Abstract
The establishment of the coronary circulation is critical for the development of the embryonic heart. Over the last several years there has been tremendous progress in elucidating the pathways that control coronary development. Interestingly, many of the pathways that regulate the development of the coronary vasculature are distinct from those governing vasculogenesis in the rest of the embryo. It is becoming increasingly clear that coronary development depends on a complex communication between the epicardium, the sub-epicardial mesenchyme, and the myocardium mediated in part by secreted growth factors. This communication coordinates the growth of the myocardium with the formation of the coronary vasculature. This review will summarize our current understanding of the role of these growth factors in the regulation of coronary development. Continued progress in this field holds the potential to lead to novel therapeutics for the treatment of patients with coronary artery disease.
Keywords: Epicardium, myocardium, coronary vessel, embryogenesis, growth factors, transcription factors
Cardiovascular disease is still the leading cause of death in the United States despite progress in reducing mortality over the recent years. Coronary artery disease accounts for over half of all these cardiovascular deaths and often leads to progressive heart failure due to recurrent myocardial ischemia and/or infarction.1 Pursuit of effective therapies to treat and prevent coronary artery disease will be aided by a more complete understanding of the molecular pathways that regulate coronary development. It is clear that the coronary vasculature develops in a unique manner from other vascular beds in the developing embryo and that this process is critically dependent on interactions between the myocardium and epicardium.
Significant progress has been achieved over the last decade in elucidating the morphological events in the formation of the coronary arteries and this has been the subject of some excellent recent reviews.2-6 Cells destined to form the coronary vasculature are thought to originate in the proepicardium, a structure located dorsal to the developing heart tube just caudal to the future atrioventricular junction.7, 8 These cells migrate and attach to the looped heart tube at the dorsal aspect of the atrioventricular junction beginning around embryonic day (E) 9.0 in the mouse (see Figure 1).9 Subsequently, these cells form the epicardium, an epithelial sheath that completely envelops the heart by E11.0. Once the epicardial cell layer is formed, a subset of cells of the epicardium undergoes a transformation to become mesenchyme and migrate into the space beneath the epicardium (the sub-epicardium), while some cells migrate further into the myocardium. Cells of the sub-epicardial mesenchyme are thought to give rise to cardiac fibroblasts, coronary vascular smooth muscle, and coronary endothelial cells.7, 8, 10, 11 Using genetic lineage tracing, two groups have recently reported cells of the epicardium may also give rise to cardiomyocytes.12, 13 The differentiation of the sub-epicardial mesenchyme into each of these lineages may be influenced by factors produced by the myocardium and epicardium.
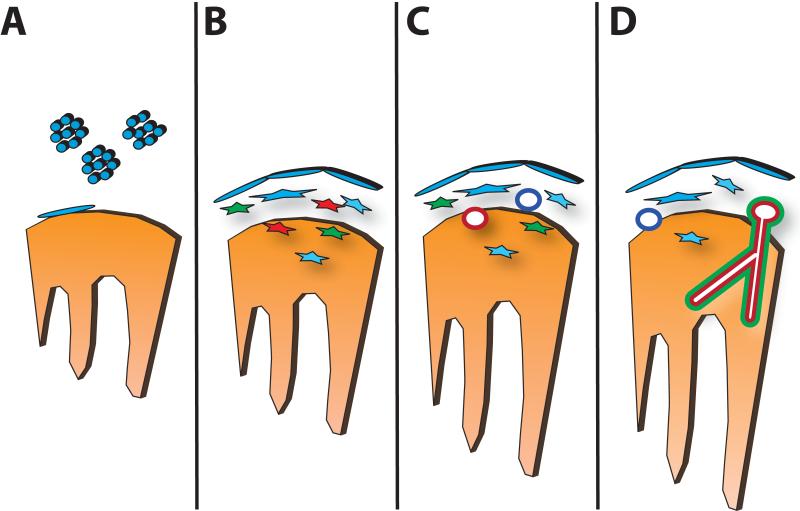
Figure 1. Steps in Coronary Vascular Development.
In (A), the epicardium is formed by migration of proepicardial cells (blue) to the surface of the heart tube (orange), forming an epithelial sheet. In (B), epithelial-to-mesenchymal transformation produces cells with hemangioblast (red), smooth muscle (green) and fibroblast (blue) cell fates. In (C), a primitive endothelial plexus forms from the endothelial progenitors, with venous-fated endothelial tubes (dark blue) forming subepicardially and arterial-fated endothelial tubules forming intramyocardially (red). In (D), the endothelial plexus remodels and recruits vascular smooth muscle and other adventitial cells to form the mature coronary arteries (red) and veins (dark blue).
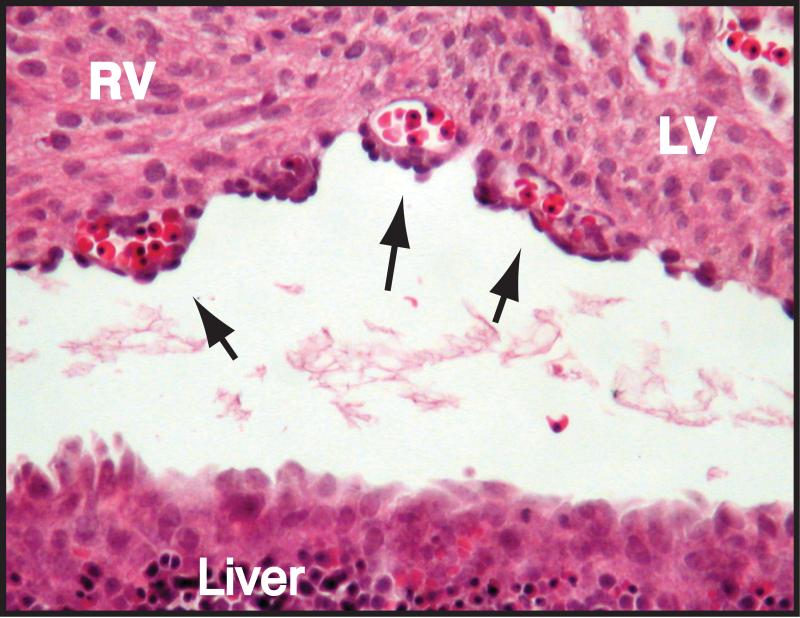
Coronary endothelial cells are likely derived, at least in part, from precursors present in the sub-epicardial mesenchyme. It is still somewhat unclear if coronary endothelial cell progenitors are hemangioblasts, progenitors capable of giving rise to cells of the hematopoietic as well as vascular lineages, or are restricted solely to the vascular lineage (i.e., angioblasts). Hemangioblasts that contribute to the systemic circulation are found in the developing yolk sac, aorta, and embryonic liver.14 The evidence to support the existence of sub-epicardial hemangioblasts is based on three observations. First, we and others have noted the presence of hematopoietic cells in the lumen of the developing coronary vascular plexus at a time prior to the connection of this plexus to the aortic circulation.15, 16 Shown in Figure 2 is a histologic section of an E12.5 mouse heart in which multiple sub-epicardial endothelial lumens are filled with erythrocytes. Since the coronary vascular plexus is not connected to the systemic circulation at this time in development, for these hematopoietic cells to be present within the lumens of the plexus, they are likely to have been derived from a progenitor that also gave rise to the vascular endothelium. Second, lineage-tracing experiments demonstrated a proepicardial origin of the erythrocytes found in the early subepicardial vascular plexus.15 Third, Levine and colleagues demonstrated the existence of sub-epicardial cells that express SCL, CD45, and Sca-1, all markers of the hemangioblast in other tissues.16 Taken together, these observations suggest the existence of a sub-epicardial hemangioblast, but further work is necessary to support this idea.
Figure 2. Evidence for a Sub-epicardial Hemangioblast.
Shown above is a transverse section of a wild-type embryonic day 12.5 heart at the level of the interventricular groove stained with hematoxylin and eosin. Arrows indicate red blood cell-filled lumens of the primitive endothelial plexus.
Once vascular progenitor cells are present in the sub-epicardium, they undergo vasculogenesis as the next step in coronary vascular development. Vasculogenesis is the process whereby vascular endothelial cell progenitors form a de novo vascular network. The sub-epicardial endothelial cells coalesce to form interconnected vascular tubes in the sub-epicardial space beginning at approximately E11.5. Plexus formation begins at the atrioventricular junction on the inferior surface of the heart and spreads toward the apex to nearly completely cover the right and left ventricles by E13.5. At this time in development, before connecting to the systemic circulation, the plexus remodels by the sprouting of new vessels from existing ones, a process termed angiogenesis. During this remodeling phase, smooth muscle cells and pericytes are recruited to form more mature coronary vessels. The coronary arteries then connect to the base of the aorta through the right and left coronary ostia to establish the coronary circulation by approximately E14.5.17 The vessels continue to remodel over the next 1-2 days and produce the mature coronary vascular bed.
As can be appreciated from the above description, the development of the coronary vasculature is a complex process, with multiple cell types interacting in a temporally specific fashion to generate the mature coronary tree. Given this complexity, it is not surprising that current work in the field is uncovering a robust and extensive signaling network between cell types within the developing heart. This review will focus on some of the pathways that illustrate the importance of ‘cross-talk’ between the myocardium and epicardium in directing coronary vascular development.
Epicardial-derived Signals Directing Coronary Development
The signals that influence coronary development can be classified into those originating from the epicardium or epicardial-derived cells, and those from cardiomyocytes. Many of these signals are growth factors that act in an autocrine or paracrine fashion, some of which have been described to be important in directing vasculogenesis within other vascular beds (see Table 1). The purpose of these signals is likely to coordinate the development of the epicardium and coronary vasculature with that of the underlying myocardium. In this section, we will review those signals originating from the epicardium.
