min and room temperature for 69 h, and AcOH (500 µL) was added at 0 °C. The mixture was stirred at 0 °C for 10 min, and solid NaHCO3 and MeOH were added. The mixture was stirred at 0 °C for 30 min, and the suspension was filtered through Celite and washed with acetone. The combined filtrate and washing were evaporated, and the residue was purified by silica gel column chromatography (25% AcOEt in hexane) to give 8 (5.57 g, 86%) as a colorless oil: 1H-NMR (300 MHz, CDCl3) δ 7.37–7.68 (m, 10H, Ph × 2), 3.69–3.98 (m, 8H, acetal-CH2 × 2, CH2OH, CH2OTBDPS), 2.74–2.78 (m, 1H, OH), 1.91–1.95 (m, 4H, CH2CH2O × 2), 1.04 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 135.52, 133.55, 129.63, 127.65, 111.09, 64.50, 59.86, 58.76, 39.29, 38.61, 26.76, 19.04. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C23H32NaO4Si: 423.19621. Found: 423.19611.
6.4. 1-O-(tert-Butyldiphenylsilyl)-1,5-dihydroxy-3-pentanone (9)
A mixture of 8 (2.45 g, 6.11 mmol) and pyridinium p-toluenesulfonate (PPTS, 6.14 g, 24.4 mmol) in acetone (55 mL) and H2O (5.5 mL) was stirred with reflux for 4 h. The mixture was diluted with AcOEt and washed with H2O, brine, dried with Na2SO4, and evaporated. The residue was purified by silica gel column chromatography (30% AcOEt in hexane) to give 9 (2.09 g, 96%) as a colorless oil: 1H-NMR (300 MHz, CDCl3) δ 7.37–7.66 (m, 10H, Ph × 2), 3.84–3.97 (m, 4H, CH2OH, CH2OTBDPS), 2.63–2.75 (m, 4H, CH2CH2O × 2), 2.49 (br-s, 1H, OH), 1.03 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 210.61, 135.44, 133.15, 129.70, 127.66, 59.48, 57.71, 45.82, 45.26, 26.66, 19.03. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C21H28NaO3Si: 379.16999. Found: 379.16975.
6.5. 1-O-(tert-Butyldiphenylsilyl)-5-O-methoxymethyl-1,5-dihydroxy-3-pentanone (10)
A mixture of 9 (114 mg, 320 µmol), MOMCl (49 µl, 640 µmol), and DIPEA (223 µl, 1.28 mmol) in CH2Cl2 (3 mL) was stirred at room temperature for 90 min, and then MeOH was added. The mixture was stirred for 10 min and evaporated. The residue was partitioned between AcOEt and ice cooled aqueous 0.1 M HCl, and the organic layer was washed with H2O, brine, dried with Na2SO4, and evaporated. The residue was dried under vacuum to give 10 (128 mg, 100%) as a colorless oil: 1H-NMR (400 MHz, CDCl3) δ 7.37–7.66 (m, 10H, Ph × 2), 4.60 (s, 2H, MOM-CH2), 3.79–3.97 (m, 4H, CH2OMOM, CH2OTBDPS), 3.34 (s, 3H, MOM-CH3), 2.66–2.76 (m, 4H, CH2CH2O × 2), 1.03 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 207.60, 135.41, 133.25, 129.58, 127.58, 96.39, 62.33, 59.40, 55.10, 45.79, 43.38, 26.62, 18.99. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C23H32NaO4Si: 423.19621. Found: 423.19487.
6.6 Grignard reaction
A mixture of ketone 10, Grignard reagent (2.0 eq. for 11i, 2.5 eq. for 11g, 3.0 eq. for 11a–d, f, j, k, 4.0 eq. for 11e) and CeCl3 (1.5 eq.) in dry THF (as a 1 mM solution) was stirred at −78 °C for 3–6 h (except for 11j and 11k) or at 0 °C for 2 h and room temperature for 4 h (for 11j and 11k). Aqueous saturated NH4Cl was added to the mixture at −78 °C (except for 11j and 11k) or at room temperature (for 11j and 11k), and the mixture was stirred at room temperature for 30 min and extracted with AcOEt. The organic layer was washed with H2O, brine, dried with Na2SO4, and evaporated. The residue was purified by silica gel column chromatography (5% AcOEt in hexane for 11g, 10% AcOEt in hexane except for 11g) to give the corresponding Grignard products.
6.7. 1-O-(tert-Butyldiphenylsilyl)-5-O-methoxymethyl-3-vinylpentan-1,3,5-triol (11a)
Compound 11a (2.02 g, 94%) was obtained as a colorless oil from 10 (2.00 g, 5.00 mmol): 1H-NMR (300 MHz, CDCl3) δ 7.36–7.68 (m, 10H, Ph × 2), 5.75–5.84 (m, 1H, CH2=CH), 5.41–5.47, 5.19–5.23 (each m, each 1H, CH2=CH), 4.60 (s, 2H, MOM-CH2), 4.19 (s, 1H, OH), 3.59–3.94 (m, 4H, CH2OMOM, CH2OTBDPS), 3.35 (s, 3H, MOM-CH3), 1.62–2.06 (m, 4H, CH2CH2O × 2), 1.04 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 142.51, 135.53, 135.51, 132.80, 129.81, 129.79, 127.75, 127.71, 113.80, 96.51, 75.26, 64.20, 61.49, 55.26, 41.54, 40.59, 26.72, 18.95. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C25H36NaO4Si: 451.22751. Found: 451.22721.
6.8. 1-O-(tert-Butyldiphenylsilyl)-3-ethyl-5-O-methoxymethylpentan-1,3,5-triol (11b)
Compound 11b (1.14 g, 87%) was obtained as a colorless oil from 10 (1.21 g, 3.02 mmol): 1H-NMR (300 MHz, CDCl3) δ 7.38–7.69 (m, 10H, Ph × 2), 4.61 (s, 2H, MOM-CH2), 3.65–3.88 (m, 5H, CH2OMOM, CH2OTBDPS, OH), 3.36 (s, 3H, MOM-CH3), 1.76–1.85 (m, 4H, CH2CH2O × 2), 1.52–1.58 (m, 2H, Et-CH2), 1.05 (s, 9H, t-Bu), 0.86 (t, 3H, Et-CH3, J = 7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ 135.48, 132.92, 129.73, 127.68, 96.42, 73.81, 64.18, 61.09, 55.24, 39.32, 37.59, 31.86, 26.69, 18.92, 8.19. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C25H38NaO4Si: 453.24316. Found: 453.24342.
6.9. 1-O-(tert-Butyldiphenylsilyl)-3-(2-methoxymethyloxyethyl)hexan-1,3,-diol (11c)
Compound 11c (2.04 g, 92%) was obtained as a colorless oil from 10 (2.00 g, 5.00 mmol): 1H-NMR (300 MHz, CDCl3) δ 7.38–7.69 (m, 10H, Ph × 2), 4.60 (s, 2H, MOM-CH2), 3.65–3.87 (m, 5H, CH2OMOM, CH2OTBDPS, OH), 3.36 (s, 3H, MOM-CH3), 1.76–1.85 (m, 4H, CH2CH2O × 2), 1.45–1.50 (m, 2H, CH3CH2CH2), 1.26–1.41 (m, 2H, CH3CH2CH2), 1.05 (s, 9H, t-Bu), 0.89 (t, 3H, CH3CH2CH2, J = 7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ 135.54, 132.96, 132.93, 129.79, 127.73, 96.47, 73.66, 64.26, 61.17, 55.29, 41.93, 39.86, 38.24, 26.76, 18.96, 17.05, 14.69. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C26H40NaO4Si: 467.25881. Found: 467.25863.
