Abstract
Recent studies indicate that microtubules (MTs) may play an important role in spine development and dynamics. Several imaging studies have now documented the exploration of dendritic spines by dynamic MTs in an activity-dependent manner. Furthermore, it was found that alterations of MT dynamics by pharmacological and molecular approaches exert profound influence on the development and plasticity of spines associated with neuronal activity. It is reasonable to speculate that dynamic MTs may be responsible for targeted delivery of specific cargos to a selected number of spines and/or for interacting with the actin cytoskeleton to generate the structural changes of spines associated with synaptic modifications.
Keywords: Synapse, postsynaptic terminals, synaptic plasticity, cytoskeleton, cytoskeletal dynamics, actin
Dendritic spines and synaptic plasticity
Dendritic spines are tiny membrane protrusions that serve as the postsynaptic terminals for most of the excitatory synapses in the vertebrate brain. The spine head contains the postsynaptic density (PSD) that features clustered neurotransmitter receptors, scaffolding components, and signaling proteins, all of which are positioned on the immediate opposite side of presynaptic terminals for effective reception of neurotransmitters. There is an overwhelming amount of evidence to show that synapses are plastic and undergo short- and long-term modifications during developmental refinement of neural circuits, as well as in learning and memory [1–3]. Furthermore, many neurological disorders have been associated with alterations of synaptic connections [3, 4][see also articles byArikkath and by Deng and Dunaevsky, this issue]. Therefore, a better understanding of the molecular and cellular mechanisms underlying synaptic plasticity is of importance to our understanding of brain development and functions under both physiological and pathological conditions.
Modulation of synaptic strength involves both pre- and post-synaptic terminals, of which the presynaptic changes are often associated with an adjustment in the probability of neurotransmitter release [5]. On the postsynaptic side, modifications of the number and activity of surface neurotransmitter receptors are considered to be a key event underlying synaptic modification. For example, the intracellular domain of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) is phosphorylated to increase ion conductance during early long term potentiation (LTP) [6]. To generate a long-lasting LTP, however, it is necessary to increase the number of postsynaptic glutamate receptors on the postsynaptic surface. These postsynaptic changes appear to be reversed during long term depression (LTD), including dephosphorylation of AMPARs and their removal from the postsynaptic membrane. While changes in transmitter receptors directly contribute to the modification of synaptic strength, many studies have reported that morphological changes of the postsynaptic terminals are equally important for postsynaptic modulation of synaptic strength. There is a large amount of literature documenting the enlargement of spine heads as well as the emergence of new spines after the induction of LTP, whereas spine shrinkage and elimination are considered to be a key step in LTD [7–9], which have been postulated to be the structural basis of memory [10–12]. Importantly, these morphological/structural changes in spines have been observed during development, aging, learning and neurodegenerative diseases [10–12].
The cytoskeletal architecture of dendritic spines
A hallmark of dendritic spines is their enrichment in actin, which is believed to be the major player for spine morphology and dynamics. Live-cell imaging studies in vitro and in vivo have established that spines are plastic and undergo activity-dependent changes in morphology, which are believed to be controlled by the actin cytoskeleton. Actin polymerization is coupled with spine enlargement/formation during LTP, whereas LTD involves spine shrinkage through actin depolymerization [8, 9, 13]. Furthermore, many actin-associated regulatory proteins, as well as signaling pathways targeting the actin cytoskeleton, have been implicated in spine dynamics and development [14–16][see several other articles in this issue].