Table 1. Growth Factors in Coronary Development.
| Molecule | Expression | Functions | Reference |
|---|---|---|---|
| TGFβ1-3 | Epicardial, Myocardial |
Supports epicardial EMT; maintains epicardial/myocardial adhesion; promotes smooth muscle cell recruitment |
25, 48-51 |
| FGF-1 | Epicardial, Myocardial |
Promotes epicardial EMT | 63 |
| FGF-2 | Epicardial, Myocardial |
Promotes epicardial EMT; induces endothelial cell differentiation from epicardial-derived cells; promotes vascular tube formation; required for coronary ostia formation |
63, 67, 72 |
| FGF-7 | Myocardial | Promotes epicardial EMT | 63 |
| FGF-9,-16,-20 | Epicardial | Supports myocardial proliferation; indirectly promotes coronary plexus formation via induction of Shh, VEGF, and Angiopoietin expression |
59, 64 |
| VEGF | Myocardial | Induces epicardial EMT; promotes endothelial cell tubulogenesis and proliferation; required for coronary ostia formation |
15, 72, 91, 92 |
| PDGF | Myocardial | Promotes epicardial EMT; supports smooth muscle cell differentiation and recruitment; promotes endothelial cell tubulogenesis? |
77, 78, 84 |
| Wnt/β-catenin | Epicardial | Promotes epicardial EMT; supports coronary vessel remodeling; Smooth muscle cell differentiation, recruitment |
28, 46 |
| Retinoic Acid | Epicardial | Maintains myocardial/epicardial adhesion; promotes epicardial EMT; indirectly promotes cardiomyocyte proliferation and differentiation |
28, 35 |
| Erythropoietin | Epicardial | Indirectly promotes cardiomyocyte proliferation; induces coronary vascular plexus formation |
35, 38 |
| Sonic Hedgehog | Epicardial | Promotes epicardial EMT; induces VEGF and Ang2 expression in myocardium |
16, 64 |
| Ang2 | Myocardial | Rescues coronary vascular plexus formation in combination with VEGF when Shh signaling is blocked |
64 |
Adhesion Molecules in Epicardial/Myocardial Signaling
As described above, the first event in coronary development is the establishment of an intact epicardium. The failure to establish the epicardium blocks coronary vascular development as has been demonstrated in the chick by the physical inhibition of proepicardial migration to the developing heart.18 Close apposition of myocardium and epicardium is essential for communication between these tissues, as each is dependent on signaling cues from the other during development. Accordingly, molecules that mediate myocardial/epicardial cell attachment have been demonstrated to be an essential first step for coronary vessel development. One such molecule, α4 integrin, is a cell-surface protein that is involved in cell-cell and cell-matrix adhesion. Homozygous integrin α4 mutant embryos fail to survive beyond E15.5 (see Table 2). One of the defects observed in these mutants is the absence of a ventricular epicardial sheath. Consequently these animals fail to generate sub-epicardial mesenchyme or a coronary plexus.19 Mice harboring mutations in the gene encoding a binding partner of α4 integrin, vascular cell adhesion molecule (VCAM)-1, display a phenotype remarkably similar to that of α4 integrin nulls.20 VCAM-1 is expressed in cardiomyocytes at E11.5. As reported in α4 integrin null embryos, VCAM-1 mutant embryos succumb between E12.5 and E13.5, fail to maintain a ventricular epicardium, and do not elaborate a coronary plexus. The similarity between the α4 integrin and VCAM-1 null mice, and their reciprocal expression patterns in affected tissues, support the hypothesis that these molecules are required for epicardial cells to remain attached to the myocardium and support myocardial and coronary vessel development.
Table 2. Animal Models with Coronary Vascular Defects.
| Gene | Animal Model |
Coronary Phenotype | Reference |
|---|---|---|---|
| α4 Integrin | Itga4−/− | Does not maintain epicardium | 19 |
| VCAM-1 | Vcam1−/− | Does not form epicardium | 20 |
| WT-1 | Wt1−/− | Does not form complete epicardium | 21 |
| Angiopoietin1 | MHC-Ang1 Transgenic |
Failure to maintain an intact epicardium | 95 |
| Podoplanin | Podo−/− | Delayed epicardial formation; partially detached epicardium |
26 |
| TGFβ Receptor III | Tgfbr3−/− | Excessive epicardial EMT; capillary plexus formation reduced |
25 |
| RXRα | RXRαf/f; GATA5-Cre |
Partially detached epicardium; thin sub-epicardial mesenchymal layer; reduced coronary branching |
28 |
| Erythropoietin Receptor |
EpoR−/− | Partially detached epicardium; PECAM-positive cells unable to form subepicardial vascular plexus |
38 |
| BAF180 | Pbrm1−/− | Attenuated epicardial EMT; PECAM-positive sub- epicardial nodules |
108 |
| Thymosin β4 | Tb4shRNAf; Nkx2.5-Cre |
Partially detached epicardium; Tie2-positive cells unable to form plexus; failed recruitment of coronary smooth muscle |
85 |
| Connexin 43 | Cx43−/− | Partially detached epicardium; decreased epicardial EMT and migration; perturbed capillary plexus remodeling |
88, 89 |
| FOG-2 | Zfpm2−/− | Epicardium intact; Does not form capillary plexus | 106, 107 |
| GATA4 | GATA4ki/ki | Epicardium intact; Does not form capillary plexus | 119 |
| p300 | p300+/AS | Delayed epicardial and capillary plexus formation | 109 |
| FGF9 | FGF9−/− | Delayed formation of the capillary plexus | 64 |
| FGF Receptors | FGFR1f/−; FGFR2f/−; Mlc2v-Cre |
Delayed formation of the capillary plexus | 64 |
| Hedgehog Signaling |
Smof/−; Mlc2v-Cre |
Absent sub-epicardial mesenchyme; failure to form coronary veins (EphB4-positive sub-epicardial vessels) |
16 |
| Smof/−; Dermo1-Cre |
Reduced intramyocardial arterial vessels (Ephrin B2- positive vessels) |
16 | |
| PDGF Receptor | Pdgfrb−/− | Decreased epicardial migration; no coronary vascular smooth muscle |
78, 83 |
| β-Catenin | Ctnnb1f/f; GATA5-Cre |
Thin sub-epicaridal mesenchymal layer; decreased migration of sub-epicardial mesenchymal cells; impaired differentiation of coronary arterial smooth muscle cells |
46 |
| ALK5 | Alk5f/−; GATA5-Cre |
Partially detached epicardium; increased capillary density; failed recruitment of coronary smooth muscle |
27 |
| Vangl2 | Lp/Lp | Reduced smooth muscle cell recruitment; aberrant sub-epicardial vessels |
87 |
| Smad6 | Smad6−/− | Dilated coronary vessels with reduced smooth muscle | 55, 56 |
| Tbx-1 | Tbx-1−/− | Abnormal patterning of proximal coronary arteries | 112 |
Other mouse mutants have demonstrated a failure of epicardial cells to maintain attachment to the myocardium and the underlying subepicardial layer. The transcription factor Wilms’ tumor (WT)-1 is essential for proper epicardial formation and coronary vessel development. The epicardium begins to form in WT-1 mutant animals, but does not extend beyond the caudal-most portion of the heart.21 In WT-1 mutant hearts at E12.5, α4 integrin expression is reduced approximately 50%.22 This reduced level of α4 integrin alone, however, does not explain the phenotype of the WT-1 null animal as α4 integrin heterozygous animals are viable.19, 23 Likely the loss of other, as-yet unidentified downstream targets is critical in mediating the phenotype of the WT-1 null animal.24 Similar separation between the epicardium and myocardium was also reported in mice with a complete knockout of the Type III TGFβ receptor25, podoplanin26, epicardial-specific knockout of ALK527 and epicardial-specific knockout of the retinoic acid receptor RXRα28 Whether the gap between the cell layers in these animals is due to mis-expression of adhesion molecules, or secondary to the cardiac insufficiency seen in these animals, remains to be determined.
As the cells of the proepicardium migrate onto the heart and form the epicardium, the epicardium assumes a role as a “signaling center” for the developing heart. This center coordinates the growth of the myocardium with the development of the coronary vasculature. As a first step, the epicardium begins to express signaling molecules, including retinoic acid, erythropoietin, and fibroblast growth factors, which are important in inducing proliferation of the underlying myocardium either directly or indirectly. Consistent with this notion, a gradient of myocyte proliferation from epicardium to endocardium has been observed, with highest rates of proliferation occurring closest to the epicardium.29 Thus, epicardial formation and subsequent development of the coronary vasculature are coupled to the proliferation of the myocardium.
Retinoic Acid
Retinoid signaling, within a discrete range of activity, is critical for normal embryogenesis and cardiac function. Quail embryos deprived of vitamin A develop thin-walled hearts that fail to undergo looping.30-32 Studies in mouse also demonstrate a requirement for retinoid signaling in the mammalian embryo. Targeted disruption of Raldh2, a gene critical for retinoid metabolism and signaling, results in early embryonic death due to cardiac insufficiency.33 The heart tube forms in Raldh2 deficient embryos, but is thin-walled, dilated, and fails to loop properly. In contrast, exposing embryos to high levels of retinoic acid through dietary vitamin A supplementation of pregnant dams also results in embryonic cardiovascular defects (reviewed in 34), making it clear that levels of retinoic acid must be tightly controlled during embryogenesis.
Studying signaling between different cardiac cell populations in a slice-culture system, Stuckmann and colleagues found that an epicardial-derived soluble factor was required for cardiac myocyte proliferation.35 Blocking retinoic acid signaling in slice cultures that included both myocardium and epicardium inhibited cardiac myocyte proliferation. Myocyte proliferation was similarly affected by removing the epicardium from slices before placing them into culture. Addition of exogenous retinoic acid to epicardial-denuded slices failed to rescue myocyte proliferation. However, conditioned medium from cultured epicardial cells treated with retinoic acid restored myocyte proliferation in denuded slice cultures. Thus, while the target of retinoic acid signaling in the heart is the epicardium, it functions to support myocardial proliferation through directing the production of a soluble proliferation factor from epicardial cells.
Retinoic acid signals are transduced by two families of nuclear hormone receptors, the retinoic acid receptors (RARs) and retinoid X receptors (RXRs). Targeted deletion of RXRα in mice results in myocardial hypoplasia and, eventually, death of the embryo from cardiac insufficiency. These findings are consistent with the phenotypes observed in vitamin A deficient quail embryos36, although the ability of RXR to heterodimerize with other nuclear hormone receptors – including the vitamin D receptor, thyroid hormone receptor and peroxisome proliferation activator receptor – complicates the interpretation of these results.