6.10. 1-O-(tert-Butyldiphenylsilyl)-3-(2-methoxymethyloxyethyl)heptan-1,3,-diol (11d)
Compound 11d (248 mg, quant.) was obtained as a colorless oil from 10 (200 mg, 500 µmol): 1H-NMR (400 MHz, CDCl3) δ 7.38–7.69 (m, 10H, Ph × 2), 4.60 (s, 2H, MOM- CH2), 3.84–3.89, 3.63–3.70 (each m, each 2H, CH2OTBDPS, CH2OMOM), 3.78 (s, 1H, OH), 3.36 (s, 3H, MOM- CH3), 1.73–1.88 (m, 4H, CH2CH2O × 2, CH3CH2CH2), 1.45–1.51 (m, 2H, CH3CH2CH2CH2), 1.21–1.29 (m, 4H, CH3CH2CH2CH2, CH3CH2CH2CH2), 1.05 (s, 9H, t-Bu), 0.89 (t, 3H, CH3CH2CH2CH2, J = 6.4 Hz); 13C-NMR (100 MHz, CDCl3) δ 135.54, 132.96, 132.94, 129.79, 127.74, 96.47, 73.68, 64.26, 61.18, 55.29, 39.87, 39.25, 38.23, 26.76, 25.99, 23.26, 18.96, 14.05. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C27H42NaO4Si: 481.27446. Found: 481.27322.
6.11. 1-O-(tert-Butyldiphenylsilyl)-3-(2-methoxymethyloxyethyl)-5-methylhexan-1,3,-diol (11e)
Compound 11e (2.73 g, 85%) was obtained as a colorless oil from 10 (2.80 g, 7.00 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.38–7.69 (m, 10H, Ph × 2), 4.60 (s, 2H, MOM- CH2), 3.84–3.87, 3.63–3.71 (each m, each 2H, CH2OTBDPS, CH2OMOM), 3.35 (s, 3H, MOM-CH3), 1.71–1.89 (m, 5H, CH2CH2O × 2, (CH3)2CHCH2), 1.39–1.42 (m, 2H, (CH3)2CHCH2), 1.05 (s, 9H, t-Bu), 0.92–0.96 (m, 6H, (CH3)2CHCH2); 13C-NMR (100 MHz, CDCl3) δ 135.44, 132.83, 132.82, 129.69, 127.64, 96.36, 74.10, 64.30, 61.16, 55.18, 47.90, 40.32, 38.81, 26.66, 24.68, 23.79, 18.86. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C27H42NaO4Si: 481.27446. Found: 481.27385.
6.12. 1-O-(tert-Butyldiphenylsilyl)-5-O-methoxymethyl-3-cyclopropyl-pentan-1,3,5-triol (11f)
Compound 11f (0.43 g, 89%) was obtained as a colorless oil from 10 (0.96 g, 3.90 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.67–7.70 (m, 4H, Ph), 7.38–7.44 (m, 6H, Ph), 4.61 (s, 2H, MOM-CH2), 3.83–3.89, 3.75–3.81 (each m, each 2H, CH2OTBDPS, CH2OMOM), 3.76 (s, 1H, OH), 3.36 (s, 3H, MOM-CH3), 1.93-1.87 (m, 2H, CH2CH2O), 1.78-1.75 (m, 2H, CH2CH2O), 1.04 (s, 9H, t-Bu), 0.69 (m, 1H, CH of cyclopropyl), 0.52-0.43 (m, 2H, CH2 of cyclopropyl), 0.34-0.32 (d, 2H, CH2 of cyclopropyl , J=8.5 Hz): 13C-NMR (125 MHz, CDCl3) δ 135.74, 133.14, 129.99, 127. 93, 96.71, 71.69, 64.82, 61.86, 60.56, 55.49, 42.25, 41.30, 26.98, 19.13. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C26H38NaO4Si: 465.24316. Found: 465.24308.
6.13. 1-O-(tert-Butyldiphenylsilyl)-5-O-methoxymethyl-3-(1-methylvinyl)pentan-1,3,5-triol (11g)
Compound 11g (2.52 g, 85%) was obtained as a colorless oil from 10 (2.67 g, 6.66 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.37–7.67 (m, 10H, Ph × 2), 5.24, 4.99 (each s, each 1H, CH2=C), 4.60 (s, 2H, MOM-CH2), 4.28 (br-s, 1H, OH), 3.53–3.87 (m, 4H, CH2OTBDPS, CH2OMOM), 3.35 (s, 3H, MOM-CH3), 1.71–2.03 (m, 4H, CH2CH2O × 2), 1.61 (s, 3H, Me), 1.03 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 147.11, 135.43, 135.41, 132.72, 132.70, 129.68, 129.65, 127.63, 127.58, 112.20, 96.46, 77.34, 64.06, 61.32, 55.13, 39.89, 38.72, 26.60, 19.53, 18.84. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C26H38NaO4Si: 465.24316. Found: 465.24369.
6.14. 1-O-(tert-Butyldiphenylsilyl)-3-(2-methoxymethyloxyethyl)-5-hexen-1,3,-diol (11i)
Compound 11i (1.78 g, quant.) was obtained as a light yellow oil from 10 (1.60 g, 4.00 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.38–7.69 (m, 10H, Ph × 2), 5.81–5.83 (m, 1H, CH2=CH), 5.02–5.08 (m, 2H, CH2=CH), 4.60 (s, 2H, MOM-CH2), 3.83–3.91, 3.69–3.73 (each m, each 2H, CH2OTBDPS or CH2OMOM), 3.35 (s, 3H, MOM-CH3), 2.31–2.32 (m, 2H, CH2=CHCH2), 1.75–1.87 (m, 4H, CH2CH2O × 2), 1.05 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 135.42, 134.06, 132.80, 129.71, 127.65, 117.79, 96.32, 73.27, 63.93, 60.94, 55.14, 44.22, 39.81, 38.21, 26.67, 18.86. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C26H38NaO4Si: 465.24316. Found: 465.24243.
6.15. 1-O-(tert-Butyldiphenylsilyl)-3-ethynyl-5-O-methoxymethylpentan-1,3,5-triol (11j)
Compound 11j (1.55 g, 79%) was obtained as a light yellow oil from 10 (1.83 g, 4.58 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.36–7.73 (m, 10H, Ph × 2), 4.80 (s, 1H, OH), 4.63 (s, 2H, MOM-CH2), 4.20–4.25, 3.81–3.97 (each m, 1H and 3H, CH2OTBDPS or CH2OMOM), 3.37 (s, 3H, MOM-CH3), 2.50 (s, 1H, ethynyl-H), 2.06–2.12, 1.97–2.02, 1.83–1.87 (each m, 2H, 1H, and 1H, CH2CH2O × 2), 1.05 (s, 9H, t-Bu); 13C-NMR (125 MHz, CDCl3) δ 135.49, 135.42, 132.62, 129.73, 129.71, 127.66, 127.61, 96.36, 85.44, 73.04, 70.34, 64.43, 61.79, 55.19, 42.64, 41.43, 26.63, 18.87. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C25H34NaO4Si: 449.21186. Found: 449.21144.