Unlike actin filaments, microtubules (MTs) are not believed to play a direct role in spine dynamics, primarily due to the fact that they, although concentrated in dendritic shafts, are rarely seen in spines by standard light and electron microscopy. MTs are polarized hollow tubules formed from a head-to-tail assembly of the α/β-tubulin heterodimers. The plus end of MT favors polymerization whereas the minus end favors disassembly; the later however is often capped by centrosomal or other minus end capping proteins. As a result, MTs generally grow from their plus ends in cells but they often switch from growth to rapid shrinkage and vice versa. It is believed that this feature of MT plus ends, termed dynamic instability, represents an important mechanism by which MTs could reassemble into different structures underlying various cell functions. For example, the dynamic instability could enable MTs to explore various cellular locations for potential interacting structures and signaling components; a productive interaction with specific cellular structures/signals could result in stabilization of the MTs and further recruitment of MTs and other cellular structures. In cells, the structure and dynamics of MTs are regulated by a large number of MT-associated proteins (MAPs) that often bind along the entire length of MTs. Significantly, recent studies have discovered several classes of proteins that specifically bind to the plus ends of MTs to regulate their stability, as well as to enable coupling to other cellular structures [17]. In neurons, MTs in both axonal and dendritic processes are present as parallel bundles crosslinked by MAPs, which not only provide the structural foundation for the polarized neuronal morphology but also serve as the tracks for MT-motor-based long-range transport of cellular cargos. MTs in axons have unidirectional arrangement with the plus end pointing to the distal end of the axon, whereas MTs in dendrites exist in mix polarity. It is known that these MT parallel bundles are quite stable and could be easily preserved by traditional fixation and staining methods. However, dynamic MTs such as the fast growing plus ends, which might be exploring dendritic spines and other cellular regions, are often destroyed by traditional fixation conditions if no specific MT stabilization and preservation approaches are used. It thus remains open whether MTs, especially dynamic MTs, are present in dendritic spines and play a role in spine development and plasticity.
Recent literature has shown that MTs participate in many actin-dependent motile activities and interact with the actin cytoskeleton [18]. For example, MTs can interact with the actin cytoskeleton to direct its polymerization and membrane protrusion in migrating cells, in which MTs grow at the leading edge but shorten at the cell body and the rear [19]. In fibroblasts, the growth of MTs at the leading edge was found to activate Rac1 GTPase, which in turn induces actin polymerization for lamellipodial protrusion [20]. In nerve growth cones, early immunostaining studies suggested that MTs do not enter the actin-rich peripheral region of the growth cone consisting of filopodia and lamellipodia, leading to a similar view that the actin cytoskeleton is primarily responsible for growth cone steering [21]. However, live imaging of fluorescently tagged tubulin proteins in growth cones showed that MTs enter and explore the peripheral region of the growth cone [22]. Further studies using sophisticated high-resolution live microscopy have convincingly shown that MTs enter filopodia and actively interact with actin filaments in growth cone filopodia to influence growth cone motility [18, 23]. Furthermore, direct local modification of MT dynamics has been shown to be sufficient to induce growth cone turning through the Rho GTPases and actin cytoskeleton [24]. While migrating cells and growth cones may be different from spines in respect to their cytoskeletal dynamics and regulation, it is tempting to speculate that the actin-based spine dynamics may be influenced by MTs.
MTs in dendritic spines: early evidence
The notion that MTs might be present in postsynaptic terminals first came from electron microscopic studies of synapses in brain tissues. With the aid of enhanced MT preservation techniques, Westrum and Gary showed that MTs appear to associate with the postsynaptic density [25]. Their subsequent EM study further revealed that some MTs are present between disks of smooth endoplasmic reticulum (ER), a special organelle referred to as spine apparatus, in large spines of some pyramidal neurons [26]. MTs were also found in the proximal apical dendritic spines of hippocampal CA3 pyramidal neurons, which are usually large and branched, and contain subcellular organelles including ribosomes, multivesicular bodies (MVB), and mitochondria [27]. In the CA1 region of hippocampal slices, MTs were also detected in dendritic spines, but it was thought to be a possible artifact from recovery after slice preparation [28]. The possible existence of MTs in spines was also supported by immuno-EM detection of β-tubulin and the MT-associated protein 2 (MAP2) in postsynaptic density, although whether these tubulin proteins polymerize into MT structures was unknown [29].
