Abstract
Aims
Our studies were designed to understand the role of the gut hormones incretins GLP-1 and GIP on diabetes remission after gastric bypass surgery (GBP).
Methods
Morbidly obese patients with type 2 diabetes (T2DM) were studied before and 1, 6, 12, 24 and 36 months after GBP. A matched group of patients were studied before and after a diet-induced 10 kg weight loss, equivalent to the weight loss 1 month after GBP. All patients underwent an oral glucose tolerance test and an isoglycaemic glucose intravenous challenge to measure the incretin effect.
Results
Post-prandial GLP-1 and GIP levels increase after GBP and the incretin effect on insulin secretion normalizes to the level of non diabetic controls. In addition, the pattern of insulin secretion in response to oral glucose changes after GBP, with recovery of the early phase, and post-prandial glucose levels decrease significantly. These changes were not seen after an equivalent weight loss by diet. The changes in incretin levels and effect observed at 1 month are long lasting and persist up to 3 years after the surgery. The improved insulin release and glucose tolerance after GBP were shown by others to be blocked by the administration of a GLP-1 antagonist in rodents, demonstrating that these metabolic changes are, in part, GLP-1 dependent.
Conclusion
Although sustained and significant weight loss is likely to be the key mediator of diabetes remission after GBP, the changes of incretins improve the early phase of insulin secretion and post-prandial glucose levels, and contribute to the better glucose tolerance.
Keywords: GLP-1, GIP, Incretin effect, Diabetes, Gastric bypass, Review
1. Introduction
One of the major benefits of surgical weight loss is the resolution of type 2 diabetes (T2DM) in 50–80% of cases [1,2]. The rapidity of the onset and the magnitude of the effect of gastric bypass surgery on diabetes remain largely unexplained.
Some determinants of impaired insulin secretion in T2DM, such as glucose or lipid toxicity [3,4], likely improve as a result of weight loss per se. In contrast, the change of the gut hormone incretins after GBP [5,6], and their resulting effect on insulin or on glucagon secretion, could be the mediator of the greater improvement of glucose levels after GBP [5,6] compared to diet or to gastric banding.
2. What are the incretins?
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1), secreted respectively from the upper (duodenum K cells) and the lower intestine (ileum L cells) [7–9], are the two incretins responsible for approximately 50% of post-prandial insulin secretion [10–13]. The incretin effect is described as the greater insulin response after oral glucose compared to an equivalent dose of intravenous glucose [11,14]. In addition to its insulinotropic effect, GLP-1 delays gastric emptying [15], decreases appetite and promotes weight loss [15,16], inhibits glucagon [17], and improves insulin sensitivity [18]. GLP-1 and GIP are rapidly inactivated by the enzyme dipeptidyl peptidase IV (DPP-IV). The incretin effect on insulin secretion is impaired in patients with T2DM [19]. GLP-1 analogues and DPP-IV inhibitors are currently used as anti-diabetic agents [20].
3. Change of incretins after bypass surgeries for weight loss
Reports of increase incretin levels after bypass surgeries started in the late 1970’s early 1980’s, at a time when no commercial assays were available. GLP-1 consistently increased after jejuno ileal bypass, biliopancreatic diversion or gastric bypass [21–23]. More recent reports, including ours, confirm a significant increase of GLP-1 levels by a factor 5 to 10 after a meal [24] or oral glucose [5] after GBP. The results of bypass surgeries on changes of GIP levels are less consistent with either elevated or decreased levels after the same types of surgery [21,23,25–27]. In morbidly obese patients with T2DM, we reported an increase of GIP levels 1 month after GBP [5]. In addition to the increase post-prandial levels of incretins, we have shown that the incretin effect on insulin secretion, blunted in patients with diabetes, normalized to the levels of non-diabetic controls as early as 1 month after gastric bypass surgery [5]. These patients had diagnosed diabetes for less than 5 years, were well controlled (mean HbA1c 6%) and on minimum therapy. Recent data in Gato-Kakizaki (GK) rats show that the increased GLP-1 secretion and improved glucose tolerance after duodeno jejunal bypass (DJB), is reversed by the administration of a GLP-1 receptor antagonism. This proof-of-concept study provides direct evidence that improvement of glucose tolerance following a gastric bypass-like surgery is mediated, at last in part, by enhanced GLP-1 action [28].