6.16. 1-O-(tert-Butyldiphenylsilyl)-3-(2-methoxymethyloxyethyl)-4-hexyn-1,3,-diol (11k)
Compound 11k (1.36 g, 77%) was obtained as a colorless oil from 10 (1.60 g, 4.00 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.38–7.74 (m, 10H, Ph × 2), 4.63 (s, 2H, MOM-CH2), 4.18–4.23, 3.79–3.95 (each m, 1H and 3H, CH2OTBDPS or CH2OMOM), 3.37 (s, 3H, MOM-CH3), 2.03–2.08, 1.92–1.96, 1.78–1.83 (each m, 2H, 1H, and 1H, CH2CH2O × 2), 1.85 (s, 3H, Me), 1.05 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 135.52, 135.47, 132.80, 132.71, 129.72, 129.68, 127.67, 127.64, 96.37, 81.00, 80.82, 70.52, 64.72, 62.03, 55.12, 43.03, 41.83, 26.65, 18.89, 3.47. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C26H36NaO4Si: 463.22751. Found: 463.22726.
6.17. Deprotection of the MOM group
A mixture of the Grignard products and ZrCl4 (0.5 eq.) in i-PrOH (as a 1 mM solution) was stirred at 90 °for 30 min. The mixture was partitioned between AcOEt and H2O, and the organic layer was washed with brine, dried with Na2SO4, and evaporated. The residue was purified by silica gel column chromatography (25% AcOEt in hexane) to give the corresponding alcohols.
6.18. 1-O-(tert-Butyldiphenylsilyl)-3-vinylpentan-1,3,5-triol (12a)
Compound 12a (1.42 g, 78%) was obtained as a colorless oil from 11a (2.02 g, 4.72 mmol): 1H-NMR (300 MHz, CDCl3) δ 7.39–7.68 (m, 10H, Ph × 2), 5.76 (dd, 1H, CH2=CH, Jtrans = 22.8 Hz, Jcis = 13.6 Hz), 5.56 (dd, 1H, CH2=CH (trans), Jvicinal = 22.8 Hz, Jgeminal = 1.6 Hz), 5.33 (dd, 1H, CH2=CH (cis), Jvicinal = 13.6 Hz, Jgeminal = 1.6 Hz), 4.72 (s, 1H, OH), 3.73–3.96 (m, 4H, CH2OH, CH2OTBDPS), 3.32 (br-s, 1H, OH), 1.52–2.14 (m, 4H, CH2CH2O × 2), 1.04 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 142.01, 135.45, 135.42, 132.26, 132.22, 129.91, 129.87, 127.79, 127.71, 114.72, 78.04, 61.90, 59.50, 41.96, 40.71, 26.60, 18.82. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C23H32NaO3Si: 407.20129. Found: 407.20051.
6.19. 1-O-(tert-Butyldiphenylsilyl)-3-ethylpentan-1,3,5-triol (12b)
Compound 12b (1.09 g, 68%) was obtained as a light yellow oil from 11b (1.79 g, 4.17 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.40–7.69 (m, 10H, Ph × 2), 3.81–3.93 (m, 4H, CH2OTBDPS, CH2OH), 1.65–1.93 (m, 6H, CH2CH2O × 2, Et- CH2), 1.05 (s, 9H, t-Bu), 0.84 (t, 3H, Et- CH3, J = 7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ 135.52, 132.51, 132.43, 129.97, 129.96, 127.85, 127.84, 76.34, 61.41, 59.50, 38.74, 38.20, 31.68, 26.73, 18.92, 8.61. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C23H34NaO3Si: 409.21694. Found: 409.21610.
6.20. 1-O-(tert-Butyldiphenylsilyl)-3-(2-hydroxyethyl)hexan-1,3,-diol (12c)
Compound 12c (1.20 g, 67%) was obtained as a light yellow oil from 11c (1.99 g, 4.47 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.40–7.69 (m, 10H, Ph × 2), 4.31 (s, 1H, OH), 3.82–3.93 (m, 4H, CH2OTBDPS, CH2OH), 1.63–1.94 (m, 6H, CH2CH2O × 2, CH3CH2CH2), 1.16–1.31 (m, 2H, CH3CH2CH2), 1.05 (s, 9H, t-Bu), 0.91 (t, 3H, CH3CH2CH2, J = 7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ 135.54, 132.49, 132.40, 129.98, 127.84, 76.39, 61.44, 59.57, 41.68, 39.39, 38.73, 26.75, 18.92, 17.50, 14.67. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H36NaO3Si: 423.23259. Found: 423.23204.
6.21. 1-O-(tert-Butyldiphenylsilyl)-3-(2-hydroxyethyl)heptan-1,3,-diol (12d)
Compound 12d (1.70 g, 90%) was obtained as a colorless oil from 11d (2.10 g, 4.57 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.40–7.70 (m, 10H, Ph × 2), 3.83–3.93 (m, 4H, CH2OTBDPS, CH2OH), 1.58–1.95 (m, 6H, CH2CH2O × 2, CH3CH2CH2CH2), 1.28–1.33 (m, 4H, CH3CH2CH2CH2, CH3CH2CH2CH2), 1.05 (s, 9H, t-Bu), 0.88–0.92 (m, 3H, CH3CH2CH2CH2); 13C-NMR (100 MHz, CDCl3) δ 135.52, 132.48, 132.37, 129.96, 127.85, 127.83, 76.42, 61.44, 59.55, 39.37, 38.96, 38.70, 26.73, 26.41, 23.23, 18.91, 14.00. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C25H38NaO3Si: 437.24824. Found: 437.24760.
6.22. 1-O-(tert-Butyldiphenylsilyl)-3-(2-hydroxyethyl)-5-methylhexan-1,3,-diol (12e)
Compound 12e (1.96 g, 82%) was obtained as a colorless oil from 11e (2.65 g, 5.78 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.40–7.69 (m, 10H, Ph × 2), 3.86–3.97 (m, 4H, CH2OTBDPS, CH2OH), 1.65–1.99 (m, 5H, CH2CH2O × 2, (CH3)2CHCH2), 1.48–1.60 (m, 2H, (CH3)2CHCH2), 1.05 (s, 9H, t-Bu), 0.97, 0.92 (each d, each 3H, (CH3)2CHCH2, each, J =6.8 Hz); 13C-NMR (100 MHz, CDCl3) δ 135.55, 132.46, 132.37, 129.99, 127.87, 127.85, 76.86, 61.47, 59.63, 47.89, 40.07, 39.41, 26.74, 24.79, 24.75, 24.06, 18.92. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C25H38NaO3Si: 437.24824. Found: 437.24788.
6.23 1-O-(tert-Butyldiphenylsilyl)-3-(1-methylvinyl)pentan-1,3,5-triol (12g)
Compound 12g (251 mg, 80%) was obtained as a light yellow oil from 11g (349 mg, 790 µmol): 1H-NMR (400 MHz, CDCl3) δ 7.38–7.68 (m, 10H, Ph × 2), 5.41, 5.13 (each s, each 1H, CH2=C), 4.84 (br-s, 1H, OH), 3.71–3.87 (m, 4H, CH2OTBDPS, CH2OH), 3.37 (br-s, 1H, OH), 1.70–2.11 (m, 4H, CH2CH2O × 2), 1.66 (s, 3H, Me), 1.04 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 146.83, 135.41, 135.38, 132.23, 132.17, 129.86, 129.81, 127.74, 127.70, 113.13, 80.41, 61.90, 59.56, 40.16, 38.96, 26.55, 19.50, 18.78. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H34NaO3Si: 421.21694. Found: 437.21606.
