Abstract
Background
Fish and seafood n-3 fatty acids may prevent or delay the progression of prostate cancer, but epidemiologic studies do not uniformly support this hypothesis.
Objective
To examine the relation of fish and seafood n-3 fatty acid intakes with prostate cancer incidence and mortality.
Design
We conducted a prospective cohort study among 20,167 men participating in the Physician’s Health Study who were free of cancer in 1983.
Results
During 382,144 person-years of follow-up, 2,161 men were diagnosed with prostate cancer and 230 died of prostate cancer. Fish intake was unrelated to prostate cancer incidence. Survival analysis among the men diagnosed with prostate cancer revealed that those consuming fish ≥5 times/week had a 48% lower risk of prostate cancer death than men consuming fish less than once weekly (RR = 0.52 [0.30–0.91; p, trend = 0.05]). A similar association was found between seafood n-3 fatty acid intake and prostate cancer mortality (RRQ5 vs. Q1 = 0.64 [0.42, 0.99]; p, trend =0.02). These associations became stronger when analyses were restricted to clinically detected cases.
Conclusions
These results suggest that fish intake is unrelated to prostate cancer incidence but may improve prostate cancer survival.
INTRODUCTION
More than 180,000 men in the United States are expected to be newly diagnosed with prostate cancer and 28,660 are expected to die of this disease in 2008 (1). Several aspects of diet may be important in preventing or slowing the progression of prostate cancer (2, 3). In vitro and animal studies suggest that long chain n-3 fatty acids may inhibit prostate cancer growth (4, 5). However, epidemiologic studies examining the association between intake of these fatty acids or fish, their main dietary source (6), and the incidence of prostate cancer are inconsistent (7). In addition, data relating fish or long chain n-3 fatty acid intake to prostate cancer progression or survival are sparse but encouraging (3).
We previously reported an inverse association, in a subsample of this cohort, between whole blood levels of long chain n-3 fatty acids, a biomarker of intake, and prostate cancer risk (8). This association was particularly strong for clinically aggressive tumors, suggesting that these nutrients may either reduce the incidence of lethal disease or improve prostate cancer survival. To follow-up on these findings, we investigated whether fish intake was related to prostate cancer incidence and mortality among all men in the Physician’s Health Study who reported their fish intake in 1983.
SUBJECTS AND METHODS
Study population
This study is based in the Physician’s Health Study (9, 10), a randomized trial of aspirin and beta-carotene in the prevention of heart disease and cancer among 22,071 male physicians, aged 40–84 in 1982. The aspirin component of the trial was terminated early in 1988 due to the benefits of aspirin on myocardial infraction (9). The beta-carotene component of the trial was terminated in 1995 (10). At baseline, participants completed two mailed questionnaires, where they provided information on medical history and several life style factors. Follow-up questionnaires were mailed at 6 and 12 months after randomization and yearly thereafter. Participants were asked to report newly diagnosed diseases of interest, including prostate cancer, in the yearly follow-up questionnaires.
Whenever a participant reported a diagnosis of prostate cancer, we requested hospital records and pathology reports to confirm the diagnosis and determine tumor stage, grade and other clinical characteristics at diagnosis. Histologic grade was recorded as well, moderately, or poorly differentiated tumors, or following the Gleason scoring system, depending on the information available in the pathology reports. Low grade tumors were defined as Gleason <7 or well or moderately differentiated. High grade tumors were defined as Gleason ≥ 7 or poorly differentiated. Stage was recorded according to the modified Whitemore-Jewett classification scheme (11). Localized disease was defined as stages A and B and advanced disease was defined as stages C and D. Cases without pathologic staging were classified as undetermined stage unless there was clinical evidence of distant metastases. Cases were divided according to the clinical presentation as prostate specific antigen (PSA)-screening detected, clinical symptoms, metastatic symptoms, abnormal digital rectal exam (DRE), or other form of presentation using the clinical information available in the medical records. We were notified of deaths by family members and postal authorities, and through periodic systematic searches of the National Death Index. We obtained death certificates and detailed medical records to determine cause of death, which was assigned by the End Point Committee of three physicians. Follow-up for this cohort is more than 99% complete for morbidity and mortality.
Dietary assessment
On the 12 month questionnaire participants completed an abbreviated food frequency questionnaire (FFQ) that did not allow the estimation of total energy intake (TEI). This questionnaire asked about the average intake during the previous year of: 1) canned tuna fish, 2) dark meat fish (e.g. mackerel, salmon, sardines, bluefish, and swordfish), 3) other fish, and 4) shrimp, lobster, or scallops as a main dish. For each food, the questionnaire offered seven options for frequency of intake ranging from rarely/never to two or more times per day. The reproducibility and validity of these questions has not been assessed in this cohort but is available from a similar population of male health professionals (12). As a measure of reproducibility, the correlations between two FFQs completed one year apart were 0.54 for canned tuna fish, 0.63 for dark meat fish, 0.48 for other fish, and 0.67 for shrimp, lobster, and scallops (12). As a measure of validity, the correlations between prospectively collected diet records and intakes from the FFQ were 0.56 for canned tuna fish, 0.42 for dark meat fish, 0.39 for other fish and 0.23 for shrimp, lobster, and scallops (12). As an additional measure of validity we calculated the correlations between fish and seafood n-3 fatty acid intakes and whole blood levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) among 436 members of this cohort who had complete data on fish intake and served as controls in a previous case-control study of prostate cancer (8) (Table 1). These correlations are generally high and higher for types of fish known to have higher EPA and DHA content suggesting reasonable validity of fish and seafood n-3 fatty acid intakes among these men.
