Abstract
The culture of human natural killer (NK) cell clones has traditionally been a long, laborious process with an efficiency of only 1–2%. Recently, a stem cell growth medium (SCGM) has been described to expand preferentially polyclonal NK cells from peripheral blood. We have tested SCGM in a single cell sorting system and shown a 4–5 fold increase in the number of proliferating NK clones compared to standard RPMI media. The cloning efficiency was further enhanced by the provision of irradiated feeder cells derived from multiple donors combined with the addition of the anti-CD3 antibody, OKT3. The combination of SCGM, single cell sorting and these multiple optimisations enhanced NK cloning efficiency by more than tenfold to greater than 20% for short-term cultures when deriving 105 cells and as high as 10% for longer term cultures when deriving more than 2×106 cells. This novel system thus facilitates the generation of NK clones and allows larger scale studies of NK function that were beyond the scope of previous methodology.
Keywords: Natural killer cells, Cloning
1. Introduction
Natural killer (NK) cells were identified by their ability to kill virus-infected and tumour cells (Takasugi et al., 1973; Trinchieri and Santoli, 1978). Major advances over the last 10 years have identified many of the cell surface receptors that determine NK function. These include the constitutively expressed killer immunoglobulin-like receptors (KIR), which are specific for the highly polymorphic major histocompatibility complex (MHC) class I proteins (reviewed by Parham, 2005). NK cells also express other receptors specific for MHC class I including the leukocyte inhibitory receptor (LIR-1) and CD94/NKG2 receptors (reviewed by Lanier, 1998; Moretta et al., 2000, 2001). Unlike the KIRs which are specific for particular class I allotypes, the CD94/NKG2 receptors are specific for the non-classical MHC class I molecule HLA-E (Braud et al., 1998; Lanier, 1998; Vales-Gomez et al., 1999). Individual NK cells express between 1 and 6 inhibitory and between 0 and 5 stimulatory MHC class I receptors and it is the combination of these receptors which defines the responsiveness of any particular NK cell (Valiante et al., 1997). In addition, a number of activating and inhibitory receptors that recognise other non-MHC class I ligands have been identified. These include NKG2D and the NK-specific natural cytotoxicity receptors (NCR), NKp30, NKp44, NKp46 (reviewed by Moretta and Moretta, 2004) as well as a number of receptors with shared expression on T-cells, such as the Leukocyte associated Ig-like receptors, LAIR-1 and LAIR-2 (Meyaard et al., 1997) and the inhibitory receptor protein 60 (IRp60) (Cantoni et al., 1999). In this context it is becoming more important to understand the role of each of the inhibitory and stimulatory molecules in determining the NK response.
Analysis of NK clones permits dissection of the NK response in the context of inhibitory and stimulatory receptor expression. However, current methods of NK cloning are inefficient and laborious. The principles of NK cloning have remained relatively unchanged since Yssel et al. first described a method for cloning both NK and T cells in 1984 (Yssel et al., 1984). In this method, NK cells are isolated by limiting dilution and stimulated with irradiated Epstein Barr virus-immortalised lymphoblastoid B cell lines, peripheral blood mononuclear cells (PBMC) and the polyclonal activating lectin, phytohaemaglutinnin. NK cells are then cultured in Yssel’s medium, a serum free medium containing bovine serum albumin, transferrin, insulin, linoleic-oleic and palmitic acid, penicillin and streptomycin. Whilst Yssel’s medium is shown to be better than RPMI for NK clone culture, NK cloning using these methods are still inefficient (Spits and Yssel, 1996). More recently, Carlens et al. (2000) demonstrated that the commercially available serum free medium, SCGM is capable of supporting the expansion of polyclonal NK cells within PBMC (Carlens et al., 2000, 2001). Additionally, they demonstrated that the culture of PBMC in vitro in SCGM with the anti-CD3 specific antibody, OKT3, boosted the number of NK cells within PBMC, although they did not investigate these conditions in an NK cloning environment. Here, we report a novel system of NK cloning using SCGM and multiple optimisations, which boosts cloning efficiency by over tenfold without the use of activating lectins such as phytohaemaglutinin.
2. Methods
2.1. Cell culture media and reagents
Cellgro SCGM serum-free medium was purchased from CellGenix (Freiburg, Germany) and RPMI 1640 was purchased from Gibco (Paisley, UK). The media were supplemented with 5% heat-inactivated, human AB serum (Rhydlafa, UK), penicillin (1×105 IU/ml) (Gibco) and streptomycin (100 mg/ml) (Gibco). IL-2 was purchased from Cetus (Emeryville, USA) and the monoclonal antibody OKT3 grown from the J.CAM1.6 hybridoma from ATCC (Middlesex, UK).
2.2. Isolation of PBMC
Informed consent was obtained from all donors (designated D#). Blood was collected in tubes containing preservative-free heparin (Monoparin) (CP Pharmaceuticals Ltd., Clwydd) (5 IU/ml blood). PBMCs were isolated by density gradient centrifugation, using Histopaque-1077 (Gibco).
