Abstract
NK cell-mediated cytotoxicity of target cells is the result of a balance between the activating and inhibitory signals provided by their respective ligand-receptor interactions. In our current study, we have investigated the significance of CD59 on human target cells in modulating this process. A range of CD59 site-specific Abs were used in NK cytotoxicity blocking studies against the CD59-expressing K562 target cell line. Significantly reduced cytotoxicity was observed in the presence of Abs previously shown to lack blocking capacity for C-mediated lysis. We investigated the consequences for alternative membrane attachment modalities, namely bis-myristoylated-peptidyl (BiMP) and GPI anchoring, on CD59-negative U937 cells. Expression of GPI-anchored CD59 either via transfection or incorporation rendered U937 targets more susceptible to NK cytotoxicity, whereas incorporation of CD59 via a BiMP anchor to similar levels did not alter susceptibility to NK cytotoxicity. Localization of both BiMP- and GPI-anchored CD59 proteins was shown to be within the lipid raft microdomain. A role for the GPI anchor and independence from glycosylation status was confirmed by expression of transmembrane-anchored CD59 or unglycosylated CD59 and by testing in NK cytotoxicity assays. To investigate mechanisms, we compared the signaling capacity of the various forms of expressed and incorporated CD59 following Ab cross-linking in calcium flux assays. GPI-anchored CD59, with or without glycosylation, mediated activation events, whereas CD59 forms lacking the GPI anchor did not. The data show that the increased susceptibility of target cells expressing CD59 to NK cytotoxicity requires GPI anchor-mediating signaling events, likely mediated by interactions between GPI-anchored CD59 on targets and NK receptors.
Protection of cells from complement is attributable to membrane-associated C regulatory (CReg)4 proteins, a battery of inhibitory proteins that includes the GPI-anchored molecules CD55 and CD59 (1). CD59, the sole cell surface inhibitor of the membrane attack complex on human cells, is broadly distributed, present in most tissues, and on all circulating cells. Addition of the GPI anchor is a posttranslational modification (2). Cells from patients with the hematological disorder paroxysmal nocturnal hemoglobinuria are defective in the machinery for GPI anchor synthesis and hence lack surface expression of both CD55 and CD59 and are highly susceptible to the lytic effects of the membrane attack complex (3, 4). Down-regulation of CD59 expression has been described previously in hematological malignancies such as promyelocytic leukemia (5). Indeed, the widely used U937 cell line was derived from the pleural effusion of a histiocytic lymphoma patient (6) and has been shown to lack CD59 expression despite normal GPI-anchoring capacity (7).
Several recent studies have implicated CReg in aspects of cellular immunity. CD46 is involved in the acquisition of a regulatory cell phenotype in CD4+ T cells (8), and CD55 has been implicated as a negative regulator of T cell responses in the mouse (9). Roles for CReg in cell-mediated cytotoxicity have also been proposed. Expression of CD55 on K562 cells protected these targets from killing by NK cells and blocking of CD55 enhanced NK cytotoxicity (10, 11). More recently, expression of CD55 on porcine endothelial cells was shown to protect against both C and NK cytotoxicity (12). Interestingly, deletion of short consensus repeat 4 in CD55 caused loss of protection from NK, but C protection was preserved, suggesting that these activities resided in different parts of the molecule (13). Contrasting roles for CD55 and CD59 expressed on the NK effector cell in cytotoxicity have been reported. NK expression of CD55 inhibited cytotoxicity (11), whereas CD59 expression on NK cells enhanced killing (14). In this latter study, CD59 was associated with the known NK cytotoxicity receptors NKp30 and NKp46. The target cells used, the P815 murine mastocytoma line, lacked CD59 expression, and the potential role of CD59 on the target was not explored.
We were interested in exploring whether expression of CD59 on target cells influenced NK-mediated cytotoxicity. With the aid of Ab blocking experiments using the CD59-positive line K562 as targets, we were able to show that expression of CD59 could enhance NK cytotoxicity and that the region involved is distinct from its C regulating region. Using the CD59-negative U937 cell line as targets and introducing CD59 in various ways we confirmed that expression of GPI-anchored CD59 on target cells indeed enhanced NK cytotoxicity, which is directly dependent on the context of this protein. We demonstrate in this study that, although a bis-myristoylated-peptidyl (BiMP)-tagged CD59 is able to efficiently bind to cells and migrate to lipid rafts, it is unable to mediate signaling capacity or the enhanced NK cytotoxicity afforded by its GPI-anchored counterpart. This functional difference between the CD59-tagged molecules was not reflected in C-mediated lysis, in which the presence of each form of CD59 efficiently protected against C lysis. These data indicate that lipid raft association and the signaling capacity conferred by the GPI-anchor are essential for CD59-mediated enhancement of NK cytotoxicity, and implicate NK receptors as potential CD59 ligands.
Materials and Methods
Abs and reagents
Unless otherwise indicated all reagents were obtained from Sigma-Aldrich. Abs used in this study included BRIC229 anti-CD59 (International Blood Group Reference Laboratory (IBGRL)), MEM43 and MEM43/5 anti-CD59 (both supplied by V. Horejsi, Academy of Sciences of the Czech Republic, Prague, Czech Republic), and YTH-53.1 anti-CD59 (supplied by H. Waldmann, Oxford University, Oxford, U.K.). Mouse IgG1a was purified in house.
Cell cultures
The human U937 and K562 cell lines were obtained from European Collection of Animal Cell Culture and maintained in RPMI 1640 supplemented with 10% FCS, 4 mM L-glutamine, 2 mM sodium pyruvate, 100 IU/ml streptomycin, and 100 IU/ml penicillin at 37°C/5% CO2. The human NK leukemia (NKL) cell line (15) was maintained under the described conditions in the presence of 200 IU/ml human IL-2 (Proleukin; Chiron).
Flow cytometry
Harvested cells were washed twice in FACS buffer (PBS/2% BSA/0.1% NaN3/10 mM EDTA) and resuspended to 106/ml. Staining was conducted at 4°C for 30 min with a final primary Ab concentration of 5 μg/ml. Following two wash steps in FACS buffer, cells were incubated in FITC- or PE-labeled secondary Ab (The Binding Site) at 4°C for 30 min. Samples were washed a further three times in FACS buffer, and data were acquired on a BD FACScan (BD Biosciences) provided by the Cardiff University Central Biotechnology Services.