6.24. 1-O-(tert-Butyldiphenylsilyl)-3-hydroxy-3-(1-methylethyl)pentan-1,5-diol (12h)
A mixture of 12g (1.47 g, 3.68 mmol) and Pd-C (400 mg) in i-PrOH (35 mL) was stirred at room temperature for 18 h under a hydrogen atmosphere. The mixture was filtered off, and the filtrate was evaporated. The residue was purified by silica gel column chromatography (33% AcOEt in hexane) to give 12h (1.51 g, quant.) as a colorless oil: 1H-NMR (400 MHz, CDCl3) δ 7.40–7.69 (m, 10H, Ph × 2), 4.32 (br-s, 1H, OH), 3.83–3.96 (m, 4H, CH2OTBDPS, CH2OH), 3.68 (br-s, 1H, OH), 1.61–2.03 (m, 5H, CH2CH2O × 2, (CH3) 2CH), 1.05 (s, 9H, t-Bu) , 0.95, 0.88 (each d, each 3H, (CH3) 2CH, each J = 6.6 Hz); 13C-NMR (125 MHz, CDCl3) δ 135.53, 132.51, 132.42, 129.99, 129.96, 127.85, 127.83, 78.28, 61.32, 59.43, 35.40, 35.31, 34.68, 26.74, 17.25, 16.83. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H36NaO3Si: 421.23259. Found: 423.23154.
6.25. 1-O-(tert-Butyldiphenylsilyl)-3-(2-hydroxyethyl)-5-hexen-1,3,-diol (12i)
Compound 12i (1.34 g, 86%) was obtained as a colorless oil from 11i (1.74 g, 3.93 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.38–7.69 (m, 10H, Ph × 2), 5.69–5.77 (m, 1H, CH2=CH), 5.03–5.08 (m, 2H, CH2=CH), 3.82–3.98, (m, 4H, CH2OTBDPS, CH2OMOM), 2.47, 2.41 (each dd, each 1H, CH2=CHCH2, Jvicinal = 7.5 Hz, Jgeminal = 14.0 Hz), 1.88–1.94, 1.65–1.83 (each m, 1H and 3H, CH2CH2O × 2), 1.06 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 135.41, 133.70, 132.34, 132.24, 129.88, 127.76, 118.25, 75.83, 61.25, 59.32, 43.87, 39.45, 38.71, 26.64, 18.81. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H34NaO3Si: 421.21694. Found: 421.21837.
6.26. 1-O-(tert-Butyldiphenylsilyl)-3-ethynylpentan-1,3,5-triol (12j)
Compound 12j (1.40 g, 93%) was obtained as a light yellow oil from 11j (1.67 g, 3.92 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.37–7.74 (m, 10H, Ph × 2), 5.19 (s, 1H, OH), 4.31–4.36, 4.10–4.18, 3.85–3.90 (each m, 1H, 1H, and 2H, CH2OTBDPS or CH2OMOM), 2.96 (br-s, 1H, OH), 2.56 (s, 1H, ethynyl-H), 2.13–2.17, 2.03–2.08, 1.86–1.91, 1.69–1.74 (each m, each 1H, CH2CH2O × 2), 1.05 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 135.51, 135.41, 132.16, 132.13, 129.90, 129.85, 127.76, 127.67, 85.37, 73.46, 72.57, 62.29, 59.89, 43.75, 41.95, 26.58, 18.82. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C23H30NaO3Si: 405.18564. Found: 405.18531.
6.27. 1-O-(tert-Butyldiphenylsilyl)-3-(2-hydroxyethyl)-4-hexyn-1,3,-diol (12k)
Compound 12k (1.12 g, 81%) was obtained as a light yellow oil from 11k (1.53 g, 3.47 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.37–7.75 (m, 10H, Ph × 2), 5.01 (s, 1H, OH), 4.29–4.34, 4.11–4.15, 3.83–3.89 (each m, 1H, 1H, and 2H, CH2OTBDPS or CH2OMOM), 3.07 (br-s, 1H, OH), 2.09–2.15, 1.99–2.04, 1.82–1.86, 1.65–1.71 (each m, each 1H, CH2CH2O × 2), 1.88 (s, 3H, Me), 1.05 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 135.60, 135.55, 132.45, 132.30, 129.96, 129.89, 127.84, 127.78, 81.64, 80.90, 72.83, 62.53, 60.17, 44.12, 42.44, 26.68, 18.90, 3.56. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H32NaO3Si: 419.20129. Found: 419.20104.
6.27. Oxidation from the alcohol to the carboxylic acid
A mixture of the alcohol and IBX (1.5 eq.) in DMSO (as a 0.1 M solution, except for 12c) or the alcohol and Dess-Martin periodinane (3.0 eq.) in CH2Cl2 (as a 0.1 M solution, for 12c) was stirred at room temperature for 15 h. Aqueous 0.1 M Na2S2O3 was added to the mixture at room temperature, and the mixture was stirred for 10 min and extracted with AcOEt. The organic layer was washed with H2O, brine, dried with Na2SO4, and evaporated. A mixture of the crude product, NaClO2 (3.0 eq.), NaH2PO4·H2O (1.0 eq.), and 2-methyl-2-butene (4.0 eq.) in acetone/H2O = 3:1 (as a 0.1 M solution) was stirred at room temperature for 4 h. The mixture was diluted with AcOEt, washed with ice cooled aqueous 0.1 M HCl, H2O, and brine, dried with Na2SO4, and evaporated. The residue was purified by silica gel column chromatography (25–75% AcOEt in hexane) to give the corresponding carboxylic acids.
6.28. 5-O-(tert-Butyldiphenylsilyl)-3,5-dihydroxy-3-vinylpentanoic acid (13a)
Compound 13a (1.12 g, 76%) was obtained as a colorless oil from 12a (1.42 g, 3.70 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.41–7.65 (m, 10H, Ph × 2), 5.83 (dd, 1H, CH2=CH, Jtrans = 16.8 Hz, Jcis = 10.4 Hz), 5.55 (d, 1H, CH2=CH (trans), J = 16.8 Hz), 5.33 (d, 1H, CH2=CH (cis), J = 10.4 Hz), 3.91–3.97, 3.78–3.80 (each m, each 1H, CH2OTBDPS), 2.67, 2.58 (each d, each 1H, CH2CO2H, J = 15.6 Hz), 2.05–2.13, 1.66–1.70 (each m, each 1H, CH2CH2O), 1.05 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 172.58, 140.04, 135.45, 135.42, 131.96, 131.90, 130.18, 130.11, 127.97, 127.89, 115.52, 74.96, 61.70, 45.64, 39.48, 26.69, 18.89. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C23H30NaO4Si: 421.18056. Found: 421.18084.
6.29. 5-O-(tert-Butyldiphenylsilyl)-3,5-dihydroxy-3-ethylpentanoic acid (13b)
Compound 13b (700 mg, 78%) was obtained as a colorless oil from 12b (871 mg, 2.25 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.39–7.67 (m, 10H, Ph × 2), 3.85–3.98 (m, 2H, CH2OTBDPS), 2.59, 2.53 (each d, each 1H, CH2CO2H, J = 15.6 Hz), 1.66–1.88 (m, 4H, CH2CH2O, Et- CH2), 1.06 (s, 9H, t-Bu), 0.91 (t, 3H, Et- CH3, J = 7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ 174.85, 135.46, 135.42, 132.29, 132.22, 130.00, 129.98, 127.85, 127.82, 74.16, 61.09, 42.80, 38.15, 32.00, 26.69, 18.87, 8.08. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C23H32NaO4Si: 423.19621. Found: 423.19677.