4. Effect of weight loss versus bypass on incretins
Previous data suggested that a diet-induced weight loss (−18.8 kg) increase the incretin levels in response to a test meal [29]. To address the question of the possible role of weight loss on the change in incretin levels and effect after GBP surgery, we designed a prospective study with a surgical group studied before and 1 month after GBP and a matched diet group studied before and after a diet-induced equivalent weight loss. Our working hypothesis was that the increase in incretin levels and incretin effect would be greater after GBP surgery than after equivalent weight loss by diet.
The inclusion criteria for the surgical group and the diet group were identical: morbidly obese patients with BMI >35kg/m2, with T2DM of less than 5 years duration, not on insulin, thiazolidinedione, exenatide or DPP-IV inhibitor, with HbA1c < 8%, age < 60 years, of all ethnic background. The GBP group was studied first. Participants in the diet group were recruited afterwards, fit the same inclusion criteria, and were matched for body weight, BMI, age, diabetes control and duration with patients from the surgical group. The diet consisted of meal replacement, about 1000 to 1200 kcal/d, given on weekly outpatient visits. The duration of weight loss was not set but the expectation was that the participants would lose 10 kg of weight in 4 to 8 weeks. The patients were kept on negative energy balance while retested after diet weight loss. The diabetes management during diet included self glucose monitoring by the patients and the adjustment of medications to avoid hypoglycaemia. At baseline and after weight loss, patients were studied off diabetes medications for 72 hours. The results of these experiments have been published elsewhere [6]. In brief, patients in GBP and diet group lost the same amount of weight (~ 10 kg). Diabetes medications were discontinued at the time of surgery for all GBP patients, and were decreased or stopped for diet patients, using algorithm based on American Diabetes Association (ADA) criteria of target glucose control. There was a significant and similar decrease of fasting glucose and fasting insulin after diet and after GBP. However, the recovery of the early phase insulin secretion in response to oral glucose and the improvement in incretin levels and effect were observed only after GBP and not after diet. Recent clinical studies of various types of bypass surgeries and/or ileal transposition in humans with BMI less than 35 kg/m2 suggest that the diabetes can be improved without weight loss [30]. Data on gut hormones in these patients has not been provided.
5. Long-term changes of incretins
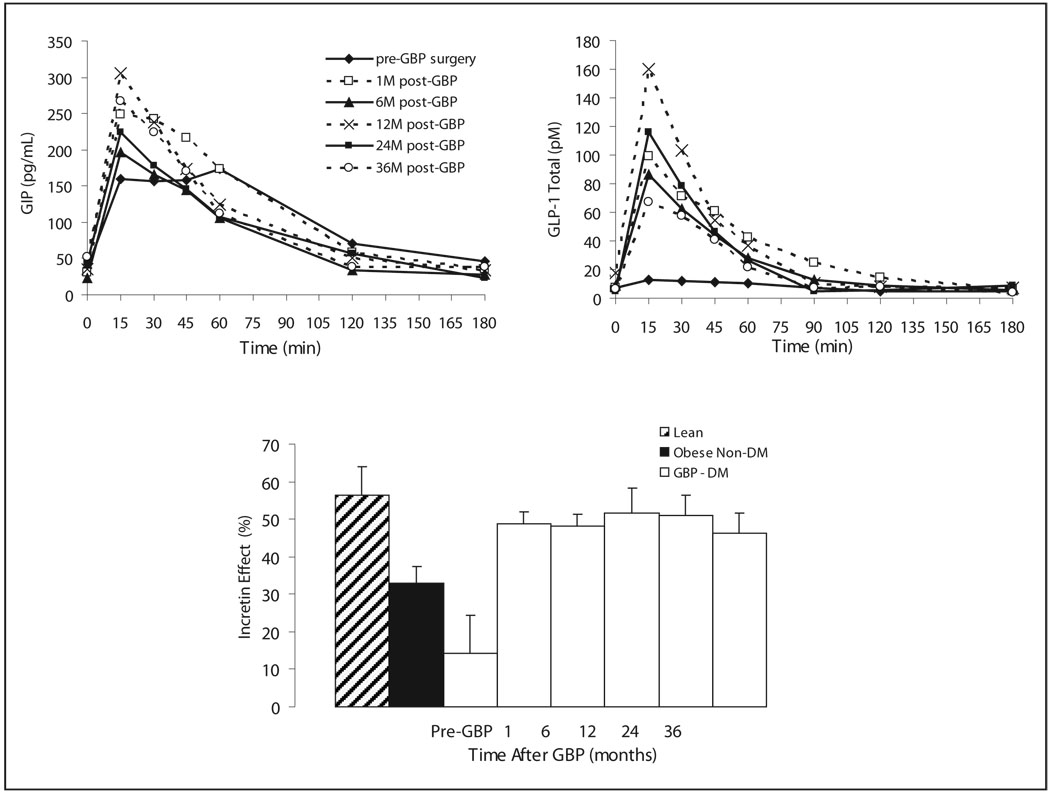
Do the incretin changes observed early after gastric bypass persist overtime? Cross sectional data from Naslund show a persistent increase fasting and post-prandial GLP-1 and GIP levels 20 years after DJB compared to obese non-operated controls [31]. Our own data show persistent increased GLP-1 response to oral glucose and GIP up to 3 years after GBP, after ~30kg weight loss, in patients with diabetes remission and normal incretin effect (Fig. 1). Off notes, the incretin levels and effect, the early phase insulin release during the OGTT and the insulinogenic index all improve rapidly (1 month) after GBP without further change at 6 and 12 months, in spite of continuous weight loss. On the contrary, other variables such as glucose levels improve as a function of weight loss up to one year. This suggests that some changes occur as a result of the surgery, independently of weight loss, while other changes are clearly weight loss related.