Ab blocking
In some experiments, before NK cytotoxicity and C-mediated lysis assays, washed cells were incubated at 37°C for 15 min with mAbs against CD59 at 10 μg/ml per 2 × 107 cells. Unbound Ab was removed by washing cells twice in RPMI 1640. Control cells were incubated with mouse IgG1a at the same concentration or with RPMI 1640 alone.
Isolation of primary mononuclear cells and CD3 depletion
PBMC were obtained from heparinized blood samples of healthy volunteers. PBMC were isolated via density gradient centrifugation using Lymphoprep (Invitrogen Life Technologies) and washed three times in PBS. Cells were resuspended at 2.5 × 105/ml in PBS/2% human AB serum and stained with mouse monoclonal CD3-FITC (Serotec) for 30 min at 4°C. Following two washes, 50 μl of CD3-stained cells were retained for predepletion assessment, and the remainder incubated with prewashed sheep anti-mouse Ig-coupled magnetic dynabeads (Dynal Biotech) for 2 h at 4°C. CD3-depleted cells were washed in PBS/2% AB serum and stained with CD56-PE (BD Biosciences) for 30 min at 4°C. Using flow cytometry analysis, CD3 depletion of the lymphocyte population was shown to be 90–96% efficient, and the CD3−/CD56+ subpopulation was used to determine the percentage of NK cells in calculating the various E:T ratios described. Lymphocytes were resuspended in RPMI 1640/5% AB serum with 1000 U/ml IFN-α and incubated overnight at 37°C/5% CO2 and subsequently used in a 4-h cytotoxicity assay to be described.
NK-mediated cytotoxicity
The cytolytic activity of the NKL cell line and primary NK cells were assessed in standard sodium chromate (51Cr; Amersham Biosciences) release assays. Target cells, with incorporated or expressed CD59 proteins where appropriate, were washed three times in PBS and loaded in excess with 5 μCi of 51Cr per 5 × 105 cells for 1 h at 37°C. NKL cells in semilogarithmic growth and primary NK cells were resuspended in RPMI 1640/2% human AB serum to final cell numbers of 9, 3, and 1 × 104 cells in quadruplicate per reaction in 96-well plates. Following washing, 51Cr-loaded target cells were plated into the relevant wells at 2000 cells/well and incubated at 37°C for 4 h before centrifuging and harvesting supernatants. Minimum and maximum 51Cr release was assessed in supernatants from cells incubated with medium alone and 2% Triton X-100 in medium, respectively. Supernatants (20 μl) were diluted in scintillant (150 μl), and 51Cr present in the reaction mixture was assessed by scintillation analysis of beta emission (MicroBeta TriLux; Wallac). Percentage of release for each condition was calculated with reference to minimum and maximum levels.
C-mediated lysis
Inhibitory activity of expressed or incorporated CD59 was assessed in a C lysis assay in triplicates in 96-well plates. Cells were washed, resuspended to 2 × 106/ml in serum-free medium, and sensitized using an anti-CD15 IgM mAb (TG1 at 100 μg/ml; a gift of Prof. P. Beverley, Edward Jenner Institute, Compton, U.K.) for 40 min at 37°C. After extensive washing in veronal-buffered saline, cells were incubated with veronal-buffered saline-diluted normal human serum in a dilution range between 1/2 and 1/256 (v/v) for 1 h at 37°C. Cells were washed twice in cold FACS buffer, resuspended in cold FACS buffer containing 2 μg/ml propidium iodide, and their percentage of viability determined by flow cytometry. Heat-inactivated normal human serum was used as control.
Whole cell lysis and Western blotting
Cells were lysed in 1% Nonidet P-40 buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA) in the presence of protease inhibitors (1 μg/ml aprotinin, 5 μg/ml leupeptin, 1 μg/ml pepstatin A) and phosphotyrosine inhibitors (50 mM NaF, 1 mM Na3VO4). Postnuclear supernatants were prepared by centrifugation (16,000 × g at 4°C for 20 min), subjected to nonreduced SDS-PAGE, and probed with the mentioned Abs in 5% skim milk in PBS/0.1% Tween 20.
Creation of stable cell lines
Stable transfectants of U937 cells were obtained using the pDR2ΔEF1α expression vector carrying the hygromycin resistance gene (16). GPI-anchored CD59 expression in this vector has been previously described (17). Cells were washed and resuspended in Dulbecco’s PBS to a final density of 3 × 107/ml and placed on ice for 10 min. Plasmid DNA was then added to a final concentration of 50 μg/ml, and 0.5 ml of the DNA/cell suspension was pulsed at 1050 μF and 280 V using the Gene Pulser (Bio-Rad). After electroporation, the chamber was placed on ice for 10 min, and cells were harvested and resuspended in complete growth medium for 10 min at room temperature. After 36 h, cell viability was assessed by trypan blue exclusion and selection maintained for 2 wk using hygromycin at 200 μg/ml (U937−CD59). Control cells were transfected with the empty vector and selected in an identical manner (U937−pDR2).
CD59 protein preparation and incorporation
GPI-anchored CD59 was purified from human erythrocytes by isolating membranes, butanol extraction, and affinity purification using a MEM43 immunoaffinity column essentially as previously described (18). The yield of pure CD59 from 1.5 liters of packed erythrocytes was 0.25 mg. Coomassie-stained SDS-PAGE and Western blotting confirmed the purity and integrity of the protein (data not shown). U937−pDR2 cells were used for incorporation of CD59 proteins. BiMP CD59 was generated from bacterially expressed recombinant CD59 by the addition of a synthetic tag, with a novel tag structure for increased stability in membranes essentially as previously described (19, 20). This agent was the gift of Inflazyme Pharmaceuticals. The expressed protein was refolded as described and purified by ammonium sulfate precipitation. Purity was confirmed at >99% with >95% as monomer by SDS-PAGE analysis; endotoxin levels were <2 EU/mg. The tagged molecule is stable on storage and does not aggregate (data not shown). Cells washed and resuspended in PBS at 1 × 107/ml were incubated at 37°C with 25 μg/ml either BiMP CD59 or GPI-anchored CD59 for 15 min with constant mixing. Unincorporated protein was removed by washing three times in PBS at room temperature. Efficiency of incorporation was assessed by Western blotting and by flow cytometry as previously described. Incorporation life-span was determined by incubating CD59-incorporated cells in RPMI 1640 with 5% FCS and at 37°C/5% CO2 for various times up to 15 h. CD59 expression was determined by flow cytometry as described.