Table 1.
Spearman correlation coefficients between whole blood levels of long chain n-3 fatty acids and intakes of fish and n-3 fatty acids from seafood (N = 436).
| Seafood n-3 fatty acid intake |
Total fish intake | Canned tuna intake |
Dark meat fish intake |
Other fish intake | Shrimp, lobster or scallop intake |
|
|---|---|---|---|---|---|---|
| Blood EPA 1 | 0.19 | 0.18 | 0.10 | 0.17 | 0.13 | 0.05 |
| Blood DHA 2 | 0.44 | 0.44 | 0.27 | 0.34 | 0.34 | 0.11 |
| Blood EPA+DHA | 0.38 | 0.38 | 0.23 | 0.31 | 0.29 | 0.10 |
EPA = eicosapentaenoic acid
DHA = docosahexaenoic acid
Intakes of the four fish items were summed to obtain the average daily fish intake. We estimated the average daily intake of n-3 fatty acids from fish by multiplying the n-3 fatty acid content per serving of each item (0.69g for canned tuna fish, 1.37 g for dark meat fish, 0.17g for other fish and 0.46g for shellfish) by the frequency of intake. The n-3 fatty acid content for the specified portion sizes was obtained from the US Department of Agriculture (13). Men were divided into groups according to their intakes of total fish and specific fish intake, as well as into quintiles according to their intake of n-3 fatty acids from fish.
Statistical Analyses
Men who died or reported a cancer diagnosis before the 12 month follow-up questionnaire and men who did not report fish intake in this questionnaire were excluded, leaving 20,167 men for analyses. For the prostate cancer incidence analyses, men were followed from the date of return of the 12 month questionnaire until the date of prostate cancer diagnosis, the date of death or the end of follow-up (March 1, 2006), whichever came first. We also analyzed prostate cancer mortality among the 2,161 men diagnosed with prostate cancer during follow-up. For the mortality analyses, men were followed from the date of prostate cancer diagnosis until the date of death or the end of follow-up, whichever came first.
The relative risks of prostate cancer and death from prostate cancer were estimated using Cox proportional-hazards regression models, using the lowest intake category as the reference group. Tests for linear trend were performed using the median intake values in each category as a continuous variable. Multivariate models included terms for body mass index (BMI), physical activity, smoking status, race, use of multivitamins and vitamin E supplements, random assignement to aspirin or beta-carotene and intakes of dairy foods, meat, alcohol and tomato products. Separate multivariate models for prostate cancer incidence were fit for cases according to grade at diagnosis, stage at diagnosis, lethality, diagnosis before or after the widespread use of PSA for prostate cancer screening (cutoff date: December 31, 1990) and clinical presentation of the case. Multivariate models for death from prostate cancer had additional terms for age at diagnosis, tumor stage and grade at diagnosis and whether or not the tumor was detected through PSA screening. Additional mortality analyses were performed after excluding cases detected by PSA screening and by limiting the analysis to cases detected by an abnormal DRE or clinical manifestations. All analyses were performed in SAS version 9.1 (SAS Institute, Cary, NC). All p-values are two-sided.
RESULTS
We confirmed 2,161 incident cases of prostate cancer diagnosed among 20,167 men followed for 382,144 person-years accrued through 2006. The mean follow-up time was 19 years. At baseline, fish intake was positively related to the intake of tomato products and alcohol, the use of multivitamin and vitamin E supplements and vigorous physical activity, and inversely related to intake of whole milk and meats. Men with a high fish intake were more likely to be Caucasian and less likely to be smokers. Fish intake was unrelated to age at enrollment, height, BMI, intake of reduced fat milk and randomization group (Table 2). Most prostate cancer cases presented as localized (71.6%), low grade (62.3%) disease and were diagnosed during the era of widespread PSA screening (84.3%). PSA screening was the most common presentation mode (61.9%) followed by clinical or metastatic symptoms (19.8%) and abnormal DRE findings (16.9%). The median age at diagnosis was 70 years.
Table 2.
Baseline characteristics by levels of baseline fish intake (N = 20,167).
