SUMMARY
SETTING
Kisenyi slum in peri-urban Kampala, Uganda.
OBJECTIVES
Using chronic cough (≥2 weeks) inquiry as a screening tool to identify undetected smear-positive tuberculosis (TB) cases and to describe the characteristics of smear-positive TB cases detected by active case finding.
DESIGN
A house-to-house survey was conducted in five randomly selected villages in Kampala between June and August 2005. A sample of households was visited; adults aged ≥15 years were consecutively interviewed to identify those with chronic cough. Three sputum specimens were collected and examined by smear microscopy.
RESULTS
Among 930 individuals, we identified 189 (20%) chronic coughers. Of these, we found 33 (18%) undiagnosed smear-positive cases. The newly detected cases had an even sex distribution (P = 0.47), a median age of 30 years, a median cough duration of 1 month and 55% had acid-fast bacilli 1+ sputum smear grade.
CONCLUSION
These findings suggest that active case finding could supplement DOTS to yield additional smear-positive TB cases, lead to early diagnosis and thus shorten the duration of infectiousness before effective chemotherapy is initiated. In communities such as Kisenyi, this is a feasible strategy that may prove useful for TB control, but its cost-effectiveness needs to be evaluated. Early health care seeking for cough should be emphasized.
Keywords: tuberculosis, active case finding, case detection, chronic cough, smear-positive
NATIONAL TUBERCULOSIS (TB) Control Programs worldwide employ passive case finding as the standard strategy for case detection. Emphasis is placed on patients with smear-positive TB because they are responsible for up to 90% of the transmission occurring in the community.1 The passive approach relies on the voluntary presentation of symptomatic patients to the health system for diagnosis of TB and initiation of effective chemotherapy.2 Although passive case finding implemented through the DOTS strategy has been shown to have a positive impact on case detection in high-burden countries such as Vietnam, Peru and Ethiopia,3,4 its usefulness is hampered by patient and health system delays and perhaps underlying human immunodeficiency virus (HIV) infections which often lead to delays in diagnosis.5–8 The delay poses public health concerns because it increases the risk of TB transmission to patients’ contacts and death, especially in TB-HIV co-infected individuals.8–11 Active case finding (ACF) can be employed as a supplementary approach to curtail diagnostic delay in high TB burden settings.
ACF is an initiative taken by health care workers to identify symptomatic patients for medical evaluation and facilitate early entry into TB care.2 This approach has been shown to be useful in increasing case detection in both clinical and community settings, especially where HIV prevalence is high.12–15 Early diagnosis of smear-positive cases potentially breaks the chain of transmission of Mycobacterium tuberculosis to contacts. In an extensive review of case finding studies performed in both the pre- and post-chemotherapy era, it was evident that ACF resulted in identification of additional TB cases and reduction in morbidity and mortality from disease complications.2,16 In Uganda, two studies employing ACF within urban HIV voluntary testing centers reported similar findings.12,17 Our study explored the utility of community-based ACF in detecting smear-positive TB cases in high-risk communities such as slums.
The Uganda National TB Program (NTP) employs passive case detection to identify smear-positive TB cases. In 2006, the World Health Organization (WHO) estimated that Uganda detected only 44% of smear-positive TB cases, falling short of the recommended 70% case detection target.18 Kampala city, with a population of about 2.5 million people,19 reports the highest TB caseload, contributing 20% of the total cases notified.20 Studies conducted in Kampala show that significant delays occur between onset of TB symptoms and presentation of patients to the health facilities.7,21
This study assessed the feasibility of ACF in a periurban setting in Kampala by estimating the prevalence of chronic cough (≥2 weeks), the yield of undetected smear-positive TB cases and their characteristics.
METHODS
Study setting and population
A cross-sectional house-to-house survey was conducted in Kisenyi slum, Kampala, between 1 June and 15 August 2005. The slum is located in Kisenyi Parish II, an administrative unit that is composed of 10 small villages of about 1000 persons each. The slum is served by a public health clinic located about 5 km from its centre. The clinic offers free primary health care, including TB diagnostic and treatment services.