Sucrose gradient ultracentrifugation and immunofluorescence microscopy for assessment of raft-associated proteins
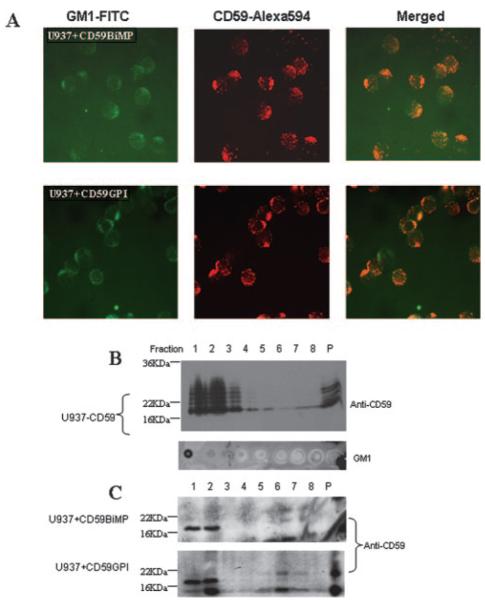
Lipid raft association of incorporated or transfected CD59 proteins was assessed by solubilization of cells (5 × 107) at various intervals after incorporation and analysis by sucrose gradient ultracentrifugation as previously described (21). Briefly, washed cells were lysed for 30 min at 4°C in 1% Nonidet P-40 (Calbiochem). Lysed cells were placed in ultracentrifuge tubes and made 40% (w/v) sucrose in 0.5 ml, overlaid with 1.75 ml of 35% sucrose and finally by 0.25 ml of 1% Nonidet P-40. Ultracentrifugation was conducted at 18,000 × g for 18 h at 4°C. Eight equal fractions were harvested; top fractions (1-3) of the standard gradient represent lipid-raft associated proteins. The nuclear pellet was also retained for analysis. For immunofluorescence staining, cells maintained at 37°C for 2 h postincorporation were washed twice in PBS/1% BSA/2 mM EDTA and double stained using GM1-FITC (1/100; Sigma-Aldrich) and BRIC229 (1/200; IBGRL) with anti-mouse Alexa Fluor 594 (Molecular Probes) at 4°C for 30 min. Cytospin preparations of twice washed cells on slides were imaged by fluorescent microscopy, and images were captured electronically (Leica).
Cross-linking and intracellular calcium flux
Cells were harvested, resuspended in RPMI 1640 with 10% FCS to 2.5 × 107/ml, and preloaded with 2 μM fura 2-AM (Molecular Probes) for 1 h at 37°C. After washing and resuspension in Krebs-HEPES buffer (20 mM HEPES, 128 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 20 mM glucose (pH 7.4)) samples were incubated with BRIC229 (2 μg/ml final) at 4°C for 15 min. Cells were washed, resuspended in Krebs-HEPES buffer, and aliquoted to a 96-well flat-bottom plate. Cross-linking was induced by addition of 20 μg/ml F(ab’)2 rabbit anti-mouse Ab at 37°C to individual wells and changes in intracellular-free Ca2+ levels were measured at 37°C using the FluoStar Optima fluorometer (BMG Lab Technologies). Fluorescence measurements were acquired every 0.5 s.
Results
Ab blocking of CD59 on K562 targets inhibits NKL lysis
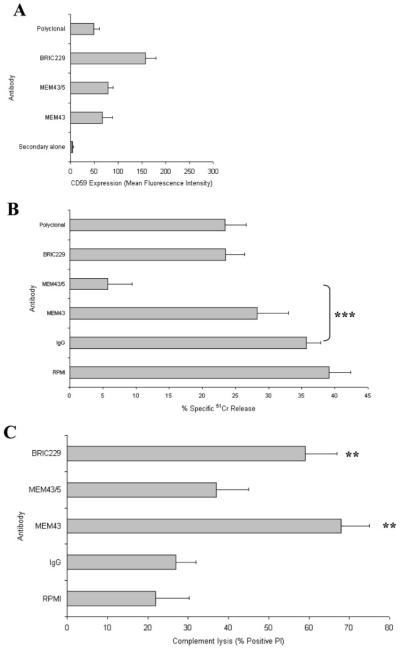
To investigate the role of CD59 in NK-mediated cytotoxicity we tested the effect of Ab blocking on this activity. The K562 cell line, derived from the pleural effusion of a chronic myeloid leukemia patient in blast crisis (22), is widely used as a highly sensitive target for in vitro NK-mediated cytotoxicity (23). We have previously mapped a panel of mAbs on CD59 and characterized their capacity to block CD59-mediated protection during complement attack (24). In this study we examined the capacity of selected mAb to modulate susceptibility of the K562 line to cytotoxicity mediated by the CD59-negative NKL cell line. Three mAbs were tested, two of which target the putative “active site” for C inhibition (BRIC229 and MEM43) and one that binds a mapped epitope remote from this site (MEM43/5). All mAb bound to CD59-positive K562 as assessed by flow cytometry; BRIC229 consistently gave 2-fold higher staining than the other mAb, likely reflecting its known high affinity for CD59 (Fig. 1A). Only MEM43/5 significantly altered NK cytotoxicity, reducing lysis from 37% in non-immune Ig controls to 7% after mAb pretreatment (Fig. 1B). C lysis experiments performed in parallel were consistent with previous observations (24-26), in that BRIC229 and MEM43 significantly enhanced C lysis, whereas MEM43/5 was without significant effect (Fig. 1C). These data suggest that CD59 on the target influences NK cytotoxicity through sites distinct from those modulating C lysis.
FIGURE 1.