Besides EM studies, a number of biochemical studies have also implicated the presence of MTs in dendritic spines. For example, proteomic studies using two-dimensional gel electrophoresis of tryptic peptides in conjunction with different identification methods found tubulin in postsynaptic density fractions from whole brain [30–33]. In addition, tubulin mRNA was found in synaptosomal preparations enriched for dendritic spines [34]. The physical interaction between soluble forms of tubulin, such as tubulin heterodimers, and postsynaptic proteins, such as NMDAR subunits, has also been presented [35]. Other indirect evidence came from the detection of MAP2 in the postsynaptic region, and that activation of NMDAR decreased its phosphorylation [36]. However, evidence for the lack of MAP2 in spines was also reported [37, 38]. It is possible that different fixation conditions employed in these studies might have contributed to these conflicting results. In addition to MAP2, several other MT-binding proteins were also implicated to participate in postsynaptic events. Light chain 2 of MAP1 is a strong interactor in the postsynaptic stargazin-AMPA receptor complex, possibly involved in AMPA receptor trafficking prior to insertion at synapse [39]. Another protein, CRIPT (cysteine-rich interactor of PDZ three), was identified as a PSD component and binds specifically to PSD95. With its ability to bind tubulin, CRIPT may regulate PSD95 interaction with MTs [40]. Finally, the observation of several types of membranous organelles (e.g. mitochondria) in spines also suggests a possible presence of MTs in spines since many of these organelles are primarily transported by MT-dependent motors. It should be noted, however, that many membraneous organelles can also move along actin filaments via myosin motors. It is thus plausible that MT-based long-range transport of these organelles could be switched to actin/myosin-based trafficking for spine delivery of these organelles. Further studies are clearly required to elucidate the precise trafficking mechanisms for many of these organelles and vesicles.
MTs in dendritic spines: new evidence
A recent EM study found that strong tetanic stimulation used for LTP induction caused a significant increase in MTs entering dendritic spines of hippocampal CA1 neurons [41]. These MTs were seen to bear a characteristic hollow tubular shape with a diameter of 25 nm, indicating that they are indeed polymerized MTs. The marked increase of MTs in spines after tetanic stimulation also suggests a potential function for MTs in activity-dependent modification of postsynaptic structures and functions. However, the limitation of EM made it difficult to quantify the extent of dendritic spines that were visited by MTs, as well as the temporal nature of these MTs. It was also not clear if the observed redistribution of MTs into spines had any significant effects on the spine dynamics and synaptic functions. These important questions have now been partially addressed by three recent studies that employed high-resolution fluorescence live microscopy in conjunction with pharmacological and molecular manipulations of MT dynamics [42–44].
The first major conclusion from the three studies is that dendritic spines do contain MTs, but the percentage of spines containing MTs at a given time point is quite low, likely due to their very dynamic nature. In our study, we used confocal imaging to examine the presence of MTs in spines of cultured hippocampal neurons expressing both GFP-tubulin and mOrange [42]. The z-sectioning and 3D reconstruction by confocal imaging allowed us to more accurately capture MTs in spines at different focal planes of a specific segment of the dendrite. To assure that we are detecting polymerized MTs, we excluded the GFP-tubulin signals in thin spines because we were unable to determine if they represent soluble GFP-tubulin proteins that filled up the thin spines. With this stringent selection criterion, we consistently detected MTs in a small but significant number of spines: about 4% of mushroom spines. The low percentage of spines containing MTs is consistent with the other two studies involving live imaging of GFP-tagged tubulin or EB3, a member of MT Plus-end Tracking Proteins (+TIPs) associated with the plus ends of growing MTs [45]. While only a small number of spines were seen to contain MTs at a given time point, long term time lapse imaging using total internal reflectance fluorescent microscopy (TIRFM) showed that a much larger portion, if not all of dendritic spines, were explored by dynamic MTs over time [43]. Importantly, these MTs only transiently stayed in spines with an average time of a few minutes. It is this transient nature of dynamic MTs in spines that likely contributed to the small percentage of spines captured containing MTs at a fixed time. Interestingly, it was found that the invasion of MTs into spines was stimulated by neuronal activity. Repetitive membrane depolarization using KCl increased the number of dendritic protrusions containing MTs, as well as the average time of MTs staying in spines. These results are consistent with the EM study and suggest that MT distribution in dendritic spines is regulated by neuronal activity.
