Abstract
To investigate post-concussive symptoms (PCS) following mild pediatric traumatic brain injury (mTBI), 8- to 15-year old children with mTBI (n=186) and a comparison group with uncomplicated orthopedic injuries (OI, n=99) were recruited from two emergency departments. Parent and child ratings of PCS and symptom counts were obtained within 3 weeks after injury (baseline) and at 1, 3, and 12 months post injury. The mTBI group also completed magnetic resonance imaging (MRI) at baseline. Group differences were examined using growth modeling, controlling for age at injury, sex, socioeconomic status (SES), and (for parent-based measures) preinjury symptom levels. Relative to the OI group, the mTBI group had higher ratings of somatic PCS and parent counts of PCS at the initial assessments, but higher parent ratings of cognitive PCS and child counts of PCS throughout follow-up. Higher levels of PCS in the mTBI group were associated with motor-vehicle-related trauma, loss of consciousness, neuroimaging abnormalities, and hospitalization. The findings validate both transient and persistent PCS in children with mTBI and document associations of symptoms with injury and non-injury factors.
Keywords: mild traumatic brain injury, children, post-concussive symptoms, prospective studies
Introduction
Traumatic brain injury (TBI) in children ages birth to 14 years accounts for approximately 39,000 hospitalizations and 484,000 emergency department (ED) visits annually based on U.S. population estimates (Cassidy et al., 2004; Guerrero, Thurman, & Sniezek, 2000; Langois, Rutland-Brown, & Thomas, 2006). The rate of ED visits, most commonly for falls and motor-vehicle-related injuries, are higher in this age range than in other age groups. Given that 80%-90% of children treated in an ED for TBI are not hospitalized and >80% of those hospitalized are diagnosed with mild brain injury (Kraus, 1995; Langois et al., 2006; Thurman & Guerrero, 1999), the vast majority of these cases involve mild TBI (mTBI). In light of the large numbers of children with mTBI, any negative consequences of these injuries on child health and behavior is a significant public health concern. To the extent that mTBI has adverse consequences, information about the nature and predictors of these effects is beneficial in identifying and treating the affected children.
Although children with mTBI have more favorable cognitive, achievement, and behavior outcomes than children with moderate to severe TBI (Anderson et al., 1997; Brown, Chadwick, Shaffer, Rutter, & Traub, 1981; Chadwick, Rutter, Brown, Shaffer, & Traub, 1981; Ewing-Cobbs et al., 2004; Fletcher, Ewing-Cobbs, Miner, Levin, & Eisenberg, 1990; Max et al., 1999), mTBI can also have adverse effects. Relative to pre-injury status, uninjured children, or groups with other non-head trauma, children with mTBI are at increased risk for post-injury cognitive deficits, behavior problems, and post-concussive symptoms (PCS) (Asarnow, Satz, Light, Lewis, & Neumann, 1991; Kirkwood et al., 2008; Massagli et al., 2004; McKinlay, Dalrymple-Alford, Horwood, & Fergusson, 2002; Mittenberg, Miller, & Luis, 1997; Overweg-Plandsoen et al., 1999). PCS are especially prominent immediately after mTBI and include complaints of headache, dizziness, fatigue, depressed or anxious mood, sleep disturbance, light sensitivity, forgetfulness, and concentration difficulties. Apart from evidence for persistent PCS and other behavior problems in a minority of children with mTBI (Kashluba, Paniak, & Casey, 2008; Ponsford et al., 1999; Yeates et al., 1999), sequelae appear to resolve in large part within the few first months post injury (Carroll et al., 2004; Fay et al., 1993; Levin et al., 1987; Light et al., 1998).
However, methodological problems preclude any firm conclusions about long-term outcomes of mTBI (Beers, 1992; Carroll et al., 2004; Satz, Zaucha, McCleary, Light, & Asarnow, 1997; Satz et al., 1999). One limitation is that children with mTBI are frequently compared to uninjured children rather than to children with other traumatic injuries. Two studies of behavior outcomes report more problems in children with mTBI relative to uninjured children but not relative to children with other traumatic injuries (Asarnow et al., 1995; Bijur & Haslum, 1995). These findings raise the possibility that the behavior problems resulting from mTBI are precipitated by traumatic injuries more generally and thus may not be secondary to brain insult (Satz et al., 1999). An alternative interpretation is that differences in behavior between mTBI and uninjured groups reflect a general tendency for children with traumatic injuries to be at elevated risk for pre-trauma behavior problems (Goldstohm & Arrfa, 1995; Light et al., 1998). In either instance, evidence for sequelae related to brain trauma per se requires inclusion of a control group with other injuries.
Additional methodological problems include the failure of many past studies to take into account problems present prior to injury, follow children longitudinally and utilize both child self-report and parent report to assess behavior change, examine risk factors for negative outcomes, or consider potential moderators of injury effects, such as age at injury. Potential biases in sample recruitment and lack of a uniform definition of mTBI may further contribute to inconsistent findings. Studies that consider only children hospitalized for mTBI or that recruit participants from retrospective reviews of medical records or head injury clinics may yield unrepresentative samples. Definitions also vary in inclusion and exclusion criteria (Asarnow et al., 1995; Bigler, 2008; Kirkwood et al., 2008). Similarly, some studies include children with TBI-related neuroimaging abnormalities or skull fractures, whereas others exclude these cases. Adding to the confusion, several alternative terms for mTBI are used, including “minor head injury,” “mild closed head injury,” and “concussion.” Regardless of terminology, the presence of brain injury is most often inferred rather than documented and more research is needed to identify the minimum criteria for adverse neurobehavioral consequences (Kibby & Long, 1996; Satz et al., 1997).
A further limitation is that past research has focused on collective symptom counts or ratings rather different types of symptoms. The problem with this approach is that it ignores evidence from adult and child studies for distinct groupings of PCS—for example, into somatic, cognitive, and emotional symptoms (Ayr, Yeates, Taylor & Browne, 2009; Meares et al., 2006). In a longitudinal study of children with moderate to severe TBI, Yeates et al. (2001) found different patterns of change over time post injury for cognitive/somatic versus emotional/behavioral symptoms. Although both symptom types were more common in the children with TBI than in children with orthopedic injuries, the TBI group showed a relative decline over follow-up in cognitive/somatic symptoms and a relative increase in emotional/behavioral symptoms. Differential associations of the two symptoms clusters with children's preinjury behavior and family functioning also confirm suspicions that different symptoms types may have different antecedents (Gasquoine, 1997; Yeates & Taylor, 2005).
Finally, past studies have often failed to examine factors that may contribute to increased risks for poor outcomes. Based on previous research with adults and children, higher risks for adverse consequences of mTBI may be associated with multiple injury and non-injury risk factors, including LOC, initial disorientation, a longer period of PTA, TBI-related abnormalities in neuroimaging, motor-vehicle-related trauma, hospitalization, younger age at injury, limited social support or family stressors, lower IQ, pre-injury behavior or learning problems, and involvement in litigation (Binder, 1986; Falk, Cederfjall, von Wendt, & Klang, 2006; Gronwald, Wrightson, & McGinn, 1997; Kashluba et al., 2008; Kirkwood et al., 2008; Levin et al., 2008; Luis, Vanderploeg, & Curtiss, 2003; McKinlay et al., 2002; Ponsford et al., 1999; Ponsford et al., 2000; Williams, Levin, & Eisenberg, 1990). Recognition of the factors that place children at high risk for persisting problems would be useful in determining which children are most deserving of close monitoring or more comprehensive clinical evaluations (Kirkwood et al., 2008).
The present study was designed to examine PCS in children with mTBI across the first year post injury using methods that addressed some of the shortcomings of previous research in this area (Yeates & Taylor, 2005). Children with mTBI and a comparison group of children with traumatic orthopedic injuries (OI) were recruited from consecutive ED visits using criteria similar to those employed by Asarnow et al. (1991). Information on different types of PCS was collected from both parents and the injured children shortly after injury and at three subsequent contacts. At the initial visit, parents also provided information on PCS prior to injury.
We recently applied finite mixture modeling to our data to identify distinct longitudinal trajectories in a composite parent rating of PCS across the first year post injury (Yeates et al., 2009). The findings indicated that children with mTBI were more likely than those with OI to be classified into subsets showing high initial levels of PCS and persistent symptoms. The results also revealed that these differences were more pronounced for children with mTBI at higher injury risk, as defined by a composite of medical risk factors. The primary objective of modeling, however, was to classify children based on change. The results did not provide an indication of overall group differences in PCS across follow-up. Moreover, we did not examine all available markers of head injury severity or assess these markers in relation to the full continuum of PCS. In further contrast to the previous report, the present study examined environmental factors as moderators of the effects of mTBI and considered different types of parent-rated PCS as well as child self-reports of PCS.