Effects of Ab blocking of CD59 on NK cytotoxicity and C lysis. A, Flow cytometry confirmed CD59 recognition by anti-CD59 Abs on K562 cells. K562 cells preincubated with CD59-blocking Abs were used in NK cytotoxicity (B) and C-mediated lysis (C) assays. Results are the mean of triplicate determinations from at least three independent experiments at a single NK E:T ratio or C dilution. Error bars represent SD. **, p < 0.01; ***, p < 0.001.
Stable incorporation of anchored CD59 into a CD59-negative cell line
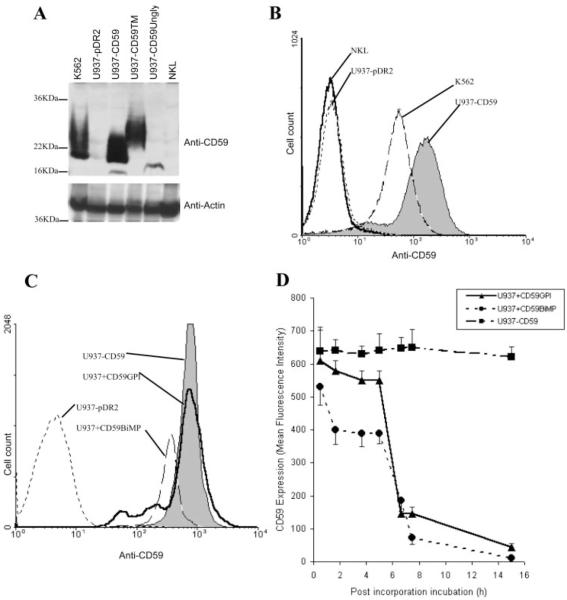
To further dissect the role of CD59 on targets during NK-mediated cytotoxicity, we next explored methods of incorporating and expressing CD59 in the CD59-negative U937 cell line. We have previously described expression by transfection of CD59 in U937 and in this study show that stable expression is achieved at a level ~4-fold higher than that of the endogenous expression level on the CD59-positive cell line K562 (Fig. 2, A and B). Two additional U937 mutant lines demonstrating stable CD59 expression of the transmembrane form (U937−CD59TM) and the unglycosylated mutant (N18→A; U937−CD59Ungly) were created; U937−CD59TM showed a similar expression level to U937−CD59 while expression of U937−CD59Ungly was ~2-fold lower by flow cytometry (data not shown). Western blot analyses showed a broad glycosylation pattern of CD59 expression of the transmembrane, resembling that of GPI-anchored CD59, whereas expression of the unglycosylated mutant showed a distinct single band of ~16 kDa (Fig. 2A). Of note, the NKL effector cell line was negative for CD59 in these assays (Fig. 2, A and B). GPI-anchored CD59 purified from erythrocyte membranes incorporated efficiently into U937, as judged by flow cytometry, reaching levels comparable with those in the CD59-transfected cells (Fig. 2C, U937+CD59GPI). A recombinant form of CD59 expressing a BiMP tag to allow membrane incorporation also incorporated efficiently into U937 cells, reaching a level ~50% of that on CD59-transfected cells (Fig. 2C, U937+CD59BiMP). Cells transfected with CD59 or with incorporated protein were then incubated at 37°C and residual CD59 expression measured at intervals by flow cytometry over 16 h. As expected, levels on the transfected line were unaltered; in contrast, GPI-anchored CD59 was lost slowly over the first 4 h in culture but then dropped rapidly, likely reflecting cell division in culture (Fig. 2D). BiMP-tagged CD59 displayed similar kinetics of loss, although loss in the first 4 h was more rapid and CD59 had almost disappeared by 7 h postincorporation (Fig. 2D).
FIGURE 2.
Characterization of the cell line models. A, CD59 expression was determined by Western blotting demonstrating strong expression on K562 and on U937 transfected with the various forms of CD59, and no expression on vector-transfected U937 and the NKL cell line. B, Flow cytometry showed strong expression by U937 transfected with CD59 (U937−CD59) as compared with endogenous levels expressed by K562. The empty vector control (U937−pDR2) and the NKL effector cell line were negative. C, Incorporated proteins were also measured by flow cytometry, illustrating comparable levels between U937 transfected CD59, GPI-anchored CD59, and BiMP CD59 (U937−CD59, U937+CD59GPI, and U937+CD59BiMP). U937 transfected with the empty vector (U937−pDR2) is shown as control. D, Time course analysis of the incorporated proteins was determined by flow cytometry.
GPI anchoring but not glycosylation is essential for enhanced NKL-mediated cytotoxicity
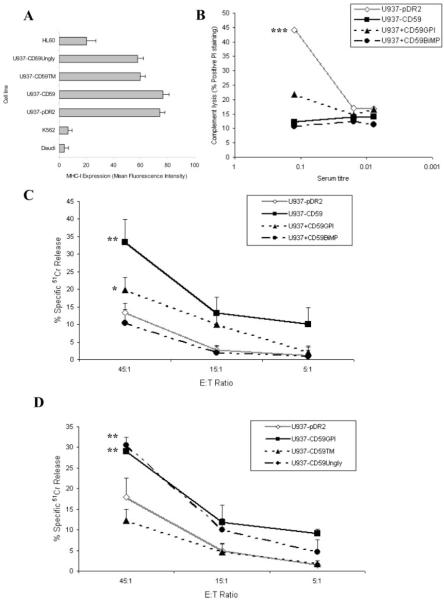
The effect on NK-mediated cytotoxicity was subsequently examined among the different forms of CD59-expressing cells. Because of the importance of target MHC class I expression in NKL killing, we checked expression of MHC class I on CD59-transfected U937 and vector controls; expression levels were identical (Fig. 3A). To confirm the functional capacity of CD59 expressed in these different ways on U937 we examined protection from C lysis (Fig. 3B). GPI-anchored (transfected or incorporated) CD59 strongly protected against C lysis, and BiMP-anchored CD59 was equally active in this assay, demonstrating that the GPI anchor was not necessary for C inhibition. In respect of NK cytotoxicity, when GPI-anchored CD59 was expressed by transfection in U937, susceptibility to NKL-mediated cytolysis was significantly and dramatically enhanced as compared with vector-transfected controls; 33 ± 6% in contrast to 13 ± 3% killing at the E:T ratio of 45:1 (Fig. 3C; p < 0.01). Incorporation of GPI-anchored CD59 caused a smaller, albeit significant, increase in cytolysis (20 ± 3%; p < 0.05), but incorporation of BiMP-anchored CD59 had no effect (11 ± 3%; Fig. 3C). To eliminate the possibility that lack of glycosylation on the recombinant BiMP-anchored CD59 was responsible for these differences, we tested the effects of the expressed GPI-anchored unglycosylated mutant; cells expressing unglycosylated GPI-anchored CD59 demonstrated a similar degree of enhanced susceptibility toward NKL-mediated cytolysis (30 ± 2%; p < 0.01). To examine the role of the GPI anchor we tested transmembrane-anchored glycosylated CD59 and showed that it was without effect in the system (Fig. 3D). These data demonstrate that CD59 modulates NKL-mediated killing and imply that the GPI anchor is essential for the observed effect, whereas the glycosylation state of this protein has no influence on NK-mediated cytotoxicity. The data show that the inhibition of C lysis by CD59 is mechanistically distinct from the effects on NK-mediated cytotoxicity.