Adults aged ≥18 years or 15–17-year-old emancipated minors (persons below legal age but heading a household or parents) and residents of Kisenyi Parish II were eligible for the study. Potential participants were excluded if they did not speak English or Luganda, were reported to have psychiatric illnesses by family members, refused to give written consent or were absent from home on the day of the survey and after two consecutive attempts to trace them.
The Institutional Review Boards at University Hospitals Health Systems (UHHS) of Cleveland, Ohio and Makerere University School of Public Health in Kampala approved the study protocol.
Sampling
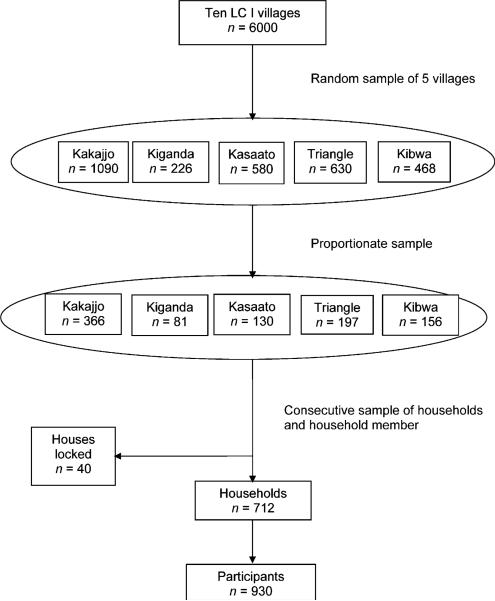
Kisenyi Parish II was purposively selected because it is over-crowded and therefore potentially at high risk for communicable diseases such as TB. Five of 10 villages were randomly selected, and the sample enrolled per village was calculated using proportions weighted upon population size. Consecutive households were visited starting from a road junction located closest to the centre of the village or a drainage channel where no road junction existed. The research assistants moved from the starting point in different directions. All eligible members in a household were enrolled until the required village sample was obtained (Figure 1).
Figure 1.
Sampling procedure. LC I = Local Council I (smallest government administrative unit).
Study measurements
Informed consent was administered in either English or Luganda, as preferred by the participants.
Trained interviewers conducted face-to-face interviews to assess socio-demographics, presence and duration of cough and TB treatment history. Participants were asked to volunteer information on whether they had ever had an HIV test. This was a sensitive question to ask in a household setting because disclosure of HIV status among couples remains problematic. Participants reporting chronic cough were requested to submit three sputum specimens (one on the survey day and two the next morning) for smear microscopy. On each day, the specimens were collected from the households by community assistants and transported in a cool box to the TB Reference Laboratory in Kampala.
Laboratory methods
Smear microscopy was performed using conventional Ziehl-Neelsen staining on un-concentrated sputum and read by two independent technicians. Positive smears were quantified using the International Union Against Tuberculosis and Lung Disease standards.22 A laboratory technologist verified a 20% random sample of the negative slides. To follow the routine NTP's protocol for new TB suspects, sputum cultures were not performed. Sputum results were entered into the laboratory database and a laboratory clerk completed result slips for each subject. Active TB was defined as at least two smear-positive results. Participants with one positive smear were requested to submit another sputum specimen.
Referrals for TB treatment and medical evaluation
Identified smear-positive TB cases were transported from their homes to Kisenyi Health Centre or Mulago TB clinic for notification and free TB treatment. Chronic coughers with negative smear results and household contacts of the smear-positive cases were not evaluated by the study due to limited resources, but were referred to the public health centre for further medical evaluation.
Analytic strategy
Data were entered into Epi Info 6.02 version (Centers for Disease Control and Prevention, Atlanta, GA, USA) and analyzed using Statistical Analysis Software (SAS) version 9.0 (SAS Institute, Cary, NC, USA). Categorical variables were compared using the χ2 test and Student's t-test for continuous variables. Univariate associations between age, sex, duration of cough and smear positivity were examined. The level of significance was defined as P < 0.05.