| < 1/wk | 1/wk | 2 – 4/wk | ≥ 5 wk | P 1 | |
|---|---|---|---|---|---|
| N | 4143 | 5093 | 8711 | 2220 | |
| Fish intake (servings/day) | 0.10 | 0.22 | 0.41 | 0.97 | |
| Age (years) 2 | 53.7 (9.83) | 53.3 (9.37) | 53.6 (9.23) | 53.3 (8.99) | 0.28 |
| Height (m) 2 | 1.78 (0.07) | 1.79 (0.07) | 1.78 (0.07) | 1.78 (0.07) | 0.81 |
| BMI (kg/m2) 2 | 24.7 (2.81) | 24.8 (2.74) | 24.8 (2.77) | 24.7 (2.78) | 0.50 |
| Skim or low fat milk (servings/day) 2 | 0.43 (0.57) | 0.40 (0.53) | 0.42 (0.54) | 0.45 (0.55) | 0.24 |
| Whole milk (servings/day) 2 | 0.21 (0.42) | 0.19 (0.39) | 0.17 (0.36) | 0.14 (0.33) | <0.001 |
| Meat intake (servings/day) 2 | 0.72 (0.47) | 0.73 (0.43) | 0.68 (0.42) | 0.61 (0.48) | <0.001 |
| Tomato and tomato juice (servings/day) 2 | 0.53 (0.44) | 0.56 (0.42) | 0.64 (0.44) | 0.72 (0.49) | <0.001 |
| Alcohol (drinks/day) 2 | 0.42 (0.46) | 0.51 (0.47) | 0.53 (0.45) | 0.51 (0.44) | <0.001 |
| White/Caucasian, % | 82.7 | 84.9 | 86.8 | 85.3 | <0.001 |
| Current smoker, % | 12.4 | 12.1 | 10.3 | 8.1 | <0.001 |
| Current multivitamin use, % | 18.1 | 18.9 | 20.1 | 23.3 | <0.001 |
| Current vitamin E supplement use, % | 4.3 | 4.0 | 5.5 | 7.1 | <0.001 |
| Vigorous exercise twice per week or more, % | 49.7 | 52.0 | 55.5 | 59.9 | <0.001 |
| Randomization group, % | 0.37 | ||||
| Placebo alone | 24.7 | 24.9 | 24.9 | 25.4 | |
| Aspirin alone | 24.5 | 25.1 | 24.8 | 25.9 | |
| Beta-carotene alone | 26.3 | 25.5 | 24.5 | 25.0 | |
| Aspirin and beta-carotene | 24.5 | 24.6 | 25.8 | 23.7 |
For continuous variables, from a linear regression model where fish intake (with the median intake in each category modeled as a continuous variable) was the explanatory variable. For categorical variables, from the Chi-square test.
Values are presented as mean (standard deviation)
Total fish intake was unrelated to prostate cancer risk. Most specific fish types were also unrelated to prostate cancer with the exception of “other” non-specified fish which was positively related to this malignancy (Table 3). Fish intake remained unrelated to prostate cancer risk when this association was examined separately according to different tumor characteristics (stage, grade, lethality, date of diagnosis) (data not shown). The positive association between “other” fish intake and prostate cancer was stronger among cases detected through PSA screening (as reported from medical records) and cases diagnosed after the widespread use of PSA screening for prostate cancer. The multivariate-adjusted relative risk for PSA screening detected prostate cancer comparing men in the highest to the lowest category of “other” fish intake was 1.37 (1.05, 1.79) (p, trend = 0.01). The corresponding relative risk for cases detected during the PSA-screening era was 1.37 (1.15, 1.65) (p, trend < 0.001). The associations of fish and seafood n-3 fatty acid intakes with prostate cancer incidence were not modified by baseline BMI or random assignment to aspirin or beta-carotene in the trial (range p, interaction = 0.27 – 0.75).
Table 3.
Relative risk (95% CI) for prostate cancer by intake of fish and seafood n-3 fatty acids (N = 20,167).
| P, trend 1 | ||||||
|---|---|---|---|---|---|---|
| All fish | < 1/wk | 1/wk | 2 – 4/wk | ≥ 5/wk | ||
| Cases / person-years | 430 / 77,923 | 550 / 96,255 | 926 / 165,970 | 255 / 41,997 | ||
| Age-adjusted | 1.00 (referent) | 1.06 (0.94, 1.21) | 1.01 (0.90, 1.14) | 1.13 (0.97, 1.33) | 0.31 | |
| Multivariate-adjusted 2 | 1.00 (referent) | 1.04 (0.91, 1.18) | 0.98 (0.87, 1.10) | 1.11 (0.95, 1.30) | 0.46 | |
| Canned tuna | < 1/mo | 1 – 3/mo | 1/wk | ≥ 2/wk | ||
| Cases / person-years | 582 / 101,641 | 895 / 157,526 | 469 / 85,609 | 215 / 37,367 | ||
| Age-adjusted | 1.00 (referent) | 1.03 (0.93, 1.15) | 1.03 (0.91, 1.16) | 1.13 (0.97, 1.32) | 0.14 | |
| Multivariate-adjusted 2 | 1.00 (referent) | 1.01 (0.91, 1.12) | 1.00 (0.89, 1.13) | 1.12 (0.95, 1.31) | 0.17 | |
| Dark meat fish | < 1/mo | 1 – 3/mo | ≥ 1/wk | |||
| Cases / person-years | 1004 / 178,306 | 914 / 158,536 | 243 / 45,302 | |||
| Age-adjusted | 1.00 (referent) | 1.01 (0.92, 1.10) | 0.91 (0.79, 1.04) | 0.21 | ||
| Multivariate-adjusted 2 | 1.00 (referent) | 0.99 (0.91, 1.09) | 0.91 (0.79, 1.05) | 0.20 | ||
| Other fish | < 1/mo | 1 – 3/mo | 1/wk | ≥ 2/wk | ||
| Cases / person-years | 257 / 50,507 | 858 / 156,294 | 729 / 129,564 | 317 / 45,780 | ||
| Age-adjusted | 1.00 (referent) | 1.17 (1.02, 1.35) | 1.13 (0.98, 1.30) | 1.37 (1.16, 1.61) | 0.001 | |
| Multivariate-adjusted 2 | 1.00 (referent) | 1.14 (0.99, 1.31) | 1.09 (0.94, 1.26) | 1.33 (1.13, 1.58) | 0.001 | |
| Shrimp, lobster or scallops | < 1/mo | 1 – 3/mo | ≥ 1/wk | |||
| Cases / person-years | 734 / 123,670 | 1181 / 211,118 | 246 / 47,356 | |||
| Age-adjusted | 1.00 (referent) | 1.02 (0.93, 1.12) | 0.94 (0.81, 1.08) | 0.44 | ||
| Multivariate-adjusted 2 | 1.00 (referent) | 1.00 (0.91, 1.09) | 0.93 (0.80, 1.08) | 0.35 | ||
| Seafood n-3 fatty acids | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Cases / person-years | 424 / 77,907 | 438 / 75,469 | 446 / 76,049 | 407 / 75,986 | 446 / 76,734 | |
| Age-adjusted | 1.00 (referent) | 1.10 (0.96, 1.25) | 1.09 (0.95, 1.24) | 0.98 (0.86, 1.13) | 1.11 (0.97, 1.26) | 0.45 |
| Multivariate-adjusted 2 | 1.00 (referent) | 1.07 (0.93, 1.22) | 1.05 (0.92, 1.21) | 0.95 (0.83, 1.09) | 1.09 (0.95, 1.25) | 0.55 |
Calculated in a separate regression model with the median intake in each category as a continuous variable
Adjusted for age, BMI, physical activity, intakes of alcohol, tomato products, dairy products and meat, smoking, race, use of multivitamins, use of vitamin E supplements and random assignment to aspirin or beta-carotene.