Using tissue-specific promoters to remove RXRα from discrete populations of cells within the heart has clarified somewhat the role of this gene during heart development and coronary vessel formation. Conditional deletion of RXRα in ventricular myocytes reveals that myocyte proliferation and ventricular chamber maturation do not require cell-autonomous RXRα activity.37 Conditional deletion of RXRα in epicardial-derived cells using Gata5-Cre has an incompletely penetrant embryonic lethal phenotype. The hearts in these mice have a detached epicardium and thin sub-epicardial mesenchymal layer, thought to be secondary to decreased FGF-2 production by the epicardium.28 The coronary arteries were also abnormal, with reduced branching patterns suggestive of a defect in coronary angiogenesis. Notably, the ventricular cells in these animals abnormally maintain expression of amhc2, a gene downregulated in ventricular myocytes as cardiogenesis proceeds. This observation allows the possibility that epicardial cells not only support myocardial proliferation, but instruct myocardial differentiation as well. Together these data produce a model where retinoid signaling through RXRα is required to support cardiac development by acting on the epicardium, not by directly signaling to cardiac myocytes.
Erythropoietin
Targeted deletion of the gene encoding the cytokine erythropoietin or its receptor (EpoR) produces a thin-walled myocardial phenotype similar to the phenotype observed with mutations in the retinoic acid metabolism pathway.38 EpoR is expressed in the epicardium, endocardium and endocardial cushions of the developing heart between E10.5 and E13.5, but not in cardiomyocytes. Mouse embryos deficient in erythropoietin or the erythropoietin receptor die in mid gestation of anemia and heart defects. The cardiac defects observed include a thin walled ventricle and a partially detached epicardium. In addition, the coronary vascular plexus does not form appropriately, as only nodules of PECAM-positive cells were observed in the sub-epicardium of erythropoietin receptor deficient mice.38 This defect could be secondary to a role for erythropoietin in regulating coronary vasculogenesis, although in other vascular beds, erythropoietin seems to be required for angiogenesis rather than vasculogenesis.39
As was observed with retinoic acid, erythropoietin stimulates myocardial proliferation only when myocytes are co-cultured with epicardial cells or are given conditioned medium from epicardial cells treated with erythropoietin.35 These results suggest that erythropoietin acts within the epicardium to induce proliferation of cardiomyocytes indirectly. Interestingly, blocking erythropoietin signaling using an anti-EpoR antibody in the myocardial slice system resulted in decreased cardiomyocyte proliferation that could be rescued by the addition of retinoic acid. Further, blocking retinoic acid signaling in this system reduced cardiomyocyte proliferation and this could be rescued by the addition of erythropoietin. Together, these results suggest that retinoic acid and erythropoietin act on the epicardium in interdependent, parallel pathways to produce signals that induce proliferation of the underlying myocardium.
Wnt Signaling
The Wnt/wingless signaling pathway is critical for heart development in organisms from flies to mice.40-42 Wnts that signal through the canonical pathway stabilize β-catenin protein, whereas signaling via the β-catenin-independent non-canonical pathway involves small GTPases and PKCδ43 Some evidence suggests that the pathway through which Wnt ligands signal is based on the complement of ligands and receptors expressed by inductive and responsive tissues.43, 44 A number of Wnts and their receptors are expressed in the heart during cardiac development.45
Epicardial-restricted deletion of the β-catenin gene in mice results in embryonic death between E15.5 and birth.46 These mice present with a thin compact zone myocardium due to decreased myocyte proliferation. The sub-epicardial mesenchymal layer is also thin, suggesting diminished epicardial EMT. While a primitive capillary plexus forms in the subepicardial space and the coronary venous system remodels appropriately, the coronary arterial vessels fail to form in the hearts from mice with epicardial-deleted β-catenin. These vessels failed to recruit smooth muscle cells, and explanted epicardial cells failed to express smooth muscle cell markers when treated with TGFβ1. These results suggest a primary failure of β-catenin-null epicardial cells to differentiate into smooth muscle cells, as opposed to a failure of the vessels to recruit smooth muscle cells due to their small size. It is also possible that β-catenin functions in the epicardium to maintain cell-cell adhesion. In cultured rat epicardial cells, β-catenin and E-cadherin localization to adherens junctions is disrupted in cells treated with TGFβ3 (which is pharmacologically similar to TGFβ1), resulting in weakened intercellular adhesion.47 Further study will be required to determine the extent to which β-catenin mediates essential adhesion events vs. transcriptional events during epicardial formation and maintenance.
Epicardial Wnt expression may be downstream of the retinoic acid signaling pathway.28 A screen for gene products mis-regulated in RXRα-null epicardial cells demonstrated that both FGF-2 and Wnt9b are significantly down-regulated. Consistent with this observation, β-catenin protein levels are also reduced in RXRα-null epicardial cells. In addition, FGF-2 can activate epicardial Wnt signaling through the induction of Wnt9b in the epicardium. Taken together, these data are consistent with a model in which epicardial retinoic acid signaling actives FGF-2 expression directly in the epicardium or indirectly in the myocardium, resulting in the induction of epicardial Wnt9b expression and epicardial or epicardial-derived cell β-catenin activation. Activated β-catenin in epicardial-derived cells then promotes the differentiation of these cells into the coronary smooth muscle lineage and subsequent contribution to the coronary arterial tree (see Figure 3).
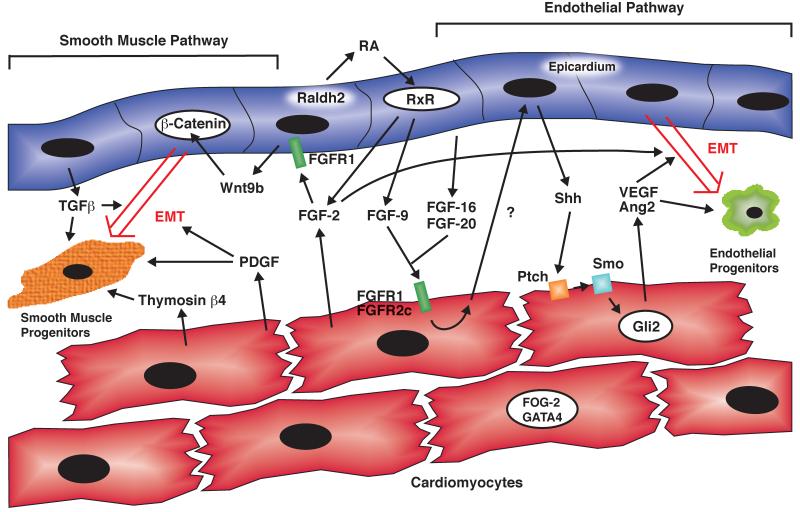
Figure 3. Epicardial-Myocardial Signaling Pathways in Coronary Vascular Development.
Shown above is a schematic of the cross talk between the epicardium (blue) and myocardium (red) during the early stages of coronary development. An FGF-Shh-VEGF/Ang2 dependent pathway is important for the generation of coronary endothelial progenitors (right), while β-catenin, PDGF, TGFβ, and Thymosin β4 are important for the development of coronary vascular smooth muscle (left).
Transforming Growth Factor β
The transforming growth factor β (TGFβ) family of signaling molecules may also be an important epicardial-derived signal regulating coronary vasculogenesis. Throughout the period of coronary vessel development, TGFβ ligands are expressed in the epicardium or the compact zone of the myocardium in an incompletely overlapping pattern.48, 49 TGFβ1 is expressed in a non-contiguous pattern throughout the epicardium at E12.5 in the mouse and later becomes focused in the outflow tract region. TGFβ2 is expressed in the proepicardium and epicardium from E9.5 through E10.5. However, its expression becomes diminished in the epicardium and by E12.5 is more highly expressed in the compact zone myocardium. TGFβ3 is not expressed until E11.5, at which time its expression is noted in the epicardium (but not myocardium) and continues to be expressed until at least E15.5.
In vitro collagen gel invasion assays have provided evidence that TGFβ ligands stimulate epithelial-mesenchymal transformation of proepicardial and epicardial cells.49-51 However, mice lacking either TGFβ1 or TGFβ3 form intact hearts without obvious signs of morphological or physiological defects.52, 53 TGFβ2 knockout mice have a pleiotropic cardiac phenotype that includes a highly penetrant ventricular septal defect, as well as a dual outlet right ventricle and dual inlet left ventricle, both of which are seen at much lower frequency. However, coronary vessel abnormalities have not been described in TGFβ2 knockout mice. This lack of a coronary phenotype in the individual TGFβ knockout mice is somewhat surprising given the in vitro data and may be due to functional redundancy of these ligands in the developing epicardium.54
Although targeted gene disruption of the TGFβ ligands has not been fruitful in elucidating the role of TGFβ signaling in coronary development, experiments disrupting the TGFβ receptor genes and downstream signaling components that mediate TGFβ signaling have provided clues into the role of TGFβ signaling during coronary vascular formation. The type III TGFβ receptor (Tgfbr3) is an extensively glycosylated transmembrane protein with a large extracellular domain that seems to mediate non-redundant roles during embryogenesis. Tgfbr3 is expressed in both the epicardium and myocardium, and mice lacking Tgfbr3 die by E14.5.25 The pattern of defects in Tgfbr3 null animals suggests unique requirements for this receptor in mediating epicardial EMT. The epicardium in Tgfbr3 null animals develops aberrantly, thinner and more widely separated from the myocardium than the epicardium in wild-type animals. Further, an overabundance of mesenchymal cells is observed in the subepicardial space of Tgfbr3 null hearts, and coronary vessel formation is partially disrupted. Although vessels form in Tgfbr3 null animals, they are less numerous and smaller than in wild-type animals. Several regions that appear to be blood islands are observed on the surface of Tgfbr3 null hearts, but PECAM-positive cells were seen less frequently in the subepicardium and myocardium. These data suggest that Tgfbr3 may negatively regulate EMT, with its loss leading to excessive EMT from the epicardium but diminished invasion into the subepicardial and myocardial compartments. However, vascular development proceeds normally in vascular fields other than the coronary system, suggesting unique roles for Tgfbr3 and TGFβ signaling during coronary vascular development.