RESULTS
Of 753 households visited, 712 had at least an eligible adult, while 40 (5%) houses were found locked during the survey period. In the 712 households, there were 1000 potential participants, of whom 930 successfully completed study interviews, generating a response rate of 93%. The 7% non-response rate comprised 52% men who refused to participate in the study, while others were students or individuals away at work.
Description of study participants
Two thirds of the participants were women, and more than half of the subjects (52%) were married. Participants’ age ranged from 16 to 86 years, with a median of 29 years (interquartile range [IQR] 25–39) (Table 1). The predominant tribe was Muganda (38%), while about 15 different tribes made up the remaining 62% of the study population, reflecting the tribal/ethnic heterogeneity of the study population. Twenty-one per cent of the households had more than five members. Participants had a generally low level of education: more than half (59%) had ≤7 years of schooling. Of the 664 (71%) participants who volunteered to be asked about HIV testing, only 30% reported ever to have been tested.
Table 1.
Characteristics of study participants in Kisenyi, Kampala, Uganda, June–August 2005
| Characteristics | Cough (n = 321) n (%) | No cough (n = 609) n (%) | Overall (N = 930) n (%) | P value |
|---|---|---|---|---|
| Age, years* | ||||
| 15–24 | 89 (28) | 194 (32) | 283 (31) | 0.370 |
| 25–34 | 124 (39) | 231 (38) | 355 (38) | |
| 35–44 | 57 (18) | 111 (18) | 168 (18) | |
| ≥45 | 49 (15) | 72 (12) | 121 (13) | |
| Sex | ||||
| Female | 202 (63) | 426 (70) | 305 (33) | 0.029 |
| Male | 119 (37) | 183 (30) | 625 (67) | |
| Marital status | ||||
| Married | 153 (48) | 332 (55) | 485 (52) | 0.002 |
| Previously married | 105 (33) | 136 (22) | 241 (26) | |
| Never married | 61 (20) | 141 (23) | 202 (22) | |
| Tribe | ||||
| Muganda | 106 (33) | 247 (41) | 353 (38) | 0.027 |
| Others | 214 (67) | 362 (59) | 576 (62) | |
| Religion | ||||
| Catholic | 123 (39) | 165 (27) | 288 (31) | 0.004 |
| Protestant | 79 (25) | 130 (22) | 209 (23) | |
| Muslim | 93 (29) | 232 (38) | 325 (35) | |
| Others | 25 (8) | 81 (13) | 106 (12) | |
| Family size† | ||||
| ≤5 people | 186 (58) | 302 (50) | 560 (79) | 0.016 |
| >5 people | 135 (42) | 306 (50) | 152 (21) | |
| Educational level | ||||
| None | 63 (20) | 77 (13) | 140 (15) | <0.0001 |
| 1–7 years | 160 (50) | 248 (41) | 408 (44) | |
| >7 years | 98 (30) | 283 (46) | 381 (41) | |
| Employed | ||||
| Yes | 206 (65) | 375 (62) | 582 (63) | 0.309 |
| No | 111 (35) | 234 (38) | 348 (37) | |
| Average weekly income | ||||
| None | 114 (36) | 237 (39) | 351 (38) | 0.030 |
| ≤15 000 Ugshs‡ | 163 (51) | 258 (42) | 421 (45) | |
| >15 000 Ugshs‡ | 44 (13) | 114 (19) | 158 (17) | |
| Smoking | ||||
| Yes | 74 (23) | 43 (7) | 117 (13) | <0.0001 |
| No | 243 (77) | 565 (93) | 809 (87) | |
| Alcohol use | ||||
| Yes | 135 (42) | 115 (19) | 250 (27) | <0.0001 |
| No | 186 (58) | 493 (81) | 679 (73) | |
| Ever had an HIV test | ||||
| Yes | 80 (33) | 121 (30) | 201 (30) | 0.524 |
| No | 160 (67) | 294 (70) | 454 (70) | |
| Unknown§ | 237 |
Median age (IQR) = 29 (25, 39).
