Abstract
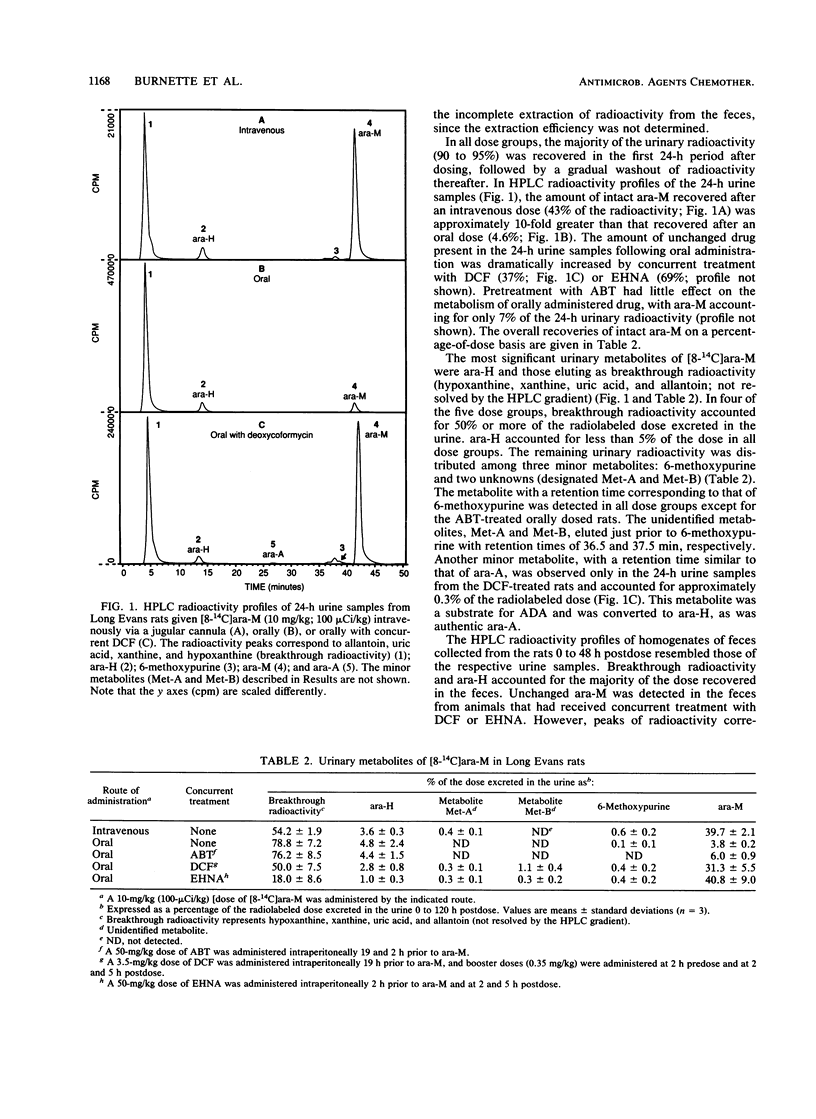
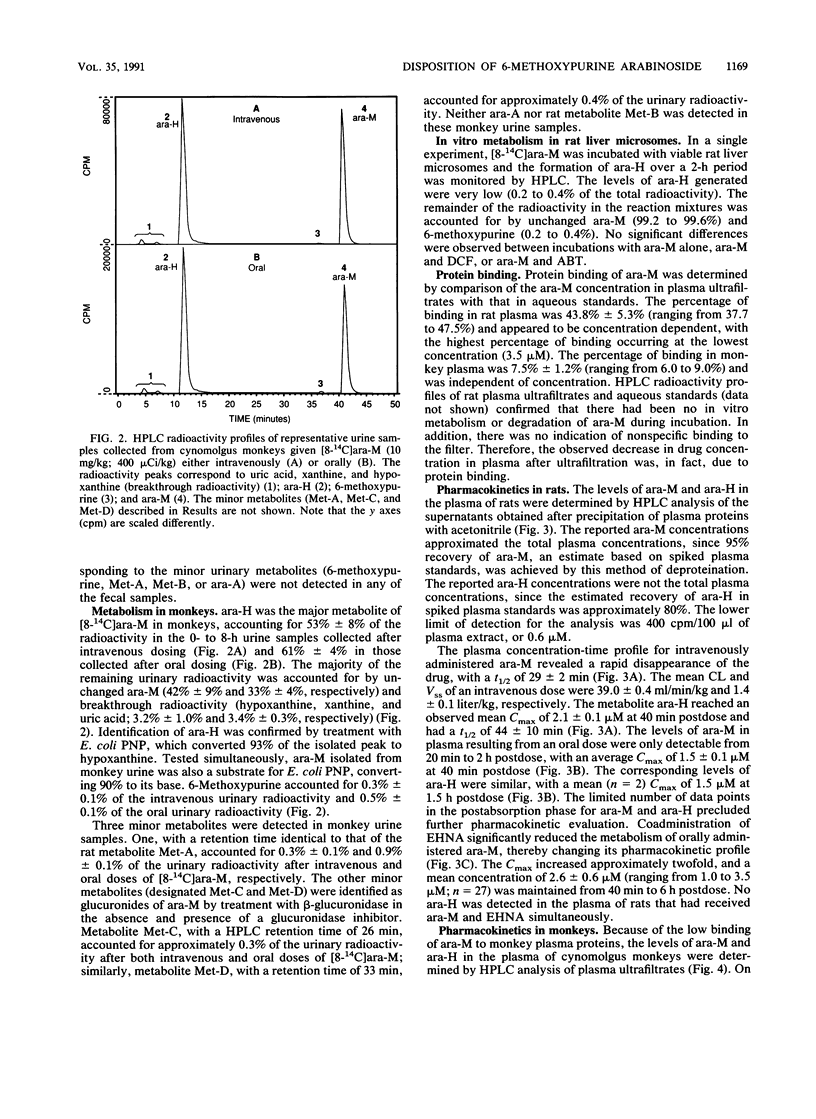
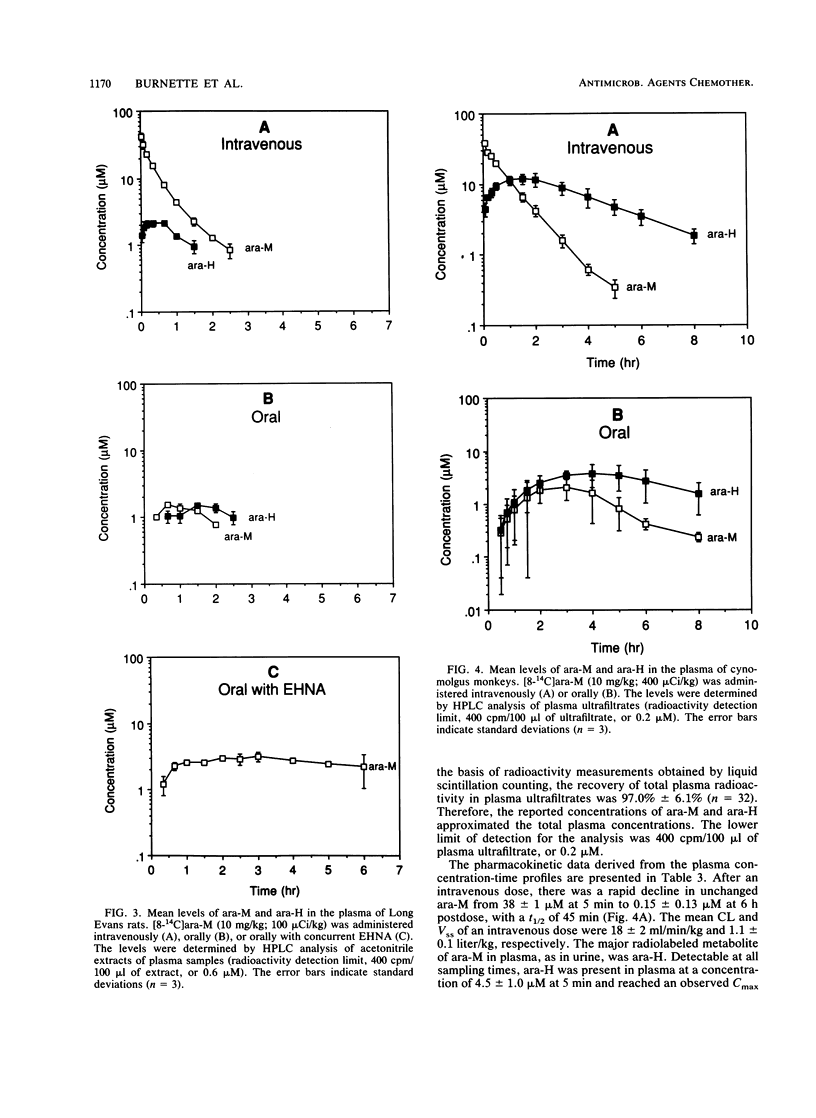
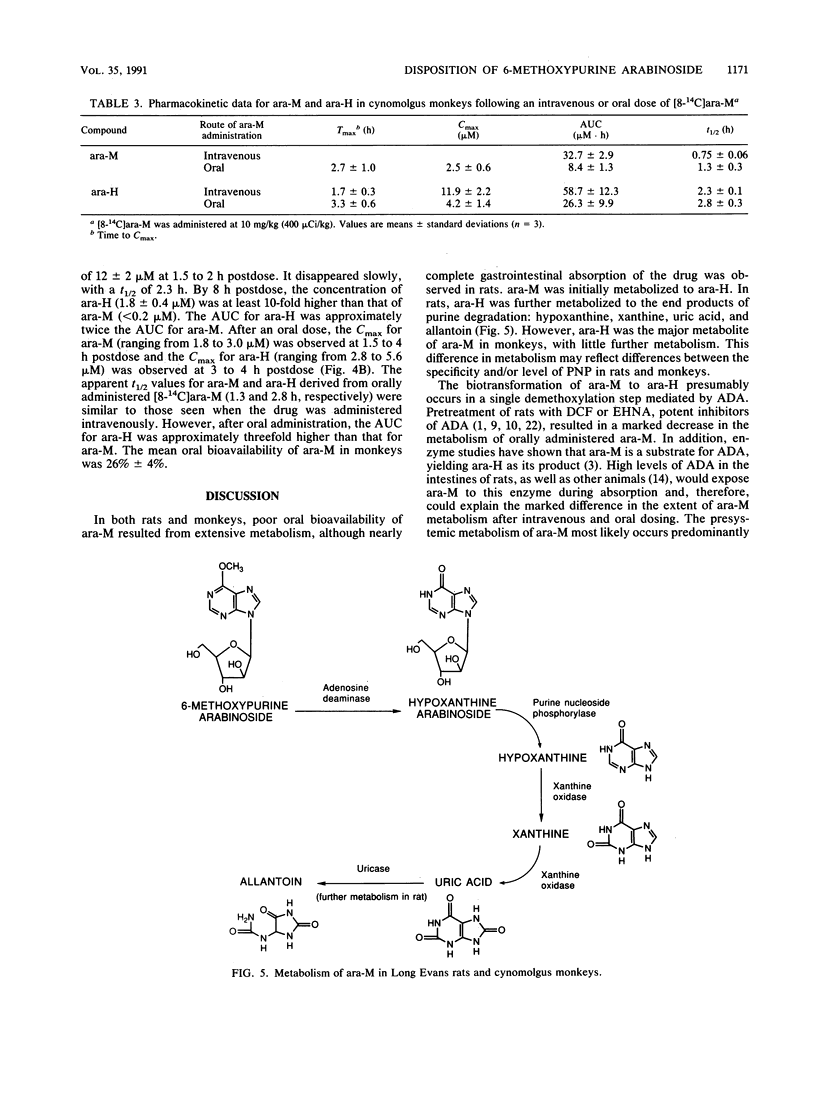
The metabolism and pharmacokinetics of 6-methoxypurine arabinoside (ara-M), a potent and selective inhibitor of varicella-zoster virus, were investigated in rats and monkeys. In Long Evans rats, orally administered [8-14C]ara-M (10 mg/kg) was well absorbed but extensively metabolized to hypoxanthine arabinoside (ara-H), hypoxanthine, xanthine, uric acid, and allantoin. Only 4% of an oral dose was recovered in the urine as unchanged drug, compared with 40% of an intravenous dose, indicating significant presystemic metabolism. Pretreatment of rats with 1-aminobenzotriazole, an inhibitor of cytochrome P-450, did not alter this metabolism. Pretreatment with deoxycoformycin or erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride, inhibitors of adenosine deaminase, resulted in a marked decrease in ara-M metabolism, indicating that adenosine deaminase plays a major role in the biotransformation of ara-M. In cynomolgus monkeys, [8-14C]ara-M (10 mg/kg) administered intravenously or orally was extensively metabolized to ara-H. Several minor urinary metabolites were detected in both rats and monkeys. However, adenine arabinoside was not found in urine or plasma from either rats or monkeys after administration of ara-M, except for a very low level detected in the urine of rats pretreated with deoxycoformycin. The elimination half-lives of intravenously administered ara-M in rats and monkeys were 29 and 45 min, respectively. The corresponding