In addition to the type III TGFβ receptor, a type I TGFβ receptor also may play a role in coronary development. Viral over-expression of the type I TGFβ receptor activin-like receptor kinase -5 (ALK5) promotes loss of epithelial character in avian epicardial cells cultured on collagen gels.50 Further, cultured murine epicardial cells lacking ALK5 maintain an epithelial morphology when treated with TGFβ3, suggesting that ALK5 is necessary for epicardial EMT.27 However, in mouse embryos with a deletion of the ALK5 TGFβ receptor in the epicardium, epicardial EMT does not appear to be disrupted, although attachment of the epicardium to the myocardium is perturbed, perhaps due to the down-regulation of N-cadherin. The coronary vessels do form in these animals, but they are smaller and blood-filled, failing to undergo proper remodeling during development. Markers of differentiated smooth muscle cells were less abundantly expressed in these vessels, consistent with a role for TGFβ signaling in coronary smooth muscle cell recruitment or differentiation.
In addition to the TGFβ receptors, downstream effectors of TGFβ signaling, the Smads, have also been implicated in regulating coronary development. Mice lacking Smad6 (an inhibitory Smad that inhibits signaling via the BMP-responsive Smads 1, 5 and 8) present with an incompletely penetrant phenotype of dilated coronary vessels that lack sufficient smooth muscle cell contribution.55, 56 Given that ALK5 signals through the Smad2/Smad3 pathway,57, 58 this resemblance to the ALK5 epicardial null phenotype suggests that down-regulation of BMP signaling in the context of TGFβ activation of ALK5 may be a necessary component of smooth muscle cell differentiation during coronary vessel remodeling.
Epicardial-Expressed Fibroblast Growth Factors
Another important class of signaling molecules produced by the epicardium is the fibroblast growth factors (FGF). It is clear that FGFs constitute at least one component of the epicardial signal that induces myocyte proliferation. A number of FGF family members have been reported to be expressed in the epicardium including FGF-1, -2, -4, -9, -16, and -20.59-63 In mice, FGF-9, -16 and -20 are all expressed in the epicardium in overlapping but distinct patterns beginning at E10.5.59 FGF-9-deficient mice die at birth due to a mildly hypoplastic left ventricle secondary to decreased myocyte proliferation.64 Further, FGF-9 is induced by retinoic acid in cultures of primary epicardial cells, providing the link between retinoic acid signaling in the epicardium with myocardial proliferation.
Embryonic cardiomyocytes express two FGF receptors, FGFR1 and FGFR2c, and thus are competent to receive FGF signals.59 Cardiomyocyte-specific ablation of both FGFR1 and FGFR2 results in embryos with a severely hypoproliferative myocardium, consistent with the notion that FGF signaling is required for cardiomyocyte proliferation. In addition, the loss of either FGF-9 in the embryo or FGFR1 and 2 specifically in the myocardium results in a delay in coronary vascular plexus formation.64 These results suggest that the epicardium signals to the myocardium via FGFs as part of the signaling pathway regulating coronary vasculogenesis. However, since loss of FGF signaling to cardiomyocytes through inactivation of FGFR1 and 2 was only able to delay – rather than completely abrogate – plexus formation, and since neither FGFR3 nor FGFR4 is expressed in the embryonic ventricle,61, 65 there are likely other parallel pathways that can partially compensate for the loss of FGF receptors in cardiomyocytes during coronary vasculogenesis.
Hedgehog Signaling
One of the downstream targets of FGF signaling during coronary vasculogenesis is sonic hedgehog (Shh). Of the three known hedgehog ligands, Shh is the most highly expressed in the heart during coronary vasculogenesis.64 At E12.5 in the mouse, Shh is expressed in the epicardium at the atrioventricular groove and the base of the heart, and then spreads throughout the rest of the epicardium over the following embryonic day. The hedgehog receptor Patched is also expressed in the atrioventricular groove and base of the heart within the cardiomyocyte compartment at this time in development, suggesting that epicardial-derived Shh signals to cardiomyocytes. Patched expression is also activated in a dynamic fashion from the base to the apex of the heart, similar to Shh. In both FGF-9−/− and FGFR1/2 conditional knockout hearts, epicardial Shh expression is delayed. Organ culture of FGFR1/2 conditional knockout hearts in the presence of exogenous Shh can rescue the delay of coronary plexus formation. Further, cyclopamine, a hedgehog signaling antagonist, blocks plexus formation in cultured wild-type hearts. Disruption of hedgehog signaling through conditional inactivation of smoothened (a transducer of hedgehog signaling downstream of Patched) in embryonic cardiomyocytes resulted in a decrease of subepicardial mesenchyme and reduced density of the vascular plexus covering the ventricles at E13.5.16 Further, expression of an activated form of Gli2 (one of the downstream transcriptional effectors of hedgehog signaling) in cardiomyocytes of FGFR1/2 knockout hearts resulted in the rescue of subepicardial vessel formation. Finally, disruption of hedgehog signaling in adult cardiomyocytes results in heart failure secondary to a loss of coronary vessel density, suggesting that this pathway is critical to maintenance as well as development of the coronary vasculature.66 Taken together, these observations suggest a pathway regulating coronary vasculogenesis in which epicardial FGFs activate myocardial FGF receptors, which then result in the induction of Shh expression in the epicardium. The mechanism by which Shh is induced by myocardial activation of FGF receptors is currently unclear.
Recent work by Ornitz and colleagues suggests that the developing coronary vascular plexus is composed of two distinct sets of arterial and venous blood vessels as indicated by the expression of the arterial endothelial marker ephrinB2 and the venous marker ephB4.16 Disruption of hedgehog signaling in embryonic cardiac myocytes led to a loss of sub-epicardial mesenchyme and a failure to form sub-epicardial ephB4-positive vessels. However, intramyocardial vessels expressing ephrinB2 still formed. Loss of hedgehog signaling in perivascular cells within the heart led to the impaired development of the intramyocardial ephrinB2-positive vessels. By using β-galactosidase to trace cells expressing ephrinB2 or ephB4, the authors determined that venous or arterial identity of coronary vessels could be distinguished as early as e12.5, before significant vascular remodeling or coronary circulation has begun. Hedgehog signaling to the perivascular cells is not necessary for their survival, but may be required for their proper differentiation or induction of pro-angiogenic gene expression within these perivascular cells. Consistent with this notion, VEGF-A, B and C were all down-regulated in the perivascular cells in which hedgehog signaling was disrupted. Since intramyocardial vessels were not completely abolished in these mice, it was suggested that myocardial VEGF produced by neighboring cardiomyocytes might support some intramyocardial vessel growth.
Myocardial Signals to the epicardium or subepicardial mesenchyme
While the epicardial-derived signals direct growth of the underlying myocardium and prepare the epicardium for EMT, the signals from the myocardium are focused on inducing epicardial EMT, directing the differentiation of the subepicardial mesenchyme into the different components of the coronary vasculature, and inducing remodeling of the primitive coronary plexus. In this section, we will review myocardial-derived signals critical for coronary development.
Myocardial-derived FGF Signals
As discussed above, the epicardium may signal to the myocardium through the use of FGFs. However, there is also evidence suggesting that the myocardium may signal back to the epicardium via FGFs. FGFR1 is expressed in the epicardium and is up-regulated in response to myocardial FGF and following epicardial EMT in select cells of the sub-epicardium.61, 62, 67 Activation of the FGFR1 signaling pathway in chick epicardium using electroporation of a plasmid encoding a constitutively-activated FGFR1 resulted in an increase in epicardial EMT.67 Proepicardial cells transduced with a retrovirus encoding anti-sense FGFR1 were still able to undergo epicardial EMT; however, while these cells were found in the sub-epicardium, they failed to invade deeper into the myocardium. This suggests that FGFR1 signaling is necessary for myocardial invasion of epicardial-derived mesenchymal cells, but not strictly required for epicardial EMT.
Several FGFs, including FGF-1, -2, and -7, are expressed in the developing heart and have been demonstrated to promote epicardial EMT through the use of in vitro collagen gel transformation assays.63 In the avian system, FGF-1 and FGF-2 expression is enriched in the compact zone myocardium adjacent to the epicardium, but is also present in the epicardium, while FGF-7 is expressed predominately in the myocardium.62, 63 Mice deficient in FGF-2 do not have any cardiac developmental defects, suggesting possible redundancy in myocardial FGF signaling.68 In adult zebrafish, myocardial expression of FGF17b and epicardial expression of FGFR2 and FGFR4 have been suggested to regulate epicardial EMT during the process of myocardial repair following resection of a portion of the ventricle.69 However, during mouse heart development, FGF17 is not expressed in the myocardium.70 Further, mice deficient in FGF-17 are viable and have no reported cardiac abnormalities.71
The FGFs may also influence the differentiation and remodeling of the sub-epicardial mesenchyme. FGF-2 has been shown to promote coronary vasculogenesis through the induction of endothelial cells in avian heart explants grown on collagen gels.72 In addition, FGF-2 stimulates tubulogenesis of coronary endothelial cells in vitro, while anti-FGF-2 antibodies inhibit VEGF-stimulated tubulogenesis.72 Consistent with these observations, over-expression of FGFR1 in the proepicardium via a retroviral-mediated approach increases the proportion of epicardial-derived cells in the endothelial cell lineage.67 Together, these results suggest that FGF-2 signaling to cells of the sub-epicardial mesenchyme may promote the endothelial cell fate and coronary vasculogenesis.
Platelet-Derived Growth Factor Signaling
Several groups have provided evidence that Platelet-derived growth factor (PDGF) signaling is important to support epicardial EMT. PDGFs are encoded by four different genes that produce monomeric peptides, designated PDGF-A, -B, -C and -D. Active PDGF ligands are dimers of these monomers that exist in one of five forms: AA, AB, BB, CC and DD. Two different transmembrane tyrosine kinase receptors bind PDGF ligands with high affinity. PDGFRα binds all the PDGF dimers except for PDGF-DD, whereas PDGFRβ binds only to –BB and –DD homodimers.73 Both PDGFRα and PDGFRβ are expressed in the epicardium74-76, conferring competency to these cells to respond to all PDGF ligands.