6.30. 3-{2-(tert-Butyldiphenylsilyloxy)ethyl}-3-hydroxyhexanoic acid (13c)
Compound 13c (447 mg, 59%) was obtained as a colorless oil from 12c (739 mg, 1.84 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.41–7.67 (m, 10H, Ph × 2), 3.85–3.96 (m, 2H, CH2OTBDPS), 2.59, 2.52 (each d, each 1H, CH2CO2H, J = 15.6 Hz), 1.76–1.92 (m, 2H, CH2CH2O), 1.57–1.67 (m, 2H, CH3CH2CH2), 1.31–1.39 (m, 2H, CH3CH2CH2), 1.06 (s, 9H, t-Bu), 0.92 (t, 3H, CH3CH2CH2, J = 7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ 173.31, 135.36, 135.33, 131.91, 131.79, 130.08, 130.05, 127.87, 127.83, 74.24, 61.23, 43.38, 41.64, 37.95, 26.63, 18.80, 17.02, 14.33. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H34NaO4Si: 437.21186. Found: 437.21247.
6.31. 3-{2-(tert-Butyldiphenylsilyloxy)ethyl}-3-hydroxyheptanoic acid (13d)
Compound 13d (1.31 g, 79%) was obtained as a colorless oil from 12d (1.60 g, 3.86 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.41–7.67 (m, 10H, Ph × 2), 5.38 (br-s, 1H, OH), 3.85–3.97 (m, 2H, CH2OTBDPS), 2.59, 2.53 (each d, each 1H, CH2CO2H, J =15.2 Hz), 1.77–1.92 (m, 2H, CH2CH2O), 1.64–1.67 (m, 2H, CH3CH2CH2CH2), 1.28–1.31 (m, 4H, CH3CH2CH2CH2, CH3CH2CH2CH2), 1.06 (s, 9H, t-Bu), 0.90 (t, 3H, CH3CH2CH2CH2, J = 6.8 Hz); 13C-NMR (100 MHz, CDCl3) δ 174.14, 135.45, 135.43, 132.14, 132.04, 130.10, 130.08, 127.92, 127.89, 74.15, 61.24, 43.43, 39.15, 38.30, 26.71, 25.87, 23.00, 18.89, 13.91. HRMS (pos. ion ESI) m/z calcd for (M+H)+ C29H33O4Si: 429.24242. Found: 429.24286.
6.32. 3-{2-(tert-Butyldiphenylsilyloxy)ethyl}-3-hydroxy-5-methylhexanoic acid (13e)
Compound 13e (1.63 g, 84%) was obtained as a colorless oil from 12e (1.87 g, 4.51 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.42–7.68 (m, 10H, Ph × 2), 5.50 (br-s, 1H, OH), 3.87–3.99 (m, 2H, CH2OTBDPS), 2.54–2.62 (m, 2H, CH2CO2H), 1.73–1.92 (m, 3H, CH2CH2O, (CH3)2CHCH2), 1.51–1.63 (m, 2H, (CH3)2CHCH2), 1.06 (s, 9H, t-Bu), 0.99, 0.94 (each d, each 3H, (CH3)2CHCH2, each J =6.6 Hz); 13C-NMR (100 MHz, CDCl3) δ 173.00, 135.37, 135.32, 131.80, 131.66, 130.12, 130.10, 127.90, 127.86, 74.77, 61.29, 47.50, 43.96, 38.42, 26.62, 24.49, 24.37, 23.87, 18.79. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C25H36NaO4Si: 451.22751. Found: 451.22784.
6.33. 5-O-(tert-Butyldiphenylsilyl)-3,5-dihydroxy-3-(1-methylvinyl)pentanoic acid (13g)
Compound 13g (1.35 g, 73%) was obtained as a colorless oil from 12g (1.79 g, 4.48 mmol): 1H-NMR (400 MHz, CDCl3) δ 7.39–7.65 (m, 10H, Ph × 2), 5.32, 5.09 (each s, each 1H, CH2=C), 3.84–3.89, 3.73–3.78 (each m, each 1H, CH2OTBDPS), 2.72, 2.67 (each d, each 1H, CH2CO2H, J = 15.4 Hz), 2.01–2.08, 1.82–1.86 (each m, each 1H, CH2CH2O), 1.72 (s, 3H, Me), 1.05 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 172.30, 145.36, 135.35, 135.32, 131.86, 131.79, 130.05, 129.99, 127.86, 127.77, 113.32, 77.27, 61.58, 44.41, 37.80, 26.57, 19.13, 18.80. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H32NaO4Si: 435.19621. Found: 435.19619.
6.34. 5-O-(tert-Butyldiphenylsilyl)-3,5-dihydroxy-3-(1-methylethyl)pentanoic acid (13h)
Compound 13h (1.51 g, 99%) was obtained as a light yellow oil from 12h (1.48 g, 3.70 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.39–7.67 (m, 10H, Ph × 2), 3.95–3.99, 3.88–3.92 (each m, each 1H, CH2OTBDPS), 2.58, 2.51 (each d, each 1H, CH2CO2H, J = 15.8 Hz), 2.01–2.06 (m, 1H, (CH3)2CH), 1.88–1.93, 1.78–1.83 (each m, each 1H, CH2CH2O), 1.07 (s, 9H, t-Bu), 0.99, 0.90 (each d, each 3H, (CH3)2CH, J = 6.8 Hz); 13C-NMR (100 MHz, CDCl3) δ 170.53, 135.35, 135.32, 131.88, 131.84, 130.11, 130.07, 127.89, 127.84, 76.72, 61.12, 39.90, 35.01, 34.89, 26.63, 18.81, 17.00, 16.80. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H34NaO4Si: 437.21186. Found: 437.21238.
6.35. 3-{2-(tert-Butyldiphenylsilyloxy)ethyl}-3-hydroxy-5-hexenoic acid (13i)
Compound 13i (995 mg, 74%) was obtained as an yellow oil from 12i (1.30 g, 3.27 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.39–7.67 (m, 10H, Ph × 2), 5.74–5.80 (m, 1H, CH2=CH), 5.08–5.15 (m, 2H, CH2=CH), 3.95–4.00, 3.86–3.91, (each m, each 2H, CH2OTBDPS or CH2OMOM), 2.60, 2.56 (each d, each 1H, CH2CO2H, J = 15.8 Hz), 2.47, 2.41 (each dd, each 1H, CH2=CHCH2, Jvicinal = 7.5 Hz, Jgeminal = 14.0 Hz), 1.88–1.93, 1.78–1.83 (each m, each 1H, CH2CH2O), 1.06 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 175.51, 135.29, 132.89, 132.38, 132.36, 129.76, 127.65, 118.79, 73.12, 60.69, 43.86, 42.80, 39.15, 26.57, 18.74. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H32NaO4Si: 435.19621. Found: 435.19639.
6.36. 5-O-(tert-Butyldiphenylsilyl)-3,5-dihydroxy-3-ethynylpentanoic acid (13j)
Compound 13j (908 mg, 64%) was obtained as a light yellow oil from 12j (1.36 g, 3.55 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.39–7.72 (m, 10H, Ph × 2), 4.22–4.27, 3.89–3.93 (each m, each 1H, CH2OTBDPS), 2.89, 2.84 (each d, each 1H, CH2CO2H, J = 15.8 Hz), 2.55 (s, 1H, ethynyl-H), 2.14–2.16, 1.90–1.94 (each m, each 1H, CH2CH2O), 1.06 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 174.71, 135.33, 135.28, 132.33, 129.73, 129.71, 127.72, 127.62, 84.08, 73.27, 68.17, 61.29, 45.96, 41.65, 26.54, 18.74. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C23H28NaO4Si: 419.16491. Found: 419.16488.