half-lives of the primary metabolite, ara-H, were 44 min and 2.3 h. Plasma profiles of orally administered ara-M in both rats and monkeys demonstrated the poor oral bioavailability of this arabinoside. The results of these studies indicate that ara-M is not well suited for oral administration because of extensive presystemic metabolism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P. Inhibitors of adenosine deaminase. Pharmacol Ther. 1982;17(3):399–429. doi: 10.1016/0163-7258(82)90023-7. [DOI] [PubMed] [Google Scholar]
- Averett D. R., Koszalka G. W., Fyfe J. A., Roberts G. B., Purifoy D. J., Krenitsky T. A. 6-Methoxypurine arabinoside as a selective and potent inhibitor of varicella-zoster virus. Antimicrob Agents Chemother. 1991 May;35(5):851–857. doi: 10.1128/aac.35.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard J. Evaluation of the relative efficacy of various techniques for deproteinizing plasma samples prior to high-performance liquid chromatographic analysis. J Chromatogr. 1981 Dec 11;226(2):455–460. doi: 10.1016/s0378-4347(00)86080-6. [DOI] [PubMed] [Google Scholar]
- Frieden C., Kurz L. C., Gilbert H. R. Adenosine deaminase and adenylate deaminase: comparative kinetic studies with transition state and ground state analogue inhibitors. Biochemistry. 1980 Nov 11;19(23):5303–5309. doi: 10.1021/bi00564a024. [DOI] [PubMed] [Google Scholar]
- Geiger J. D., Lewis J. L., MacIntyre C. J., Nagy J. I. Pharmacokinetics of 2'-deoxycoformycin, an inhibitor of adenosine deaminase, in the rat. Neuropharmacology. 1987 Sep;26(9):1383–1387. doi: 10.1016/0028-3908(87)90103-1. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Krenitsky T. A. Purine nucleoside phosphorylase: purification using an ether-linked formycin B/sepharose 6B resin with unusual properties. Prep Biochem. 1990;20(1):75–85. doi: 10.1080/00327489008050178. [DOI] [PubMed] [Google Scholar]
- Ho D. H., Pincus C., Carter C. J., Benjamin R. S., Freireich E. J., Bodey G. P., Sr Distribution and inhibition of adenosine deaminase in tissues of man, rat, and mouse. Cancer Treat Rep. 1980 Apr-May;64(4-5):629–633. [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Koszalka G. W., Chen I. S., Beacham L. M., 3rd, Rideout J. L., Elion G. B. Deoxycytidine kinase from calf thymus. Substrate and inhibitor specificity. J Biol Chem. 1976 Jul 10;251(13):4055–4061. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambe C. U., Nelson D. J. Pharmacokinetics of inhibition of adenosine deaminase by erythro-9-(2-hydroxy-3-nonyl)adenine in CBA mice. Biochem Pharmacol. 1982 Feb 15;31(4):535–539. doi: 10.1016/0006-2952(82)90156-3. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Mico B. A., Mathews J. M., Kunze K. L., Miwa G. T., Lu A. Y. Selective inactivation of cytochrome P-450 isozymes by suicide substrates. Arch Biochem Biophys. 1981 Sep;210(2):717–728. doi: 10.1016/0003-9861(81)90239-3. [DOI] [PubMed] [Google Scholar]
- Perrier D., Mayersohn M. Noncompartmental determination of the steady-state volume of distribution for any mode of administration. J Pharm Sci. 1982 Mar;71(3):372–373. doi: 10.1002/jps.2600710332. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Schwender C. F. Enzyme inhibitors. 26. Bridging hydrophobic and hydrophilic regions on adenosine deaminase with some 9-(2-hydroxy-3-alkyl)adenines. J Med Chem. 1974 Jan;17(1):6–8. doi: 10.1021/jm00247a002. [DOI] [PubMed] [Google Scholar]