Fig. 1.
GLP-1 and GIP levels during an oral glucose tolerance test and incretin effect on insulin secretion before and at 1, 6, 12, 24 and 36 months after gastric bypass surgery in patients with type 2 diabetes.
Whether the improved post-prandial insulin and glucose levels observed one month after GBP is responsible for the later development of hyperinsulinemic hypoglycaemia with [32,33] or without [34] nesidioblastosis is unknown. Although GLP-1 has been shown to preserve human islet in vitro [35] and prevent beta cell apoptosis in rodents [36] there is no human data to suggest that GLP-1 increases beta cell mass after gastric bypass in humans.
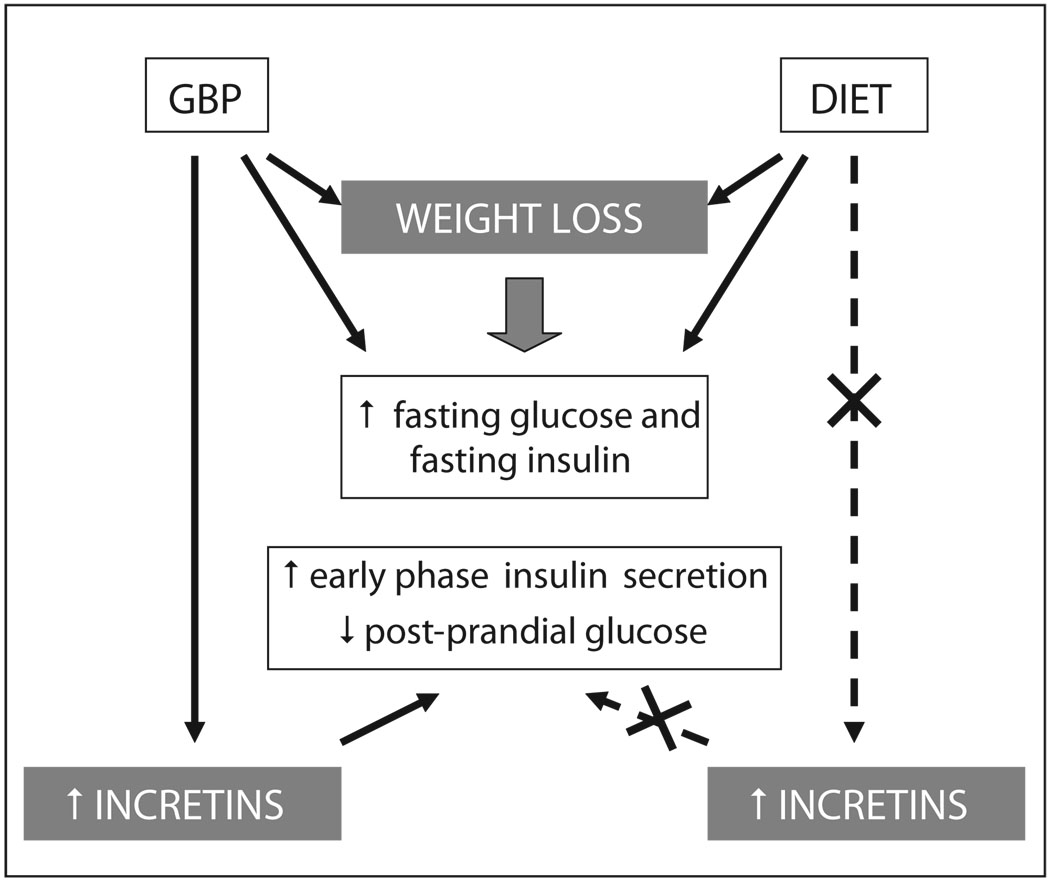
6. Mechanisms of incretin release after gastric bypass (Fig. 2)
Fig. 2.