FIGURE 3.
Expression of GPI-anchored CD59 on targets caused increased NK-mediated cytotoxicity. No significant difference in MHC class I expression was observed between U937−pDR2 and U937−CD59 as assessed by flow cytometry (A), although different from other cell lines commonly used in NK cytotoxicity assays. C-mediated protection was determined using normal human serum (B), representative of five independent experiments. The various U937 cell lines were preloaded with 51Cr and used in a 4-h assay against NKL cells at the indicated ratios (C and D). Measurements are the mean of triplicate determinations from at least three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
GPI-anchored CD59 on U937 target cells significantly enhances human primary NK cell-mediated cytotoxicity
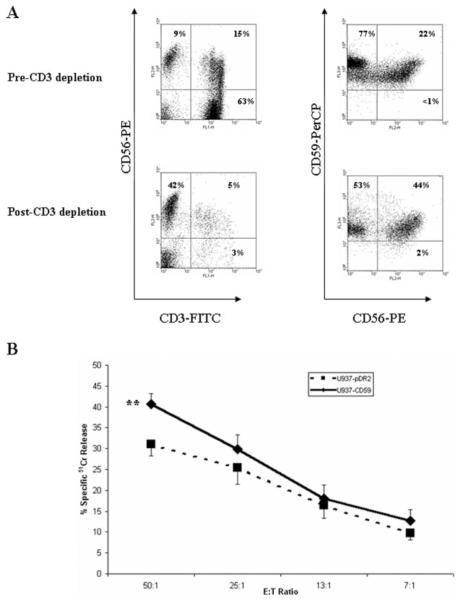
To substantiate the role of CD59 on target cells in NK activity, freshly isolated human primary NK cells were used in cytotoxicity assays against the U937 mutant cell line targets. Triple-color flow cytometry was used to determine the proportion of NK cells in the lymphocyte population, characterized by the CD3−/CD56+ markers (27) and the percentage of cells expressing CD59. CD3 depletion was successfully achieved with 90–96% efficiency (92% in the representative plot shown in Fig. 4A), whereas 37–45% of the lymphocyte population (42% in the representative plot; Fig. 4A) were NK cells. Interestingly, 97–99% of the lymphocyte population were identified as CD59-positive whether depleted of CD3 or not. Consistent with the NKL-mediated cytotoxicity data, primary NK-mediated cytotoxicity is enhanced in the presence of GPI-anchored CD59 on target cells as compared with empty-vector control cells, although the enhancement was statistically significant only at the 50:1 E:T ratio ( p < 0.05; Fig. 4B).
FIGURE 4.
Expression of GPI-anchored CD59 on targets enhanced cytotoxicity mediated by freshly isolated human primary NK cells. A, PBMC were depleted of CD3 and proportions of NK cells (CD3−/CD56+) and CD59high populations quantified by flow cytometry. B, Following overnight activation in the presence of IFN-α, 4-h 51Cr release cytotoxicity assay was conducted against preloaded U937 cell lines at the indicated E:T ratios. Measurements are the mean of triplicate determinations. Error bars represent SE of the mean from nine independent experiments with samples from four healthy volunteers. **, p < 0.01.
Both GPI-anchored and BiMP-anchored CD59 are localized in lipid rafts after incorporation in U937
To investigate the reasons for the differences between the two forms of CD59 in modulating susceptibility of targets to NKL cytotoxicity, we examined the location of the incorporated BiMP- and GPI-anchored CD59 in U937 target cells. Immunofluorescence microscopy demonstrated a patchy distribution of both forms of CD59 and colocalization with ganglioside GM1, a marker of lipid rafts (Fig. 5A). Location in rafts was confirmed by sucrose gradient ultracentrifugation, both forms of CD59 localizing in early fractions from the gradient along with specific raft markers, and substantially lower levels present in nonraft fractions under saturating conditions (Fig. 5B). Banding patterns on the Western blot of GPI-anchored CD59 reflect the well-described heterogeneity of glycosylation, apparent as a “laddering” pattern in the mass range of 18–25 kDa, with intervals corresponding to addition of high mannose units (28). The bacterially expressed BiMP CD59 is unglycosylated and shown as a sharp, single band of mass 17 kDa. These data show that both BiMP-anchored and GPI-anchored CD59 locate in lipid rafts (Fig. 5C), an environment that has been implicated in signaling with integral roles in functional activity in diverse cells (29).
FIGURE 5.
Incorporation of BiMP CD59 and GPI-anchored CD59 into U937 cells. A, Cocapping is illustrated by immunofluorescence analysis of CD59 colocalization with the cholera toxin-ganglioside GM1 raft protein. Magnification is ×400. Sucrose gradient ultracentrifugation of the stable CD59-expressing cell line (B) and incorporated CD59 lines (conducted as described in Materials and Methods) (C) confirm the localization of CD59 in the detergent insoluble (lipid raft) fractions. The nuclear pellet (P) was resuspended by brief sonication on ice. Fractions containing GM1 were identified by dot blotting to confirm the location of lipid rafts in the gradient (B).