The major aim of the present paper was to characterize the natural history of PCS for the total mTBI group relative to children with OI and to examine group differences across the continuum of symptoms. Additional aims were to explore potential moderating effects of age at injury and demographic factors on group differences, as well as associations of several injury characteristics with outcomes within the mTBI group. We hypothesized that PCS would be more pronounced and persistent in the mTBI group, that these group differences would be more pronounced in younger children, and that markers of greater injury severity would predict higher levels of PCS after mTBI. Based on previous research with more severely injured children (Yeates et al., 2001), we additionally anticipated different patterns of change for different types of PCS, with somatic symptoms showing more rapid recovery than cognitive or emotional symptoms.
Method
Participants
Children in the mTBI and OI groups were recruited from consecutive visits to the emergency departments of two children's hospitals in Ohio, Nationwide Children's Hospital in Columbus and Rainbow Babies and Children's Hospital in Cleveland. Only children who were 8-15 years of age at the time of injury were enrolled. Eligibility criteria for the mTBI group included a blunt head trauma and evidence for at least one of the following indications of concussion: an observed LOC; a Glasgow Coma Scale (Teasdale & Jennett, 1974) score of 13 or 14; or note of at least two of the following acute signs and symptoms of concussion: persistent post-traumatic amnesia (PTA), transient neurological deficits, vomiting, nausea, headache, diplopia, dizziness, disorientation, and other mental status changes. Children with mTBI were excluded if they had a LOC >30 minutes, a GCS score of less than 13, delayed neurological deterioration, or any medical contraindication to magnetic resonance imaging (MRI). Potential participants were not excluded if they required hospitalization or demonstrated intracranial lesions or skull fractures on acute computerized tomography (CT) scans.
Children were eligible for the OI group if they had sustained upper or lower extremity fractures associated with a score of 3 or less on the Abbreviated Injury Scale (AIS; American Association for Automotive Medicine, 1990). They were excluded if they displayed any evidence of head injury or symptoms of concussion. Criteria for exclusion from both groups included neurosurgical or surgical intervention; an injury that would interfere with neuropsychological testing (e.g., fracture of preferred upper extremity); hypoxia, hypotension, or shock during or following the injury; ethanol or drug ingestion involved with the injury; documented history of previous head injury requiring medical treatment; premorbid neurological disorder or mental retardation; injury determined to be the result of child abuse or assault; or a history of severe psychiatric disorder requiring hospitalization.
The participation rates for children in the mTBI and OI groups who met inclusion and exclusion criteria were 47% and 35%, respectively. Participants did not differ significantly from eligible non-participants in age, gender, or ethnic/racial minority status, or in census tract measures of socioeconomic status (mean family income, percentage of minority heads of household, and percentage of households below the poverty line) obtained using the Federal Financial Institutions Examinations Council Geocoding System (2003, 2005).
The total sample included 186 children with mTBI and 99 with OI. As shown in Table 1, the groups did not differ significantly in age at injury, time between injury and the baseline assessment, sex or race distribution, or socioeconomic status (SES) as assessed by averaging sample z scores for years of maternal education, median family income for census tract, and parent occupational status as measured by the Duncan Socioeconomic Index (Stevens & Cho, 1985). Comparison of the groups on a short form of the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, 1999) consisting of the Vocabulary and Matrix Reasoning subtests also failed to reveal significant differences in IQ.
Table 1. Sample Characteristics.
| Variable | mTBI Group (n = 186) |
OI Group (n = 99) |
|---|---|---|
| Age at injury in years, M (SD) | 11.96 (2.22) | 11.76 (2.23) |
| Days between injury and baseline assessment, M (SD) | 11.43 (3.33) | 11.21 (3.59) |
| Males, n (%) | 132 (71.0) | 64 (64.6) |
| White, n (%) | 132 (71.0) | 64 (64.6) |
| SES, M (SD) | .05 (.91) | -.09 (1.15) |
| WASI Short Form IQ, M (SD) | 99.66 (13.83) | 98.90 (15.08) |
| Modified Injury Severity Scale, M (SD) | ||
| 1-3 | 65 (34.9) | 28 (28.3) |
| 4-8 | 94 (50.5) | 70 (70.7) |
| 9-15 | 14 (7.5) | 1 (1.0) |
| 16-25 | 13 (7.0) | 0 (0) |
| Cause of injury, n (%) | ||
| Sports/Recreation | 106 (57.0) | 62 (62.6) |
| Falls | 36 (19.4) | 21 (21.2) |
| Motor Vehicle | 31 (16.7) | 3 (3.0) |
| Other | 31 (7.0) | 13 (13.1) |
| Loss of consciousness, n (%) | 74 (39.8) | --- |
| CT abnormality, n (%) | 11 (8.8) | |
| MRI abnormality, n (%) | 32 (17.2) | --- |
| Non-head injury | 47 (25.3) | --- |
mTBI = mild traumatic brain injury; OI = orthopedic injury; WASI = Wechsler Abbreviated Intelligence Scale; SES = socioeconomic status; CT = computed tomography; MRI = magnetic resonance imaging. SES was assessed by averaging sample z scores for years of maternal education, median family income for census tract, and the Duncan Socioeconomic Index (a measure of occupational status).
Injury severity, defined according to the Modified Injury Severity Scale (Mayer, Matlak, Johnson, & Walker, 1980) as the sum of the squares of the three most affected body regions, was higher for the mTBI group. Recreational and sports-related accidents and falls were the most common cause of injury. Motor-vehicle-related trauma, though less common, was a more frequent cause of mTBI. Table 1 also lists other injury characteristics. LOC typically was brief in duration (median = 1 minute, range = <1-15 minutes). Acute computed tomography (CT) scans were available for 125 children in the mTBI group, with abnormalities in 11 (9%), including 6 with parenchymal lesions or edema and 5 with extra-axial lesions only. Thirty two children with mTBI had MRI abnormalities (17%), including 25 with parenchymal contusions or hemorrhages, 5 with extra-axial (subdural or subarachnoid) hemorrhages only, and 2 with mild atrophy only that provided less definitive evidence for TBI-related brain insult. Parenchymal lesions on MRI varied widely by location and were often multi-focal. Forty seven (25%) children with mTBI had other (non-head), including fractures to the ribs or extremities, or lacerations, abrasions, hematomas, or contusions to the body surface. Fifty nine (37%) children with mTBI were hospitalized for 1-7 days (90% of those were for 1-2 days) compared with none of the children with OI.
Procedures
Children who met study criteria and whose parents agreed to participate were scheduled for an initial assessment within 3 weeks after injury. Parents and children were again contacted by phone at 1 month post injury, and they came in for additional follow-up visits at 3 and 12 months post injury. Institutional review board approval and informed parental consent and child assent were obtained prior to participation.
At the initial assessment, children completed measures of their current PCS. Parents completed the same measures first retrospectively based on children's status prior to injury and then based on their current status. Parents also provided information on family background. Although this report focused on measures of PCS, the short-form WASI and other assessments of the children's postinjury cognitive and academic skills and standardized child behavior ratings were completed during this and other visits as part of the larger study (Yeates & Taylor, 2005).
Either on the same day as the baseline visit or shortly thereafter, the children completed an MRI. The pulse sequence for the MRI included sagittal T1-weighted spin echo images, axial T2-weighted and proton density fast spin echo images, coronal 2-dimensional gradient echo images, coronal fluid attenuated inversion recovery (FLAIR) images, and axial diffusion-weighted echo planar images. Board-certified radiologists specializing in pediatric radiology who were blinded as to group status and the results of other assessments rated the scans for TBI-related intracranial abnormalities.
At 1, 3, and 12 months post injury, the PCS measures were again administered to the children and their parents based on children's status at those times. The PCS measures were administered by phone for the 1-month follow-up and as part of face-to-face return visits at 3 and 12 months post injury. Attrition was relatively low, with retention of 183 (98%) and 98 (99%) children in the mTBI and OI groups, respectively, at the 1-month follow-up; 178 (96%) and 90 (91%) children at the 3-month follow-up; and 169 (91%) and 84 (85%) at the 12-month follow-up. Four children in the mTBI group did not complete an MRI. Children who dropped out before the final follow-up were more often non-white and had lower SES than those who completed the study. However, children from the sample who dropped out did not differ from those who completed follow-up on measures of preinjury or baseline symptoms, age at injury, or sex.