What is the consequence of dynamic MTs entering dendritic spine? This question was partially answered by the time-lapse live imaging in which the entry of fluorescently-tagged MTs into spines was found to be followed by growth and enlargement of the particular spines [43, 44]. Further support for MTs on spine shape came from pharmacological and molecular manipulation of MT dynamics. Inhibition of MT polymerization or EB3 knockdown reduced the number of mushroom-shaped spines, while increasing the number of filopodia-like dendritic protrusions, without affecting the total number of dendritic protrusions. On the other hand, overexpression EB3-GFP in neurons resulted in an opposite effect: an increase in mushroom-shaped spines together with a reduction in dendritic filopodia-like protrusions. Since dendritic spines are thought to develop and mature from filopodia-like protrusions to mushroom-like spines during synaptogenesis [46], these data suggest that MTs play an important role in spine development and maturation. Indeed, our data showed that shRNA knockdown of EB3 reduced the number of mature spines in hippocampal neurons, suggesting the importance of MT dynamics in spine formation.
The development of spines and synapses is regulated by a variety of extracellular factors and neuronal activity [10]. Brain-derived neurotrophic factor (BDNF) is one of the neurotrophins known to promote spine development and synapse formation [47]. To test a role of MTs in spine development, we used MT-specific drugs (nocodazole for disrupting and taxol for stabilizing dynamic MTs, respectively) to manipulate MT dynamics and examined the spine development of a particular neuron under the influence of BDNF over several days by live confocal imaging. To avoid a gross disruption of MTs in the cells, both nocodazole and taxol were used at very low concentrations (in nanomolar range), which have been shown to primarily affect the dynamic ends of MTs without causing disruption of the overall integrity of existing MT networks [24]. The observation that the baseline level of spine formation in hippocampal cultures was not affected by these MT-drugs at low concentrations support the notion that MTs were not substantially disrupted. Interestingly, we found that the low dose of taxol further potentiated the effects of BDNF on spine formation, leading to about 30% more spines than those treated with BDNF alone. On the other hand, nocodazole completely blocked the effect of BDNF. Taxol with BDNF also significantly increased spine size, implying an acceleration of spine maturation by MT stabilization during BDNF exposure. These findings suggest that MTs play an important role in spine development under neurotrophic influence.
The mechanism by which MTs increase spine size and formation is believed to be mediated by the actin cytoskeleton [44]. It was shown that EB3 regulation of spine morphology was correlated with changes in actin polymerization within the spines. Furthermore, knockdown of EB3 caused a loss of F-actin from dendritic protrusions and conversely, overexpression of EB3 increased F-actin abundance. Moreover, treatment of neurons with jasplakinolide, a drug that binds and stabilizes actin filaments, rescued the EB3 knockdown phenotype to increase mushroom-shaped spines. On the other hand, treatment of neurons with latrunculin B, an actin monomer-sequestering protein that inhibits actin polymerization, further increased the number of filopodia-like protrusions and decreased the number of spines in EB3-knockdown cells. The link between EB3 and the actin cytoskeleton appears to be p140Cap, a Src-binding protein that inhibits Src kinase activity. It was proposed that EB3-MT delivery of p140Cap to spines enables the inhibition of Src kinase phosphorylation of cortactin molecules to increase actin polymerization for spine growth. It should be noted that MT regulation of actin polymerization has been observed in fibroblasts and in nerve growth cones [18]. It is thus exciting to speculate that similar MT-to-actin pathways may be conserved and could be utilized (with possible variations, though) for different neuronal motile activities associated with growth cone migration and synaptic plasticity.
Unsolved issues, future directions, and link to diseases
It is clear that these recent studies have presented evidence to show that dynamic MTs enter dendritic spines and may regulate spine dynamics and morphology. The imaging work together with pharmacological and molecular manipulations of MT dynamics has also indicated a potential role for dynamic MTs in spine development and synaptic plasticity. However, it remains to be determined about the precise functions of the MTs exploring spines. It should be noted, however, that all the manipulations on MT dynamics described (either MT-drug treatment or EB3 knockdown) were performed globally to the entire cultures. As a result, MTs throughout the entire neurons, including those in dendritic shafts, as well as in the presynaptic terminals, could be affected by these manipulations. Such global manipulation of MT dynamics could affect MT-based cellular functions, for instance, vesicle delivery in dendrites and presynaptic terminals, to generate secondary effects on dendritic spines. Adefinite determination of a functional role for MTs in spines requires spine-restricted manipulation of MT dynamics, such as the use of local photoactivation of caged MT-drugs (e.g. caged taxol) [24] or chromophore-assisted laser inactivation of MT components [48].