6.37. 3-{2-(tert-Butyldiphenylsilyloxy)ethyl}-3-hydroxy-4-hexynoic acid (13k)
Compound 13k (686 g, 62%) was obtained as a light yellow oil from 12k (1.07 g, 2.71 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.40–7.73 (m, 10H, Ph × 2), 4.25–4.30, 3.86–3.90 (each m, each 1H, CH2OTBDPS), 2.89, 2.84 (each d, each 1H, CH2CO2H, J = 15.5 Hz), 2.11–2.15, 1.77–1.81 (each m, each 1H, CH2CH2O), 1.85 (s, 3H, Me), 1.06 (s, 9H, t-Bu); 13C-NMR (100 MHz, CDCl3) δ 174.61, 135.41, 135.37, 132.42, 132.33, 129.79, 129.75, 127.68, 127.65, 81.60, 79.41, 68.67, 61.72, 46.52, 41.89, 26.57, 18.80, 3.41. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C24H30NaO4Si: 433.18056. Found: 433.18014.
6.38. Deprotection of the TBDPS group
A solution of the carboxylic acid in acetone/aqueous 1.0 M HCl = 1:1 (as a 1 mM solution) was stirred at room temperature for 24 h and evaporated. The residue was purified by silica gel column chromatography (75% AcOEt in hexane) to give the corresponding lactones.
6.39. 4-Hydroxy-2-oxo-4-vinyltetrahydropyrane (1a)
Compound 1a (32 mg 85%) was obtained as a colorless oil from 13a (104 mg, 261 µmol): 1H-NMR (400 MHz, CDCl3) δ 5.97 (dd, 1H, CH2=CH, Jtrans = 17.6 Hz, Jcis = 10.4 Hz), 5.33 (d, 1H, CH2=CH (trans), J = 17.6 Hz), 5.22 (d, 1H, CH2=CH (cis), J = 10.4 Hz), 4.61–4.68, 4.35–4.40 (each m, each 1H, CH2O), 2.66 (s, 2H, CH2CO2), 1.93–2.06, 1.89–1.92 (each m, each 1H, CH2CH2O); 13C-NMR (100 MHz, CDCl3) δ 170.50, 141.83, 114.03, 70.01, 65.88, 42.52, 33.93. Anal. Calcd for C7H10O3: C, 59.14; H, 7.09. Found: C, 59.33; H, 6.99.
6.40. 4-Ethyl-4-hydroxy-2-oxotetrahydropyrane (1b)
Compound 1b (92 mg 72%) was obtained as a colorless oil from 13b (354 mg, 883 µmol): 1H-NMR (400 MHz, CDCl3) δ 4.58–4.64, 4.33–4.38 (each m, each 1H, CH2O), 2.61, 2.52 (each d, each 1H, CH2CO2H, J = 17.6 Hz), 1.83–1.91 (m, 2H, CH2CH2O), 1.59–1.66 (m, 2H, Et- CH2), 0.98 (t, 3H, Et- CH3, J = 7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ 171.45, 70.18, 66.01, 42.61, 34.79, 33.41, 7.12. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C7H12NaO3: 167.06787. Found: 167.06792.
6.41. 4-Hydroxy-2-oxo-4-propyltetrahydropyrane (1c)
Compound 1c (124 mg 87%) was obtained as a light yellow oil from 13c (372 mg, 897 µmol): 1H-NMR (400 MHz, CDCl3) δ 4.57–4.64, 4.32–4.37 (each m, each 1H, CH2O), 2.62, 2.53 (each d, each 1H, CH2CO2, J = 17.6 Hz), 1.83–1.96 (m, 2H, CH2CH2O), 1.52–1.60 (m, 2H, CH3CH2CH2), 1.38–1.43 (m, 2H, CH3CH2CH2), 0.98 (t, 3H, CH3CH2CH2, J = 7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ 171.36, 70.06, 65.97, 44.60, 43.02, 33.89, 16.11, 14.24. Anal. Calcd for C8H14O3: C, 60.74; H, 8.92. Found: C, 60.32; H, 9.00.
6.42. 4-Butyl-4-hydroxy-2-oxotetrahydropyrane (1d)
Compound 1d (144 mg 84%) was obtained as a light yellow oil from 13d (429 mg, 1.00 mmol): 1H-NMR (400 MHz, CDCl3) δ 4.57–4.64, 4.32–4.37 (each m, each 1H, CH2O), 2.62, 2.52 (each d, each 1H, CH2COO, J =17.2 Hz), 1.84–1.95 (m, 2H, CH2CH2O), 1.57–1.59 (m, 2H, CH3CH2CH2CH2), 1.35–1.37 (m, 4H, CH3CH2CH2CH2, CH3CH2CH2CH2), 0.93 (t, 3H, CH3CH2CH2CH2, J =6.6 Hz); 13C-NMR (100 MHz, CDCl3) δ 171.54, 69.80, 65.97, 42.88, 41.88, 33.73, 24.82, 22.73, 13.80. Anal. Calcd for C9H16O3: C, 62.77; H, 9.36. Found: C, 62.86; H, 9.50.
6.43. 4-Hydroxy-4-(2-methylpropyl)-2-oxotetrahydropyrane (1e)
Compound 1e (149 mg 88%) was obtained as a light yellow oil from 13e (421 mg, 982 µmol): 1H-NMR (400 MHz, CDCl3) δ 4.58–4.64, 4.32–4.37 (each m, each 1H, CH2O), 2.66, 2.52 (each d, each 1H, CH2COO, J =17.2 Hz), 1.83–1.90 (m, 3H, CH2CH2O, (CH3)2CHCH2), 1.48–1.58 (m, 2H, (CH3)2CHCH2), 1.02, 1.00 (each s, each 3H, (CH3)2CHCH2); 13C-NMR (100 MHz, CDCl3) δ 171.45, 70.25, 65.93, 50.63, 43.46, 34.28, 24.48, 24.44, 23.35. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C9H16NaO3: 195.09917. Found: 195.09929.
6.44. 4-Hydroxy-4-(1-methylvinyl)-2-oxotetrahydropyrane (1g)
Compound 1g (99 mg 70%) was obtained as an yellow oil from 13g (371 mg, 899 µmol): 1H-NMR (500 MHz, CDCl3) δ 5.04, 4.96 (each s, each 1H, CH2=C), 4.63 (ddd, 1H, CH2O, J = 0.8, 3.8, 9.0 Hz), 4.36 (ddd, 1H, CH2O, J = 4.0, 4.0, 5.5 Hz), 2.75, 2.68 (each d, each 1H, CH2COO, J = 17.0 Hz), 2.08–2.14, 1.91–1.96 (each m, each 1H, CH2CH2O), 1.84 (s, 3H, Me); 13C-NMR (100 MHz, CDCl3) δ 171.18, 147.58, 111.20, 71.73, 65.94, 42.01, 32.65, 18.09. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C8H12NaO3: 179.06787. Found: 179.06769.
6.45. 4-Hydroxy-4-(1-methylethyl)-2-oxotetrahydropyrane (1h)
Compound 1h (157 mg 80%) was obtained as a light yellow oil from 13h (520 mg, 1.25 mmol): 1H-NMR (500 MHz, CDCl3) δ 4.60 (ddd, 1H, CH2O, J = 4.0, 11.0, 11.0 Hz), 4.35 (ddd, 1H, CH2O, J = 3.5, 3.5, 10.5 Hz), 2.60, 2.55 (each d, each 1H, CH2COO, J = 17.0 Hz), 1.93(ddd, 1H, CH2CH2O, J = 4.0, 5.5, 10.5 Hz), 1.79–1.84 (m, 1H, (CH3)2CH), 1.70–1.77 (m, 1H, CH2CH2O), 0.98, 0.96 (each s, each 3H, (CH3)2CH); 13C-NMR (100 MHz, CDCl3) δ 172.11, 72.37, 65.92, 40.52, 37.46, 31.30, 16.37, 16.13. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C8H14NaO3: 181.08352. Found: 181.08354.