Mean family size (SD) = 5 (2.3).
15 000 Ugshs = $9.38.
Missing data = information not volunteered.
HIV = human immunodeficiency virus; IQR = interquartile range; SD = standard deviation; Ugshs = Ugandan shillings.
Prevalence of chronic cough
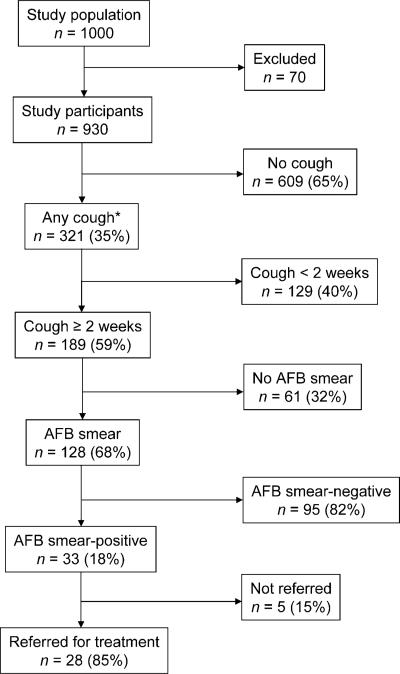
Three hundred and twenty-one (35%, 95% confidence interval [CI] 31–38), participants reported any cough, while 189 (20%, 95%CI 18–23) had chronic cough at the time of the household visits. Approximately one third (61, 32%) of chronic coughers did not submit sputum (Figure 2) for the following reasons: dry cough (n = 39), receiving TB treatment (n = 9), not at home at time of sputum collection (n = 5) and refused sputum test (n = 8). The chronic coughers who did not submit sputum were similar to those who submitted sputum in terms of median age (29 years), but were more likely to be female (65%) and had a longer mean duration of cough (8 vs. 6 weeks) than those who did submit sputum.
Figure 2.
Study profile. * Information regarding duration of cough not available for three subjects. AFB = acid-fast bacilli.
Prevalence of active TB among chronic coughers
Among 930 participants, 42 individuals (4.5%) were identified as having TB disease, of whom 33 (3.5%) were through ACF and nine (1%) through passive case finding (they were already on TB treatment). Among the 189 people reporting chronic cough, the prevalence of active TB was 22% (95%CI 16–28), including cases detected through active and passive case finding, but when we considered only those detected by ACF the prevalence was 18% (95%CI 12–23). In the survey, we did not find multiple TB cases from a single household. One in 28 adults had active TB, whereas approximately one in five adults with chronic cough had active TB. All chronic coughers not in care were included in this analysis, as they were potentially suspects for active TB at the time of the survey, although some did not submit sputum specimens for examination.
Characteristics of smear-positive TB cases
Of the 128 participants who underwent sputum examinations, 33 were smear-positive. The positive smear grade distribution was skewed towards lower grade (Table 2). The sputum smear-positive cases had a significantly shorter median duration of cough compared with chronic coughers with negative smears (1 vs. 2 months). The smear-positive cases were significantly older than the smear-negative cases; however, there was no difference in sex distribution. The 95 (74%) smear-negative chronic coughers were not evaluated further, but were offered advice by the research assistants to seek care from the local public health clinic.
Table 2.
Sputum results for chronic coughers (≥2 weeks), N = 128
| Variable | Smear positive (n = 33) n (%) | Smear negative (n = 95) n (%) | P value |
|---|---|---|---|
| Smear grades | |||
| 1+ | 18 (55) | NA | |
| 2+ | 12 (36) | NA | |
| 3+ | 3 (9) | NA | <0.001 |
| Cough duration, months, median (IQR) | 1.0 (0.6–5.6) | 1.8 (0.8–7.6) | 0.034 |
| Age, years, median (IQR) | 30 (23–44) | 26 (22–34) | 0.046 |
| Sex† | |||
| Male | 16 (48) | 39 (41) | 0.47 |
| Female | 17 (52) | 56 (59) |
NA = not applicable; IQR = interquartile range.