Measures of PCS
Measures of PCS included both rating scales and symptom counts. To obtain ratings of PCS, we administered the Health and Behavior Inventory (HBI, Ayr et al., 2009) to parents as well as to the injured child. The HBI is comprised of 50 items pertaining to a variety of cognitive, somatic, and emotional/behavioral symptoms, each of which is rated on a 4-point scale in terms of frequency of occurrence over the last week (0 = never, 1 = rarely, 2 = sometimes, 3 = often). Although the HBI was originally developed to assess neurobehavioral symptoms in children with moderate to severe TBI (Barry, Taylor, Klein, & Yeates, 1996), an earlier version of the HBI was used in a previous study of PCS in children with mTBI (Yeates et al., 1999) and item content is similar to that found in PSC checklists used with adults (Axelrod et al, 1996; Cicerone & Kalmar, 1995; Gouvier, Cubic, Jones, Brantley, & Cutlip, 1992; Gerber & Schraa, 1995). Except for rewording some items from the parent version to make them appropriate for child self-report, the parent and child versions were identical. Parents and children completed the HBI in separate locations. Most children and parents completed the ratings in written form, though research assistants read items orally to participants who had difficulty reading them.
After discarding infrequently endorsed items, factor analysis of ratings from the parent version of the HBI using targeted rotation indicated that items from the parent PCS ratings could be grouped into scales for somatic PCS [9 items, internal reliability (IR) for total sample = .89)], cognitive PCS (11 items, IR = .95), and emotional PCS (12 items, IR = .89) (Ayr et al., 2009). Applying similar procedures to the child self-ratings, items were grouped into somatic PCS (9 items, IR = .86) and cognitive PCS (11 items, IR = .89) identical to those in the adult scales. Analysis of child self-ratings did not yield evidence for a distinct emotional or behavioral symptom cluster, hence this dimension of PCS was assessed only by parents.
Counts of PCS were obtained using the Post-Concussive Symptom Interview, which was based on a similar measure used in previous research on mTBI and PCS in children (Mittenberg et al., 1997; Mittenberg, Wittner, & Miller, 1997). The interview assesses the number of 15 somatic, cognitive, and emotional symptoms that have been present during the preceding week. The symptoms are similar to the criteria for Post-Concussion Syndrome in the International Classification of Diseases (ICD-10; World Health Organization, 1992) and for Post-concussional Disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994). The reliability and validity of the PSC Interview has been demonstrated in these previous studies, and IR for the total sample in this study was satisfactory for both the baseline parent and child versions (.81 and .70, respectively).
Scores included in analysis were the sum ratings for each of the PSC rating scales and the total number or count of symptoms present from the interview measure. Table 2 presents correlations between the PCS measures at the baseline assessment. The results show significant correlations between child- and parent-based measures somatic and cognitive PCS ratings and counts of PCS. Correlations between different measures of child and parent PCS were also significant at each follow-up, indicating positive associations of different types of PCS with total PCS both within and across child and parent informants.
Table 2. Correlations of Measures of PCS.
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Child rating of somatic PCS | --- | ||||||
| 2. Child rating of cognitive PCS | .63** | --- | |||||
| 3. Child report of # of PCS | .71** | .66* | --- | ||||
| 4. Parent rating of somatic PCS | .36** | .17** | .34** | --- | |||
| 5. Parent rating of cognitive PCS | .24** | .31** | .29** | .42** | --- | ||
| 6. Parent rating of emotional PCS | .14* | .13* | .18** | .48** | .55** | --- | |
| 7. Parent report of # of PCS | .31** | .18** | .38** | .70** | .53** | .56* | --- |
p < .05;
p < .01.
Data Analysis
Group comparisons of PCS across the four assessments (baseline and 1-, 3-, and 12-month follow-ups) were conducted using general linear mixed model analysis, or growth modeling (SAS Proc Mixed; Singer, 1998). To examine group differences in the evolution of PCS, our primary analytic models included the factors group, age at injury, and time since injury (modeled in terms of both linear and quadratic change). Sex, race, SES, and age at injury were included as covariates in the models. The corresponding measure of preinjury symptoms (i.e., same symptoms as those listed in PCS rating or interview) was also included as a covariate in analysis of parent-reported PCS; thus the dependent measures in these instances reflect changes in symptoms relative to preinjury levels. To explore potential moderating effects of demographic status on the group differences, the models also included interactions of sex, race, SES, and age at injury with group, time since injury, and group × time since injury. Non-significant higher- and then lower-order interactions were trimmed to identify the most parsimonious models and reduce risks of over-fitting.
The effects of injury-related factors on PCS ratings and on interview-based symptom counts were examined using additional mixed model analysis of data from the mTBI group. The factors included in these analyses were the same as those used in comparing the mTBI and OI groups, with substitution of the presence/absence of each of several injury factors for group. The factors LOC, TBI-related abnormality on CT and MRI scans, other (non-head) injury, hospitalization, and motor-vehicle-related trauma (MVRT) were each considered separately in these analyses. The small number of children with CT or MRI abnormalities precluded analysis of the effects of specific lesion location. The primary analysis of MRI findings compared children with and without any abnormality. However, in view of evidence presented by Williams et al. (1990), a secondary analysis was also conducted in which MRI abnormality was defined as parenchymal contusions or hemorrhages (other abnormalities excluded from analysis).
To control for Type I error, we applied a family-wise alpha for the parent and child measures of PCS, with alpha set at .0125 for each of the four parent measures and at .0167 for each of the three child measures. Unstandardized beta weights and standard errors (se's) for significant main or simple effects indicate group differences in scores and provide estimates of effect size. Examination of the distributions of PCS scores suggested non-normality for some of the measures. Normality was improved using square root transformations, but because results for transformed and untransformed analyses were essentially the same, only findings from the latter analyses are reported.
Results
Group Differences in PCS and Non-Injury Predictors for the Total Sample
Parent report
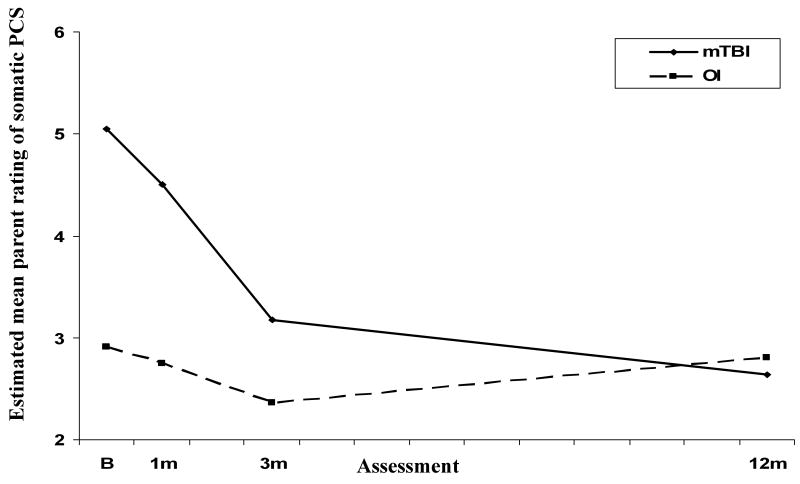
Analysis of parent ratings of somatic PCS revealed a group × time since injury effect (see Table 3). Figure 1 plots the estimated mean ratings for the two groups across the four assessments. Simple effects tests showed that ratings of somatic PCS were higher in the mTBI group than in the OI group at baseline and 1 month, but not at 3 months or 12 months. Decreases in ratings over follow-up were significant for the mTBI group only.
Table 3.