6.46. 4-Hydroxy-2-oxo-4-(2-propenyl)tetrahydropyrane (1i)
Compound 1i (129 mg 82%) was obtained as a light yellow oil from 13i (413 mg, 1.00 mmol): 1H-NMR (500 MHz, CDCl3) δ 5.81–5.89 (m, 1H, CH2=CH), 5.17–5.34 (m, 2H, CH2=CH), 4.57–4.65 (m, 1H, CH2O), 4.35 (ddd, 1H, CH2O, J = 3.5, 3.5, 5.5 Hz), 2.61, 2.55 (each d, each 1H, CH2COO, J = 17.5 Hz), 2.31–2.49 (m, 1H, CH2=CHCH2), 1.91–1.97, 1.83–1.88 (each m, each 1H, CH2CH2O); 13C-NMR (100 MHz, CDCl3) δ 171.26, 131.57, 120.13, 69.31, 65.85, 46.35, 42.49, 33.49. . HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C8H12NaO3: 179.06787. Found: 179.06769.
6.47. 4-Ethynyl-4-hydroxy-2-oxotetrahydropyrane (1j)
Compound 1j (109 mg 77%) was obtained as a colorless oil from 13j (397 mg, 1.00 mmol): 1H-NMR (500 MHz, CDCl3) δ 7.39–7.72 (m, 10H, Ph × 2), 4.55–4.61, 4.40–4.46 (each m, each 1H, CH2O), 2.95, 2.86 (each d, each 1H, CH2COO, J = 17.5 Hz), 2.64 (s, 1H, ethynyl-H), 2.21–2.27, 2.14–2.19 (each m, each 1H, CH2CH2O); 13C-NMR (100 MHz, CDCl3) δ 169.33, 84.18, 73.54, 65.57, 63.58, 44.35, 35.42. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C7H8NaO3: 179.03657. Found: 163.03694.
6.48. 4-Hydroxy-2-oxo-4-(2-propynyl)tetrahydropyrane (1k)
Compound 1k (32 mg 85%) was obtained as a colorless oil from 13k (104 mg, 261 µmol): 1H-NMR (500 MHz, CDCl3) δ 4.52–4.57, 4.40–4.44 (each m, each 1H, CH2O), 2.91, 2.80 (each d, each 1H, CH2COO, J = 17.5 Hz), 2.08–2.20 (m, 2H, CH2CH2O), 1.86 (s, 3H, Me); 13C-NMR (100 MHz, CDCl3) δ 169.61, 81.67, 79.98, 65.84, 63.89, 44.98, 35.97, 3.25. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C8H10NaO3: 177.05222. Found: 177.05199.
6.49. 3-Cyclopropyl-1-O-(methoxymethyl)pentan-1,3,5-triol (14)
A mixture of 11f (885 mg, 2.00 mmol) and TBAF (1.0 M in THF, 2.40 mL, 2.40 mmol) in THF (10 mL) was stirred at room temperature for 5 h and evaporated. The residue was purified by silica gel column chromatography (75% AcOEt in hexane) to give 14 (350 mg, 86%) as a colorless oil: 1H-NMR (400 MHz, CDCl3) δ 4.63 (s, 2H, MOM- CH2), 3.80–4.02 (m, 4H, CH2OH, CH2OMOM), 3.49 (s, 1H, OH), 3.39 (s, 3H, MOM- CH3), 3.21 (br-s, 1H, OH), 1.75–2.10 (m, 4H, CH2CH2O × 2), 0.40–0.75 (m, 5H, cyclopropyl). 13C-NMR (125 MHz, CDCl3) δ 96.40, 72.72, 64.77, 59.44, 55.39, 41.86, 39.95, 18.68, −0.56, −0.60. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C10H20NaO4: 227.12538. Found: 227.12558.
6.50. Methyl 3-cyclopropyl-3-hydroxy-5-(methoxymethoxy)pentanoate (15)
A mixture of 14 (490 mg, 2.40 mmol) and IBX (1.01 g, 3.60 mmol) in DMSO (24 mL) was stirred at room temperature for 15 h. Aqueous 0.1 M Na2S2O3 was added to the mixture at room temperature, and the mixture was stirred for 10 min and extracted with AcOEt. The organic layer was washed with H2O, brine, dried with Na2SO4, and evaporated. A mixture of the crude product, NaClO2 (651 mg, 7.20 mmol), NaH2PO4·H2O (331 mg, 2.40 mmol), and 2-methyl-2-butene (1.00 mL, 9.60 mmol) in acetone (18 mL) and H2O (6 mL) was stirred at room temperature for 3h. The mixture was diluted with AcOEt, and washed with ice cooled aqueous 0.1 M HCl, H2O, and brine. The water layer was extracted with AcOEt/acetone = 1:1 (3 times), and the combined organic layers were dried with Na2SO4, and evaporated. The crude mixture and K2CO3 (719 mg, 5.20 mmol) in anhydrous DMF (8.7 mL) was stirred at 0 °C for 20 min. MeI (0.16 mL, 2.60 mmol) was added to the mixture at 0 °C, and the mixture was stirred at room temperature for 8h. MeOH (1 mL) was added to the mixture at 0 °C, and the mixture was stirred at room temperature for 10 min. The mixture was partitioned between AcOEt and H2O, and the organic layer was washed with brine, dried with Na2SO4, and evaporated. The residue was purified by silica gel column chromatography (10% AcOEt in hexane) to give 15 (257 mg, 46%) as a colorless oil: 1H-NMR (500 MHz, CDCl3) δ 4.62 (s, 2H, MOM- CH2), 3.82–3.86, 3.75–3.80 (each m, each 1H, CH2OMOM), 3.70 (s, 3H, CO2CH3), 3.54 (br, 1H, OH) 3.37 (s, 3H, MOM- CH3),2.61 (s, 2H, CH2CO2CH3), 1.93–2.02 (m, 2H, CH2CH2O), 0.85–0.90 (m, 1H, cycloproyl), 0.31–0.49 (m, 4H, cyclopropyl). 13C-NMR (125 MHz, CDCl3) δ 172.71, 96.37, 70.12, 64.28, 55.25, 51.45, 45.02, 39.92, 18.89, 0.09, −0.57. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C11H20NaO5: 255.12029. Found: 255.12039.
6.51. 4-Cyclopropyl-4-hydroxy-2-oxotetrahydropyrane (1f)
A mixture of 15 (85mg, 0.37 mmol) and ZrCl4 (43 mg, 0.18 mmol) in i-PrOH (37 mL) was stirred at 90 °C for 1.5 h and evaporated. The residue was purified by silica gel column chromatography (50% AcOEt in hexane) to give 1f (10 mg,18%) as a colorless oil: 1H-NMR (400 MHz, CDCl3) δ 4.56–4.62, 4.33–4.41 (each m, each 1H, CH2O), 2.58, 2.51 (each, d, each 1H, CH2COO, J = 17.6 Hz), 1.81–1.96 (m, 2H, CH2CH2O), 0.42–0.94 (m, 5H, cyclopropyl); 13C-NMR (100 MHz, CDCl3) δ 171.15, 68.88, 65.99, 42.65, 33.81, 20.94, 0.38, −0.05. HRMS (pos. ion ESI) m/z calcd for (M+Na)+ C8H12NaO3: 179.06787. Found: 179.06762.