Next, we examined the association between baseline fish intake and prostate cancer mortality among the 2,161 men diagnosed with prostate cancer. Baseline fish intake was inversely related to prostate cancer death (Table 4). The association was similar for most types of fish and for n-3 fatty acids from seafood, apart from the category of shrimp, scallops and lobster and was not modified by BMI or assignment to aspirin or beta-carotene (range p, interaction = 0.36 – 0.99)
Table 4.
Relative risk (95% CI) for prostate cancer death by fish and seafood n-3 fatty acid intakes (N = 2,161, 230 deaths).
| P, trend 1 | ||||||
|---|---|---|---|---|---|---|
| All fish | < 1/wk | 1/wk | 2 – 4/wk | ≥ 5/wk | ||
| Deaths / person-years | 54 / 3,264 | 59 / 4,332 | 100 / 7,127 | 17 / 2,056 | ||
| Age-adjusted | 1.00 (referent) | 0.86 (0.59, 1.24) | 0.89 (0.64, 1.23) | 0.53 (0.31, 0.92) | 0.05 | |
| Multivariate-adjusted 2 | 1.00 (referent) | 0.73 (0.50, 1.08) | 0.76 (0.54, 1.08) | 0.52 (0.30, 0.91) | 0.05 | |
| Canned tuna | < 1/mo | 1 – 3/mo | 1/wk | ≥ 2/wk | ||
| Deaths / person-years | 78 / 4,400 | 91 / 7,198 | 45 / 3,620 | 16 / 1,561 | ||
| Age-adjusted | 1.00 (referent) | 0.74 (0.55, 1.00) | 0.75 (0.52, 1.08) | 0.62 (0.36, 1.06) | 0.11 | |
| Multivariate-adjusted 2 | 1.00 (referent) | 0.65 (0.47, 0.89) | 0.85 (0.58, 1.25) | 0.57 (0.33, 0.99) | 0.13 | |
| Dark meat fish | < 1/mo | 1 – 3/mo | ≥ 1/wk | |||
| Deaths / person-years | 109 / 7,839 | 102 / 7,022 | 19 / 1,918 | |||
| Age-adjusted | 1.00 (referent) | 1.04 (0.80, 1.37) | 0.70 (0.43, 1.14) | 0.23 | ||
| Multivariate-adjusted 2 | 1.00 (referent) | 0.76 (0.57, 1.02) | 0.64 (0.39, 1.04) | 0.04 | ||
| Other fish | < 1/mo | 1 – 3/mo | 1/wk | ≥ 2/wk | ||
| Deaths / person-years | 40 / 2,002 | 83 / 6,521 | 81 / 5,682 | 26 / 2,574 | ||
| Age-adjusted | 1.00 (referent) | 0.67 (0.46, 0.98) | 0.75 (0.51, 1.09) | 0.55 (0.34, 0.90) | 0.09 | |
| Multivariate-adjusted 2 | 1.00 (referent) | 0.66 (0.45, 0.98) | 0.70 (0.47, 1.03) | 0.58 (0.35, 0.97) | 0.17 | |
| Shrimp, lobster or scallops | < 1/mo | 1 – 3/mo | ≥ 1/wk | |||
| Deaths / person-years | 84 / 5,832 | 112 / 9,020 | 34 / 1,926 | |||
| Age-adjusted | 1.00 (referent) | 0.88 (0.67, 1.17) | 1.30 (0.87, 1.94) | 0.27 | ||
| Multivariate-adjusted 2 | 1.00 (referent) | 0.85 (0.63, 1.15) | 1.10 (0.72, 1.67) | 0.73 | ||
| Seafood n-3 fatty acids | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Deaths / person-years | 50 / 3,291 | 53 / 3,443 | 54 / 3,386 | 37 / 3,210 | 36 / 3,448 | |
| Age-adjusted | 1.00 (referent) | 1.02 (0.69, 1.50) | 1.03 (0.70, 1.52) | 0.78 (0.51, 1.20) | 0.70 (0.46, 1.08) | 0.04 |
| Multivariate-adjusted 2 | 1.00 (referent) | 0.98 (0.66, 1.46) | 0.78 (0.52, 1.17) | 0.65 (0.41, 1.02) | 0.65 (0.42, 0.99) | 0.02 |
Calculated in a separate regression model with the median intake in each category as a continuous variable
Adjusted for age at prostate cancer diagnosis, BMI, physical activity, intakes of alcohol, tomato products and dairy products, smoking, race, use of multivitamins, use of vitamin E supplements, random assignment to aspirin or beta-carotene, tumor stage and grade at diagnosis, and clinical presentation of case.