Results of Mixed Models Analysis of Measures of PCS
| Measures | Effect | Estimate | Std Error | df | t | p |
|---|---|---|---|---|---|---|
| Parent Rating of Somatic PCS | Premorbid Rating | 0.50 | 0.05 | 280 | 10.22 | <.001 * |
| Age at Injury | -0.01 | 0.07 | 282 | -0.19 | 0.846 | |
| SES | -0.39 | 0.17 | 274 | -2.29 | 0.023 | |
| Race (Minority) | -0.67 | 0.37 | 287 | -1.81 | 0.072 | |
| Gender (Female) | 0.48 | 0.34 | 280 | 1.42 | 0.156 | |
| Group × Time Since Injury [F (1,309) = 6.51, p = .001] | ||||||
| Group at baseline | 2.14 | 0.50 | 310 | 4.26 | <.001 * | |
| Group at 1 month | 1.76 | 0.41 | 290 | 4.32 | <.001 * | |
| Group at 3 months | 0.80 | 0.47 | 307 | 1.72 | 0.086 | |
| Group at 12 months | -0.16 | 0.42 | 263 | -0.39 | 0.697 | |
| Parent Rating of Cognitive PCS | Group | 1.51 | 0.53 | 287 | 2.84 | 0.005 * |
| Premorbid Rating | 0.70 | 0.03 | 285 | 20.49 | <.001 * | |
| Age at Injury | -0.15 | 0.11 | 287 | -1.27 | 0.206 | |
| SES | -0.48 | 0.28 | 284 | -1.73 | 0.084 | |
| Race (Minority) | 0.24 | 0.59 | 288 | 0.41 | 0.686 | |
| Gender (Female) | 0.62 | 0.55 | 286 | 1.13 | 0.259 | |
| Time Since Injury [F (2,282) = 7.11, p = .001] | ||||||
| Parent Rating of Emotional PCS | Group | 0.29 | 0.51 | 281 | 0.56 | 0.575 |
| Premorbid Rating | 0.63 | 0.04 | 282 | 16.19 | <.001 * | |
| Age at Injury | -0.15 | 0.11 | 279 | -1.36 | 0.175 | |
| SES | -0.54 | 0.26 | 274 | -2.07 | 0.040 | |
| Race (Minority) | -0.14 | 0.57 | 283 | -0.25 | 0.804 | |
| Gender (Female) | 0.26 | 0.52 | 278 | 0.49 | 0.624 | |
| Time Since Injury [F (2,286) = 1.07, p = .345] | ||||||
| Parent Count of PCS | Premorbid Count | 0.55 | 0.07 | 287 | 7.95 | <.001 * |
| Age at Injury | -0.15 | 0.05 | 283 | -2.82 | 0.005 * | |
| SES | -0.26 | 0.13 | 278 | -2.06 | 0.040 | |
| Race (Minority) | 0.07 | 0.27 | 287 | 0.26 | 0.792 | |
| Gender (Female) | 0.62 | 0.25 | 282 | 2.52 | 0.012 * | |
| Group × Time Since Injury [F (2,288) = 4.41, p = .013] | ||||||
| Group at baseline | 1.36 | 0.33 | 300 | 4.12 | <.001 * | |
| Group at 1 month | 1.13 | 0.28 | 289 | 4.05 | <.001 * | |
| Group at 3 months | 0.55 | 0.29 | 291 | 1.89 | 0.060 | |
| Group at 12 months | 0.23 | 0.31 | 267 | 0.74 | 0.461 | |
| Child Rating of Somatic PCS | Age at Injury | -0.20 | 0.12 | 283 | -1.63 | 0.104 |
| SES | -0.73 | 0.29 | 279 | -2.49 | 0.013 * | |
| Race (Minority) | 0.39 | 0.63 | 286 | 0.61 | 0.544 | |
| Gender (Female) | 1.69 | 0.58 | 282 | 2.9 | 0.004 * | |
| Group × Time Since Injury (linear) [F (1,309) = 6.51, p = .011] | ||||||
| Group at baseline | 2.11 | 0.66 | 288 | 3.19 | 0.002 * | |
| Group at 1 month | 1.63 | 0.61 | 283 | 2.68 | 0.008 * | |
| Group at 3 months | 0.50 | 0.69 | 293 | 0.73 | 0.465 | |
| Group at 12 months | 0.59 | 0.68 | 274 | 0.87 | 0.387 | |
| Child Rating of Cognitive PCS | Group | 0.85 | 0.76 | 285 | 1.11 | 0.266 |
| Race (Minority) | -1.16 | 0.85 | 286 | -1.36 | 0.174 | |
| Gender (Female) | 1.50 | 0.78 | 283 | 1.92 | 0.056 | |
| Age at Injury × Time Since Injury [F (2,278) = 4.99, p = .007] | ||||||
| SES × Time Since Injury [F (2,280) = 4.60, p = .011] | ||||||
| Child Count of PCS | Group | 0.73 | 0.30 | 283 | 2.48 | 0.014 * |
| SES | -0.32 | 0.15 | 280 | -2.12 | 0.035 | |
| Race (Minority) | 0.11 | 0.33 | 284 | 0.34 | 0.736 | |
| Gender (Female) | 1.16 | 0.30 | 281 | 3.84 | <.001 * | |
| Age at Injury × Time Since Injury (linear) [F (1,314) = 7.76, p = .006] | ||||||
Note: Asterisk indicates significance at Bonferroni-adjusted alpha level. Model estimates for group effects are group differences in raw scores. Model estimates are not given for time since injury, as this represents the conjoint effects of linear and quadratic terms. SES=Socioeconomic Status. Metrics: premorbid (raw score), age at injury (years), SES (z-score).
Figure 1.
Estimated mean parent ratings of somatic PCS across follow-up for the mTBI and OI groups.
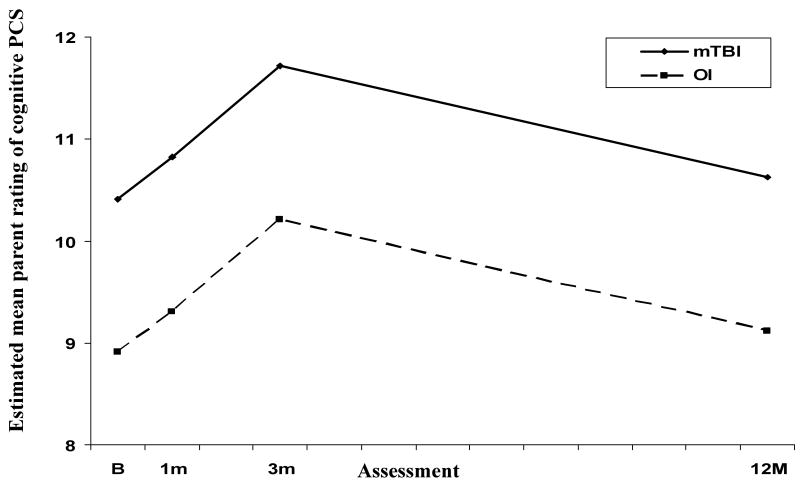
Analysis also revealed a group main effect for ratings of cognitive PCS, with higher PCS in the mTBI across follow-up (see Figure 2). A main effect for time since injury reflected significant increases in these ratings from baseline to 1 month and from 1 month to 3 months, and a significant decrease from 3 to 12 months.
Figure 2.
Estimated mean parent ratings of cognitive PCS across follow-up for the mTBI and OI groups.
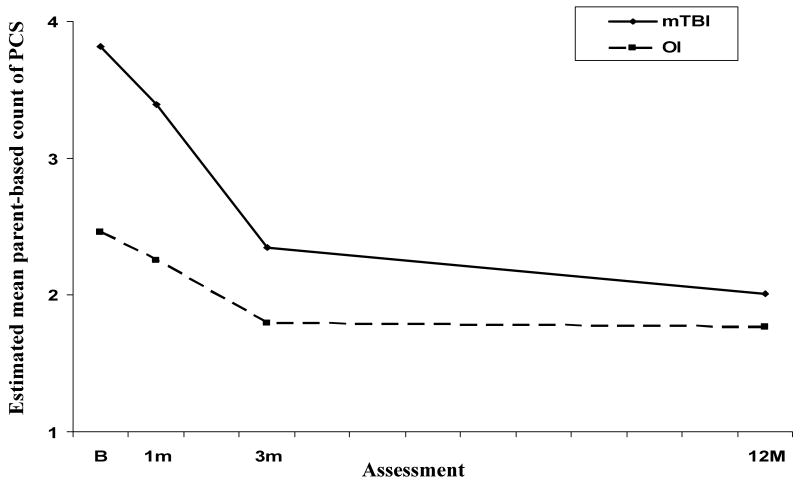
Findings for parent-based counts of PCS indicated a group × time since injury effect that was just shy of significance using Bonferroni corrected alpha. Because this effect indicated variations in group differences across the assessment that would have been obscured by examining only the main effect of group (see Figure 3), follow-up tests of group differences were conducted at each assessment. The results of these tests indicated higher counts of PCS at baseline and 1 month but not at 3 months or 12 months. Further analysis revealed significant decreases in counts of PCS across follow-up for both the mTBI and OI groups. The latter finding, together with the fact that counts of PCS were higher at baseline relative to preinjury status even in the OI group [paired t (98) = 6.77, p < .001], suggested postinjury changes in this group. Group differences in parent ratings of emotional PCS were not significant.