6.52. Recyclization analysis
A solution of the lactone in aqueous 2.0 M LiOH (1 mL) was stirred at room temperature for 1 h, and evaporated. The residue was purified by silica gel column chromatography (25% MeOH in CH2Cl2) to give the corresponding hydrolyzed carboxylic acids. They were kept as a dry form or a solution of MeOH, H2O, or a buffer (50 mM HEPES and 1 mM MgCl2, pH 8.0) at room temperature. Only the buffer solution resulted in no recyclization after a week.
6.53. Preparation of linearized mevalonate analogues
Lactones 1a–1h were dissolved in 2.0 M aqueous LiOH and incubated for 1 hour at 37 °C. Each solution was titrated to pH 7.0 using concentrated HCl to give 2a–2h. Compounds were diluted to a final concentration of 2 mM for storage at −80 °C.
6.54. In vitro enzyme assay
Recombinant S. pneumoniae MK (Uniprot: Q8DR51) and PMK (Uniprot: Q8DR49) were overexpressed in E. coli as dual-tagged His6-GST fusion proteins. The purification and removal of affinity tags was carried out as previously described.11 The phosphorylation of substrates generates ADP, which can be monitored continuously using the well established pyruvate kinase/lactate dehydrogenase coupled assay system 11 (see Figure 2 legend). MK reactions were initiated by the addition of racemic compound (2a–2k) to 200 µM (100 µM in each enantiomer), and reaction progress was monitored at 339 nm (−340 = 6.22 mM−1). Following completion of the MK reaction (0.2–4 hours), PMK was added to the cuvette, along with additional PK, LDH, PEP, and NADH, and phosphorylation of compounds 3a–c, e, f, i, j was monitored. Three progress curves were obtained for each compound and were averaged to produce the traces shown in Figure 2. The reproducibility of the replicate curves was assessed statistically using the pooled standard deviation (Mev: 5.41, 243 points; 2a: 6.23,1286 points; 2b: 5.03, 1847 points; 2c: 4.33, 484 points; 2e: 1.82, 310 points; 2f: 0.41, 3642 points; 2i: 5.20, 242 points; 2j: 2.51, 2894 points; Pmev: 2.05, 76 points; 3a: 4.94, 493 points; 3b: 0.83, 284 points; 3c: 3.70, 301 points; 3e: 3.26, 561 points; 3f: 1.69, 508 points; 3i: 2.58, 218 points; 3j: 1.51, 847 points).
Acknowledgments
The authors are grateful to the National Institutes of Health (AI 068989) for financial support of this research.
Abbreviations used
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- DPM-DC
diphosphomevalonate decarboxylase
- DTT
dithiothreitol
- GHMP
galactokinase, homoserine kinase, mevalonate kinase, phosphomevalonate kinase
- HEPES
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- IPTG
isopropyl-1-thio-β-D-galactopyranoside
- LDH
lactate dehydrogenase
- 2-ME
β-mercaptoethanol
- Mev
mevalonate
- Pmev
phosphomevalonate
- DPM
diphosphomevalonate
- HMG-CoA
3-hydroxy-3-methyl-glutaryl-CoA
- IPP
isopentenyl diphosphate
- MK
mevalonate kinase
- NADH
nicotinamide adenine dinucleotide, reduced form
- Pi
inorganic phosphate
- PEP
phosphoenolpyruvate
- PK
pyruvate kinase
- PMK
phosphomevalonate kinase
- Tris
tris(hydroxymethyl)aminomethane
- unit
1 µmol of product formed per minute at a saturating concentration of substrate(s)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schuchat A, Robinson K, Wenger JD, Harrison LH, Farley M, Reingold AL, Lefkowitz L, Perkins BA. N Engl J Med. 1997;337:970. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 2.Obaro S, Adegbola R. J Med Microbiol. 2002;51:98. doi: 10.1099/0022-1317-51-2-98. [DOI] [PubMed] [Google Scholar]
- 3.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. Pediatrics. 2009;124:e1. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Nature. 1999;399:590. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 5.Sung H, Shin HB, Kim MN, Lee K, Kim EC, Song W, Jeong SH, Lee WG, Park YJ, Eliopoulos GM. J Clin Microbiol. 2006;44:3524. doi: 10.1128/JCM.00558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Bambeke F, Reinert RR, Appelbaum PC, Tulkens PM, Peetermans WE. Drugs. 2007;67:2355. doi: 10.2165/00003495-200767160-00005. [DOI] [PubMed] [Google Scholar]
- 7.Doern GV, Richter SS, Miller A, Miller N, Rice C, Heilmann K, Beekmann S. Clin Infect Dis. 2005;41:139. doi: 10.1086/430906. [DOI] [PubMed] [Google Scholar]
- 8.Carbon C, Isturiz R. Drugs. 2002;62:1289. doi: 10.2165/00003495-200262090-00001. [DOI] [PubMed] [Google Scholar]
- 9.Fuller JD, Low DE. Clin Infect Dis. 2005;41:118. doi: 10.1086/430829. [DOI] [PubMed] [Google Scholar]
- 10.Wilding EI, Brown JR, Bryant AP, Chalker AF, Holmes DJ, Ingraham KA, Iordanescu S, So CY, Rosenberg M, Gwynn MN. J Bacteriol. 2000;182:4319. doi: 10.1128/jb.182.15.4319-4327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreassi JL, 2nd, Dabovic K, Leyh TS. Biochemistry. 2004;43:16461. doi: 10.1021/bi048075t. [DOI] [PubMed] [Google Scholar]
- 12.Hinson DD, Chambliss KL, Toth MJ, Tanaka RD, Gibson KM. J Lipid Res. 1997;38:2216. [PubMed] [Google Scholar]
- 13.Voynova NE, Rios SE, Miziorko HM. J Bacteriol. 2004;186:61. doi: 10.1128/JB.186.1.61-67.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuthbert JA, Lipsky PE. J Biol Chem. 1990;265:18568. [PubMed] [Google Scholar]
- 15.Cuthbert JA, Lipsky PE. Cancer Res. 1995;55:1732. [PubMed] [Google Scholar]
- 16.Voynova NE, Fu Z, Battaile KP, Herdendorf TJ, Kim JJ, Miziorko HM. Arch Biochem Biophys. 2008;480:58. doi: 10.1016/j.abb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhe-Paganon S, Magrath J, Abeles RH. Biochemistry. 1994;33:13355. doi: 10.1021/bi00249a023. [DOI] [PubMed] [Google Scholar]
- 18.Byres E, Alphey MS, Smith TK, Hunter WN. J Mol Biol. 2007;371:540. doi: 10.1016/j.jmb.2007.05.094. [DOI] [PubMed] [Google Scholar]
- 19.Fritz G. Curr Cancer Drug Targets. 2009;9:626. doi: 10.2174/156800909789057033. [DOI] [PubMed] [Google Scholar]
- 20.Wiemer AJ, Hohl RJ, Wiemer DF. Anticancer Agents Med Chem. 2009;9:526. doi: 10.2174/187152009788451860. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Singh SM, Labrie F. Tetrahedron Lett. 1994;35(8):1157. [Google Scholar]
- 22.Tchen TT. J Biol Chem. 1958;233:1100. [PubMed] [Google Scholar]
- 23.Andreassi JL, 2nd, Bilder PW, Vetting MW, Roderick SL, Leyh TS. Protein Sci. 2007;16:983. doi: 10.1110/ps.072755707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreassi JL, 2nd, Vetting MW, Bilder PW, Roderick SL, Leyh TS. Biochemistry. 2009;48:6461. doi: 10.1021/bi900537u. [DOI] [PMC free article] [PubMed] [Google Scholar]