DISCUSSION
The main aim of our study was to detect undiagnosed active TB cases in the community. We found 33 (18%) cases of undiagnosed smear-positive TB among chronic coughers, suggesting that additional TB cases can be identified using ACF. Studies conducted elsewhere found relatively lower yields of TB cases among chronic coughers, ranging from 4% in South Africa to 13% in Ethiopia.13,23 However, it is important to note that our study was conducted in a slum, and the high yield observed from ACF could be due to high underlying TB and perhaps HIV prevalence in a potentially high-risk setting. Nonetheless, our findings highlight the need to target ACF strategies to similar crowded settings, such as prisons and inner cities.
The overall prevalence of TB in the study community was 4.5%, with nine cases already on treatment and 33 newly detected. The observed overall prevalence (4500/100 000) was almost seven times as high as the estimated prevalence of TB in the Ugandan general population, of 651/100 000.20 The difference between our study findings and the national estimates may be explained by the fact that national figures include all age groups and all forms of TB (not only smear-positive). Second, differences may to some extent be due to inadequate recording and reporting systems; the national notification data are therefore often incomplete, leading to underestimation. Third, the higher prevalence observed may be an indicator of ‘pockets’ of high TB prevalence areas existing within Kampala city, notably the slums. For Kampala District, where 27% of its population resides in slums,19 ACF could be an applicable strategy.
The observed 20% prevalence of chronic cough in the study community supports use of cough symptom inquiry as a simple and applicable tool for ACF, consistent with similar studies in Ethiopia.23,24 Previous studies used tools such as mass radiographs, physical examinations and TB symptom surveys, with varying sensitivity.25,26 These tools, used either singly or in combination, were shown to be effective in detecting 60–82% of active TB cases.2 Chronic cough inquiry was highly effective when used to identify TB cases in primary health care facilities and in household-based studies in Zimbabwe and Mexico, respectively.27–29 In Zimbabwe, a study in two urban primary health care clinics showed a high prevalence of TB among chronic coughers (43%, N = 544), 71% of whom had smear-positive disease. The prevalence of HIV infection was nearly twice as high as that of TB in the same group of patients. This finding suggested that chronic cough may also be a useful surrogate marker for HIV infection.28 Given the inextricable link between TB and HIV, chronic cough could become an increasingly valuable community screening tool for both TB and HIV in low-resource settings.
In this study, the smear-positive cases that were detected were not different in age and sex from the smear-negative patients with cough, consistent with a study in Ethiopia.23 The duration of cough was relatively short for smear-positive coughers compared to coughers whose smears were negative. A possible explanation could be that the more ill smear-positive cases had already been passively detected by the time of the survey. However, the average duration of cough for clinic-based studies of smear-positive patients attending public health clinics in Ethiopia and in Kampala, Uganda, was 3 to 4 months before presenting for care.5,7,21,30
The smear grade of the cases detected by the ACF survey was generally lower than that of cases typically diagnosed at a referral TB clinic in Kampala (1+, 55% vs. 10%).7,21 These findings suggest that ACF could potentially enhance earlier detection of TB cases, and reduce the duration of morbidity and the risk of transmission to others before diagnosis or initiation of chemotherapy. Moreover, patients detected through passive case finding often have advanced TB disease due to patient and/or health system delays in the diagnosis of symptomatic patients.21
The smear-negative coughers should ideally have been evaluated further in the routine health system to rule out smear-negative TB, which is fairly likely in high HIV settings such as Uganda. Second, rigorous clinical evaluation would benefit these patients with respiratory symptoms, as recommended under the new WHO Practical Approach to Lung Health (PAL) strategy.31
Nine cases were already receiving treatment for active TB under DOTS compared to 33 who were not in care. This means that for every one passively detected case there were at least three new cases found. This is higher than the 1:2 cases found in a similar study in southern Ethiopia.23 Through the ACF strategy, additional active TB cases not in care were identified, highlighting its potential to increase TB case detection in high TB burden settings.15 However, it is important to strengthen the health system to handle the increased workload. Rapid expansion of TB microscopy services to the lowest primary health care level could be a valuable intervention. Community TB education and awareness programs should continue to emphasize early health care seeking for cough.