- Schenkman J. B., Wilson B. J., Cinti D. L. Dimethylaminoethyl 2,2-diphenylvalerate HCl (SKF 525-A)--in vivo and in vitro effects of metabolism by rat liver microsomes--formation of an oxygenated complex. Biochem Pharmacol. 1972 Sep 1;21(17):2373–2383. doi: 10.1016/0006-2952(72)90389-9. [DOI] [PubMed] [Google Scholar]
- Sophianopoulos J. A., Durham S. J., Sophianopoulos A. J., Ragsdale H. L., Cropper W. P., Jr Ultrafiltration is theoretically equivalent to equilibrium dialysis but much simpler to carry out. Arch Biochem Biophys. 1978 Apr 15;187(1):132–137. doi: 10.1016/0003-9861(78)90015-2. [DOI] [PubMed] [Google Scholar]
- Spector T. Mammalian adenylosuccinate lyase. Participation in the conversion of 2'-dIMP and beta-D-arabinosyl-IMP to adenine nucleotides. Biochim Biophys Acta. 1977 Apr 12;481(2):741–745. doi: 10.1016/0005-2744(77)90308-4. [DOI] [PubMed] [Google Scholar]
- Spector T., Miller R. L. Mammalian adenylosuccinate synthetase. Nucleotide monophosphate substrates and inhibitors. Biochim Biophys Acta. 1976 Sep 14;445(2):509–517. doi: 10.1016/0005-2744(76)90104-2. [DOI] [PubMed] [Google Scholar]
- Upton R. A. Simple and reliable method for serial sampling of blood from rats. J Pharm Sci. 1975 Jan;64(1):112–114. doi: 10.1002/jps.2600640123. [DOI] [PubMed] [Google Scholar]
- Whitlam J. B., Brown K. F. Ultrafiltration in serum protein binding determinations. J Pharm Sci. 1981 Feb;70(2):146–150. doi: 10.1002/jps.2600700208. [DOI] [PubMed] [Google Scholar]