Model of the mechanisms of diabetes control after weight loss by gastric bypass surgery (GBP) and diet. Both diet and GBP induce weight loss and decrease similarly fasting glucose and insulin. GBP, but not diet increases incretin levels and effect, improves early phase insulin secretion and decreases post-prandial glucose.
As a result of GBP, a small gastric pouch of about 30cc is anastomosed directly to the distal part of the ileum (alimentary limb). The rest of the stomach, including the pylorus, with the duodenum and part of the jejunum is shunted from the nutrients and reattached to the very distal par of the ileum to allow gastrointestinal and pancreatic juice to be excreted (biliopancreatic (BP) limb). Elegant studies in a rat model of diabetes suggest that the exclusion of the upper gut (foregut hypothesis), rather than weight loss, benefits glucose tolerance [37,38]. Rats after gastrojejunal bypass have better glucose tolerance than sham-operated pair-fed control animals with equivalent body weight [37]. The hindgut hypothesis suggests that the rapid stimulation of the distal ileum by nutrients is responsible for increased GLP-1 and beneficial effect on glucose tolerance, as suggested by studies of ileal transposition in rodents [39–41]. There are few data in humans to support these hypotheses. After GBP, the emptying of the gastric pouch is faster for liquids but delayed for solids [24,42]. The increased GE and intestinal transit time for liquid [24] may result in rapid release of GLP-1 by the distal ileum rapidly in contact with nutrients GLP-1. It is likely that both duodenal exclusion (foregut hypothesis) [43] and the rapid exposure of the distal ileum to undigested nutrients (hindgut hypothesis) [39,40] are possible mechanisms that may contribute to incretin levels increase after GBP, but this has not yet been studied in humans.
What is the place of incretins in the remission of type 2 diabetes after gastric bypass surgery? Data show clearly beneficial changes of incretin levels and effect after GBP, resulting in better profile of insulin secretion and decreased post-prandial glucose. However it is likely that weight loss by its magnitude (~40%) and its duration (years) is the major contributor to glucose control after GBP, via mechanisms other than incretins, such as decreased inèammation, decreased liver fat, intramyocellular fat, insulin resistance and changes in adipokines.
Acknowledgements
This work was funded by grants from the American Diabetes Association CR-7-05 CR-18, NIH R01-DK67561, GCRC 1 UL1 RR024156-02, ORC DK-26687, DERC DK-63068-05, Merck and Amylin Investigator Initiated Studies Program.
Footnotes
Conflicts of interests
The authors have reported no conèict of interests.
References
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 3.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 4.Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care. 1992;15:442–455. doi: 10.2337/diacare.15.3.442. [DOI] [PubMed] [Google Scholar]
- 5.Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 8.Holst JJ, Orskov C. Incretin hormones--an update. Scand J Clin Lab Invest Suppl. 2001;61 suppl 234:75–85. [PubMed] [Google Scholar]
- 9.Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550–E559. doi: 10.1152/ajpendo.00326.2004. [DOI] [PubMed] [Google Scholar]
- 10.Ebert R, Creutzfeldt W. Gastrointestinal peptides and insulin secretion. Diabetes Metab Rev. 1987;3:1–26. doi: 10.1002/dmr.5610030101. [DOI] [PubMed] [Google Scholar]
- 11.Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 12.Vilsboll T, Krarup T, Madsbad S, Holst J. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regulatory Peptides. 2003;114:115–121. doi: 10.1016/s0167-0115(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 13.Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest. 2004;113:635–645. doi: 10.1172/JCI20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- 15.Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25:781–792. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- 16.Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276(5 Pt 2):R1541–R1544. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 17.Naslund E, Bogefors J, Skogar S, Gryback P, Jacobsson H, Holst JJ, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277(3 Pt 2):R910–R916. doi: 10.1152/ajpregu.1999.277.3.R910. [DOI] [PubMed] [Google Scholar]