PDGF stimulates cultured epicardial cells to undergo the initial steps of EMT. Epicardial cells, cultured as epithelial sheets on solid substratum, elongate and migrate when treated with PDGF ligands. This effect of PDGF is blocked by treating explants with inhibitors of p160 Rho-kinase, implicating the PI3K pathway as the downstream effector of PDGF-stimulated EMT. Further, as the PDGF-BB homodimer induces this activation behavior much more potently than either PDGF-AB or PDGF-AA, this EMT-inducing property of PDGF is likely mediated by PDGFRβ77 Powerful in vivo evidence supporting the notion that PDGF signaling through PDGFRβ is required for EMT comes from studies of Pdgfrb−/− mice crossed to the transgenic reporter line capsulinlacZ that labels proepicardial, epicardial, and epicardial-derived cells via LacZ expression. In mice lacking PDGFRβ fewer lacZ-expressing cells were observed in the subepicardial space and myocardium, indicating a defect in epicardial EMT.78 It was noted that Pdgfrb−/− epicardial cells express the mesenchymal marker vimentin more abundantly than wild-type epicardial cells. As vimentin is up-regulated in epicardial cells undergoing EMT79, this could indicate that PDGF signaling is required for delamination and migration of epicardial cells into the myocardium.
In addition to its role in regulating epicardial EMT, PDGF may be another one of the factors that directs the differentiation and remodeling of the sub-epicardial mesenchyme. Recent work has suggested that vascular tube formation by cultured chick epicardium is stimulated 3-fold by the addition of PDGF-BB.80 Further, injection of anti-PDGF antisera in ovo diminished myocardial capillary density by approximately 20%. Together, these observations suggest that PDGF may play a modest role in endothelial tube formation, although it is possible these results may be due to PDGF’s ability to stimulate epicardial EMT rather than vascular tube formation.
PDGF is also a required signal for the differentiation and recruitment of smooth muscle cells and pericytes to the coronary vascular bed. PDGF-BB potently induces smooth muscle cell differentiation in explanted proepicardia.77 Since neither PDGF-AA nor -AB induces smooth muscle cell differentiation in proepicardial explants, it is likely that PDGF-BB-induced smooth muscle differentiation is mediated through PDGFRβ. In support of that notion, mice with a targeted deletion of the Pdgfb gene die perinatally with widespread hemorrhage, likely due to a loss of microvascular pericytes that reinforce the capillary wall81, 82. Subsequent reports described more extensive cardiovascular malformations in Pdgfb−/− mice including a deficiency or lack of vascular smooth muscle cells surrounding sub-epicardial and intramyocardial coronary arteries, respectively.83 This reduction in smooth muscle cells undoubtedly contributes to the dilated coronary vessels seen in Pdgfb mutants.84 Targeted disruption of Pdgfrb only in the epicardium produces a similar deficiency of coronary vascular smooth muscle cells and pericytes.78, 81 Taken together, these observations demonstrate the importance of PDGF signaling to epicardially derived cells for the development of the coronary vasculature.
Thymosin β4
Another gene that may influence the differentiation of the sub-epicardial mesenchyme is Thymosin β4.85 Thymosin β4 is an actin monomer-binding protein expressed in the myocardium, but not the epicardium. Thymosin β4 is presumed to be secreted by the myocardium and taken up by the embryonic epicardium, but the mechanism by which this occurs remains unclear. Reduction of thymosin β4 using a transgenic antisense approach in embryonic cardiomyocytes in the developing heart led to a reduction in cells expressing smooth muscle alpha actin, suggesting a defect in the differentiation of smooth muscle cells from the subepicardial mesenchyme.85 Further, the smooth muscle cells that were generated also showed a migration defect, remaining localized in the epicardium or sub-epicardial space and not migrating deep into the developing myocardium.
The Planar Cell Polarity Signaling Pathway
Vangl2, a component of the planar cell polarity pathway, is also critically important for heart and coronary vascular development. The mouse Looptail (Lp) mutant maps to the Vangl2 locus, and studies of homozygous Lp mutants have demonstrated that Vangl2 is involved in muscularization of the outflow tract septum.86 Closer examination of the hearts of Lp mutants revealed a coronary vascular patterning defect.87 Despite making successful connection to the aorta at E15.5, the vascular plexus appears disorganized and by E17.5 is composed of distended subepicardial vessels. Smooth muscle marker expression in the coronary vasculature is delayed as compared to wild-type or Lp heterozygous animals, and staining along the coronary arteries is patchy at E18.5, as opposed to the strong, continuous staining seen along the length of vessels from wild-type and heterozygous mice. Consistent with a role for Vangl2 in mediating planar cell polarity signaling, epicardial cells from Lp homozygous mutant animals lack a polarized morphology and have a reduced capacity to undergo EMT. What is especially striking about this phenotype is the lack of expression of Vangl2 in epicardial cells or their derivatives, suggesting that the effect of Vangl2 mutation on epicardial cells is due to alterations in the adjacent myocytes. Similarly, mice deficient in connexin 43 also have defects in epicardial cell polarization, migration, and early remodeling of the coronary vascular plexus.88, 89 However, aberrant expression of planar cell polarity pathway components was not detected in the connexin 43 knockout hearts, so it is currently unclear if and how connexin 43 interacts with the planar cell polarity pathway.
Vascular Endothelial Growth Factor (VEGF)
The VEGF family likely plays multiple roles in the development of the coronary vasculature. There are 5 ligands (VEGF-A, -B, -C, -D, and Placental Growth Factor) with multiple splice isoforms and three receptors (VEGFR1, VEGFR2, and VEFGR3) in this family.90 In developing mouse hearts at E13.5, VEGF-A, VEGF-B, and VEGF-D are expressed in cardiomyocytes, while VEGF-C is expressed in vascular pericytes.64 The VEGF receptors are expressed in the epicardium and sub-epicardium, suggesting that this growth factor family is involved in myocardial-epicardial signaling. Further, VEGF may act directly on the sub-epicardial angioblasts/hemangioblasts as these cells are known to express Flk-1 (VEGFR2).14 One result of VEGF-mediated signaling may be to induce epicardial EMT, as VEGF-A has been shown to promote epicardial EMT when added to an in vitro heart culture system.91 In addition, VEGF acts potently to induce coronary endothelial cell proliferation, migration and tube formation in conjunction with FGF-2.15, 72, 92 Blocking VEGF function using a soluble VEGFR1/VEGFR2 fusion protein significantly reduces coronary vascular bed tubulogenesis, as does treatment with anti-VEGF-B antibodies.15
Yet another role for VEGF in coronary development involves the connection of the coronary plexus with the aortic root.15 Expression of VEGF is particularly abundant at the region of the coronary sinus, and functional experiments have demonstrated a role for this growth factor during ingrowth of the coronary capillaries into the aortic root. Blocking VEGF-B signaling using antisera or with a soluble form of VEGFR2 inhibits coronary ostia formation when injected into chicks 1-3 days before the ostia develop.
Not surprisingly, VEGF expression in developing hearts is regulated by a number of factors. In mice with a heart-specific knockdown of Thymosin β4, the coronary vascular plexus failed to form and VEGF expression was reduced.85 Importantly, there were sub-epicardial “nodules” present composed of vascular endothelial cells forming lumens filled with erythrocytes, likely derived from hemangioblasts that differentiated down both the vascular and hematopoietic lineages. These vascular progenitors, however, failed to link together to form a plexus, perhaps due in part to the absence of VEGF. Sonic hedgehog signaling also appears to be upstream of VEGF expression. Activation of hedgehog signaling in cardiomyocytes leads to the induction of VEGF-A, -B, -C and Angiopoietin-2 expression in cultured heart slices.64 Further, loss of hedgehog signaling to cardiomyocytes led to decreased VEGF-A and B expression within developing hearts and the failure to form a sub-epicardial vascular plexus.
Angiopoietins
The angiopoietin family of growth factors contains 4 members (Ang1-4) that all bind to the Tie2 receptor.93 During cardiac development, Ang1 and Ang2 appear to be the most important. Ang1 is highly expressed in the myocardium at E11.5, while Tie2 is expressed in the developing epicardium and endocardium, suggesting that myocardial Ang1 signals to the developing epicardium and endocardium during heart development.94, 95 In other vascular beds, Tie2 activation by Ang1 promotes the survival of endothelial cells, stimulates migration of activated endothelial cells, and promotes capillary organization and vessel stablization.93 Mouse embryos deficient in Tie2 die of vascular malformations thought secondary to defects in angiogenesis at approximately E10.5, before the formation of the coronary vascular plexus.96 Mice lacking Ang1 die approximately 2 days later in development with a thin myocardium, disrupted endocardium, and generalized vascular defects similar to those seen in the Tie2-deficient mice.94 Epicardial and coronary plexus formation was not specifically examined in these embryos; however, given its role in vascular development, it would not be surprising to find coronary defects. Further, myocardial over-expression of Ang1 during development disrupted epicardial development and subsequent coronary plexus formation, suggesting that the levels of this growth factor must be tightly regulated during coronary vasculogenesis.95
The other major ligand for the Tie2 receptor, Ang2, may also be involved in coronary vascular development. Recent work suggests that Ang2 is a weak Tie2 agonist in the absence of Ang1, and an antagonist in the presence of Ang1.97 Mice deficient in Ang2 have defects in postnatal vascular remodeling and lymphatic development, suggesting that Ang2 is not required for coronary development.98 However, over-expression of Ang2 and VEGF in the myocardium led to a synergistic increase in capillary density.99 Further, both of these factors are downstream of Sonic hedgehog signaling in the developing heart and can rescue coronary plexus formation in hearts in which Sonic hedgehog signaling is blocked.64
Prokineticin
Prokineticins are small, secreted peptides that are expressed in the nervous, gastrointestinal and cardiovascular systems.100 Prokineticin-1, also called endocrine-gland VEGF, and prokineticin-2, which shares significant homology with a component of Black Mamba snake venom, signal through two different G-protein coupled receptors, Prokineticin receptor (PKR)-1 and -2. In the heart, prokineticin-2 and PKR-1 may act cooperatively to support coronary vessel development and protect cardiomyocytes after ischemic injury.101 Prokineticin-2 promotes tubulogenesis in cultured coronary vascular endothelial cells in a PKR-1 dependent manner, while infecting cardiomyocytes with a PKR-1 adenovirus increases capillary density in a coronary vessel ligation model of myocardial infarction.101 In mouse hearts transgenically overexpressing PKR-1, coronary capillary density is increased compared to wild-type animals, suggesting that prokineticin signaling may support coronary vessel development during embryogenesis.102 Epicardial cells were more highly proliferative and more abundant in transgenic hearts. Further, epicardial cells from juvenile or adult mice cultured in the presence of prokineticin-2 differentiated into endothelial or smooth muscle cells. As prokineticin signaling both protects against ischemic damage in cardiomyocytes and promotes differentiation of epicardial cells to vascular lineages, this pathway represents a promising target for future clinical studies.