Figure 3.
Estimated mean parent-based counts of PCS across follow-up for the mTBI and OI groups.
Child self-report
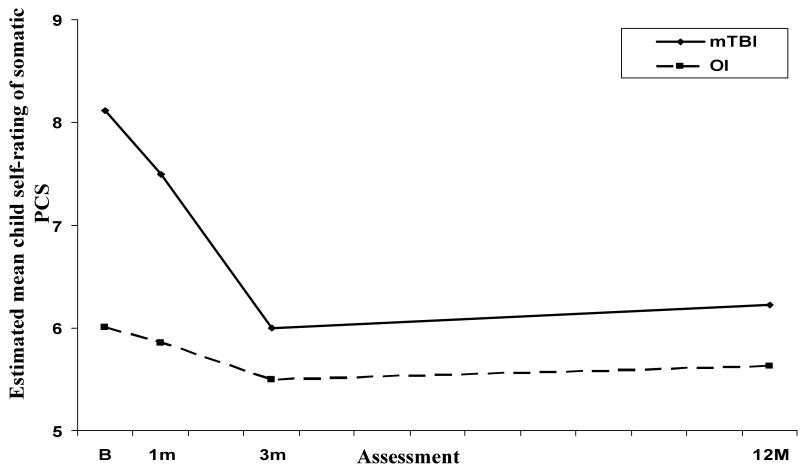
Results for self-ratings of somatic PCS were similar to those for the parent ratings (see Figure 4). Analysis of this measure revealed a group × time since injury (linear) effect, with simple effects tests indicating higher ratings for the mTBI group than for the OI group at baseline and 1 month, but not at 3 months or 12 months. Self-ratings of somatic PCS decreased significantly for the mTBI group from baseline to 1 month and from 1 month to 3 months, but did not vary significantly over follow-up for the OI group.
Figure 4.
Estimated mean child self-ratings of somatic PCS across follow-up for the mTBI and OI groups.
A main effect of group was found for child-reported counts of PCS, indicating a higher number of PCS in the mTBI group throughout the follow-up period. Estimated means for the mTBI group were 4.84 at baseline, 4.58 at 1 month, 3.96 at 3 months, and 4.20 at 12 months, with means for OI group 0.73 less at each assessment. Counts decreased significantly from baseline to 1 month and from 1 to 3 months, but did not change significantly from 3 to 12 months. For this variable, time since injury interacted with age at injury, reflecting steeper decreases in symptom counts from baseline through 3 months for older children in the sample. Group differences in child self-ratings of cognitive PCS were not significant.
Predictors of PCS other than group
As evident in Table 3, group differences were not moderated by pre-injury symptoms or any demographic factors. However, higher levels of parent measures of PCS were associated with higher levels of the corresponding parent estimates of pre-injury symptoms. Higher parent counts of PCS were also associated with younger age at injury and female sex; and higher child self-ratings of somatic PCS with lower SES and female sex. Older age at injury was related to higher child self-ratings of cognitive PCS but not at all assessments. Lower SES was also related to higher self-ratings of cognitive PCS but more so at the earlier assessments.
Association of Injury Factors with PCS in Children with mTBI
Analysis of data from the mTBI group revealed several main effects of injury factors on PCS. LOC was associated with higher parent ratings of cognitive PCS [F (1,185) = 7.43, beta (se) = 1.71 (0.63), p = .007]; and MVRT predicted higher parent-reported counts of PCS [F (1,190) = 9.87, beta (se) = 1.16 (0.37), p = .002]. Marginally significant findings included associations of hospitalization with higher parent ratings of cognitive PCS [F (1,186) = 5.17, beta (se) = 1.52 (0.67), p =.024], MVRT with higher parent ratings of emotional PCS [F (1,183) = 6.23, beta (se) = 1.92 (0.77), p = .014], and non-head injuries with higher parent-reported counts of PCS [F (1,188) = 4.17, beta (se) = 0.65 (0.32), p = .043].
Analysis also suggested that some injury-related effects varied across follow-up. The effects of MVRT on parent ratings of cognitive PCS varied with the linear component of time since injury [F (1,203) = 7.02, p =.009], with simple effects tests indicating higher PCS in the children with MVRT only at 3 months [t (190) = 4.18, beta (se) = 4.56 (1.09), p < .001] and 12 months [t (176) = 2.62, beta (se) = 3.52 (1.35), p = .010]. The effect of hospitalization on parent ratings of somatic PCS diminished across follow-up [F (2,194) = 6.44, p = .002], with higher ratings reported for hospitalized children only at baseline [t (198) = 2.58, beta (se) = 0.33 (0.13), p = .011]. A similar effect of hospitalization was observed for parent ratings of emotional PCS [F (2,186) = 5.18, p = .006], with marginally significant effects of this variable at baseline [t (196) = 2.25, beta (se) = 0.31 (0.14), p = .025] and 1 month [t (187) = 2.27, beta (se) = 0.26 (0.11), p = .024]. The effect of LOC on parent counts of PCS was likewise transitory. For this outcome, effects varied with both age at injury and time since injury [F (2,187) = 4.75, p = .010]. Tests of simple effects conducted at age 1 SD above and below the sample mean revealed a more negative effect of LOC at the two initial assessments but only in younger children.
Finally, two non-injury characteristics moderated the effects of injury-related factors on PCS. Age at injury moderated the effects of CT abnormality on parent ratings of emotional PCS [F (1,119) = 9.05, p = .003]. SES also moderated both the effects of non-head injury on parent ratings of somatic PCS [F (1,190) = 7.55, p = .007], as well as the effect of parenchymal contusions or hemorrhages on MRI on the parent ratings of cognitive PCS, though the latter effect varied across time post injury [F (1,190) = 6.72, p = .010]. Simple effects of CT abnormality at ages at injury 1 SD above and below the sample mean revealed associations of acute brain lesions with more emotional PCS only for participants injured at a younger age [t (119) = 3.42, beta (se) = 6.25 (1.83), p < .001]. Simple effects of non-head injury at SES levels 1 SD above and below the sample mean indicated an adverse effect of this factor but only in children of lower SES [t (194) = 3.80, beta (se) = 2.75 (0.72), p < .001]. MRI evidence of parenchymal lesions were associated with higher parent ratings of cognitive PCS only at the 3-month follow-up in children of lower SES [t (178) = 2.86, beta (se) = 6.02 (2.11), p = .005]. As children with MVRT had lower SES than children with other causes of injury [χ2 (1, N = 186) = 21.19, p < .001], SES-related predisposition to MVRT may account for the moderating effects of this factor. SES also moderated the effects of hospitalization on changes across follow-up parent and child counts of PCS, but results of simple effects were not interpretable and are likely spurious.
Discussion
The present findings support the hypothesis that children with mTBI have PCS that can not be accounted for by the effects of traumatic injuries more generally or by pre-existing symptoms. Controlling for preinjury symptoms, parent ratings of somatic and cognitive PCS and parent-based counts of PCS were higher in children with mTBI than in children with minor OI. The results illustrate the benefits of examining different types of PCS and of considering variations in these effects in relation to time since injury and injury and non-injury characteristics. Group differences in parent ratings of somatic PCS and counts of PCS were most evident at the initial follow-ups and had resolved by 12 months post injury. In contrast, parent ratings of cognitive PCS in children with mTBI did not peak until the 3-month assessment and remained higher for these children than for the OI group throughout the follow-up period. The parent ratings confirm our expectations that changes in PCS after mTBI vary for different types of symptoms and suggest that some types are more persistent than others.
Similar to results for the parent-based measures, child self-report revealed higher ratings of somatic PCS in the mTBI group only at earlier in follow-up, with children in the two groups endorsing similar levels of physical complaints by 3 months post injury. However, like parent ratings of cognitive PCS, the mTBI group had higher self-reported counts of PCS than the OI group across the follow-up interval. Correlations between child and parent reports of PCS provide consensual validation for the findings. The consistency of child self-ratings of somatic PCS and counts of PCS with the corresponding parent data also helps to establish the validity of self-report in 8- to 15-year-old children.
