Abstract
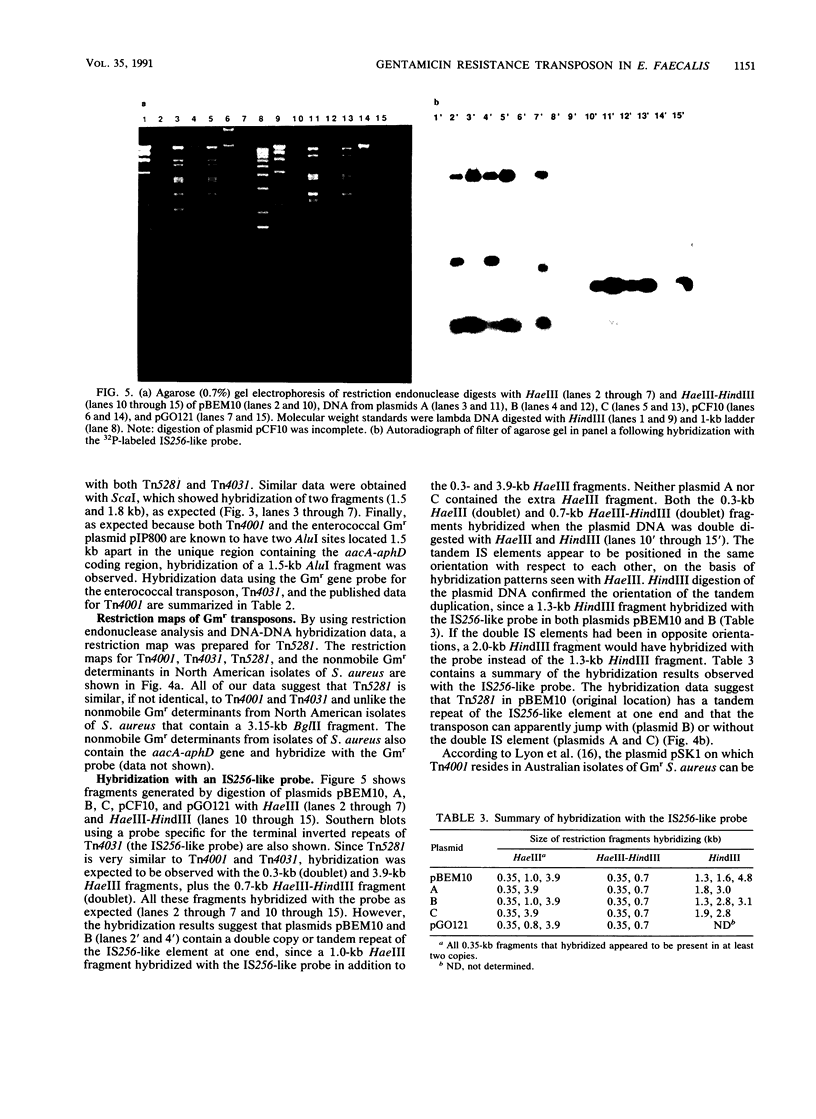
In Enterococcus faecalis, the genetic determinant encoding gentamicin resistance (Gmr) on the conjugative plasmid pBEM10 previously has been shown to be on a mobile element. In the current study, this element, termed Tn5281, was shown to relocate in the absence of homologous recombination in E. faecalis UV202. On the basis of restriction endonuclease analysis and DNA-DNA hybridization studies, Tn5281 was shown to be similar, if not identical, to the Gmr transposons Tn4001 found in Australian isolates of Staphylococcus aureus and Tn4031 found in U.S. isolates of Staphylococcus epidermidis, since all three of these transposons have symmetrically located HindIII (2.5 kb apart), ClaI (slightly more than 2.5 kb apart), and HaeIII (3.9 kb apart) sites. Restriction endonuclease digestion patterns of Tn5281 generated with HincII, ScaI, and AluI were also consistent with Tn4001 and Tn4031. By using a probe specific for the external portion of the terminal inverted repeat of Tn4031, it was determined that each terminus of Tn5281 contained a 0.35-kb HaeIII fragment and a 0.7-kb HindIII-HaeIII fragment. The sizes of these fragments are identical to those found in the staphylococcal transposons, which is a further indication that inverted repeats like IS256 are present in Tn5281. A 1-kb HaeIII fragment in pBEM10 also hybridized with this probe, which indicates that Tn5281 in pBEM10 contains a double copy of the inverted repeat at one end.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. E., Rouch D. A., Skurray R. A. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989 Sep 30;81(2):361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- Christie P. J., Dunny G. M. Identification of regions of the Streptococcus faecalis plasmid pCF-10 that encode antibiotic resistance and pheromone response functions. Plasmid. 1986 May;15(3):230–241. doi: 10.1016/0147-619x(86)90041-7. [DOI] [PubMed] [Google Scholar]
- Combes T., Carlier C., Courvalin P. Aminoglycoside-modifying enzyme content of a multiply resistant strain of Streptococcus faecalis. J Antimicrob Chemother. 1983 Jan;11(1):41–47. doi: 10.1093/jac/11.1.41. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G., Funk C., Adsit J. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid. 1981 Nov;6(3):270–278. doi: 10.1016/0147-619x(81)90035-4. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M. T., Lyon B. R., Messerotti L. J., Skurray R. A. Chromosome- and plasmid-mediated gentamicin resistance in Staphylococcus aureus encoded by Tn4001. J Med Microbiol. 