2.3. Cell sorting and flow cytometry
PBMC were stained with CD3 and CD56 for cell sorting and for analysis of NK clonality. CD3-FITC (MEM-57) was purchased from Serotec (Oxford, UK) and CD56-PE (B-A19) was purchased from Diaclone (Bensancon, France). Briefly, cells were stained with the appropriate concentration of antibodies and incubated for 20 min at 4 °C, then washed twice in PBS 2% v/v ABS. Data acquisition and analysis was performed on a FACSCalibur (BD, Mountain View, CA) using Cellquest software (BD). Single cell sorting of CD3−CD56+ cells was performed on a MoFlo (Dako-Cytomation, Cambridge, UK). CD94-PE (HP-3D9) was purchased from BD, NKG2A (Z199) was purchased from Immunotech (Marseille, France) and visualised with goat anti mouse IgG2b-FITC (R19-15) purchased from BD.
2.4. Proliferation assay
Polyclonal NK cells were plated out in triplicate at 4×104 cells/well in a U-bottomed 96 well plate (Greiner, Stonehouse, UK) and stimulated with various concentrations of IL-2 in RPMI or SCGM media. To measure the proliferation of a clone, a single clone was incubated with the described stimulations before pulsing with tritiated (3H) thymidine. 3H-thymidine (Amersham, Little Chalfont, UK) (1 μCi/well) was added to each well and the cells were incubated for 18 h at 37 °C. The radiolabelled cells were harvested onto fibreglass Titertek filtermats, by a Skatron Titertek cell harvester (Skatron, Norway). 3H-thymidine incorporation was measured on a 1450 Microbeta Trilux Liquid Scintillation Counter (Perkin-Elmer). Proliferating polyclonal NK cells were identified using a stimulation index, calculated by dividing the geometric mean of the test wells by the geometric mean of the unstimulated control wells. A stimulation index of 4 or more was considered as positive for proliferation. To measure the proliferation of NK clones, the geometric mean of 6 control samples (containing feeder cells only) was multiplied by 4 and subtracted from the test well. Test wells that gave a positive number were considered to be proliferating.
2.5. Cytotoxicity assay
Standard chromium release assays were used to measure cytotoxicity. In brief, K562 target cells were loaded with Na2 51CrO4 (Amersham) using 2 MBq for every 5×105 target cells. Following washing away of excess Na2 51CrO4, assays were performed in 200 μl reaction volumes in 96 well round bottomed plates using RPMI v/v 2% ABS and 2×103 K562 targets/well. Spontaneous Na2 51CrO4 release was determined by incubating target cells with medium only. Maximum Na2 51CrO4 release was determined by the addition of 5% (v/v) Triton X-100 (100 μl). After the addition of target cells, plates were incubated at 37 °C for 4 h before the radioactivity of a 20 μl sample was measured on a 1450 Microbeta Trilux Liquid Scintillation Counter. Percentage specific lysis was calculated using the standard equation:
2.6. Statistical analysis
To test the relationship between the 3H-thymidine incorporation of NK cells from the same individual cultured in two different media, a 1-way ANOVA was performed. A Tukey’s multiple comparison post-test was used to compare 3H-thymidine incorporation values for each medium. A 2-way ANOVA was used to test the difference in 3H-thymidine incorporation when NK cells were cultured in two different media with various concentrations of IL-2. A Bonferroni post-test was used to compare values at each concentration of IL-2. All ANOVA and post-tests were carried out using Prism 4 software (GraphPad, USA). For all tests, a p value of less than 0.05 was taken as being significant.
2.7. NK cloning
NK clones were generated from fresh PBMC by single cell sorting CD3−CD56+ cells into single wells of a 96 well plate (Greiner). NK clones were cultured at 37°C and 5% CO2 in SCGM 5% v/v ABS supplemented with IL-2 (250 IU/ml), OKT3 (50 ng/ml) (ATCC) and 5×104 irradiated (3000 rads) PBMC from three allogeneic individuals for 4 days. On day 4 half the medium was removed and replaced with fresh medium containing IL-2 (1000 IU/ml). Cells were cultured for up to 10 weeks, and restimulated with irradiated PBMC, IL-2 and OKT3 every 7–10 days. Clones were used in functional assays 10 days after restimulation with irradiated PBMC when all feeder cells were no longer viable. Trypan blue was used as an exclusion dye to ensure that the dead irradiated feeder cells were not counted when estimating the number of cells cultured for an individual NK clone.
3. Results
3.1. Culture of polyclonal NK cells in SCGM and RPMI
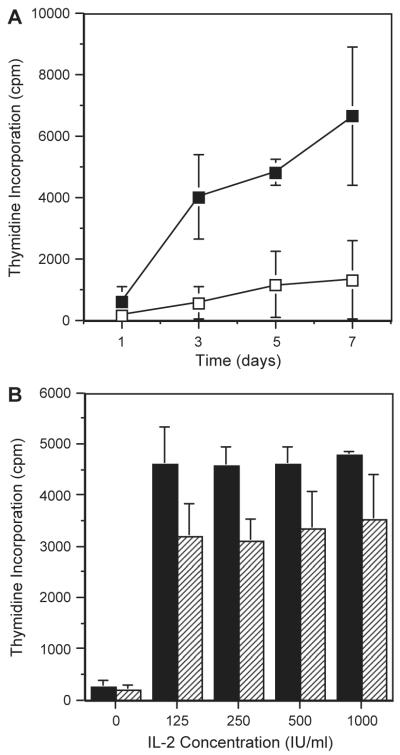
We first tested the ability of SCGM to maintain culture of peripheral blood-derived polyclonal NK cells. CD3+CD56− NK cells were cell sorted from three different donors and cultured in SCGM or RPMI supplemented with IL-2 (500 IU/ml) for 7 days and proliferation was measured on days 1, 3, 5 and 7. The combined mean 3H-thymidine incorporation values from all 3 donors are shown in Fig. 1A. 3H-thymidine incorporation was greatest in SCGM+IL-2 and this was significantly greater than for cells cultured in RPMI+IL-2 (p =0.0011).