GPI-anchored CD59 is signaling competent in U937 cells
Intracellular signaling as a consequence of Ab-mediated CD59 cross-linking has been widely reported; outputs include the generation of reactive oxygen species, induction of tyrosine phosphorylation, and the release of Ca2+ from intracellular stores (30, 31). To investigate whether signaling through the various anchoring and glycosylation forms of CD59 might explain the different activities of the proteins in NK target cell lysis, intracellular Ca2+ flux was measured following Ab cross-linking. When GPI-anchored CD59 was expressed on U937, either through transfection or incorporation, cross-linking of CD59 caused cell activation as demonstrated in this study by the rapid and transient increase in cytosolic-free Ca2+ (Fig. 6A). In contrast, when BiMP CD59 was expressed by incorporation at a similar level on U937, cross-linking did not cause any detectable change in intracellular Ca2+, demonstrating that the synthetic anchor was not capable of transducing activation events. Furthermore, U937 cells expressing the unglycosylated (but GPI-anchored) form of CD59 were also able to demonstrate intracellular Ca2+ flux at a similar level and with the same kinetics following cross-linking as compared with the glycosylation-competent expressing cells (Fig 6A, U937−CD59GPI), whereas no discernible flux was observed in cells expressing the transmembrane protein (Fig. 6B, U937−CD59TM). Therefore in correlation with the NK cytotoxicity data, these observations highlight the importance of GPI anchoring but not glycosylation in mediating signal transduction via CD59.
FIGURE 6.
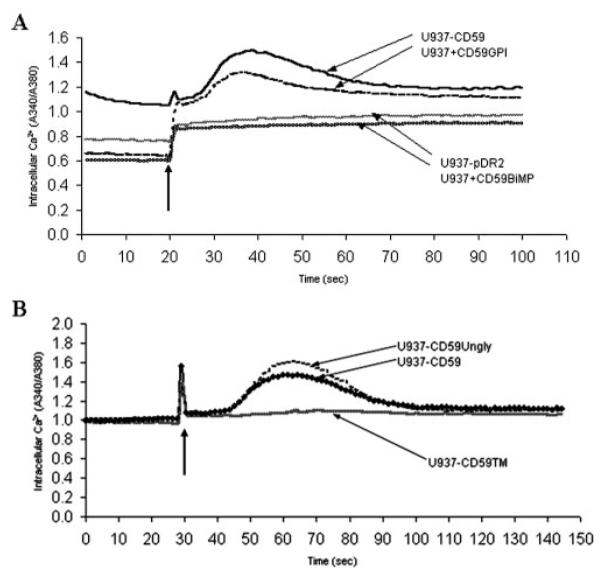
Cross-linking of GPI-anchored CD59, whether glycosylated or unglycosylated, triggers intracellular Ca2+ concentration increase in U937. CD59 (A) and CD59 mutants (B) were either incorporated into U937 or stably expressed in this line. Cells were subjected to Ab-mediated cross-linking, indicated by the arrow, and intracellular Ca2+ concentration levels were determined by measuring the spectral shift of preloaded fura 2-Ca2+ indicator. Results are representative of three independent experiments.
Discussion
We set out to investigate whether the expression of CD59 on target cells modulated NK cytotoxicity. To this end, we first performed Ab blocking experiments using previously mapped anti-CD59 mAb on the widely used target line K562, a line known to express CD59 at high levels (32). The effector cells used throughout this study were the well-described NKL cell line, shown in this study to be CD59-negative. Preincubation of K562 with mAb MEM43/5 strongly inhibited NKL-mediated cytotoxicity. This mAb has previously been shown to have no effect on CReg activity of CD59 and maps to an epitope at or close to Leu33 (24). Two mAb previously shown to block the CReg activity of CD59 and bind at or close to Trp40 and Arg53 (adjacent in the folded protein) had no effect on NKL cytotoxicity in these assays.
To further investigate the contribution of target cell CD59 expression on NKL cytotoxicity we chose the U937 cell, a line we have shown is CD59-negative but expresses other GPI-anchored molecules and can express CD59 after transfection (33). U937 were susceptible to cytotoxicity by NKL and expression of GPI-anchored CD59, either through transfection or through incorporation of the erythrocyte-derived protein, markedly increased lytic susceptibility. In marked contrast, incorporation of a form of CD59 bearing a synthetic BiMP anchor, comprising a charged peptide stretch and two myristoyl residues (19), had no effect on sensitivity of U937 to NKL cytotoxicity. The GPI-anchored and BiMP forms of CD59 incorporated to similar levels (a 2-fold difference) and were both retained on the cell surface for hours. Additional CD59 mutant U937 cell lines stably expressing the transmembrane-anchored and unglycosylated forms of CD59 at similar levels demonstrated the essential role of the GPI anchor while eliminating a role for CD59-glycosylation in NK-mediated cytotoxicity. Furthermore, findings using the NKL cell line were further substantiated by using primary NK effectors, which also demonstrated enhanced killing capacity toward targets expressing GPI-anchored CD59 as compared with vector control cells. The smaller effects in NK cytotoxicity at lower E:T ratios using primary cells may reflect homotypic interactions of CD59, as primary NK cells were strongly positive for CD59.
To explore the reasons for the apparent GPI-dependency of the observed effects, we first investigated the location of the two forms of CD59 on the U937 cell after incorporation. GPI-anchored proteins accumulate in lipid rafts, cholesterol-rich microdomains in the membrane that play key roles in signaling events in diverse cell types (34-36). We have previously shown that GPI-anchored CD59 incorporated in U937 accumulates in lipid rafts over the course of a few hours, and once in rafts, acquires signaling capacity absent in the newly incorporated nonraft-associated protein (37). In this study we confirmed this observation; GPI-anchored CD59, expressed or incorporated, was almost 100% raft-associated as demonstrated by raft fractionation techniques supported by microscopy and the spatial association with the raft marker GM1. BiMP CD59 also associated with lipid rafts. Proteins naturally myristoylated at the N terminus are known to associate in lipid rafts (38). However, this association has not previously been documented for proteins modified at the C terminus, but is consistent with previous observations of colocalization of other myristoylpeptidyl and BiMP-anchored proteins with markers of lipid rafts (S. H. Ridley and R. A. G. Smith, unpublished observations). Despite their similar location in the membrane, cross-linking of these two forms of CD59 revealed key differences. Whereas GPI-anchored CD59 was signaling competent, triggering Ca2+ flux in the cell, cross-linking of BiMP-anchored CD59 did not cause any detectable Ca2+ flux, indicating that it was signaling incompetent. Signaling through GPI-anchored proteins requires association with adaptor molecules in the membrane (39, 40). Our preliminary data indicate that, although GPI-anchored CD59 associates with an adaptor after incorporation in U937 to acquire signaling capacity, BiMP-anchored CD59 does not acquire such associations, likely explaining the failure to signal (data not shown). Another difference between the BiMP CD59 and the GPI-anchored forms of CD59 is that the former is bacterially expressed and lacks the large N-glycan group. However, we have shown that expressed unglycosylated CD59 efficiently mediates signaling as measured by Ca2+ flux analysis, whereas the glycosylated transmembrane protein is unable to signal on cross-linking. The data suggest that CD59-specific signaling mechanisms may mediate the enhanced NK-mediated cytotoxicity toward CD59-expressing targets.