Consistent with previous studies and with high base rates of PCS in the general population, PCS were evident in children with OI as well as in those with mTBI (Bijur & Haslum, 1995; Gasquoine, 1997; Goldstrohm & Arffa, 1995; Kashluba, Casey, & Paniak, 2006; Meares et al., 2006; Light et al., 1998; Wong, Regennitter, & Barrios, 1994). Early in follow-up, both groups showed increases in parent ratings of cognitive PCS and decreases in ratings of somatic PCS and in counts of PCS, suggesting that some symptom changes after mTBI reflect responses to injury more generally rather than effects of brain insult.
Also in keeping with past studies, PCS were related both to injury characteristics and non-injury factors (Kirkwood et al., 2008). Higher levels of preinjury symptoms predicted higher scores on all corresponding measures of PCS. Other associations included younger age and female sex with higher parent counts of PCS; lower SES and female sex with higher child self-ratings of somatic PCS; and lower SES and older age at injury with higher child self-ratings of cognitive PCS. Although these associations were evident for both groups of children, they underscore the importance of considering non-injury background characteristics in evaluating PCS in children with mTBI.
Several injury characteristics assumed to reflect greater risk for brain insult predicted higher levels of PCS within the mTBI, including LOC, acute CT scan abnormality, parenchymal lesion on MRI, hospitalization, MVRT, and injuries to body regions other than the head (Falk et al, 2006; Levin et al., 2008; Luis et al., 2003; McKinley et al., 2002; Ponsford et al., 2000; Yeates et al., 2009). The effects of LOC and MVRT were observed throughout the follow-up interval, suggesting that persisting symptoms are more likely in children with higher-risk head injuries. These findings highlight the need for further investigation of ways to grade injury severity in this population and are consistent with suggestions that a minimal level of severity may be necessary for head-injury-specific PCS (Asarnow et al., 1995; Satz et al., 1997).
We recently found that MRI abnormalities were associated with higher parent ratings of cognitive PCS, but only in children with lower cognitive ability (Fay et al., in press). The present findings indicate that this association may extend to the broader mTBI sample when abnormalities are defined in terms of parenchymal lesions. These findings are also consistent with adult data showing greater cognitive deficits following complicated compared with uncomplicated mTBI (Williams et al., 1990). It is unclear why parenchymal lesions on MRI predicted more cognitive PCS only at the 3-month assessment, but higher rates of MVRT (implicating more severe mTBI) in children with lower SES may explain why the association of brain lesions with cognitive PCS was evident only in this subset of the TBI group. The association of acute CT scan abnormalities with higher parent ratings of emotional PCS is also consistent with previous findings (de Andrade et al., 2006; Levin et al., 2008). In the present study, both this association and that between LOC and parent counts of PCS were more evident for children injured at younger ages. This age-at-injury effect is similar to that observed in studies of other outcomes of pediatric mTBI (Gronwald et al., 1997; McKinlay et al., 2002) and suggests that younger age at injury increases risks for PCS.
The results of this study accord well with the hypothesis that both neurological and psychological factors contribute to PCS in children with mTBI (Alexander, 1997; Binder, 1986). An implication of post-injury changes in PCS in both injury groups is that the experience of trauma or factors not specific to head injury, such as physical discomfort or difficulties in adjusting to the effects of injury, are likely to contribute to PCS after mTBI. Consistent with findings from both animal and human studies (Bigler, 2008), neurological disruption may result in problems in functioning that precipitate or add to these effects, at least in a minority of children with mTBI (Kasluba et al., 2008; Massagli et al., 2004; Ponsford et al., 1999; Ponsford et al., 2000; Yeates et al., 1999). An alternative possibility is that group differences reflected greater expectancies for behavior change after injury to the head than after other injuries (Ferguson, Mittenberg, Barone, & Schneider, 1999; Gunstad & Suhr, 2001), though the association of injury factors to PCS within the mTBI group argues against such an interpretation. More definitive support for a neurogenic component of PCS will require investigation of associations of subjective symptoms with residual injury-related changes in brain structure or function (Bigler, 2008; Kibby & Long, 1996). Potential influences on PCS of children's coping skills and of neural “reserve” (Dennis, Yeates, Taylor, & Fletcher, 2007) may also need to be considered, as these characteristics may obscure the effects of demonstrable brain insults.
A major limitation of the study is that information regarding the children's status at the time they presented to the ED was determined by retrospective review of medical records rather than via structured procedures for intake and evaluation. Substantial inter-individual variability likely exists among ED health care providers in methods of evaluating children with minor traumatic injuries and in details regarding the injury and the child's current medical status included in the medical record. Reliance on these data constrained our ability to assess injury factors in a more uniform and probing manner and to detect relations between injury factors and PCS. A further weakness is the lack of a non-injury comparison group (Satz et al., 1997). Inclusion of an uninjured group would have been useful in clarifying child and family characteristics associated with minor traumatic injuries and in providing a yardstick against which to assess the extent of post-injury PCS in both the mTBI and OI groups. A third limitation is that the MRI procedures employed in this study, although selected to be the most sensitive available at the time the study was designed, may have been insensitive to some of the subtle insults associated with mTBI. Scans conducted soon after injury or methods more sensitive to white matter injury, such as diffusion tensor imaging or susceptibility-weighted scans, may provide for a more sensitive assessment of neuropathology (Bigler, 2008).
Although we have recently demonstrated that persisting effects of PCS occur in a minority of children with mTBI (Yeates et al., 2009), further research is needed to find ways to identify these children, isolate the neural and cognitive correlates of their problems, and determine the implications of these symptoms for children's behavior, learning, and daily functioning. A focus on individual symptoms that distinguish children with mTBI from other traumatic injuries at a point at which more transient symptoms have resolved may be one useful strategy (Kashluba et al., 2006: Overweg-Plandsoen et al., 1999). Another way to advance this goal is to examine other potential risk factors. As an example of this approach, we are currently examining pre-injury child behavior and the family environment as moderators of group differences in PCS in the present sample. Investigation of the mechanisms responsible for persistent PCS is also essential. Persistent cognitive deficits that are subtle and present in only a minority of children may help to account for the largely negative results of well-controlled studies of the cognitive outcomes mTBI (Beers, 1992; Cassidy et al., 2004; Satz et al., 1997). Another possibility is that children have problems coping with initial the cognitive or physical effects of injury, and that these adjustment difficulties perpetuate problems in functioning even in the absence of brain-based cognitive weaknesses (Gasquoine, 1997).
Research on the psychosocial outcomes of mTBI would also profit from closer examination of family consequences of these injuries. Study of family outcomes at baseline and 3 months in the present sample revealed only a temporary increase in burden among families of children with mTBI compared to those of children with OI (Ganesalingam et al., 2008). However, because higher levels of PCS at baseline were associated with greater family burden and distress in both injury groups, PCS may be of clinical concern even if not specific to head trauma. Additional research is required to determine if families are adversely affected by persistent PCS, if the longer-term burden of mTBI is greater than that associated with other traumatic injuries, and how to help families and children manage these symptoms.
In conclusion, these results add to the existing literature and our previous report on postinjury changes in PCS (Fay et al., in press; Yeates et al., 2009) in several ways. They reveal that: (1) while postinjury changes in PCS are observed following mild traumatic injuries other than mTBI, these changes are more pronounced in children with mTBI; (2) different patterns of change over time post injury are evident for different types of PCS; (3) negative effects of mTBI on PCS are evident on child self-report as well as on parent ratings; (4) multiple indicators of mTBI severity are associated with more PCS following mTBI, including MVRT, LOC, hospital admission, acute CT scan abnormality, parenchymal abnormality on MRI, and accompanying non-head injury; and (5) the effects of mTBI on PCS are moderated by non-injury factors such as age at injury and SES. These findings imply a need for more careful monitoring of PCS in children after mTBI, including efforts to distinguish among different types PCS, as well as for additional studies of injury and non-injury factors that place children at risk for persistent symptoms. Further research is required to investigate factors useful in identifying individual children who are most likely to experience elevations in PCS after mTBI and to examine the relation of these symptoms to learning or behavior problems. Nevertheless, our results provide initial clues regarding potentially useful injury and non-injury markers of increased risk, supporting the possibility of targeting subsets of children for closer post-injury monitoring. Larger-scale investigations of the neurological, neuropsychological, and psychosocial dimensions of PCS that employ rigorous research designs are imperative for further progress in this area (Carroll et al., 2004). As the vast majority of children with mTBI and other minor traumatic injuries are released from the ED without systematic follow-up (Hawley, 2003; Kirkwood et al., 2008), the promise of this research is improved methods to monitor, identify, and treat the sequelae of mTBI and other traumatic injuries.