Fig. 1.
Comparison of proliferation of polyclonal NK cells cultured in SCGM or RPMI. CD3−CD56+ NK clones were isolated by cell sorting and used in a standard 3H-thymidine incorporation assay following culture in either SCGM or RPMI supplemented with 5% AB serum for various numbers of days (A) and various concentrations of IL-2 (B) before overnight pulsing with 3H-thymidine. (A) Culture in SCGM (filled squares) generated increased proliferation compared to RPMI (open squares) from 3 days onwards. Two-way ANOVA showed significance at p =0.0011. (B) Culture in SCGM (filled) generated increased proliferation at all IL-2 concentrations compared to RPMI (hashed). Two-way ANOVA showed significance at p =0.0029.
3.2. Effects of IL-2 on NK cell proliferation
Polyclonal NK cells from three different donors were then cultured in SCGM or RPMI supplemented with serial dilutions of IL-2 from 125 to 1000 IU/ml for 3 days. Fig. 1B shows the combined results for all three donors. The presence of IL-2 significantly increased the 3H-thymidine incorporation for cells cultured in both SCGM and RPMI (p <0.0001). NK cells cultured in SCGM incorporated significantly more 3H-thymidine than cells cultured in RPMI (p =0.0029). IL-2 could not recover the deficit observed between the two media.
3.3. Effects of SCGM and RPMI on NK clone proliferation and cytotoxicity
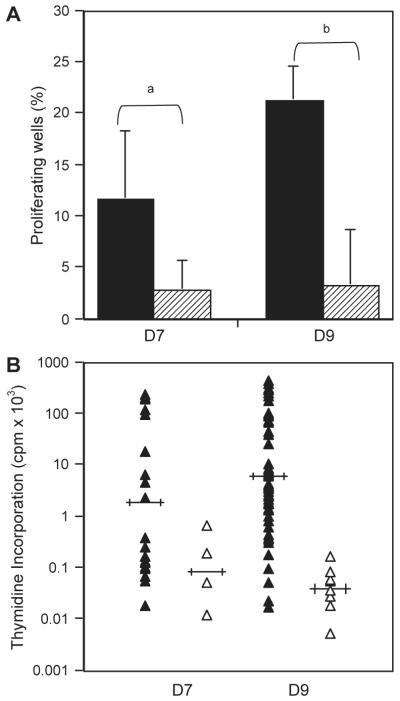
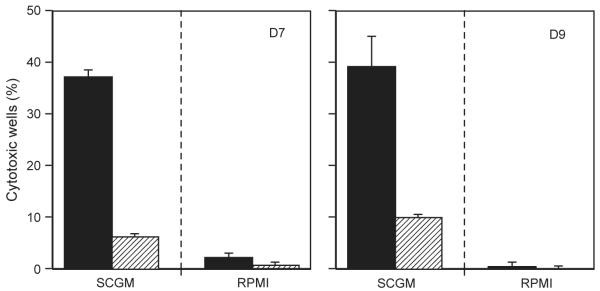
The improved proliferation of polyclonal NK cells in SCGM compared to RPMI encouraged an investigation into the benefits of SCGM for NK cell cloning. Single CD3−CD56+ cells from two donors were cultured in SCGM or RPMI supplemented with irradiated feeders from three allogeneic donors and IL-2 (500 IU/ml). Fig. 2A showed that there was at least a 400% increase in the number of proliferating wells for single cells cultured in SCGM compared to RPMI and these proliferating wells had higher 3H-thymidine incorporation values (Fig. 2B). Additionally, at least eleven times as many wells cultured in SCGM were cytotoxic against the NK-specific target cell line K562 compared to those cultured in RPMI (Fig. 3). Wells containing only irradiated feeder cells were unable to lyse K562 targets, indicating that these irradiated feeders could not be a source of NK cells. These data indicated a significant association between increased frequency of both proliferating and cytotoxic NK cells and culture in SCGM and illustrate that SCGM is not only able to support polyclonal NK expansion, but also NK clone growth in culture.
Fig. 2.
Comparison of Proliferation of NK Clones Cultured in SCGM or RPMI. NK clones were isolated using single cell sorting and used in a standard 3H-thymidine incorporation assay following culture in either SCGM or RPMI supplemented with 5% AB serum, IL-2 (500 IU/ml) and irradiated allogeneic feeders for 10 days. Positive wells were identified by setting a positive cut-off at 4× the mean 3H-thymidine incorporation values of control wells (stimulation index=4). (A) Results are expressed as a % of 180 wells for D7 or 240 wells for D9. SCGM (filled) generated between 4× and 6× more positive proliferating wells compared with RPMI (hashed). The variation indicated by the bars, represents frequencies calculated using a positive cut off of 3× the mean incorporation values of control wells. (B) The proliferating wells identified in (A) were ranked according to the magnitude of the proliferative response. Mean proliferation is indicated by horizontal bars. Wells cultured in SCGM (filled triangles) had higher incorporation values of 3H-thymidine than wells cultured in RPMI (open triangles). χ2 tests showed significant differences between the indicated results at ap =0.0011 and bp =2.3×10−9.