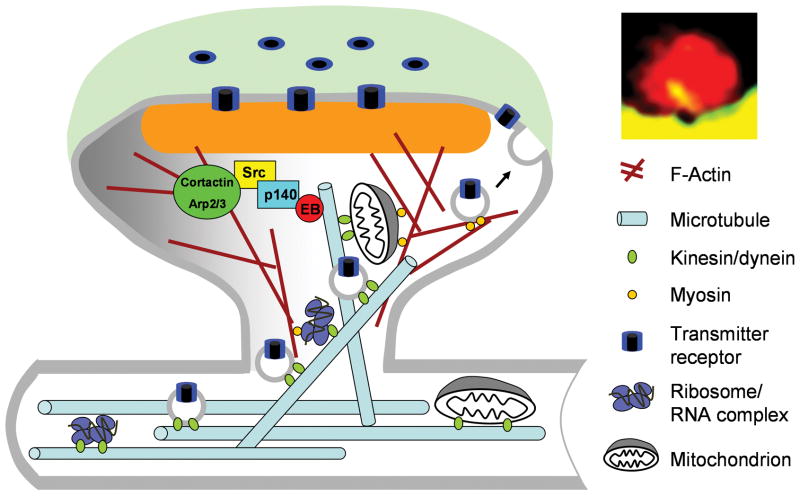
While the present evidence suggests that MTs in spines may act on the actin cytoskeleton for changes of spine shape, other potential functions for MTs in spine dynamics and synaptic plasticity remain to be explored (Figure 1). One legitimate guess is that MTs may be involved in delivery of vesicular components and cellular organelles that are essential for postsynaptic structure and function. For example, mitochondria were found in about 9% of spines and smooth ER is found in about half of the spines [49, 50]. Spine localization of mitochondria was shown to be activity-dependent. Both smooth ER and mitochondria are known to depend on MTs for their transport and cellular distribution, thus they could, in principle, be transported into spines by a MT-based mechanism. Furthermore, polyribosomes were found to be recruited into spines after LTP induction [51]. The transport of polyribosome components and target mRNAs could depend on MTs as well for their spine localization. Finally, MTs could be involved in vesicle trafficking of neurotransmitter receptors during synaptic plasticity. In support of this possibility, it was reported that a labile component of AMPA receptor-mediated synaptic transmission depends on MT motors [52]. Future experiments that employ spatiotemporally-restricted manipulation of MT components at the single spine level, in combination with high-resolution imaging of synaptic changes, could potentially provide the answers to these important questions.
Figure 1.
A schematic diagram illustrating potential functions of microtubules in dendritic spines. In addition to the proposed MT regulation of actin cytoskeleton through p140Cap and Src kinases on cortactin, MTs may also be involved in delivering membraneous organelles (e.g. mitiochondria and receptor-containing vesicles), as well as ribosome/RNA complexes, to the dendritic spine. It is likely that MTs and the actin cytoskeleton cooperate in the delivery of these cargos into spines and in the regulation of spine structure and function.
Arole for MTs in spine development and plasticity could open up a new window for our understanding of the molecular and cellular mechanisms underlying several brain disorders. Given that many brain disorders are associated with abnormal spine morphology or density, it would be interesting to see if MTs are involved in defective neuronal connections. Indeed, defects in MT associated proteins have been linked to several brain disorders. For example, the fragile X mental retardation protein (FMRP) regulates MAP1b mRNA translation and its protein level, which is important for stabilizing MT networks [53]. Lack of FMRP has been shown to result in filopodia-like immature spines and altered synaptic plasticity in fragile X-syndrome, possibly through its regulation of MAP1B translation. Disrupted-In-Schizophrenia 1 (DISC1) is a susceptibility gene for schizophrenia and bipolar disorder and it was reported that some schizophrenia patients exhibit low densities of dendritic spines in frontal and temporal cortex [54]. Recently, DISC1 protein was found to accelerate dendritic development and spine formation in adult newborn neurons [55]. Immuno-EM studies showed that DISC1 is enriched in spines and PSD, and some appeared to associate with MT structures [56, 57]. DISC1 also interacts with light chain 2 of MAP1A [58]. Although most reports studying the interaction of DISC1 and the MT structures focused on centrosome, there is a potential interplay between DISC1 and MTs in dendritic structure to regulate spine development. Finally, Williams syndrome is a developmental disorder caused by hemizygous deletion of approximately 28 genes on chromosome 7 [59]. The mild mental retardation of Williams syndrome patients is thought to be linked to two genes encoding proteins that regulate the cytoskeleton: LIM kinase 1, an upstream regulator of ADF/cofilin for actin dynamics and CLIP-115, a MT +TIP protein. Deletion of either of these two led to neurodevelopmental abnormalities in transgenic mice [60, 61]. LIMK1 knockout mice also exhibit immature spine morphology and enhanced LTP, suggesting that both actin and MT cytoskeleton are important for spine development and maturation.
