Abstract
Grafting oligodendrocyte precursor cells (OPCs) has been used as a strategy to repair demyelination of the central nervous system (CNS). Whether OPCs can promote CNS axonal regeneration remains to be tested. If so, they should be permissive to axonal growth and may express less inhibitory molecules on their surface. Here we examined the expression of two oligodendrocyte-associated myelin inhibitors Nogo-A and myelin-associated glycoprotein (MAG) during oligodendrogliogenesis and tested their abilities to promote neurite outgrowth in vitro. Whereas the intracellular domain of Nogo-A was consistently expressed throughout oligodendrocyte differentiation, MAG was expressed only at later stages. Furthermore, the membrane-associated extracellular domain of Nogo-A was not expressed in OPCs but expressed in mature oligodendrocytes. In a dorsal root ganglion (DRG) and OPC/oligodendrocyte co-culture model, significantly greater DRG neurite outgrowth onto OPC monolayer than mature oligodendrocyte was found (1042 ± 123 vs. 717 ± 342 micrometer; p = 0.011). Moreover, DRG neurites elongated as fasciculated fiber tracts and contacted directly on OPCs (133 ± 37 cells/fascicle). In contrast, few, if any, direct contacts were found between DRG neurites and mature oligodendrocytes (5 ± 3 cells/fascicle, p<0.001). In fact, acellular spaces were found between neurites and surrounding mature oligodendrocytes in contrast to the lack of such spaces in OPC/DRG coculture (51.1 ± 16.5 vs. 2.4 ± 3.9 micrometer; p<0.001). Thus, OPCs expressing neither extracellular domain of Nogo-A nor MAG are significantly more permissive than mature oligodendrocytes expressing both. Grafting OPCs may thus represent a feasible strategy to foster CNS axonal regeneration.
Keywords: Myelin inhibitors, MAG, Neurite outgrowth, Nogo-A, OPC
Introduction
Injury to the adult mammalian central nervous system (CNS) is devastating partly due to the inability of injured central axons to regenerate and to rebuild their functional connections. Neural cell transplantation has provided a great potential for investigating therapeutic strategies for human CNS injuries. Previously, we demonstrated that Schwann cells, a type of peripheral myelin-forming cells, combined with neurotrophins, supported axonal growth across and beyond a lesion gap in the injured spinal cord (Xu et al., 1997; Bamber et al., 2001). Although Schwann cells are strong promoters of axonal regeneration and have many unique growth-promoting properties (Chau et al., 2004; Oudega and Xu, 2006), they do not normally reside in the CNS, form only one myelin segment on a single axon, and produce space-filling basal lamina and extracellular matrix molecules (ECMs). These factors making Schwann cells less efficiency in tissue repair than their CNS counterparts, the oligodendrocytes.
Given their efficiency and environmental compatibility, oligodendrocytes are theoretically more appropriate donor cells for CNS transplantation than Schwann cells. Impressively, a single oligodendrocyte can myelinate up to 50 axonal segments (Peters et al., 1991; Baumann and Pham-Dinh, 2001). However, ample evidence suggests that mature oligodendrocytes and components of the CNS myelin are non-permissive substrates for neurite outgrowth in vitro (Schwab and Caroni, 1988) and axonal regeneration in vivo (Schnell and Schwab, 1990). To date, three major inhibitory components have been identified in the CNS myelin: myelin-associated glycoprotein (MAG) (McKerracher et al., 1994; Mukhopadhyay et al., 1994), Nogo-A (Chen et al., 2000; GrandPre et al., 2000; Prinjha et al., 2000), and oligodendrocyte myelin glycoprotein (OMgp) (Kottis et al., 2002; Wang et al., 2002b). Interestingly, each of these three myelin components acts through a common receptor complex consisting of the Nogo-66 receptor (NgR) (Fournier et al., 2001; Domeniconi et al., 2002; Liu et al., 2002; Wang et al., 2002b), a GPI-anchored molecule that binds each of the inhibitors, and p75, a transmembrane signal transducer previously shown to be a low-affinity receptor for the neurotrophins (Wang et al., 2002a; Wong et al., 2002).
Although mature oligodendrocytes and the CNS myelin are inhibitory to regeneration, their progenitor cells may not. During development, oligodendrocytes originated from oligodendrocyte progenitor cells (OPCs) or glial-restricted precursor cells (GRPs) (Baumann and Pham-Dinh, 2001). In the mouse spinal cord, the early OPCs originate from the pMN domain (motor neuron progenitor domain) occurring as early as embryonic day (E) 12.5, migrate out from the ventral ventricular zone, and proliferate to generate more progenitors during migration (Lu et al., 2002). Recent studies also showed a dorsal origin of OPCs from progenitor domains dp3–5 of the mouse neural tube at E14.5 (Cai et al., 2005). This indicates that the timing of OPC generation, migration and distribution in the spinal white matter occurs much earlier than the growth of many long fiber tracts into the spinal cord. For example, the growth of the corticospinal tract (CST) into the mouse spinal cord occurs postnatally with the leading axons arriving at the caudal cervical cord at postnatal day (P) 2 and lumbar cord at P9 (Gianino et al., 1999). This implies that, during development, CST axons must navigate through a white matter region within which the OPCs have already populated (Qi et al., 2001). It also implies that, during the course of CST axonal growth, OPCs are less inhibitory than their adult counterparts. If so, the OPCs at early developmental stages may express less or no myelin inhibitors. Whether myelin inhibitors are expressed at early stages of oligodendrocyte development and whether cells at these stages have different effects on neurite outgrowth remain largely unknown.