Fig. 3.
Comparison of cytotoxicity of NK clones cultured in SCGM or RPMI. NK clones were isolated using single cell sorting and cultured in either SCGM or RPMI supplemented with 5% AB serum, IL2 (500 IU/ml) and irradiated allogeneic feeders before cytotoxic NK assays using K562s as targets on day 17. Positive wells were identified as lysing targets at greater than 10%. The variation indicated by the bars, represents frequency calculated using maximum and minimum control release minus 1 SD. Results are expressed as a percentage of 180 wells for D7 or 240 wells for D9. SCGM (filled) generated between 11× and 24× more positive cytotoxic wells compared with RPMI (hashed).
3.4. Effects of OKT3 on NK clone proliferation and cytotoxicity
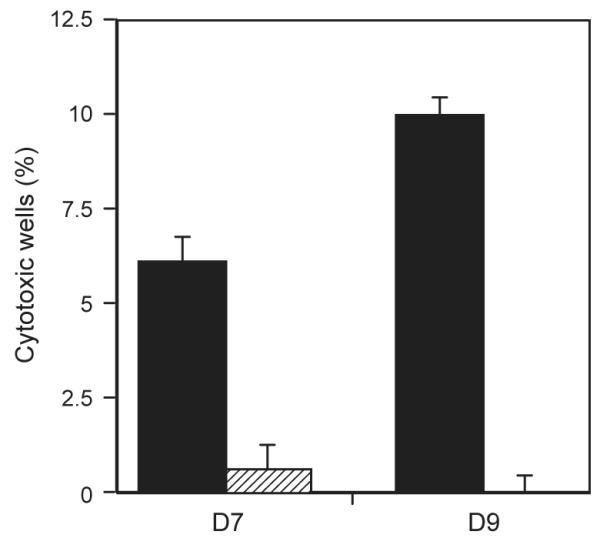
Proliferation and cytotoxicity of NK clones cultured in SCGM or RPMI with or without OKT3 (50 ng/ml) was investigated because OKT3 had previously been shown to increase the proportion of CD3−CD56+ NK cells in 3-week cultures of PBMC (Carlens et al., 2000). We observed a significant association between increased proportions of both proliferating and cytotoxic wells and culture in SCGM. As shown in Table 1, the addition of OKT3 increased the number of proliferating wells of single cell sorted NK cells cultured in SCGM by 157% for D7. Categorising the proliferating wells cultured in SCGM according to the magnitude of the proliferative response showed that the addition of OKT3 increased the number of wells proliferating in the highest category by 400%. Additionally, 4–6 times as many NK clones cultured in SCGM+OKT3 were cytotoxic against K562 targets compared to those clones cultured without OKT3 (Fig. 4).
Table 1.
Effect of OKT3 on magnitude of NK clone proliferation
| Proliferation cpm × 1000 | Positive wellsa |
|||
|---|---|---|---|---|
| SCGMb | SCGM+OKT3b | RPMIb | RPMI+OKT3b | |
| D7 | ||||
| <50 | 15/21 (71%) | 22/54 (40%) | 5/5 (100%) | 1/2 (50%) |
| 51–100 | 1/21 (5%) | 9/54 (17%) | 0/5 (0%) | 0/2 (0%) |
| 101–150 | 2/21 (10%) | 7/54 (13%) | 0/5 (0%) | 0/2 (0%) |
| 151–200 | 1/21 (5%) | 6/54 (11%) | 0/5 (0%) | 0/2 (0%) |
| 201+ | 2/21 (10%) | 10/54 (19%) | 0/5 (0%) | 1/2 (50%) |
| Total positive | 21/180 | 54/180 | 5/180 | 2/180 |
| D9 | ||||
| 50< | 37/51 (72%) | 31/91 (34%) | 4/8 (50%) | 0/0 (0%) |
| 51–100 | 6/51 (12%) | 15/91 (16%) | 3/8 (38%) | 0/0 (0%) |
| 101–150 | 0/51 (0%) | 9/91 (10%) | 0/8 (0%) | 0/0 (0%) |
| 151–200 | 1/51 (20%) | 7/91 (8%) | 1/8 (12%) | 0/0 (0%) |
| 201+ | 7/51 (14%) | 29/91 (31%) | 0/8 (0%) | 0/0 (0%) |
| Total positive | 51/240c | 91/240c | 8/240 | 0/240 |
Positive wells were identified by subtracting 4× the geometric mean of control samples (feeder cells only) from the test well. Test wells that gave a positive number were considered to be proliferating. Proliferating clones were then categorised according to the amount of their 3H-thymidine incorporation.
Cultured in IL-2 (500 IU/ml), with medium and OKT3 as indicated.