The focus of the current study was the influence of CD59 expressed on target cells on NK cytotoxicity. GPI-anchored CD59, either expressed or incorporated, was shown to enhance NK cell cytotoxicity. The expressed form always caused higher susceptibility to killing than the incorporated protein despite similar levels of expression. This result may be due to more efficient recruitment of signaling systems with endogenous expression in the target. Of note a recent elegant study demonstrated that CD59 acts as a co-activating molecule with NKp30/NKp46 on NK cells and thus influences cytotoxicity (14).
In conclusion, these data demonstrate that CD59 on target cells enhances NKL cytotoxicity and that this effect is dependent on the GPI anchor in CD59. The findings suggest that CD59 binds ligands on the NKL and that these interactions trigger signaling events in the target that contribute to killing. We have not yet identified the NKL ligand for CD59, but have found that a directly labeled recombinant soluble form of CD59 binds NKL in a specific manner suggestive of a receptor (data not shown). Identification of this putative receptor is the subject of ongoing studies. Our observation that the BiMP membrane targeting system is signaling-incompetent is of significance for the separation of CReg and signaling-dependent activities. This observation is directly pertinent to the development of replacement therapy of C inhibitory activity for paroxysmal nocturnal hemoglobinuria, in which NK cell-mediated cytotoxicity would be undesirable.
Acknowledgments
We thank Dr. Carmen W. van den Berg for CD59 constructs and U937 mutants lines and Dr. Tim R. Hughes for helpful comments and advice.
Footnotes
This work was supported by the Welcome Trust Programme Grant 068590.
- BiMP
- bis-myristoylated-peptidyl
- CReg
- C regulatory protein
Disclosures R.A.G.S. has a commercial interest in Inflazyme Pharmaceuticals, which freely provided the BiMP CD59 to the Morgan Laboratory (Cardiff, U.K.) where the experiments were conducted.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Morgan BP, Harris CL. Complement Regulatory Proteins. Academic Press; London: [Google Scholar]
- 2.Chatterjee S, Mayor S. The GPI-anchor and protein sorting. Cell. Mol. Life Sci. 2001;58:1969–1987. doi: 10.1007/PL00000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iida Y, Takeda J, Miyata T, Inoue N, Nishimura J, Kitani T, Maeda K, Kinoshita T. Characterization of genomic PIG-A gene: a gene for glycosylphosphatidylinositol-anchor biosynthesis and paroxysmal nocturnal hemoglobinuria. Blood. 1994;83:3126–3131. [PubMed] [Google Scholar]
- 4.Miyata T, Yamada N, Iida Y, Nishimura J, Takeda J, Kitani T, Kinoshita T. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 1994;330:249–255. doi: 10.1056/NEJM199401273300404. [DOI] [PubMed] [Google Scholar]
- 5.Seya T, Tejima H, Fukuda H, Hara T, Matsumoto M, Hatanaka M, Sugita Y, Masaoka T. Acute promyelocytic leukemia with CD59 deficiency. Leuk. Res. 1993;17:895–896. doi: 10.1016/0145-2126(93)90155-e. [DOI] [PubMed] [Google Scholar]
- 6.Sundström C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int. J. Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 7.Hatanaka M, Seya T, Matsumoto M, Hara T, Nonaka M, Inoue N, Takeda J, Shimizu A. Mechanisms by which the surface expression of the glycosyl-phosphatidylinositol-anchored complement regulatory proteins decay-accelerating factor (CD55) and CD59 is lost in human leukaemia cell lines. Biochem. J. 1996;314(Pt. 3):969–976. doi: 10.1042/bj3140969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD, Song WC. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J. Exp. Med. 2005;201:567–577. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seya T, Kojima A, Hara T, Hazeki K, Sugita Y, Akedo H. Enhancement of lymphocyte-mediated K562 cytotoxicity by antibodies against complement membrane cofactor protein (CD46) and decay-accelerating factor (CD55) Immunobiology. 1991;183:115–124. doi: 10.1016/S0171-2985(11)80191-9. [DOI] [PubMed] [Google Scholar]
- 11.Finberg RW, White W, Nicholson-Weller A. Decay-accelerating factor expression on either effector or target cells inhibits cytotoxicity by human natural killer cells. J. Immunol. 1992;149:2055–2060. [PubMed] [Google Scholar]
- 12.Kusama T, Miyagawa S, Moritan T, Kubo T, Yamada M, Sata H, Fukuta D, Matsunami K, Shirakura R. Downregulation of NK cell-mediated swine endothelial cell lysis by DAF (CD55) Transplant. Proc. 2003;35:529–530. doi: 10.1016/s0041-1345(02)03834-4. [DOI] [PubMed] [Google Scholar]
- 13.Miyagawa S, Kubo T, Matsunami K, Kusama T, Beppu K, Nozaki H, Moritan T, Ahn C, Kim JY, Fukuta D, Shirakura R. Delta-short consensus repeat 4-decay accelerating factor (DAF: CD55) inhibits complement-mediated cytolysis but not NK cell-mediated cytolysis. J. Immunol. 2004;173:3945–3952. doi: 10.4049/jimmunol.173.6.3945. [DOI] [PubMed] [Google Scholar]
- 14.Marcenaro E, Augugliaro R, Falco M, Castriconi R, Parolini S, Sivori S, Romeo E, Millo R, Moretta L, Bottino C, Moretta A. CD59 is physically and functionally associated with natural cytotoxicity receptors and activates human NK cell-mediated cytotoxicity. Eur. J. Immunol. 2003;33:3367–3376. doi: 10.1002/eji.200324425. [DOI] [PubMed] [Google Scholar]