In conclusion, these recent studies have added important evidence supporting the involvement of MTs in spine formation, dynamics, and plasticity that is associated with synaptic development and plasticity. Hopefully, future studies will provide a better understanding of MT functions in dendritic spines, which will enable us to dissect the molecular and cellular mechanisms underlying normal neural development and neurological disorders.
Acknowledgments
JQZ is supported by research grants from National Institutes of Health (GM083889 and GM084363).
References
- 1.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–4. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 2.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–90. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007;17:325–30. doi: 10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Zakharenko SS, Zablow L, Siegelbaum SA. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat Neurosci. 2001;4:711–7. doi: 10.1038/89498. [DOI] [PubMed] [Google Scholar]
- 6.Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–7. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- 7.Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–67. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–57. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–12. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 10.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–53. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 11.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–89. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 12.Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–84. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- 13.Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–60. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 14.Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–56. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 15.Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Schubert V, Dotti CG. Transmitting on actin: synaptic control of dendritic architecture. J Cell Sci. 2007;120:205–12. doi: 10.1242/jcs.03337. [DOI] [PubMed] [Google Scholar]
- 17.Howard J, Hyman AA. Dynamics and mechanics of the microtubule plus end. Nature. 2003;422:753–8. doi: 10.1038/nature01600. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 19.Waterman-Storer CM, Salmon E. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol. 1999;11:61–7. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
- 20.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 21.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–72. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991;115:345–63. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J Cell Biol. 2002;158:139–52. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. J Neurosci. 2002;22:9358–67. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westrum LE, Gray EG. Microtubules and membrane specializations. Brain Res. 1976;105:547–50. doi: 10.1016/0006-8993(76)90601-6. [DOI] [PubMed] [Google Scholar]
- 26.Westrum LE, Jones DH, Gray EG, Barron J. Microtubules, dendritic spines and spine appratuses. Cell Tissue Res. 1980;208:171–81. doi: 10.1007/BF00234868. [DOI] [PubMed] [Google Scholar]
- 27.Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol. 1992;325:169–82. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- 28.Fiala JC, Kirov SA, Feinberg MD, et al. Timing of neuronal and glial ultrastructure disruption during brain slice preparation and recovery in vitro. J Comp Neurol. 2003;465:90–103. doi: 10.1002/cne.10825. [DOI] [PubMed] [Google Scholar]
- 29.Caceres A, Payne MR, Binder LI, Steward O. Immunocytochemical localization of actin and microtubule-associated protein MAP2 in dendritic spines. Proc Natl Acad Sci U S A. 1983;80:1738–42. doi: 10.1073/pnas.80.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly PT, Cotman CW. Synaptic proteins. Characterization of tubulin and actin and identification of a distinct postsynaptic density polypeptide. J Cell Biol. 1978;79:173–83. doi: 10.1083/jcb.79.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li KW, Hornshaw MP, Van Der Schors RC, et al. Proteomics analysis of rat brain postsynaptic density. Implications of the diverse protein functional groups for the integration of synaptic physiology. J Biol Chem. 2004;279:987–1002. doi: 10.1074/jbc.M303116200. [DOI] [PubMed] [Google Scholar]
- 32.Walters BB, Matus AI. Tubulin in postynaptic junctional lattice. Nature. 1975;257:496–8. doi: 10.1038/257496a0. [DOI] [PubMed] [Google Scholar]