The proportion of wells proliferating in each category were compared using a χ2 test. Number of positive wells cultured in SCGM were significantly different to wells cultured in SCGM+OKT3 (p =0.00022).
Fig. 4.
Comparison of cytotoxicity of NK clones cultured in SCGM or RPMI with or without OKT3. NK clones were isolated using single cell sorting and cultured in either SCGM or RPMI supplemented with 5% AB serum, IL2 (500 IU/ml) and irradiated allogeneic feeders with or without OKT3 (50 ng/ml) before cytotoxic NK assays using K562s as targets on day 17. Positive wells were identified as lysing targets at greater than 10%. The variation indicated by the bars, represents frequency calculated using maximum and minimum control release minus 1 SD. Results from two donors (D7, D9) are shown. OKT3 (filled) generated between 4× and 6× more positive cytotoxic wells than those without OKT3 when cultured in SCGM (hashed). RPMI cultures resulted in less than 3% of wells showing cytotoxicity.
OKT3 did not significantly affect the number of proliferating wells cultured in RPMI indicating that OKT3 could not overcome the deficit between the two media. These data suggest that the ability of SCGM to support NK clone growth in culture was boosted by the addition of OKT3.
3.5. Investigation into the effect of the number of donors for feeder cells
To determine the importance of mixed lymphocyte reactions (MLR) within irradiated feeders in supporting NK clone proliferation and cytotoxicity, NK clones were cultured in SCGM supplemented with IL-2 (500 IU/ml), OKT3 (50 ng/ml) and irradiated allogeneic PBMC from either one or three different donors. Table 2 showed that 35% more NK clones proliferated when cultured with allogeneic PBMC from three donors compared to one. Categorising the clones according to the magnitude of the proliferative response showed that nearly half of the clones stimulated with one allogeneic feeder were in the lowest category of proliferation (45%). In contrast, clones stimulated with three allogeneic feeders were distributed across the proliferative range. These data are supported by the cytotoxicity data which showed that significantly more cytotoxic clones were generated when NK clones were stimulated with three donors compared to one allogeneic donor (p =0.0082) (Fig. 5). These data have allowed us to formulate a relatively efficient method for NK cloning (Fig. 6). Using this method, clones could be maintained in culture for up to 10 weeks. From a single cell, cultures containing between 7×104 and 3.3×106 cells were generated. Counting cultures of more than 2×106 cells (thus allowing cytotoxic analysis against multiple targets), 140 “clones” were derived from 30 plates (1800 wells) from four different subjects, including one donor from whom 71 clones were successfully generated from 12 plates.
Table 2.
Effect of different numbers of allogeneic, feeder donors on NK clone proliferation
| Proliferation cpm × 1000 | Positive wellsa |
|
|---|---|---|
| 1 Feederb | 3 Feederc | |
| D9 | ||
| 50< | 32/71 (45%) | 26/96 (27%) |
| 51–100 | 18/71 (26%) | 20/96 (21%) |
| 101–150 | 7/71 (10%) | 18/96 (19%) |
| 151–200 | 8/71 (11%) | 16/96 (17%) |
| 201+ | 6/71 (8%) | 16/96 (17%) |
| Total positive | 71/300 (24%)d | 96/300 (32%)d |
Positive wells were identified by subtracting 4× the geometric mean of the control samples (feeder cells only) from the test well. Test wells that gave a positive number were considered to be proliferating. Proliferating clones were then categorised according to the amount of their 3H-thymidine incorporation.
NK clones stimulated with 1×106/ml irradiated PBMC from 1 allogeneic donor.
NK clones stimulated with 1×106/ml irradiated PBMC from 3 different allogeneic donors.
χ2 test showed a significant difference at p =0.0082.
Fig. 5.
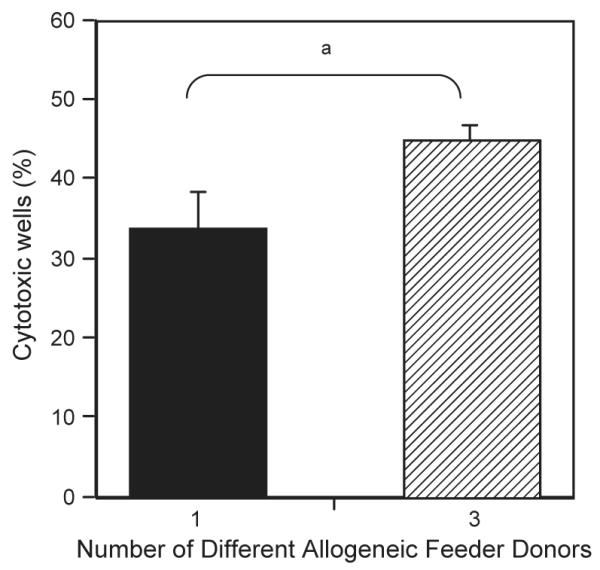
Comparison of cytotoxicity of NK clones cultured with irradiated, allogeneic PBMC feeders derived from different numbers of donors. NK clones were isolated using single cell sorting direct and cultured in SCGM supplemented with 5% AB serum and IL-2 (500 IU/ml) and OKT3 (50 ng/ml). 5×104 irradiated allogeneic feeders from different numbers of donors were used for stimulation. Cytotoxic NK assays using K562s as targets were performed on day 17. Positive wells were identified as lysing targets at greater than 10%. The variation indicated by the bars represents frequency calculated using maximum and minimum control release minus 1 SD. χ2 tests showed significant differences between the indicated results at ap= 0.0082.