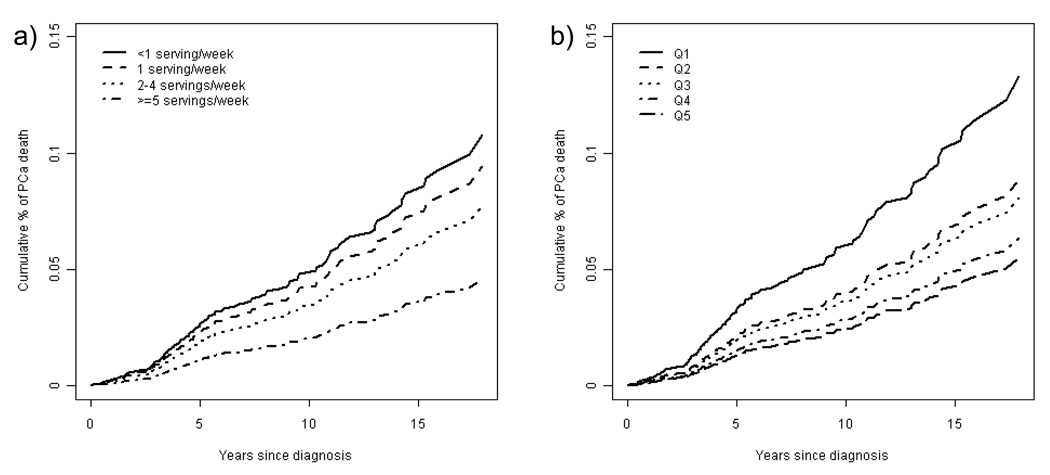
We considered the possibility that these inverse associations were due to early detection or treatment of cases detected through increased PSA screening among health-conscious men who ate more fish. We therefore repeated the analyses after excluding all PSA-detected cases and by limiting the analyses to cases presenting by clinical symptoms or abnormal DRE. In the analyses excluding PSA-detected cases, intakes of fish and n-3 fatty acids from seafood remained strongly inversely related to prostate cancer mortality. The multivariate-adjusted prostate cancer mortality ratios for increasing intake of total fish were 0.82 (0.54, 1.24) for once a week, 0.85 (0.57, 1.25) for 2 to 4 times per week and 0.51 (0.27, 0.95) for 5 or more times weekly, as compared to men consuming fish less than once a week (p, trend = 0.08). The corresponding mortality ratios for increasing quintiles of n-3 fatty acid intake were 0.88 (0.57, 1.36), 0.83 (0.54, 1.27), 0.73 (0.44, 1.18) and 0.55 (0.34, 0.91), compared to men in the lowest quintile of intake (p, trend = 0.01). These associations became stronger when analyses were restricted to men presenting with clinical symptoms or abnormal DRE (Figure 1). The multivariate-adjusted prostate cancer mortality ratio was 0.39 (95% CI 0.17, 0.88; p, trend, 0.01) comparing men consuming fish 5 or more times weekly to men consuming fish less than once a week, and 0.39 (0.21, 0.71; p, trend, 0.004) comparing top to bottom quintile of n-3 fatty acid intake from seafood.
Figure 1.
Multivariate-adjusted 1 cumulative prostate cancer mortality rates by a) fish and b) seafood n-3 fatty acid intake among DRE or clinically detected prostate cancer cases (N = 478, 122 deaths).
1 Adjusted for age at prostate cancer diagnosis, BMI, physical activity, intakes of alcohol, tomato products and dairy products, smoking, race, use of multivitamins, use of vitamin E supplements, random assignment to aspirin or beta-carotene and tumor stage and grade at diagnosis.
DISCUSSION
In this prospective study we found that fish intake was unrelated to prostate cancer incidence. Further we found that pre-diagnostic fish intake was inversely related to prostate cancer mortality. This inverse association between fish intake and prostate cancer mortality did not appear to be the result of earlier detection or treatment of PSA detected cases and was not changed after accounting for potential confounding factors.
Multiple animal and in vitro studies have shown that long chain n-3 fatty acids inhibit prostate cancer growth (4, 5, 14, 15). These findings suggest that a higher intake of fish, where these fatty acids are particularly concentrated (6), might have a role in the primary prevention of prostate cancer. However, most epidemiological studies have not found an association between fish or long chain n-3 fatty acid intake and prostate cancer risk (16–30). These null findings include most prospective cohort studies (16–18, 24–26, 30), most case control studies (19–23, 27–29) as well as most studies with questionnaire based diet assessment (16–18, 23–25, 28, 30) and biomarker assessment of fatty acids (19, 21, 22, 26, 27, 29). In contrast to our previous report (8), these findings do not support the hypothesis that fish or long chain n-3 fatty acid intake decreases prostate cancer incidence and are consistent with the results of the majority of epidemiologic studies.