1987 Sep;24(2):139–144. doi: 10.1099/00222615-24-2-139. [DOI] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Mobilization of the gentamicin resistance gene in Enterococcus faecalis. Antimicrob Agents Chemother. 1990 Jun;34(6):1278–1280. doi: 10.1128/aac.34.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Gillespie M. T., Byrne M. E., May J. W., Skurray R. A. Plasmid-mediated resistance to gentamicin in Staphylococcus aureus: the involvement of a transposon. J Med Microbiol. 1987 Mar;23(2):101–110. doi: 10.1099/00222615-23-2-101. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Gillespie M. T., Skurray R. A. Detection and characterization of IS256, an insertion sequence in Staphylococcus aureus. J Gen Microbiol. 1987 Nov;133(11):3031–3038. doi: 10.1099/00221287-133-11-3031. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193(3):554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- Martel A., Masson M., Moreau N., Le Goffic F. Kinetic studies of aminoglycoside acetyltransferase and phosphotransferase from Staphylococcus aureus RPAL. Relationship between the two activities. Eur J Biochem. 1983 Jul 1;133(3):515–521. doi: 10.1111/j.1432-1033.1983.tb07494.x. [DOI] [PubMed] [Google Scholar]
- Murray B. E., An F. Y., Clewell D. B. Plasmids and pheromone response of the beta-lactamase producer Streptococcus (Enterococcus) faecalis HH22. Antimicrob Agents Chemother. 1988 Apr;32(4):547–551. doi: 10.1128/aac.32.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouch D. A., Byrne M. E., Kong Y. C., Skurray R. A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987 Nov;133(11):3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Mobility of gentamicin resistance genes from staphylococci isolated in the United States: identification of Tn4031, a gentamicin resistance transposon from Staphylococcus epidermidis. Antimicrob Agents Chemother. 1989 Aug;33(8):1335–1341. doi: 10.1128/aac.33.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Gotoh A., Konno M. Purification and characterization of aminoglycoside-modifying enzymes from Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1984 Jun;25(6):754–759. doi: 10.1128/aac.25.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger A. R., Murray B. E. Comparison of enterococcal and staphylococcal beta-lactamase plasmids. J Infect Dis. 1990 Jan;161(1):54–58. doi: 10.1093/infdis/161.1.54. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Recombination-deficient mutant of Streptococcus faecalis. J Bacteriol. 1980 Aug;143(2):966–970. doi: 10.1128/jb.143.2.966-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]