Fig. 6.
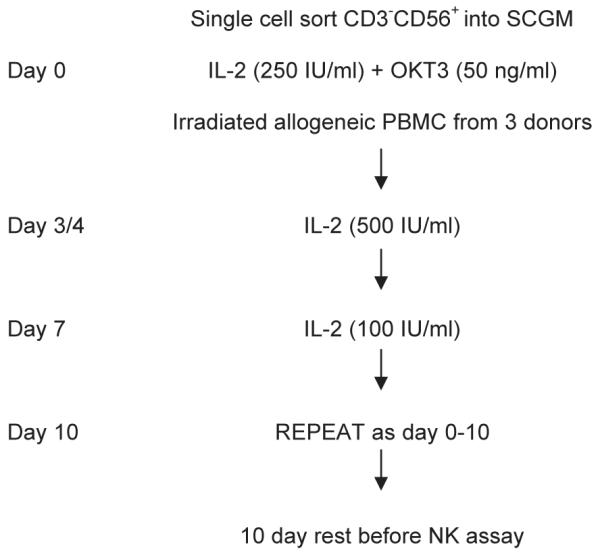
Summary method for NK cloning. NK clones were single cell sorted from PBMC into single wells of a 96 well plate and cultured at 37 °C and 5% CO2 in SCGM 5% v/v ABS supplemented with IL2 (250 IU/ml), OKT3 (50 ng/ml) and 5×104 irradiated PBMC from three allogeneic individuals for 4 days. On day 4, half the medium was removed and replaced with fresh medium containing IL2 (final concentration 500 IU/ml) and on day 7 half the medium was removed and replaced with fresh medium containing IL2 (final concentration 100 IU/ml). Clones were re-stimulated with irradiated PBMC, IL2 and OKT3 every 10 days and could be maintained for up to 10 weeks.
3.6. Investigation into the phenotype of cultured NK clones
Identification of NK clones was also confirmed and their phenotype investigated by flow cytometry. Cells were stained with CD3 and CD56 specific monoclonal antibodies and NK clones were identified as being CD3−, CD56+. Of the 140 cultures, only sixteen were excluded from further analysis, either because flow cytometric analysis indicated a double population of CD56+ cells or consisted of contaminating T cells from the cell sort. A large proportion of the clones were of a CD56dim phenotype suggesting that they expressed high levels of CD16 (Nagler et al., 1989, 1990) and a range of KIR (Jacobs et al., 2001). Further phenotyping of a proportion of the NK clones for CD94 and NKG2A and comparison of their frequency with the frequency of the same NK cells in the peripheral blood of each donor indicated that the culture conditions were better for NK cells of a CD94hi phenotype (Table 3). These NK clones have been successfully used to quantitate novel NK modulatory functions (Tomasec et al., 2005).
Table 3.
Comparison of NK clonal phenotype with NK phenotype in the peripheral blood
| Phenotype | Donor | Frequency in peripheral blooda |
Frequency in cultured clonesb |
|---|---|---|---|
| CD94hi | D3 | 42% | 85% (11/13) |
| D7 | 34% | 52% (13/25) | |
| D8 | ND | 96% (26/27) | |
| D9 | 12% | 78% (35/45) | |
| NKG2A | D9 | 25% | 27% (5/18) |
Frequency of NK cells in the peripheral blood with the denoted phenotype.
Frequency of cultured NK clones with the denoted phenotype.
4. Discussion
In this study, a novel and highly efficient system for NK cloning was developed. In contrast to current techniques, which stimulate NK clones with irradiated EBV-LCL and PHA, we used a mixture of irradiated allogeneic PBMC supplemented with IL-2 and OKT3 (Roncarolo et al., 1991; Litwin et al., 1993; Carr et al., 2002). This was sufficient to achieve cloning efficiencies of up to 10% as measured by the derivation of at least 2×106 cells for any specific clone. This represents a significant increase compared to previously published NK cloning efficiencies. Additionally, phenotyping of NK clones indicated that while this system of culture preferentially expanded CD94hi NK cells (Table 3), it still supported the growth of less common NK cells that are often lost when polyclonal NK lines are cultured such as those with a CD94lo phenotype.
Unlike PHA that has non-specific potent agglutinating and mitogenic activities, OKT3 specifically stimulates T cells by binding to CD3 on their surface (Leavitt et al., 1977). Binding of OKT3 to T cells in the irradiated PBMC may therefore have a similar role to that proposed for PHA and EBV-LCL, by stimulating the irradiated PBMC to produce IL-2 and perhaps other growth and/or co stimulatory factors, that enhance NK clone growth (Spits and Yssel, 1996).