We found a positive association between intake of “other” non-specified fish, and prostate cancer risk that was restricted to PSA-detected tumors. It is likely that the observed association does not represent a deleterious effect of fish but is rather a spurious association arising from higher prostate cancer detection through PSA screening among health-conscious individuals. Fish intake was related to several healthy behaviors in this cohort. Although we did not collect data on PSA screening habits in the entire cohort, others have reported that PSA screening is more common among men who have other health-conscious behaviors (31, 32). Because PSA screening markedly increases detection of prostate cancer, not accounting for it will likely overestimate the association between “healthy” lifestyle habits, such as fish consumption, and prostate cancer risk. This possibility is consistent with our results.
Higher intakes of fish, particularly dark meat fish, and seafood n-3 fatty acids were related to lower prostate cancer mortality among the men diagnosed with prostate cancer. This association did not appear to be the result of earlier diagnosis or treatment of PSA detected cases as it became stronger when these men were excluded from the analyses and when the analyses were restricted to cases known to be detected by abnormal DRE or clinical symptoms, suggesting that fish intake itself could be beneficial in delaying tumor progression. Few epidemiologic studies have examined whether fish or long chain n-3 fatty acid intake influence prostate cancer progression or mortality. Chan and collaborators (3) found that fish intake after prostate cancer diagnosis was inversely related to a composite outcome of prostate cancer progression, recurrence or death. Also, Freeman and colleagues (33) found that long chain n-3 fatty acid levels in non-cancerous prostate tissue of men undergoing radical prostatectomy for clinically localized prostate cancer were lower among men who later experienced biochemical recurrence of the disease compared to those without recurrence. In a recent small pilot randomized trial, men assigned to a study diet which emphasized, among other changes, increased intake of n-3 fatty acid rich fish, had an increase in PSA doubling time (34). Similarly, the studies that have previously reported fish or long chain n-3 fatty acid intake to decrease prostate cancer risk, including our previous work (8), have generally reported stronger associations with advanced stage (35–37), clinically aggressive (8), metastatic (38) or lethal disease (39), suggesting that these dietary factors may have a role in reducing disease progression or mortality.
Laboratory data also suggest a role of marine n-3 fatty acids in reducing prostate cancer progression and mortality. EPA, and to a greater extent its 15-LOX metabolite 15-HEPE, suppress proliferation of multiple prostate cancer lines and the generation of COX-2 and 5-LOX metabolites of arachidonic acid (40) known to increase proliferation, tumor cell survival and angiogenesis (41–44). Moreover, in a mouse model simulating prostate cancer recurrence after radical prostatectomy, mice fed an EPA precursor had reduced tumor recurrence, increased PSA doubling time as well as decreased proliferation and increased apoptosis in recurrent tumor cells (14) Likewise, mice fed EPA in a model of hormone ablation therapy showed improved response to therapy (higher tumor apoptosis/mitosis ratios) and decreased progression into androgen independence (45).
Our study has several limitations. First, we have only a single assessment of fish intake at baseline. Although this certainly misclassifies the true exposure information over time, the effect of this misclassification would be to attenuate the true association between fish intake and our outcomes of interest. Second, the dietary information collected was insufficient to estimate total energy intake and thus we were unable to adjust our models for energy intake. However, all our models were adjusted for body size (expressed as BMI), one of the primary determinants of TEI, and for physical activity, the most important determinant of between-person variation in TEI (46). These adjustments may indirectly account for TEI to some extent. In a similar cohort of male health professionals, the correlations of fish and seafood n-3 fatty acids with TEI are low (0.16 and 0.17, respectively) compared to other nutrients (47), and the n-3 fatty acids were not important sources of energy (0.14% of calories (range 0 – 2.96%)), further suggesting that the inability to control for TEI may not have been an important source of bias, as previous analyses of dietary factors in this cohort also suggest (48). Third, we did not collect information on screening practices among the entire cohort. However, we had data available on the clinical presentation of cases. Lastly, because all the cohort members are physicians some results from this study may not be directly generalizable. However, it is unlikely that the underlying biology of prostate cancer differs between physicians and non-physicians as previous findings from this and other cohorts of health professionals have been replicated in other populations (48–51). Strengths of this study include its prospective design and high follow-up rates for morbidity and mortality. Also, the large number of cases allowed us to examine the associations separately according to several tumor characteristics and to examine the relation of fish and long chain n-3 fatty acid intake with prostate cancer mortality.
In summary, our findings suggest that fish intake may not affect the risk of developing prostate cancer, in agreement with the majority of epidemiological evidence to date. In addition, our survival data suggests that fish intake may reduce prostate cancer mortality.
ACKNOWLEDGEMENTS
JEC, MJS and JM were responsible for the study concept and design. JEC analyzed the data and drafted the manuscript. JM obtained funding. MJS, HDS and JM contributed to the collection and assembly of data. All the authors critically reviewed the manuscript and provided important intellectual content and approved the final version of the manuscript. None of the authors has personal or financial conflicts of interest. The authors thank Dr. Weiliang Qiu for his assistance preparing the cumulative mortality figures.