CONCLUSIONS
The burden of undiagnosed TB among individuals with chronic cough in a slum setting in Kampala was high. ACF should be considered as a potential supplementary approach to the existing DOTS strategy of passive case detection. In communities such as Kisenyi, ACF is a feasible strategy that may prove useful for TB control, but its cost-effectiveness needs to be evaluated.
Acknowledgements
The authors thank NTP Manager Dr F E Adatu for his overall technical support and guidance during data collection, the technicians at the Wandegeya National Reference Laboratory for diligently performing the microscopic smear examinations, A Chiunda for his support during data analysis, research assistants S Nalumansi, H Nalubwama and E Namisango who worked tirelessly to gather the data and data management officer F Nakayima. The study was supported by funds from the Fogarty International Centre ICOHRTA training programme grant number D43 TW000011.
References
- 1.Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis. 2003;3:288–296. doi: 10.1016/s1473-3099(03)00609-1. [DOI] [PubMed] [Google Scholar]
- 2.Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9:1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO reports 10 million TB patients successfully treated under ‘DOTS’ 10 years after declaring TB a global emergency. WHO; Geneva, Switzerland: 2003. [Google Scholar]
- 4.Shargie EB, Lindtjorn B. DOTS improves treatment outcomes and service coverage for tuberculosis in South Ethiopia: a retrospective trend analysis. BMC Public Health. 2005;5:62. doi: 10.1186/1471-2458-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salaniponi FM, Harries AD, Banda HT, et al. Care-seeking behaviour and diagnostic processes in patients with smear-positive pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2000;4:327–332. [PubMed] [Google Scholar]
- 7.Kiwuwa MS, Charles K, Harriet MK. Patient and health service delay in pulmonary tuberculosis patients attending a referral hospital: a cross-sectional study. BMC Public Health. 2005;5:122. doi: 10.1186/1471-2458-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golub JE, Bur S, Cronin WA, et al. Delayed tuberculosis diagnosis and tuberculosis transmission. Int J Tuberc Lung Dis. 2006;10:24–30. [PubMed] [Google Scholar]
- 9.Whalen CC. Failure of directly observed treatment for tuberculosis in Africa: a call for new approaches. Clin Infect Dis. 2006;42:1048–1050. doi: 10.1086/501022. [DOI] [PubMed] [Google Scholar]
- 10.Lawn SD, Wood R. When should antiretroviral treatment be started in patients with HIV-associated tuberculosis in South Africa? S Afr Med J. 2007;97:412, 4–5. [PubMed] [Google Scholar]
- 11.Whalen CC, Nsubuga P, Okwera A, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aisu T, Raviglione MC, van Praag E, et al. Preventive chemotherapy for HIV-associated tuberculosis in Uganda: an operational assessment at a voluntary counselling and testing centre. AIDS. 1995;9:267–273. [PubMed] [Google Scholar]
- 13.Pronyk PM, Joshi B, Hargreaves JR, et al. Active case finding: understanding the burden of tuberculosis in rural South Africa. Int J Tuberc Lung Dis. 2001;5:611–618. [PubMed] [Google Scholar]
- 14.Kimerling ME, Schuchter J, Chanthol E, et al. Prevalence of pulmonary tuberculosis among HIV-infected persons in a home care program in Phnom Penh, Cambodia. Int J Tuberc Lung Dis. 2002;6:988–994. [PubMed] [Google Scholar]