The data presented here suggest that SCGM was not only able to support polyclonal NK growth but also efficiently supported NK clone expansion in culture for long periods. Current NK cloning techniques culture NK cells in RPMI or Yssel’s medium, a serum free medium prepared with Iscove’s modified Dulbecco’s medium supplemented with sodium bicarbonate, bovine serum albumin, transferrin, insulin, linoleic, oleic and palmitic acid (Yssel et al., 1984). As NK cloning efficiencies in Yssel’s medium are low (typically ~2%), the use of SCGM for NK cloning thus represents a major advance on current NK cloning techniques (Litwin et al., 1993; Robertson et al., 1993, Wada et al., 2004). The factors responsible for the superiority of SCGM compared to either Yssel’s medium or RPMI for culture of NK clones cannot be revealed here as the formulation of SCGM has not been fully disclosed by the manufacturers. Their data sheets suggest that SCGM does not contain any growth factors, antibiotics or undefined supplements and that it is nutritionally complete, providing a balanced environment for haematopoietic stem and progenitor cells, T cells and NK cells. Like Yssel’s medium SCGM contains albumin, insulin and transferrin, and it is therefore unlikely that any of these factors are responsible for the advantage of SCGM for NK cloning.
Proliferation of NK clones was greatest when stimulated with a combination of allogeneic PBMC feeders from multiple donors, suggesting that factors secreted from a mixed lymphocyte reaction (MLR) are able to stimulate and maintain the growth of NK clones. c-KIT ligand, IL-15 and IL-21 have all been implicated in the maintenance and/or activation of mature NK cells, but they are all growth factors and as such would not have been included in the manufactured SCGM media. However, c-KIT ligand has been shown to be relatively abundant in human serum and binding of c-KIT ligand to c-KIT+ CD56hi cells was shown to upregulate bcl-2 preventing apoptosis, thereby indicating a role for c-KIT ligand in the survival of CD56hi cells (Carson et al., 1994b). Interestingly, c-KIT ligand has also been shown to potentiate cytokine release in MLRs (Langley et al., 1993; Bluman et al., 1996) and this combined with its anti-apoptotic effect on CD56hiCD16+ NK cells may at least partly explain the observed support of NK clone growth in this culture system.
MLRs may also provide IL-15 and IL-21 to the NK cells. The main sources of IL-15 are monocytes and macrophages (Carson et al., 1995; Kennedy and Park, 1996) and binding of IL-15 to components of the IL-2 receptor was shown to activate NK cells in a similar but not identical manner to that of IL-2 (Carson et al., 1994a). Furthermore, a role for IL-15 in the survival of mature NK cells in vivo was demonstrated in adoptive transfer experiments where NK cells transferred to IL-15 deficient mice were rapidly lost (Ranson et al., 2003).
IL-21 is closely related to IL-2 and IL-15 and is expressed exclusively by activated CD4+ T cells. As such, it may also be present in the media as a consequence of stimulation through the OKT3 in our optimised media or a MLR (Lyman et al., 1994; Parrish-Novak et al., 2000). However, the literature suggests a more important role for IL-21 in inducing a maturation program rather than promoting survival of NK cells. Thus, culture of mature CD56+ cells with IL-21 enhanced lytic activity against K562 targets (Parrish-Novak et al., 2000) and triggered the upregulation of cell surface markers associated with activation (Brady et al., 2004), but blocked IL-15 induced expansion of resting NK cells (Kasaian et al., 2002). Irrespective of the potential relative contribution of such cytokines to this NK cloning system, culture in SCGM must provide either additional factors that aid NK proliferation and survival or induce higher levels of these cytokines as MLRs are also present in our comparative RPMI cultures.
In summary, we have described a novel and highly efficient method of NK cloning that uses SCGM media supplemented with IL-2 and a stimulation protocol using irradiated allogeneic PBMC from multiple donors and OKT3. This contrasts to the methods currently in use, which generally stimulate NK cells with a mixture of allogeneic PBMC, EBV-LCL and PHA in Yssel’s media. The considerable increase in NK cloning efficiency described here allows broader investigation of NK responses as this methodology makes feasible the timely generation of hundreds of NK clones (Tomasec et al., 2005). In so doing, it has the potential to revolutionise the functional characterisation of NK cells in both basic and clinical immunological research fields.
Acknowledgements
This work was funded by grants from The Wellcome Trust and Medical Research Council. We are grateful to the Flow Cytometry Facility of the Central Biotechnology Service, Wales College of Medicine, Cardiff University, for provision of cell sorting and flow cytometers for benchtop analysis.