Myocardial GATA4 and FOG-2
There are a number of transcription factors that are expressed in cardiomyocytes that likely play a role in coronary development. Of particular interest are the transcription factors FOG-2 and GATA4. Adult mice with a cardiac-specific deletion of GATA4 have reduced myocardial capillary density, while over-expression of GATA4 in adult hearts leads to an increase in myocardial capillary density.103 Gene expression analysis in these mice indicated altered expression of a number of angiogenic genes including VEGF-A and Ang1. Further work demonstrated that GATA4 directly bound and activated the VEGF-A promoter, suggesting that VEGF-A is a direct downstream target of the angiogenic activity of GATA4.
Friend of GATA 2 (FOG-2) is another myocardial transcription factor that physically interacts with GATA4 and also plays a role in the regulation of coronary development.104-106 Mice deficient in FOG-2 were found to have an intact epicardium, but the coronary vascular plexus was greatly attenuated in these mice.106 Interestingly, this defect could be rescued by transgenic expression of FOG-2 in the developing myocardium, suggesting that FOG-2 acts non-cell-autonomously in the epicardium by inducing the expression of one or more factors in cardiomyocytes that promote plexus formation. Similar to GATA4, deletion of FOG-2 in adult mice leads to a reduction in myocardial capillary density.107 In these mice, a number of angiogenic factors were down-regulated, including VEGF-A, and FGF-2, -9, -12, and -16, while a number of angiostatic factors such as Timp1 and Timp2 were up-regulated. This suggests that the GATA4/FOG-2 transcriptional complex may serve as a broad regulator of myocardial angiogenic signals.
Other transcription factors in coronary vessel development
In addition to GATA4 and FOG-2, the role of other cardiac transcription factors during coronary vessel development is an active area of research. Like FOG-2, BAF180 is a repressor of transcription that plays an important role in coronary vessel development. Although the epicardium forms in BAF180 mutants, the coronary plexus fails to remodel appropriately and fine capillaries are reduced compared to wild-type animals.108 Nodules containing a disorganized mixture of smooth muscle and endothelial cells are seen on the surface of the heart in mutant animals, similar to the phenotype noted for thymosin β4 mutants as described previously.85 Epicardial cells harvested from BAF180 mutant animals were notably impaired in their ability to undergo EMT.108 Expression of the epicardially expressed growth factors FGF, PDGF and TGFβ were all reduced in the hearts of these animals. As these factors are all important for epicardial EMT, it is likely that the combinatorial deficit of these signals led to the impaired EMT noted in the embryos. Also, since EMT was reduced in vitro, these results could implicate BAF180 in establishing an autocrine signaling loop to promote transformation of epicardial cells.
Another transcriptional regulator implicated in coronary development is p300. This transcription factor possesses an acetyltransferase activity that acts on histones to remodel chromatin to promote transcription of target genes. A targeted allele of p300 that destroys the acetyltransferase activity of the protein acts in a dominant-negative manner, causing cardiac malformations and pulmonary dysgenesis.109 While heterozygotes begin dying at E12.5, a fraction are live-born but expire quickly due to respiratory failure. Epicardial EMT and coronary plexus formation are impaired in heterozygous animals, suggesting the importance of chromatin remodeling for coronary development.
Investigations into the role of Ets-1 during coronary vessel formation suggest that this transcription factor may be required for coronary arteriogenesis. In the heart, Ets-1 expression localizes to areas of EMT, including the epicardium and subepicardium110. Partial knockdown of Ets-1 and Ets-2 using an antisense virus perturbed coronary artery formation in chick embryos111. In the most severely affected embryos one or both coronary ostia failed to form and the arteries themselves were improperly patterned.
Compelling studies suggest that T-box transcription factors are involved in epicardial and coronary vessel development. The T-box factor Tbx-1 has been shown to be required for the patterning of the proximal coronary arteries.112 Another T-box factor, Tbx18, is abundantly expressed in embryonic proepicardium and epicardium.113, 114 In zebrafish, resting epicardial cells in adult fish do not express Tbx18. However, upon injury to the myocardium, Tbx18 expression is increased in the epicardium coincident with epicardial cell invasion into the wound site and subsequent neovascularization of the newly generated myocardial tissue.69 Tbx18 may therefore be required for epicardial cell EMT or subsequent migration during coronary vasculogenesis. Tbx5, the gene mutated in Holt-Oram syndrome in humans,115, 116 may also regulate epicardial EMT. PE cells injected with a Tbx5-expressing retrovirus showed deficient migration in vitro and in vivo, with many fewer virally-transduced cells contributing to coronary vascular tissues than cells infected with an empty virus expressing only β-galactosidase.117 Tbx5 may also have a cytoplasmic intracellular localization in cells of the coronary vasculature,118 suggesting a complex regulation of its transcriptional activity. Further studies on the role of Tbx18 and Tbx5 during epicardial EMT and subsequent coronary vessel development seem warranted based on these intriguing results.
Future Directions
In summary, epicardial-myocardial signaling directing coronary vasculogenesis is complex, with multiple signaling pathways involved in each step of the process and many signaling pathways used in multiple roles (see Figure 3). The first critical step in coronary development involves the establishment of an intact epicardium through the interaction of cell adhesion molecules such as α4 integrin and VCAM-1. Autocrine signaling within the immature epicardium by erythropoietin and retinoic acid is critical for inducing cardiomyocyte proliferation and for the next step in signaling – that of epicardial FGF secretion. These FGFs, acting on the underlying myocardium, induce cardiomyocyte proliferation and likely the elaboration of other growth factors that signal back to the epicardium. The signaling back to the epicardium can then direct epicardial EMT to generate sub-epicardial mesenchyme fated to the endothelial, fibroblast, or smooth muscle cell fate. A Shh-VEGF-Ang2 pathway is important for the generation of vascular endothelial cells, while PDGF, Wnt/β-catenin, and TGFβ are important for the differentiation of coronary vascular smooth muscle. Despite the identification of several transcription factors that are necessary for coronary vessel development, understanding how the pathways are knit together from growth factor-bound receptor to DNA-bound transcription factors is an area that requires substantially more investigation.
While our understanding of this communication has increased dramatically, important questions remain: What are the downstream signaling events within the epicardium and myocardium following α4 integrin/VCAM-1 ligation and how critical are these events for coronary vasculogenesis? What other pathways compensate for the loss of FGF signaling in cardiomyocytes during coronary development? What are the myocardial-derived signals that induce Shh expression in the epicardium? What are the signals that promote a fibroblast cell fate in epicardial-derived cells? And most importantly, can these pathways be re-activated in adult hearts to regenerate the coronary vasculature in patients with coronary artery disease?
Acknowledgments
SOURCES OF FUNDING
E.C.S. is supported by NIH grant # HL71063.
LIST OF NON-STANDARD ABBREVIATIONS
- ALK
Activin receptor-Like Kinase
- Amhc2
Atrial myosin heavy chain 2
- Ang
Angiopoietin
- BAF
Brahma-related gene-1 Associated Factor
- BMP
Bone Morphogenetic Protein
- E
embryonic day
- EMT
Epithelial-Mesenchymal Transformation
- Epo/EpoR
Erythropoietin/Erythropoietin Receptor
- Ets
avian erythroblastosis virus oncogene
- FGF
Fibroblast Growth Factor
- FGFR
Fibroblast Growth Factor Receptor
- Flk
fetal liver kinase
- FOG
Friend-of-GATA
- Gli
Glioma-associated oncogene
- GTPase
Guanosine Triphosphatase
- Lp
Looptail
- PDGF
Platelet-derived Growth Factor
- PDGFR
Platelet-derived Growth Factor Receptor
- PECAM
Platelet/Endothelial Cell Adhesion Molecule
- PI3K
Phosphatidyinositol-3 Kinase
- PKC
Protein Kinase C
- PKR
prokineticin receptor
- Raldh2
Retinaldehyde dehydrogenase 2
- RAR
Retinoic Acid Receptor
- Rho
Rat sarcoma virus oncogene homolog
- RXRa
Retinoid X Receptor alpha
- SCL
Stem Cell Leukemia
- Sca-1
Stem cell antigen-1
- Shh
Sonic hedgehog
- TGFb
Transforming Growth Factor beta
- Tgfbr3
Type III Transforming Growth Factor beta receptor
- Tie
tunica internal endothelial cell kinase
- Vangl2
Van Gogh-like 2
- VCAM-1
Vascular Cell Adhesion Molecule-1
- WT-1
Wilms’ Tumor-1
- VCAM
Vascular Cell Adhesion Molecule
Footnotes
DISCLOSURES
None.