Financial support: The work reported in this manuscript was supported by the research grants CA42182, CA58684, CA90598 and the Yerby Postdoctoral Fellowship Program.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures 2008. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 2.Chan JM, Gann PH, Giovannucci EL. Role of Diet in Prostate Cancer Development and Progression. J Clin Oncol. 2005;23:8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 3.Chan JM, Holick C, Leitzmann M, et al. Diet After Diagnosis and the Risk of Prostate Cancer Progression, Recurrence, and Death (United States) Cancer Causes and Control. 2006;17:199–208. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 4.Connolly JM, Coleman M, Rose DP. Effects of dietary fatty acids on DU145 human prostate cancer cell growth in athymic nude mice. Nutr Cancer. 1997;29:114–119. doi: 10.1080/01635589709514611. [DOI] [PubMed] [Google Scholar]
- 5.Chung BH, Mitchell SH, Zhang J-S, Young CYF. Effects of docosahexaenoic acid and eicosapentaenoic acid on androgen-mediated cell growth and gene expression in LNCaP prostate cancer cells. Carcinogenesis. 2001;22:1201–1206. doi: 10.1093/carcin/22.8.1201. [DOI] [PubMed] [Google Scholar]
- 6.Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, PR H. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids. 2003;38:391–398. doi: 10.1007/s11745-003-1074-0. [DOI] [PubMed] [Google Scholar]
- 7.Astorg P. Dietary n - 6 and n - 3 Polyunsaturated Fatty Acids and Prostate Cancer Risk: A Review of Epidemiological and Experimental Evidence. Cancer Causes and Control. 2004;15:367–386. doi: 10.1023/B:CACO.0000027498.94238.a3. [DOI] [PubMed] [Google Scholar]
- 8.Chavarro JE, Stampfer MJ, Li H, Campos H, Kurth T, Ma J. A Prospective Study of Polyunsaturated Fatty Acid Levels in Blood and Prostate Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1364–1370. doi: 10.1158/1055-9965.EPI-06-1033. [DOI] [PubMed] [Google Scholar]
- 9.Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 10.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 11.Catalona WJ, Avioli LV. Diagnosis, staging, and surgical treatment of prostatic carcinoma [clinical conference] Arch Intern Med. 1987;147:361–363. [PubMed] [Google Scholar]
- 12.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 13.United States Department of Agriculture, Agriculural Research Service. USDA Nutrient Database for Standard Reference, Release 19. 2006. [Google Scholar]
- 14.Kelavkar UP, Hutzley J, Dhir R, Kim P, Allen KGD, McHugh K. Prostate Tumor Growth and Recurrence Can Be Modulated by the −6:-3 Ratio in Diet: Athymic Mouse Xenograft Model Simulating Radical Prostatectomy. Neoplasia. 2006;8:112–124. doi: 10.1593/neo.05637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose DP, Connolly JM. Effects of fatty acids and eicosanoid synthesis inhibitors on the growth of two human prostate cancer cell lines. Prostate. 1991;18:243–254. doi: 10.1002/pros.2990180306. [DOI] [PubMed] [Google Scholar]
- 16.Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in adventist men. Cancer. 1989;64:598–604. doi: 10.1002/1097-0142(19890801)64:3<598::aid-cncr2820640306>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Severson RK, Nomura AMY, Grove JS, Stemmermann GN. A Prospective Study of Demographics, Diet, and Prostate Cancer among Men of Japanese Ancestry in Hawaii. Cancer Res. 1989;49:1857–1860. [PubMed] [Google Scholar]
- 18.Hsing AW, McLaughlin JK, Schuman LM, et al. Diet, Tobacco Use, and Fatal Prostate Cancer: Results from the Lutheran Brotherhood Cohort Study. Cancer Res. 1990;50:6836–6840. [PubMed] [Google Scholar]
- 19.Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci E, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86:281–286. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- 20.Andersson S, Baron J, Wolk A, Lindgren C, Bergstrom R, Adami H. Early life risk factors for prostate cancer: a population-based case-control study in Sweden. Cancer Epidemiol Biomarkers Prev. 1995;4:187–192. [PubMed] [Google Scholar]
- 21.Godley P, Campbell M, Gallagher P, Martinson F, Mohler J, Sandler R. Biomarkers of essential fatty acid consumption and risk of prostatic carcinoma. Cancer Epidemiol Biomarkers Prev. 1996;5:889–895. [PubMed] [Google Scholar]
- 22.Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997;71:545–551. doi: 10.1002/(sici)1097-0215(19970516)71:4<545::aid-ijc7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Deneo-Pellegrini H, De Stefani E, Ronco A, Mendilaharsu M. Foods, nutrients and prostate cancer: a case-control study in Uruguay. Br J Cancer. 1999;80:591–597. doi: 10.1038/sj.bjc.6690396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuurman AG, van den Brandt PA, Dorant E, Brants HAM, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk. Cancer. 1999;86:1019–1027. [PubMed] [Google Scholar]
- 25.Schuurman AG, van den Brandt PA, Dorant E, Goldbohm RA. Animal products, calcium and protein and prostate cancer risk in The Netherlands Cohort Study. Br J Cancer. 1999;80:1107–1113. doi: 10.1038/sj.bjc.6690472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laaksonen DE, Laukkanen JA, Niskanen L, et al. Serum linoleic and total polyunsaturated fatty acids in relation to prostate and other cancers: a population-based cohort study. Int J Cancer. 2004;111:444–450. doi: 10.1002/ijc.11614. [DOI] [PubMed] [Google Scholar]