- 15.Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 1996;24:406–415. [PubMed] [Google Scholar]
- 16.Charreau B, Cassard A, Tesson L, Le Mauff B, Navenot JM, Blanchard D, Lublin D, Soulillou JP, Anegon I. Protection of rat endothelial cells from primate complement-mediated lysis by expression of human CD59 and/or decay-accelerating factor. Transplantation. 1994;58:1222–1229. [PubMed] [Google Scholar]
- 17.Hiscox S, Hallett MB, Morgan BP, van den Berg CW. GPI-anchored GFP signals Ca2+ but is homogeneously distributed on the cell surface. Biochem. Biophys. Res. Commun. 2002;293:714–721. doi: 10.1016/S0006-291X(02)00280-2. [DOI] [PubMed] [Google Scholar]
- 18.van den Berg CW, Morgan BP. Complement-inhibiting activities of human CD59 and analogues from rat, sheep, and pig are not homologously restricted. J. Immunol. 1994;152:4095–4101. [PubMed] [Google Scholar]
- 19.Fraser DA, Harris CL, Williams AS, Mizuno M, Gallagher S, Smith RA, Morgan BP. Generation of a recombinant, membrane-targeted form of the complement regulator CD59: activity in vitro and in vivo. J. Biol. Chem. 2003;278:48921–48927. doi: 10.1074/jbc.M302598200. [DOI] [PubMed] [Google Scholar]
- 20.Smith RA. Targeting anticomplement agents. Biochem. Soc. Trans. 2002;30:1037–1041. doi: 10.1042/bst0301037. [DOI] [PubMed] [Google Scholar]
- 21.van Beek J, van Meurs M, ’t Hart BA, Brok HP, Neal JW, Chatagner A, Harris CL, Omidvar N, Morgan BP, Laman JD, Gasque P. Decay-accelerating factor (CD55) is expressed by neurons in response to chronic but not acute autoimmune central nervous system inflammation associated with complement activation. J. Immunol. 2005;174:2353–2365. doi: 10.4049/jimmunol.174.4.2353. [DOI] [PubMed] [Google Scholar]
- 22.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- 23.Ortaldo JR, Oldham RK, Cannon GC, Herberman RB. Specificity of natural cytotoxic reactivity of normal human lymphocytes against a myeloid leukemia cell line. J. Natl. Cancer Inst. 1977;59:77–82. doi: 10.1093/jnci/59.1.77. [DOI] [PubMed] [Google Scholar]
- 24.Bodian DL, Davis SJ, Morgan BP, Rushmere NK. Mutational analysis of the active site and antibody epitopes of the complement-inhibitory glycoprotein, CD59. J. Exp. Med. 1997;185:507–516. doi: 10.1084/jem.185.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones J, Hallett MB, Morgan BP. Reversible cell damage by T-cell perforins: calcium influx and propidium iodide uptake into K562 cells in the absence of lysis. Biochem. J. 1990;267:303–307. doi: 10.1042/bj2670303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan BP. Effects of the membrane attack complex of complement on nucleated cells. Curr. Top. Microbiol. Immunol. 1992;178:115–140. doi: 10.1007/978-3-642-77014-2_8. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald-Bocarsly P, Herberman RB, Hercend T. A definition of natural killer cells. In: Ades E, Lopez C, editors. Natural Killer Cells and Host Defense. Karger; Basel: 1989. pp. 43–45. [Google Scholar]
- 28.Rudd PM, Morgan BP, Wormald MR, Harvey DJ, van den Berg CW, Davis SJ, Ferguson MA, Dwek RA. Roles for glycosylation in the anti-inflammatory molecule CD59. Biochem. Soc. Trans. 1997;25:1177–1184. doi: 10.1042/bst0251177. [DOI] [PubMed] [Google Scholar]
- 29.Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 30.Stefanová I, Horejsi V. Association of the CD59 and CD55 cell surface glycoproteins with other membrane molecules. J. Immunol. 1991;147:1587–1592. [PubMed] [Google Scholar]
- 31.Lund-Johansen F, Olweus J, Symington FW, Arli A, Thompson JS, Vilella R, Skubitz K, Horejsi V. Activation of human monocytes and granulocytes by monoclonal antibodies to glycosylphosphatidylinositol-anchored antigens. Eur. J. Immunol. 1993;23:2782–2791. doi: 10.1002/eji.1830231110. [DOI] [PubMed] [Google Scholar]
- 32.Jurianz K, Ziegler S, Donin N, Reiter Y, Fishelson Z, Kirschfink M. K562 erythroleukemic cells are equipped with multiple mechanisms of resistance to lysis by complement. Int. J. Cancer. 2001;93:848–854. doi: 10.1002/ijc.1406. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg CW, Williams OM, Morgan BP. Presence of a dysfunctional form of CD59 on a CD59+ subclone of the U937 cell line. Immunology. 1994;81:637–642. [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuuchi L, Gold MR. New views of BCR structure and organization. Curr. Opin. Immunol. 2001;13:270–277. doi: 10.1016/s0952-7915(00)00215-6. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 36.Brdicka T, Imrich M, Angelisova P, Brdickova N, Horvath O, Spicka J, Hilgert I, Luskova P, Draber P, Novak P, et al. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 2002;196:1617–1626. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Berg CW, Cinek T, Hallett MB, Horejsi V, Morgan BP. Exogenous glycosyl phosphatidylinositol-anchored CD59 associates with kinases in membrane clusters on U937 cells and becomes Ca2+-signaling competent. J. Cell Biol. 1995;131:669–677. doi: 10.1083/jcb.131.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcell. Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- 39.Lindquist JA, Simeoni L, Schraven B. Transmembrane adapters: attractants for cytoplasmic effectors. Immunol. Rev. 2003;191:165–182. doi: 10.1034/j.1600-065x.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 40.Stefanová I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]