Subject Codes: [6] Cardiac development, [130] Animal models of human disease
REFERENCES
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y, Subcommittee AHASCaSS Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Lavine KJ, Ornitz DM. Rebuilding the coronary vasculature: hedgehog as a new candidate for pharmacologic revascularization. Trends in Cardiovascular Medicine. 2007;17:77–83. doi: 10.1016/j.tcm.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mu H, Ohashi R, Lin P, Yao Q, Chen C. Cellular and molecular mechanisms of coronary vessel development. Vascular medicine (London, England) 2005;10:37–44. doi: 10.1191/1358863x05vm584ra. [DOI] [PubMed] [Google Scholar]
- 4.Olivey HE, Compton LA, Barnett JV. Coronary vessel development: the epicardium delivers. Trends in Cardiovascular Medicine. 2004;14:247–251. doi: 10.1016/j.tcm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Tomanek RJ. Formation of the coronary vasculature during development. Angiogenesis. 2005;8:273–284. doi: 10.1007/s10456-005-9014-9. [DOI] [PubMed] [Google Scholar]
- 6.Wessels A, Pérez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 7.Dettman RW, Denetclaw W, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Developmental Biology. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 8.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Developmental Biology. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 9.Viragh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Pomares JM, Carmona R, González-Iriarte M, Atencia G, Wessels A, Muñoz-Chápuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int. J. Dev. Biol. 2002;46:1005–1013. [PubMed] [Google Scholar]
- 11.Wilting J, Buttler K, Schulte I, Papoutsi M, Schweigerer L, Männer J. The proepicardium delivers hemangioblasts but not lymphangioblasts to the developing heart. Developmental Biology. 2007;305:451–459. doi: 10.1016/j.ydbio.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong J. Molecular and developmental biology of the hemangioblast. Dev Dyn. 2008;237:1218–1231. doi: 10.1002/dvdy.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomanek RJ, Ishii Y, Holifield JS, Sjogren CL, Hansen HK, Mikawa T. VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo. Circulation Research. 2006;98:947–953. doi: 10.1161/01.RES.0000216974.75994.da. [DOI] [PubMed] [Google Scholar]
- 16.Lavine KJ, Long F, Choi K, Smith C, Ornitz DM. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development. 2008;135:3161–3171. doi: 10.1242/dev.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-Iriarte M, Carmona R, Pérez-Pomares JM, Macías D, Costell M, Muñoz-Chápuli R. Development of the coronary arteries in a murine model of transposition of great arteries. Journal of Molecular and Cellular Cardiology. 2003;35:795–802. doi: 10.1016/s0022-2828(03)00134-2. [DOI] [PubMed] [Google Scholar]
- 18.Gittenberger-de Groot AC, Vrancken Peeters MP, Bergwerff M, Mentink MM, Poelmann RE. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circulation Research. 2000;87:969–971. doi: 10.1161/01.res.87.11.969. [DOI] [PubMed] [Google Scholar]
- 19.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 20.Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 21.Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- 22.Kirschner KM, Wagner N, Wagner KD, Wellmann S, Scholz H. The Wilms tumor suppressor Wt1 promotes cell adhesion through transcriptional activation of the alpha4integrin gene. J Biol Chem. 2006;281:31930–31939. doi: 10.1074/jbc.M602668200. [DOI] [PubMed] [Google Scholar]
- 23.Sengbusch JK, He W, Pinco KA, Yang JT. Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol. 2002;157:873–882. doi: 10.1083/jcb.200203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner N, Wagner KD, Theres H, Englert C, Schedl A, Scholz H. Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms’ tumor transcription factor Wt1. Genes & Development. 2005;19:2631–2642. doi: 10.1101/gad.346405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compton LA, Potash DA, Brown C, Barnett JV. Coronary vessel development is dependent on the type III transforming growth factor beta receptor. Circulation Research. 2007;101:784–791. doi: 10.1161/CIRCRESAHA.107.152082. [DOI] [PubMed] [Google Scholar]
- 26.Mahtab EA, Wijffels MC, Van Den Akker NM, Hahurij ND, Lie-Venema H, Wisse LJ, Deruiter M, Uhrin P, Zaujec J, Binder BR, Schalij MJ, Poelmann R, Gittenberger-De Groot A. Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: Correlation with abnormal epicardial development. Dev Dyn. 2008;237:847–857. doi: 10.1002/dvdy.21463. [DOI] [PubMed] [Google Scholar]
- 27.Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P, Kaartinen V. Signaling via the Tgf-beta type I receptor Alk5 in heart development. Developmental Biology. 2008;322:208–218. doi: 10.1016/j.ydbio.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Belmonte JC, Chien K, Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci USA. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circulation Research. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 30.Heine UI, Roberts AB, Munoz EF, Roche NS, Sporn MB. Effects of retinoid deficiency on the development of the heart and vascular system of the quail embryo. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;50:135–152. doi: 10.1007/BF02889897. [DOI] [PubMed] [Google Scholar]
- 31.Kostetskii I, Jiang Y, Kostetskaia E, Yuan S, Evans T, Zile M. Retinoid signaling required for normal heart development regulates GATA-4 in a pathway distinct from cardiomyocyte differentiation. Dev Biol. 1999;206:206–218. doi: 10.1006/dbio.1998.9139. [DOI] [PubMed] [Google Scholar]
- 32.Zile MH. Vitamin A and embryonic development: an overview. J Nutr. 1998;128:455S–458S. doi: 10.1093/jn/128.2.455S. [DOI] [PubMed] [Google Scholar]
- 33.Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 34.Pan J, Baker KM. Retinoic acid and the heart. Vitam Horm. 2007;75:257–283. doi: 10.1016/S0083-6729(06)75010-5. [DOI] [PubMed] [Google Scholar]
- 35.Stuckmann I, Evans S, Lassar AB. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Developmental Biology. 2003;255:334–349. doi: 10.1016/s0012-1606(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 36.Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Kubalak SW, Chien KR. Ventricular muscle-restricted targeting of the RXRalpha gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development. 1998;125:1943–1949. doi: 10.1242/dev.125.10.1943. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Lee SH, Gao J, Liu X, Iruela-Arispe ML. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597–3605. doi: 10.1242/dev.126.16.3597. [DOI] [PubMed] [Google Scholar]
- 39.Kertesz N, Wu J, Chen TH, Sucov HM, Wu H. The role of erythropoietin in regulating angiogenesis. Developmental Biology. 2004;276:101–110. doi: 10.1016/j.ydbio.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Park M, Wu X, Golden K, Axelrod JD, Bodmer R. The wingless signaling pathway is directly involved in Drosophila heart development. Developmental Biology. 1996;177:104–116. doi: 10.1006/dbio.1996.0149. [DOI] [PubMed] [Google Scholar]
- 41.Garriock RJ, D’Agostino SL, Pilcher KC, Krieg PA. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Developmental Biology. 2005;279:179–192. doi: 10.1016/j.ydbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien K, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 43.Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Developmental Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. Plos Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brade T, Männer J, Kühl M. The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart. Cardiovascular Research. 2006;72:198–209. doi: 10.1016/j.cardiores.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Zamora M, Männer J, Ruiz-Lozano P. Epicardium-derived progenitor cells require beta-catenin for coronary artery formation. Proc Natl Acad Sci USA. 2007;104:18109–18114. doi: 10.1073/pnas.0702415104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dokic D, Dettman RW. VCAM-1 inhibits TGFbeta stimulated epithelial-mesenchymal transformation by modulating Rho activity and stabilizing intercellular adhesion in epicardial mesothelial cells. Developmental Biology. 2006;299:489–504. doi: 10.1016/j.ydbio.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 48.Molin DG, Bartram U, Van Der Heiden K, Van Iperen L, Speer C, Hierck B, Poelmann R, Gittenberger-De-Groot A. Expression patterns of Tgfbeta1-3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Dev Dyn. 2003;227:431–444. doi: 10.1002/dvdy.10314. [DOI] [PubMed] [Google Scholar]
- 49.Olivey HE, Mundell NA, Austin AF, Barnett J. Transforming growth factor-beta stimulates epithelial-mesenchymal transformation in the proepicardium. Dev Dyn. 2006;235:50–59. doi: 10.1002/dvdy.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Compton LA, Potash DA, Mundell NA, Barnett JV. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev Dyn. 2006;235:82–93. doi: 10.1002/dvdy.20629. [DOI] [PubMed] [Google Scholar]
- 51.Austin AF, Compton LA, Love JD, Brown C, Barnett JV. Primary and immortalized mouse epicardial cells undergo differentiation in response to TGFbeta. Dev Dyn. 2008;237:366–376. doi: 10.1002/dvdy.21421. [DOI] [PubMed] [Google Scholar]
- 52.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 54.Azhar M, Schultz Jel J, Grupp I, Dorn GW, 2nd, Meneton P, Molin DG, Gittenberger-de Groot AC, Doetschman T. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev. 2003;14:391–407. doi: 10.1016/s1359-6101(03)00044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 56.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr., Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- 58.Macias-Silva M, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL. MADR2 is a substrate of the TGFbeta receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- 59.Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Developmental Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Pennisi D, Ballard VL, Mikawa T. Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart. Dev Dyn. 2003;228:161–172. doi: 10.1002/dvdy.10360. [DOI] [PubMed] [Google Scholar]
- 61.Sugi Y, Ito N, Szebenyi G, Myers K, Fallon JF, Mikawa T, Markwald RR. Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Developmental Biology. 2003;258:252–263. doi: 10.1016/s0012-1606(03)00099-x. [DOI] [PubMed] [Google Scholar]
- 62.Pennisi D, Mikawa T. Normal patterning of the coronary capillary plexus is dependent on the correct transmural gradient of FGF expression in the myocardium. Developmental Biology. 2005;279:378–390. doi: 10.1016/j.ydbio.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 63.Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Developmental Biology. 2001;234:204–215. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- 64.Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui CC, Ornitz DM. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes & Development. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cool SM, Sayer RE, van Heumen WR, Pickles JO, Nurcombe V. Temporal and spatial expression of fibroblast growth factor receptor 4 isoforms in murine tissues. Histochem J. 2002;34:291–297. doi: 10.1023/a:1023326524562. [DOI] [PubMed] [Google Scholar]