- 33.Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–11. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- 34.Chicurel ME, Terrian DM, Potter H. mRNA at the synapse: analysis of a synaptosomal preparation enriched in hippocampal dendritic spines. J Neurosci. 1993;13:4054–63. doi: 10.1523/JNEUROSCI.13-09-04054.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Rossum D, Kuhse J, Betz H. Dynamic interaction between soluble tubulin and C-terminal domains of N-methyl-D-aspartate receptor subunits. J Neurochem. 1999;72:962–73. doi: 10.1046/j.1471-4159.1999.0720962.x. [DOI] [PubMed] [Google Scholar]
- 36.Quinlan EM, Halpain S. Postsynaptic mechanisms for bidirectional control of MAP2 phosphorylation by glutamate receptors. Neuron. 1996;16:357–68. doi: 10.1016/s0896-6273(00)80053-7. [DOI] [PubMed] [Google Scholar]
- 37.Bernhardt R, Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984;226:203–21. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- 38.Kaech S, Fischer M, Doll T, Matus A. Isoform specificity in the relationship of actin to dendritic spines. J Neurosci. 1997;17:9565–72. doi: 10.1523/JNEUROSCI.17-24-09565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ives JH, Fung S, Tiwari P, Payne HL, Thompson CL. Microtubule-associated protein light chain 2 is a stargazin-AMPA receptor complex-interacting protein in vivo. J Biol Chem. 2004;279:31002–9. doi: 10.1074/jbc.M402214200. [DOI] [PubMed] [Google Scholar]
- 40.Niethammer M, Valtschanoff JG, Kapoor TM, et al. CRIPT, a novel postsynaptic protein that binds to the third PDZ domain of PSD-95/SAP90. Neuron. 1998;20:693–707. doi: 10.1016/s0896-6273(00)81009-0. [DOI] [PubMed] [Google Scholar]
- 41.Mitsuyama F, Niimi G, Kato K, et al. Redistribution of microtubules in dendrites of hippocampal CA1 neurons after tetanic stimulation during long-term potentiation. Ital J Anat Embryol. 2008;113:17–27. [PubMed] [Google Scholar]
- 42.Gu J, Firestein BL, Zheng JQ. Microtubules in dendritic spine development. J Neurosci. 2008;28:12120–4. doi: 10.1523/JNEUROSCI.2509-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci. 2008;28:13094–105. doi: 10.1523/JNEUROSCI.3074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaworski J, Kapitein LC, Gouveia SM, et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Akhmanova A, Hoogenraad CC. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr Opin Cell Biol. 2005;17:47–54. doi: 10.1016/j.ceb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–6. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Lu B. Acute and long-term synaptic modulation by neurotrophins. Prog Brain Res. 2004;146:137–50. doi: 10.1016/s0079-6123(03)46010-x. [DOI] [PubMed] [Google Scholar]
- 48.Surrey T, Elowitz MB, Wolf PE, et al. Chromophore-assisted light inactivation and self-organization of microtubules and motors. Proc Natl Acad Sci U S A. 1998;95:4293–8. doi: 10.1073/pnas.95.8.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–45. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- 52.Kim CH, Lisman JE. A labile component of AMPA receptor-mediated synaptic transmission is dependent on microtubule motors, actin, and N-ethylmaleimide-sensitive factor. J Neurosci. 2001;21:4188–94. doi: 10.1523/JNEUROSCI.21-12-04188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu R, Wang H, Liang Z, et al. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–6. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garey LJ, Ong WY, Patel TS, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–53. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duan X, Chang JH, Ge S, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishizuka K, Paek M, Kamiya A, Sawa A. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–97. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 57.Kirkpatrick B, Xu L, Cascella N, Ozeki Y, Sawa A, Roberts RC. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol. 2006;497:436–50. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- 58.Morris JA, Kandpal G, Ma L, Austin CP. DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003;12:1591–608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- 59.Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci. 2006;7:380–93. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- 60.Meng Y, Zhang Y, Tregoubov V, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–33. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 61.Hoogenraad CC, Koekkoek B, Akhmanova A, et al. Targeted mutation of Cyln2 in the Williams syndrome critical region links CLIP-115 haploinsufficiency to neurodevelopmental abnormalities in mice. Nat Genet. 2002;32:116–27. doi: 10.1038/ng954. [DOI] [PubMed] [Google Scholar]