- 15.Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comstock GW. Commentary: the first Framingham Study—a pioneer in community-based participatory research. Int J Epidemiol. 2005;34:1188–1190. doi: 10.1093/ije/dyi178. [DOI] [PubMed] [Google Scholar]
- 17.Mugisha B, Bock N, Mermin J, et al. Tuberculosis case finding and preventive therapy in an HIV voluntary counselling and testing center in Uganda. Int J Tuberc Lung Dis. 2006;10:761–767. [PubMed] [Google Scholar]
- 18.World Health Organization . Global tuberculosis control. WHO report 2008. WHO; Geneva, Switzerland: 2008. [Google Scholar]
- 19.Uganda Bureau of Statistics . 2002 Uganda population and housing census. Report. UBoS; Kampala, Uganda: 2004. [Google Scholar]
- 20.Uganda National Tuberculosis and Leprosy Program . National Tuberculosis and Leprosy Control Program: Annual Report. National Tuberculosis and Leprosy Control Program; Kampala, Uganda: 2005. [Google Scholar]
- 21.Guwatudde D, Nakakeeto M, Jones-Lopez EC, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–898. doi: 10.1093/aje/kwg227. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enarson DA, Rieder HL, Arnadottir T, Trébucq A. Management of tuberculosis: a guide for low-income countries. 5th ed. International Union Against Tuberculosis and Lung Disease; Paris, France: 2000. [Google Scholar]
- 23.Demissie M, Zenebere B, Berhane Y, Lindtjorn B. A rapid survey to determine the prevalence of smear-positive tuberculosis in Addis Ababa. Int J Tuberc Lung Dis. 2002;6:580–584. [PubMed] [Google Scholar]
- 24.Shargie EB, Morkve O, Lindtjorn B. Tuberculosis case-finding through a village outreach programme in a rural setting in southern Ethiopia: community randomized trial. Bull World Health Organ. 2006;84:112–119. doi: 10.2471/blt.05.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harries AD, Kamenya A, Subramanyam VR, et al. Screening pulmonary tuberculosis suspects in Malawi: testing different strategies. Trans R Soc Trop Med Hyg. 1997;91:416–419. doi: 10.1016/s0035-9203(97)90262-5. [DOI] [PubMed] [Google Scholar]
- 26.van Cleeff MR, Kivihya-Ndugga L, Githui W, Nganga L, Odhiambo J, Klatser PR. A comprehensive study of the efficiency of the routine pulmonary tuberculosis diagnostic process in Nairobi. Int J Tuberc Lung Dis. 2003;7:186–189. [PubMed] [Google Scholar]
- 27.Sanchez-Perez HJ, Hernan MA, Hernandez-Diaz S, Jansa JM, Halperin D, Ascherio A. Detection of pulmonary tuberculosis in Chiapas, Mexico. Ann Epidemiol. 2002;12:166–172. doi: 10.1016/s1047-2797(01)00308-8. [DOI] [PubMed] [Google Scholar]
- 28.Munyati SS, Dhoba T, Makanza ED, et al. Chronic cough in primary health care attendees, Harare, Zimbabwe: diagnosis and impact of HIV infection. Clin Infect Dis. 2005;40:1818–1827. doi: 10.1086/429912. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Perez H, Flores-Hernandez J, Jansa J, Cayla J, Martin-Mateo M. Pulmonary tuberculosis and associated factors in areas of high levels of poverty in Chiapas, Mexico. Int J Epidemiol. 2001;30:386–393. doi: 10.1093/ije/30.2.386. [DOI] [PubMed] [Google Scholar]
- 30.Demissie M, Lindtjorn B, Berhane Y. Patient and health service delay in the diagnosis of pulmonary tuberculosis in Ethiopia. BMC Public Health. 2002;2:23. doi: 10.1186/1471-2458-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization . Practical approach to lung health. WHO, STOP TB Department; Geneva, Switzerland: 2005. [Google Scholar]