- 18.D'Alessio DA, Prigeon RL, Ensinck JW. Enteral enhancement of glucose disposition by both insulin-dependent and insulin-independent processes. A physiological role of glucagon-like peptide I. Diabetes. 1995;44:1433–1437. doi: 10.2337/diab.44.12.1433. [DOI] [PubMed] [Google Scholar]
- 19.Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 20.Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologia. 2006;49:253–260. doi: 10.1007/s00125-005-0107-1. [DOI] [PubMed] [Google Scholar]
- 21.Sarson DL, Scopinaro N, Bloom SR. Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes. 1981;5:471–480. [PubMed] [Google Scholar]
- 22.Naslund E, Gryback P, Hellstrom PM, Jacobsson H, Holst JJ, Theodorsson E, et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord. 1997;21:387–392. doi: 10.1038/sj.ijo.0800418. [DOI] [PubMed] [Google Scholar]
- 23.Naslund E, Backman L, Holst JJ, Theodorsson E, Hellstrom PM. Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg. 1998;8:253–260. doi: 10.1381/096089298765554449. [DOI] [PubMed] [Google Scholar]
- 24.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 25.Sarson DL, Besterman HS, Bloom SR. Radioimmunoassay of gastric inhibitory polypeptide and its release in morbid obesity and after jejuno-ileal bypass [proceedings] J Endocrinol. 1979;81:155P–156P. [PubMed] [Google Scholar]
- 26.Lauritsen KB, Christensen KC, Stokholm KH. Gastric inhibitory polypeptide (GIP) release and incretin effect after oral glucose in obesity and after jejunoileal bypass. Scand J Gastroenterol. 1980;15:489–495. doi: 10.3109/00365528009181506. [DOI] [PubMed] [Google Scholar]
- 27.Halverson JD, Kramer J, Cave A, Permutt A, Santiago J. Altered glucose tolerance, insulin response, and insulin sensitivity after massive weight reduction subsequent to gastric bypass. Surgery. 1982;92:235–240. [PubMed] [Google Scholar]
- 28.Kindel TL, Yoder SM, Seeley RJ, D'Alessio DA, Tso P. Duodenal-Jejunal Exclusion Improves Glucose Tolerance in the Diabetic, Goto-Kakizaki Rat by a GLP-1 Receptor-Mediated Mechanism. J Gastrointest Surg. 2009;13:1762–1772. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 29.Verdich C, Toubro S, Buemann B, Lysgard MJ, Juul HJ, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety--effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 30.Cohen RV, Schiavon CA, Pinheiro JS, Correa JL, Rubino F. Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg Obes Relat Dis. 2007;3:195–197. doi: 10.1016/j.soard.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Naslund E, Backman L, Holst JJ, Theodorsson E, Hellstrom PM. Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg. 1998;8:253–260. doi: 10.1381/096089298765554449. [DOI] [PubMed] [Google Scholar]
- 32.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 33.Cummings DE. Gastric bypass and nesidioblastosis--too much of a good thing for islets? N Engl J Med. 2005;353:300–302. doi: 10.1056/NEJMe058170. [DOI] [PubMed] [Google Scholar]
- 34.Kellogg TA, Bantle JP, Leslie DB, Redmond JB, Slusarek B, Swan T, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis. 2008;4:492–499. doi: 10.1016/j.soard.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Farilla L, Bulotta A, Hirshberg B, Li CS, Khoury N, Noushmehr H, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 36.Cornu M, Yang JY, Jaccard E, Poussin C, Widmann C, Thorens B. Glucagon-like peptide-1 protects beta-cells against apoptosis by increasing the activity of an IGF-2/IGF-1 receptor autocrine loop. Diabetes. 2009;58:1816–1825. doi: 10.2337/db09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacheco D, de Luis DA, Romero A, Gonzalez SM, Conde R, Izaola O, et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am J Surg. 2007;194:221–224. doi: 10.1016/j.amjsurg.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Patriti A, Facchiano E, Annetti C, Aisa MC, Galli F, Fanelli C, et al. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg. 2005;15:1258–1264. doi: 10.1381/096089205774512573. [DOI] [PubMed] [Google Scholar]
- 40.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 41.Strader AD. Ileal transposition provides insight into the effectiveness of gastric bypass surgery. Physiol Behav. 2006;88:277–282. doi: 10.1016/j.physbeh.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 42.Horowitz M, Collins PJ, Harding PE, Shearman DJ. Gastric emptying after gastric bypass. Int J Obes. 1986;10:117–121. [PubMed] [Google Scholar]
- 43.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]