Acknowledgments
Portions of the research were presented at the meeting of the International Neuropsychological Society, Zurich, Switzerland, July, 2006, and the American Academy of Clinical Neuropsychology, Boston, MA, June, 2008. This research was funded by project grants R01 HD39834 and K02 HD44099 from the National Institute of Child Health and Human Development to Keith Owen Yeates. The authors thank Lauren Ayr, Anne Birnbaum, Amy Clemens, Taryn Fay, Amanda Lininger, Katie Pestro, Elizabeth Roth, Elizabeth Shaver, and Heidi Walker for their assistance in carrying out this project.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Alexander MP. Minor traumatic brain injury: A review of physiogenesis and psychogenesis. Seminars in Clinical Neuropsychiatry. 1997;2:177–187. doi: 10.1053/SCNP00200177. [DOI] [PubMed] [Google Scholar]
- American Association for Automotive Medicine. The abbreviated injury scale (AIS)-1990 revision. Des Plaines, IL: American Association for Automotive Medicine; 1990. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Anderson VA, Morse SA, Klug G, Catroppa C, Haritou F, Rosenfeld JV, Pentland L. Predicting recovery from head injury in young children: A prospective analysis. Journal of the International Neuropsychological Society. 1997;3:568–580. [PubMed] [Google Scholar]
- Asarnow RF, Satz P, Light R, Zaucha K, Lewis R, McCleary C. The UCLA study of mild head injury in children and adolescents. In: Michel ME, Broman S, editors. Traumatic head injury in children. New York: Oxford University Press; 1995. pp. 117–146. [Google Scholar]
- Axelrod B, Fox DD, Less-Haley PR, Earnest K, Dolezal-Wood S, Goldman RS. Latent structure of the Postconcussion Syndrome Questionnaire. Psychological Assessment. 1996;8:422–427. [Google Scholar]
- Ayr LK, Yeates KO, Taylor HG, Browne M. Dimensions of post-concussive symptoms in children with mild traumatic brain injuries. Journal of the International Neuropsychological Society. 2009;15:19–30. doi: 10.1017/S1355617708090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CT, Taylor HG, Klein S, Yeates KO. The validity of neurobehavioral symptoms reported in children after traumatic brain injury. Child Neuropsychology. 1996;2:213–226. [Google Scholar]
- Beers SR. Cognitive effects of mild head injury in children and adolescents. Neuropsychology Review. 1992;3:281–320. doi: 10.1007/BF01108414. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. Journal of the International Neuropsychological Society. 2008;14:1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- Bijur PE, Haslum M. Cognitive, behavioral, and motoric sequelae of mild head injury in a national birth cohort. In: Broman S, Michel ME, editors. Traumatic head injury in children. Oxford University Press; 1995. pp. 147–164. [Google Scholar]
- Binder LM. Persisting symptoms after mild head injury: A review of the postconcussive syndrome. Journal of Clinical and Experimental Neuropsychology. 1986;8:323–346. doi: 10.1080/01688638608401325. [DOI] [PubMed] [Google Scholar]
- Brown G, Chadwick O, Shaffer P, Rutter M, Traub M. A prospective study ofchildren with head injuries: III. Psychiatric sequelae. Psychological Medicine. 1981;11:63–78. doi: 10.1017/s0033291700053289. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, Paniak C, Pépin M. Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of Rehabilitation Medicine. 2004 43:84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- Cassidy JD, Carroll L, Peloso P, Borg J, von Holst H, Holm L, Kraus J, Coronado V. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of Rehabilitation Medicine. 2004;36:28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- Chadwick O, Rutter M, Brown G, Shaffer D, Traub M. A prospective study of children with head injuries: II. Cognitive sequelae. Psychological Medicine. 1981;11:49–61. doi: 10.1017/s0033291700053277. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. Journal of Head Trauma Rehabilitation. 1995;10:1–17. [Google Scholar]
- De Andrade AF, de Almeida AN, Bor-Seng-Shu E, Lourenco L, Mandel M, Marino R., Jr The value of cranial computed tomography in high-risk, mildly head-injured patients. Surgical Neurology. 2006;65:S1:10–S1:13. doi: 10.1016/j.surneu.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Dennis M, Yeates KO, Taylor HG, Fletcher JM. Brain reserve capacity, cognitive reserve capacity, and age-based functional plasticity after congenital and acquired brain injury in children. In: Stern Y, editor. Cognitive reserve. New York: Taylor & Francis; 2007. pp. 53–83. [Google Scholar]
- Ewing-Cobbs L, Barnes M, Fletcher JM, Levin HS, Swank PR, Song J. Modeling of longitudinal academic achievement scores after pediatric traumatic brain injury. Developmental Neuropsychology. 2004;25:107–133. doi: 10.1080/87565641.2004.9651924. [DOI] [PubMed] [Google Scholar]
- Falk A, Cederfjall C, von Wendt L, Klang B. Are the symptoms of head injury predictive of clinical findings three months later? Acta Paediatrica. 2006;95:1533–1539. doi: 10.1080/08035250600731957. [DOI] [PubMed] [Google Scholar]
- Fay GC, Jaffe KM, Polissar NL, Liao S, Martin KM, Shurtleff HA, Rivara JB, Winn JR. Mild pediatric traumatic brain injury: A cohort study. Archives of Physical Medicine and Rehabilitation. 1993;74:895–901. [PubMed] [Google Scholar]
- Fay TB, Yeates KO, Taylor HG, Bangert B, Dietrich A, Nuss KE, Rusin J, Wright M. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617709991007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Financial Institutions Examinations Council Geocoding System. 2003 Retrieved January 1, 2001—December 31, 2004, from http://www.ffiec.gov/Geocode/default.htm.
- Federal Financial Institutions Examinations Council Geocoding System. 2005 Retrieved January 1, 2005—November 1, 2005, from http://www.ffiec.gov/Geocode/default.htm.