References
- Bluman EM, Schnier GS, Avalos BR, Strout MP, Sultan H, Jacobson FW, Williams DE, Carson WE, Caligiuri MA. The c-kit ligand potentiates the allogeneic mixed lymphocyte reaction. Blood. 1996;88:3887. [PubMed] [Google Scholar]
- Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J. Immunol. 2004;172:2048. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- Braud VM, Allan DS, O’Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- Cantoni C, Bottino C, Augugliaro R, Morelli L, Marcenaro E, Castriconi R, Vitale M, Pende D, Sivori S, Millo R, Biassoni R, Moretta L, Moretta A. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur. J. Immunol. 1999;29:3148. doi: 10.1002/(SICI)1521-4141(199910)29:10<3148::AID-IMMU3148>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Carlens S, Gilljam M, Remberger M, Aschan J, Christensson B, Dilber MS. Ex vivo T lymphocyte expansion for retroviral transduction: influence of serum-free media on variations in cell expansion rates and lymphocyte subset distribution. Exp. Hematol. 2000;28:1137. doi: 10.1016/s0301-472x(00)00526-9. [DOI] [PubMed] [Google Scholar]
- Carlens S, Gilljam M, Chambers BJ, Aschan J, Guven H, Ljunggren HG, Christensson B, Dilber MS. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum. Immunol. 2001;62:1092. doi: 10.1016/s0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- Carr WH, Little AM, Mocarski E, Parham P. NK cell-mediated lysis of autologous HCMV-infected skin fibroblasts is highly variable among NK cell clones and polyclonal NK cell lines. Clin. Immunol. 2002;105:126. doi: 10.1006/clim.2002.5273. [DOI] [PubMed] [Google Scholar]
- Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994;180:1395. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson WE, Haldar S, Baiocchi RA, Croce CM, Caligiuri MA. The c-kit ligand suppresses apoptosis of human natural killer cells through the upregulation of bcl-2. Proc. Natl. Acad. Sci. U. S. A. 1994;91:7553. doi: 10.1073/pnas.91.16.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, Caligiuri MA. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Invest. 1995;96:2578. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 2001;31:3121. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, Wurster AL, Donaldson DD, Collins M, Young DA, Grusby MJ. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Park LS. Characterization of interleukin-15 (IL-15) and the IL-15 receptor complex. J. Clin. Immunol. 1996;16:134. doi: 10.1007/BF01540911. [DOI] [PubMed] [Google Scholar]
- Langley KE, Bennett LG, Wypych J, Yancik SA, Liu XD, Westcott KR, Chang DG, Smith KA, Zsebo KM. Soluble stem cell factor in human serum. Blood. 1993;81:656. [PubMed] [Google Scholar]
- Lanier LL. NK cell receptors. Annu. Rev. Immunol. 1998;16:359. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- Leavitt RD, Felsted RL, Bachur NR. Biological and biochemical properties of Phaseolus vulgaris isolectins. J. Biol. Chem. 1977;252:2961. [PubMed] [Google Scholar]
- Litwin V, Gumperz J, Parham P, Phillips JH, Lanier LL. Specificity of HLA class I antigen recognition by human NK clones: evidence for clonal heterogeneity, protection by self and non-self alleles, and influence of the target cell type. J. Exp. Med. 1993;178:1321. doi: 10.1084/jem.178.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman SD, James L, Johnson L, Brasel K, de Vries P, Escobar SS, Downey H, Splett RR, Beckmann MP, McKenna HJ. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795. [PubMed] [Google Scholar]
- Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7:283. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Human NK-cell receptors. Immunol. Today. 2000;21:420. doi: 10.1016/s0167-5699(00)01673-x. [DOI] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001;19:197. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J. Immunol. 1989;143:3183. [PubMed] [Google Scholar]
- Nagler A, Lanier LL, Phillips JH. Constitutive expression of high affinity interleukin 2 receptors on human CD-16 natural killer cells in vivo. J. Exp. Med. 1990;171:1527. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P. Immunogenetics of killer cell immunoglobulin-like receptors. Mol. Immunol. 2005;42:459. doi: 10.1016/j.molimm.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Manley TJ, Donahue C, Levine H, Ritz J. Costimulatory signals are required for optimal proliferation of human natural killer cells. J. Immunol. 1993;150:1705. [PubMed] [Google Scholar]
- Roncarolo MG, Bigler M, Haanen JB, Yssel H, Bacchetta R, de Vries JE, Spits H. Natural killer cell clones can efficiently process and present protein antigens. J. Immunol. 1991;147:781. [PubMed] [Google Scholar]
- Spits H, Yssel H. Cloning of human T and natural killer cells. Methods. 1996;9:416. doi: 10.1006/meth.1996.0047. [DOI] [PubMed] [Google Scholar]
- Takasugi M, Mickey MR, Terasaki PI. Reactivity of lymphocytes from normal persons on cultured tumor cells. Cancer Res. 1973;33:2898. [PubMed] [Google Scholar]
- Tomasec P, Wang EC, Davison AJ, Vojtesek B, Armstrong M, Griffin C, McSharry BP, Morris RJ, Llewellyn-Lacey S, Rickards C, Nomoto A, Sinzger C, Wilkinson GW. Downregulation of natural killer cell-activating ligand CD155 by human cytomegalovirus UL141. Nat. Immunol. 2005;6:181. doi: 10.1038/ni1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J. Exp. Med. 1978;147:1314. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vales-Gomez M, Reyburn HT, Erskine RA, Lopez-Botet M, Strominger JL. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J. 1999;18:4250. doi: 10.1093/emboj/18.15.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiante NM, Uhrberg M, Shilling HG, Lienert-Weidenbach K, Arnett KL, D’Andrea A, Phillips JH, Lanier LL, Parham P. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- Wada H, Matsumoto N, Maenaka K, Suzuki K, Yamamoto K. The inhibitory NK cell receptor CD94/NKG2A and the activating receptor CD94/NKG2C bind the top of HLA-E through mostly shared but partly distinct sets of HLA-E residues. Eur. J. Immunol. 2004;34:81. doi: 10.1002/eji.200324432. [DOI] [PubMed] [Google Scholar]
- Yssel H, De Vries JE, Koken M, Van Blitterswijk W, Spits H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J. Immunol. Methods. 1984;72:219. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]