- 27.Newcomer LM, King IB, Wicklund KG, Stanford JL. The association of fatty acids with prostate cancer risk. Prostate. 2001;47:262–268. doi: 10.1002/pros.1070. [DOI] [PubMed] [Google Scholar]
- 28.Kristal AR, Cohen JH, Qu P, Stanford JL. Associations of Energy, Fat, Calcium, and Vitamin D with Prostate Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2002;11:719–725. [PubMed] [Google Scholar]
- 29.Mannisto S, Pietinen P, Virtanen MJ, et al. Fatty Acids and Risk of Prostate Cancer in a Nested Case-Control Study in Male Smokers. Cancer Epidemiol Biomarkers Prev. 2003;12:1422–1428. [PubMed] [Google Scholar]
- 30.Crowe FL, Key TJ, Appleby PN, et al. Dietary fat intake and risk of prostate cancer in the Eurpean Prospective investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;87:1405–1413. doi: 10.1093/ajcn/87.5.1405. [DOI] [PubMed] [Google Scholar]
- 31.Littman A, Kristal A, White E. Recreational Physical Activity and Prostate Cancer Risk (United States) Cancer Causes and Control. 2006;17:831–841. doi: 10.1007/s10552-006-0024-8. [DOI] [PubMed] [Google Scholar]
- 32.Close D, Kristal A, Li S, Patterson R, White E. Associations of demographic and health-related characteristics with prostate cancer screening in Washington State. Cancer Epidemiol Biomarkers Prev. 1998;7:627–630. [PubMed] [Google Scholar]
- 33.Freeman V, Flanigan R, Meydani M. Prostatic fatty acids and cancer recurrence after radical prostatectomy for early-stage prostate cancer. Cancer Causes and Control. 2007;18:211–218. doi: 10.1007/s10552-006-0095-6. [DOI] [PubMed] [Google Scholar]
- 34.Carmody J, Olenedzki B, Reed G, Andersen V, Rosenzweig P. A dietary intervention for recurrent prostate cancer after definitive primary treatment: results of a randomized pilot study. Urology. 2008 Apr 7; doi: 10.1016/j.urology.2008.01.015. [Epub ahead of print] doi:10.1016/j.urology.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238–1242. doi: 10.1038/sj.bjc.6690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leitzmann MF, Stampfer MJ, Michaud DS, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 37.Freeman VL, Meydani M, Hur K, Flanigan RC. Inverse association between prostatic polyunsaturated fatty acid and risk of locally advanced prostate carcinoma. Cancer. 2004;101:2744–2754. doi: 10.1002/cncr.20676. [DOI] [PubMed] [Google Scholar]
- 38.Augustsson K, Michaud DS, Rimm EB, et al. A Prospective Study of Intake of Fish and Marine Fatty Acids and Prostate Cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:64–67. [PubMed] [Google Scholar]
- 39.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–1766. doi: 10.1016/S0140-6736(00)04889-3. [DOI] [PubMed] [Google Scholar]
- 40.Vang K, Ziboh VA. 15-lipoxygenase metabolites of [gamma]-linolenic acid/eicosapentaenoic acid suppress growth and arachidonic acid metabolism in human prostatic adenocarcinoma cells: Possible implications of dietary fatty acids. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;72:363–372. doi: 10.1016/j.plefa.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 42.Sundaram S, Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: Critical role in survival. Biochem Biophys Res Commun. 2006;339:93–98. doi: 10.1016/j.bbrc.2005.10.189. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh J, Myers CE. Arachidonic Acid Stimulates Prostate Cancer Cell Growth: Critical Role of 5-Lipoxygenase. Biochemical and Biophysical Research Communications. 1997;235:418–423. doi: 10.1006/bbrc.1997.6799. [DOI] [PubMed] [Google Scholar]
- 45.McEntee MF, Ziegler C, Reel D, et al. Dietary n-3 polyunsaturated fatty acids enhance hormone ablation theraphy in androgen-dependent prostate cancer. Am J Pathol. 2008;173:229–241. doi: 10.2353/ajpath.2008.070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willett WC, Stampfer MJ. Chapter 11: Implications of total energy intake for epidemiologic analyses. In: Willett WC, editor. Nutritional Epidemiology. Second Edition. New York: Oxford University Press; 1998. pp. 273–301. [Google Scholar]
- 47.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of a expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 48.Chan JM, Stampfer MJ, Ma J, Gann PH, Gaziano JM, Giovannucci EL. Dairy products, calcium, and prostate cancer risk in the Physicians' Health Study. Am J Clin Nutr. 2001;74:549–554. doi: 10.1093/ajcn/74.4.549. [DOI] [PubMed] [Google Scholar]
- 49.Mitrou PN, Albanes D, Weinstein SJ, et al. A prospective study of dietary calcium, dairy products and prostate cancer risk (Finland) International Journal of Cancer. 2007;120:2466–2473. doi: 10.1002/ijc.22553. [DOI] [PubMed] [Google Scholar]
- 50.Rohrmann S, Platz E, Kavanaugh C, Thuita L, Hoffman S, Helzlsouer K. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes and Control. 2007;18:41–50. doi: 10.1007/s10552-006-0082-y. [DOI] [PubMed] [Google Scholar]
- 51.Giovannucci E, Liu Y, Stampfer MJ, Willett WC. A Prospective Study of Calcium Intake and Incident and Fatal Prostate Cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:203–210. doi: 10.1158/1055-9965.EPI-05-0586. [DOI] [PubMed] [Google Scholar]