- Ferguson RJ, Mittenberg W, Barone DF, Schneider B. Postconcussion syndrome following sports-related head injury: Expectation as etiology. Neuropsychology. 1999;13:582–589. doi: 10.1037//0894-4105.13.4.582. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Ewing-Cobbs L, Miner M, Levin H, Eisenberg H. Behavioral changes after closed head injury in children. Journal of Consulting and Clinical Psychology. 1990;58:93–98. doi: 10.1037//0022-006x.58.1.93. [DOI] [PubMed] [Google Scholar]
- Ganesalingam K, Yeates KO, Ginn MS, Taylor HG, Dietrich A, Nuss K, Wright M. Family burden and parental distress following mild traumatic brain injury in children and its relationship to post-concussive symptoms. Journal of Pediatric Psychology. 2008;33:621–629. doi: 10.1093/jpepsy/jsm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasquoine PG. Postconcussion symptoms. Neuropsychology Review. 1997;7:77–85. doi: 10.1023/b:nerv.0000005945.58251.c0. [DOI] [PubMed] [Google Scholar]
- Gerber DJ, Schraa JC. Mild traumatic brain injury: Searching for the syndrome. Journal of Head Trauma Rehabilitation. 1995;10:28–40. [Google Scholar]
- Goldstrohm SL, Arffa S. Preschool children with mild to moderate traumatic brain injury: An exploration of immediate and post-acute morbidity. Archives of Clinical Neuropsychology. 2005;20:675–695. doi: 10.1016/j.acn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Gouvier WD, Cubic B, Jones G, Brantley P, Cutlip Q. Postconcussion symptoms and daily stress in normal and head-injured college populations. Archives of Clinical Neuropsychology. 1992;7:193–211. [PubMed] [Google Scholar]
- Gronwall D, Wrightson P, McGinn V. Effect of mild head injury during the preschool years. Journal of the International Neuropsychological Society. 1997;3:592–597. [PubMed] [Google Scholar]
- Guerrero JL, Thurman DJ, Sniezek JE. Emergency department visits associated with traumatic brain injury: United States, 1995-1996. Brain Injury. 2000;14:181–186. [PubMed] [Google Scholar]
- Gunstad J, Suhr JA. “Expectation as eitiology” versus “the good old days”: Postconcussion syndrome symptom reporting in athletes, headache sufferers, and depressed individuals. Journal of the International Neuropsychological Society. 2001;7:323–333. doi: 10.1017/s1355617701733061. [DOI] [PubMed] [Google Scholar]
- Hawley CA. Reported problems and their resolution following mild, moderate, and severe traumatic brain injury amongst children and adolescents in the UK. Brain Injury. 2003;17:105–129. doi: 10.1080/0269905021000010131. [DOI] [PubMed] [Google Scholar]
- Kashluba S, Casey JE, Paniak C. Evaluating the utility of ICD-10 diagnostic criteria for postconcussion syndrome following mild traumatic brain injury. Journal of the International Neuropsychological Society. 2006;12:111–118. doi: 10.1017/S1355617706060036. [DOI] [PubMed] [Google Scholar]
- Kashluba S, Paniak C, Casey JE. Persistent symptoms associated with factors identified by the WHO Task Force on Mild Traumatic Brain Injury. The Clinical Neuropsychologist. 2008;22:195–208. doi: 10.1080/13854040701263655. [DOI] [PubMed] [Google Scholar]
- Kibby MY, Long CJ. Minor head injury: Attempts at clarifying the confusion. Brain Injury. 1996;10:159–186. doi: 10.1080/026990596124494. [DOI] [PubMed] [Google Scholar]
- Kirkwood MW, Yeates KO, Taylor HG, Randolph C, McCrea M, Anderson VA. Management of pediatric mild traumatic brain injury: A neuropsychological review from injury through recovery. The Clinical Neuropsychologist. 2008;22:769–800. doi: 10.1080/13854040701543700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus JF. Epidemiological features of brain injury in children: Occurrence, children at risk, causes and manner of injury, severity, and outcomes. In: Broman SH, Michel ME, editors. Traumatic head injury in children. New York: Oxford University Press; 1995. pp. 22–39. [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2006. [Google Scholar]
- Levin HS, Hanten G, Roberson G, Li X, Ewing-Cobbs L, Dennis M, Chapman S, Max JE, Hunter J, Schachar R, Luerssen TG, Swank P. Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. Journal of Neursurgery: Pediatrics. 2008;1:461–470. doi: 10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- Levin HS, Mattis S, Eisenberg H, Marshall L, Tabbador K, High W, Frankowski R. Neurobehavioral outcome following minor head injury: A three center study. Journal of Neurosugery. 1987;66:234–243. doi: 10.3171/jns.1987.66.2.0234. [DOI] [PubMed] [Google Scholar]
- Light R, Asarnow R, Satz P, Zucha K, McCleary C, Lewis R. Mild closed-head injury in children and adolescents: Behavior problems and academic outcomes. Journal of Consulting and Clinical Psychology. 1998;66:1023–1029. doi: 10.1037//0022-006x.66.6.1023. [DOI] [PubMed] [Google Scholar]
- Luis CA, Vanderploeg RD, Curtiss G. Predictors of postconcussion symptom complex in community dwelling male veterans. Journal of the International Neuropsychological Society. 2003;9:1001–1015. doi: 10.1017/S1355617703970044. [DOI] [PubMed] [Google Scholar]
- Massagli TL, Fann JR, Burington BE, Jaffe KM, Katon WJ, Thompson RS. Psychiatric illness after mild traumatic brain injury in children. Archives of Physical Medicine and Rehabilitation. 2004;85:1428–1434. doi: 10.1016/j.apmr.2003.12.036. [DOI] [PubMed] [Google Scholar]
- Max JE, Roberts MA, Koele SL, Lindgren SD, Robin DA, Arndt S, Smith WL, Jr, Sato Y. Cognitive outcome in children and adolescents following severe traumatic brain injury: Influence of psychosocial, psychiatric, and injury-related variables. Journal of the International Neuropsychological Society. 1999;5:58–68. doi: 10.1017/s1355617799511089. [DOI] [PubMed] [Google Scholar]
- Mayer T, Matlak M, Johnson D, Walker M. The Modified Injury Severity Scale in pediatric multiple trauma patients. Journal of Pediatric Surgery. 1980;15:719–726. doi: 10.1016/s0022-3468(80)80271-5. [DOI] [PubMed] [Google Scholar]
- McKinlay A, Dalrymple-Alford JC, Horwood LJ, Fergusson DM. Long term psychosocial outcomes after mild head injury in early childhood. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73:281–288. doi: 10.1136/jnnp.73.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meares S, Shores EA, Batchelor J, Baguley IJ, Cahpman J, Gurka J, Marosszeky JE. The relationship of psychological and cognitive factors and opioids in the development of the postconcussion syndrome in general trauma patients with mild traumatic brain injury. Journal of the International Neuropsychological Society. 2006;12:792–801. doi: 10.1017/S1355617706060978. [DOI] [PubMed] [Google Scholar]
- Mittenberg W, Miller LJ, Luis CA. Postconcussion syndrome persists in children. The Clinical Neuropsychologist. 1997;11:305. [Google Scholar]
- Mittenberg W, Wittner MS, Miller LJ. Postconcussion syndrome occurs in children. Neuropsychology. 1997;11:447–452. doi: 10.1037//0894-4105.11.3.447. [DOI] [PubMed] [Google Scholar]
- Overweg-Plandsoen WCG, Kodde A, van Straaten M, van der Linden EAM, Neyens LGJ, Aldenkamp AP, Vermeulen M. Mild closed head injury in children compared to traumatic fractured bone; neurobehavioural sequelae in daily life 2 years after the accident. European Journal of Pediatrics. 1999;158:249–252. doi: 10.1007/s004310051061. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Willmott C, Rothwell A, Cameron P, Ayton G, Nelms R, Curran C, Ng KT. Cognitive and behavioral outcomes following mild traumatic head injury in children. Journal of Head Trauma Rehabilitation. 1999;14:360–372. doi: 10.1097/00001199-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Willmott C, Rothwell A, Cameron P, Kelly AM, Nelms R, Curran C, Ng K. Factors influencing outcome following mild traumatic brain injury in adults. Journal of the International Neuropsychological Society. 2000;6:568–579. doi: 10.1017/s1355617700655066. [DOI] [PubMed] [Google Scholar]
- Satz P, Alfano MS, Light R, Morgenstern H, Zaucha K, Asarnow RF, Newton S. Persistent post-concussive syndrome: A proposed methodology and literature review to determine the effects, if any, of mild head and other bodily injury. Journal of Clinical and Experimental Neuropsychology. 1999;21:620–628. doi: 10.1076/jcen.21.5.620.870. [DOI] [PubMed] [Google Scholar]
- Satz P, Zaucha K, McCleary C, Light R, Asarnow R. Mild head injury in children and adolescents: A review of studies (1970-1995) Psychological Bulletin. 1997;122:107–131. doi: 10.1037/0033-2909.122.2.107. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23:323–355. [Google Scholar]
- Stevens G, Cho JH. Socioeconomic indexes and the new 1980 census occupational classification scheme. Social Science Research. 1985;14:142–168. [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Guerrero J. Trends in hospitalization associated with traumatic brain injury. Journal of the American Medical Association. 1999;282:954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence: Test manual. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Wong JL, Regennitter RP, Barrios F. Base rate and simulated symptoms of mild head injury among normal. Archives of Clinical Neuropsychology. 1994;9:411–425. [PubMed] [Google Scholar]
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- Yeates KO, Luria J, Bartkowski H, Rusin J, Martin L, Bigler ED. Post-concussive symptoms in children with mild closed-head injuries. Journal of Head Trauma Rehabilitation. 1999;14:337–350. doi: 10.1097/00001199-199908000-00003. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG. Neurobehavioural outcomes of mild head injury in children and adolescents. Pediatric Rehabilitation. 2005;8:5–16. doi: 10.1080/13638490400011199. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Barry CT, Drotar D, Wade SL, Stancin T. Neurobehavioral symptoms in childhood closed-head injuries: Changes in prevalence and correlates during the first year post injury. Journal of Pediatric Psychology. 2001;26:79–91. doi: 10.1093/jpepsy/26.2.79. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Rusin J, Bangert B, Dietrich A, Nuss K, Wright M, Nagin DS, Jones BL. Longitudinal trajectories of post-concussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics. 2009;123:735–743. doi: 10.1542/peds.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]