- 66.Lavine KJ, Kovacs A, Ornitz DM. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. Journal of Clinical Investigation. 2008 doi: 10.1172/JCI34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pennisi DJ, Mikawa T. FGFR-1 is required by epicardium-derived cells for myocardial invasion and correct coronary vascular lineage differentiation. Dev Biol. 2009;328:148–159. doi: 10.1016/j.ydbio.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, Yin M, Coffin JD, Kong L, Kranias EG, Luo W, Boivin GP, Duffy JJ, Pawlowski SA, Doetschman T. Fibroblast growth factor 2 control of vascular tone. Nat. Med. 1998;4:201–207. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 70.Xu J, Lawshe A, MacArthur CA, Ornitz DM. Genomic structure, mapping, activity and expression of fibroblast growth factor 17. Mechanisms of Development. 1999;83:165–178. doi: 10.1016/s0925-4773(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 71.Xu J, Liu Z, Ornitz DM. Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development. 2000;127:1833–1843. doi: 10.1242/dev.127.9.1833. [DOI] [PubMed] [Google Scholar]
- 72.Tomanek RJ, Zheng W, Peters KG, Lin P, Holifield JS, Suvarna PR. Multiple growth factors regulate coronary embryonic vasculogenesis. Dev Dyn. 2001;221:265–273. doi: 10.1002/dvdy.1137. [DOI] [PubMed] [Google Scholar]
- 73.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 74.Orr-Urtreger A, Bedford MT, Do MS, Eisenbach L, Lonai P. Developmental expression of the alpha receptor for platelet-derived growth factor, which is deleted in the embryonic lethal Patch mutation. Development. 1992;115:289–303. doi: 10.1242/dev.115.1.289. [DOI] [PubMed] [Google Scholar]
- 75.Shinbrot E, Peters KG, Williams LT. Expression of the platelet-derived growth factor beta receptor during organogenesis and tissue differentiation in the mouse embryo. Dev Dyn. 1994;199:169–175. doi: 10.1002/aja.1001990302. [DOI] [PubMed] [Google Scholar]
- 76.Takakura N, Yoshida H, Ogura Y, Kataoka H, Nishikawa S, Nishikawa S. PDGFR alpha expression during mouse embryogenesis: immunolocalization analyzed by whole-mount immunohistostaining using the monoclonal anti-mouse PDGFR alpha antibody APA5. J Histochem Cytochem. 1997;45:883–893. doi: 10.1177/002215549704500613. [DOI] [PubMed] [Google Scholar]
- 77.Lu J, Landerholm TE, Wei JS, Dong XR, Wu SP, Liu X, Nagata K, Inagaki M, Majesky MW. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Developmental Biology. 2001;240:404–418. doi: 10.1006/dbio.2001.0403. [DOI] [PubMed] [Google Scholar]
- 78.Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, Ruiz FR, Pu WT, Tallquist MD. Platelet-Derived Growth Factor Receptor {beta} Signaling Is Required for Efficient Epicardial Cell Migration and Development of Two Distinct Coronary Vascular Smooth Muscle Cell Populations. Circulation Research. 2008 doi: 10.1161/CIRCRESAHA.108.176768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. Contribution of the primitive epicardium to the subepicardial mesenchyme in hamster and chick embryos. Dev Dyn. 1997;210:96–105. doi: 10.1002/(SICI)1097-0177(199710)210:2<96::AID-AJA3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 80.Tomanek RJ, Hansen HK, Christensen LP. Temporally expressed PDGF and FGF-2 regulate embryonic coronary artery formation and growth. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1237–1243. doi: 10.1161/ATVBAHA.108.166454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 82.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 83.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 84.Van Den Akker NM, Winkel LC, Nisancioglu MH, Maas S, Wisse LJ, Armulik A, Poelmann R, Lie-Venema H, Betsholtz C, Gittenberger-De Groot A. PDGF-B signaling is important for murine cardiac development: its role in developing atrioventricular valves, coronaries, and cardiac innervation. Dev Dyn. 2008;237:494–503. doi: 10.1002/dvdy.21436. [DOI] [PubMed] [Google Scholar]
- 85.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien K, Riley P. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 86.Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circulation Research. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 87.Phillips HM, Hildreth V, Peat JD, Murdoch JN, Kobayashi K, Chaudhry B, Henderson DJ. Non-cell-autonomous roles for the planar cell polarity gene Vangl2 in development of the coronary circulation. Circulation Research. 2008;102:615–623. doi: 10.1161/CIRCRESAHA.107.160861. [DOI] [PubMed] [Google Scholar]
- 88.Walker DL, Vacha SJ, Kirby ML, Lo CW. Connexin43 deficiency causes dysregulation of coronary vasculogenesis. Developmental Biology. 2005;284:479–498. doi: 10.1016/j.ydbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Rhee DY, Zhao XQ, Francis RJ, Huang GY, Mably JD, Lo CW. Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development. 2009;136:3185–3193. doi: 10.1242/dev.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovascular Research. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Nesbitt TL, Roberts A, Tan H, Junor L, Yost MJ, Potts JD, Dettman R, Goodwin RL. Coronary endothelial proliferation and morphogenesis are regulated by a VEGF-mediated pathway. Dev Dyn. 2009;238:423–430. doi: 10.1002/dvdy.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holifield J, Arlen A, Runyan R, Tomanek R. TGF-beta1, -beta2 and -beta3 cooperate to facilitate tubulogenesis in the explanted quail heart. J Vasc Res. 2004;41:491–498. doi: 10.1159/000081805. [DOI] [PubMed] [Google Scholar]
- 93.Augustin H, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 94.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 95.Ward N, Van Slyke P, Sturk C, Cruz M, Dumont DJ. Angiopoietin 1 expression levels in the myocardium direct coronary vessel development. Dev Dyn. 2004;229:500–509. doi: 10.1002/dvdy.10479. [DOI] [PubMed] [Google Scholar]
- 96.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 97.Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Molecular and Cellular Biology. 2009;29:2011–2022. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Developmental Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 99.Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci USA. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ngan ES, Tam PK. Prokineticin-signaling pathway. Int J Biochem Cell Biol. 2008;40:1679–1684. doi: 10.1016/j.biocel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 101.Urayama K, Guilini C, Messaddeq N, Hu K, Steenman M, Kurose H, Ert G, Nebigil CG. The prokineticin receptor-1 (GPR73) promotes cardiomyocyte survival and angiogenesis. Faseb J. 2007;21:2980–2993. doi: 10.1096/fj.07-8116com. [DOI] [PubMed] [Google Scholar]
- 102.Urayama K, Guilini C, Turkeri G, Takir S, Kurose H, Messaddeq N, Dierich A, Nebigil CG. Prokineticin receptor-1 induces neovascularization and epicardial-derived progenitor cell differentiation. Arterioscler Thromb Vasc Biol. 2008;28:841–849. doi: 10.1161/ATVBAHA.108.162404. [DOI] [PubMed] [Google Scholar]
- 103.Heineke J, Auger-Messier M, Xu J, Oka T, Sargent M, York A, Klevitsky R, Vaikunth S, Duncan S, Aronow B, Robbins J, Crombleholme TM, Cromblehol T, Molkentin J. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. Journal of Clinical Investigation. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Svensson EC, Tufts RL, Polk CE, Leiden JM. Molecular cloning of FOG-2: a modulator of transcription factor GATA-4 in cardiomyocytes. Proc Natl Acad Sci USA. 1999;96:956–961. doi: 10.1073/pnas.96.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tevosian S, Deconinck AE, Cantor AB, Rieff HI, Fujiwara Y, Corfas G, Orkin SH. FOG-2: A novel GATA-family cofactor related to multitype zinc-finger proteins Friend of GATA-1 and U-shaped. Proc Natl Acad Sci USA. 1999;96:950–955. doi: 10.1073/pnas.96.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tevosian S, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 107.Zhou B, Ma Q, Kong S, Hu Y, Campbell P, Mcgowan F, Ackerman K, Wu B, Zhou B, Tevosian S, Pu W. Fog2 is critical for cardiac function and maintenance of coronary vasculature in the adult mouse heart. J Clin Invest. 2009;119:1462–1476. doi: 10.1172/JCI38723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang X, Gao X, Diaz-Trelles R, Ruiz-Lozano P, Wang Z. Coronary development is regulated by ATP-dependent SWI/SNF chromatin remodeling component BAF180. Developmental Biology. 2008;319:258–266. doi: 10.1016/j.ydbio.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 109.Shikama N, Lutz W, Kretzschmar R, Sauter N, Roth J-F, Marino S, Wittwer J, Scheidweiler A, Eckner R. Essential function of p300 acetyltransferase activity in heart, lung and small intestine formation. EMBO J. 2003;22:5175–5185. doi: 10.1093/emboj/cdg502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Macias D, Perez-Pomares JM, Garcia-Garrido L, Carmona R, Munoz-Chapuli R. Immunoreactivity of the ets-1 transcription factor correlates with areas of epithelial-mesenchymal transition in the developing avian heart. Anat Embryol (Berl) 1998;198:307–315. doi: 10.1007/s004290050186. [DOI] [PubMed] [Google Scholar]
- 111.Lie-Venema H, Gittenberger-De Groot A, van Empel LJ, Boot MJ, Kerkdijk H, de Kant E, Deruiter M. Ets-1 and Ets-2 transcription factors are essential for normal coronary and myocardial development in chicken embryos. Circulation Research. 2003;92:749–756. doi: 10.1161/01.RES.0000066662.70010.DB. [DOI] [PubMed] [Google Scholar]
- 112.Théveniau-Ruissy M, Dandonneau M, Mesbah K, Ghez O, Mattei M-G, Miquerol L, Kelly RG. The del22q11.2 candidate gene Tbx1 controls regional outflow tract identity and coronary artery patterning. Circulation Research. 2008;103:142–148. doi: 10.1161/CIRCRESAHA.108.172189. [DOI] [PubMed] [Google Scholar]
- 113.Kraus F, Haenig B, Kispert A. Cloning and expression analysis of the mouse T-box gene Tbx18. Mech Dev. 2001;100:83–86. doi: 10.1016/s0925-4773(00)00494-9. [DOI] [PubMed] [Google Scholar]
- 114.Haenig B, Kispert A. Analysis of TBX18 expression in chick embryos. Dev Genes Evol. 2004;214:407–411. doi: 10.1007/s00427-004-0415-3. [DOI] [PubMed] [Google Scholar]
- 115.Li QY, Newbury-Ecob RA, Terrett JA, Wilson DI, Curtis AR, Yi CH, Gebuhr T, Bullen PJ, Robson SC, Strachan T, Bonnet D, Lyonnet S, Young ID, Raeburn JA, Buckler AJ, Law DJ, Brook JD. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet. 1997;15:21–29. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- 116.Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA, Leblanc-Straceski J, Renault B, Kucherlapati R, Seidman JG, Seidman CE. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 117.Hatcher CJ, Diman NY, Kim MS, Pennisi D, Song Y, Goldstein MM, Mikawa T, Basson CT. A role for Tbx5 in proepicardial cell migration during cardiogenesis. Physiol Genomics. 2004;18:129–140. doi: 10.1152/physiolgenomics.00060.2004. [DOI] [PubMed] [Google Scholar]
- 118.Bimber B, Dettman RW, Simon H-G. Differential regulation of Tbx5 protein expression and sub-cellular localization during heart development. Developmental Biology. 2007;302:230–242. doi: 10.1016/j.ydbio.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crispino JD, Lodish MB, Thurberg BL, Litovsky SH, Collins T, Molkentin JD, Orkin SH. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes & Development. 2001;15:839–844. doi: 10.1101/gad.875201. [DOI] [PMC free article] [PubMed] [Google Scholar]