In the present study, we sought to determine whether OPCs expressed myelin-associated inhibitors, specifically Nogo-A and MAG, during their in vitro development, and, if so, whether their expression (or lack of expression) had any effect on neurite outgrowth.
Materials and methods
Embryonic OPC isolation
OPCs were immunopanned from the embryonic day (E) 15 rat spinal cords (Sprague-Dawley; Charles River Laboratories, Inc., Wilmington, MA) using an A2B5 antibody according to a protocol described previously (Mayer-Proschel et al.,1997; Mujtaba et al.,1999; Cao et al., 2005). Briefly, E15 embryos were removed from their mothers under anesthesia and placed in dishes containing L15 (Invitrogen, Carlsbad, CA). Spinal cords were isolated, their meninges removed, and tissues incubated in HBSS-containing 0.05% trypsin/EDTA (Invitrogen) for 30 min at 4 °C. The cells were dissociated in single-cell suspension by trituration through a fire-polished Pasteur pipette and seeded into a Petri-dish to deplete astrocytes and meningeal cells followed by incubation on an A2B5- (IgM; American Type Culture Collection, ATCC, Rockville, MA) coated Petri-dish to select A2B5-positive cells. After A2B5 immunopanning at room temperature (RT) for 1 h, the binding cells were dislodged by a cell lifter (Corning Inc., Stone Mountain, GA) and seeded into a poly-L-lysine (PLL, 20 µg/ml)/laminin (20 µg/ml, Sigma, St Louis, MO) double-coated tissue culture dish (Becton Dickinson, Franklin Lakes, NJ) containing OPC-growth medium (described below). Sequential immunopanning resulted in OPC purity >94% from the E15 spinal cord.
Proliferation and in vitro differentiation of embryonic OPCs
Dissociated OPCs were allowed to proliferate in the growth medium (defined as OPC-growth medium) consisting of 1× Dulbecco's Modified Eagle's Medium/F12 (DMEM/F12, Invitrogen) supplemented with N2 and B27 supplements (each at 1×, Invitrogen), fibroblast growth factor 2 (FGF-2) (20 ng/ml, Invitrogen), and platelet-derived growth factor aa (PDGF-aa) (10 ng/ml, Sigma). The medium was changed every other day. After 5–7 days, the cells were passaged at 70% confluence by incubation in accutase (1×, Innovative Cell Technologies, Inc., San Diego, CA). The resuspended cells were replated at a density of 6× 105 cells/100 mm dish.
To induce OPCs to differentiate in vitro, the FGF-2 and PDGF-aa were removed and thyroid hormone [tri-iodothyronine (T3), 30 ng/ml, Sigma] was added to the OPC-growth medium. After the T3 introduction, A2B5-positive OPCs were induced to differentiate in vitro for 2, 4, 6, and 8 days respectively and were either lysed for Western blot or fixed for immunocytochemical analysis.
Immunocytochemistry
During the course of OPC differentiation, cells sequentially express A2B5, platelet-derived growth factor receptor-α (PDGFRα), NG2, O4, Gal-C and myelin basic protein (MBP) (Levine et al., 2001). To detect the development from OPCs to mature oligodendrocytes in vitro, the following antibodies were used: monoclonal anti-A2B5, anti-O4, anti-O1 IgM (all from ATCC, Rockville, MA, used as undiluted hybridoma supernatants), anti-receptor-interacting protein IgG (RIP, 1:20, DSHB, Iowa City, IA) and monoclonal anti-MBP antibody (1:10, Chemicon, Temecula, CA). Monoclonal anti-GFAP antibody (1:400, Sigma) was used to detect OPC-derived astrocytes. To detect at which developmental stage the two myelin inhibitors Nogo-A and MAG expressed, immunofluorescence double-labeling was performed using polyclonal anti-Nogo A (against intracellular domain; 1:200, Santa Cruz Biotechnol., Santa Cruz, CA), polyclonal anti-Nogo-A (against extracellular domain; 1:50, Chemicon, Temecula, CA) and anti-MAG (1:100, Zymed Lab., South San Francisco, CA) with the above-mentioned cell specific markers.
For live labeling with antibodies against A2B5, O4, O1 and Nogo-A extracellular domains, cells were incubated for 40 min at 4 °C with the respective hybridoma supernatants, rinsed and then fixed with 4% paraformaldehyde (PFA, Sigma). After blocking with 10% heat-inactivated donkey serum-0.3% Triton X-100/PBS, cells were then incubated with anti-Nogo-A or anti-MAG antibodies diluted in blocking solution at 4 °C overnight. The RIP, MBP, and GFAP immunolabeling was performed similarly on fixed cells without the live labeling steps. The primary antibodies were detected by secondary antibodies coupled to donkey anti-mouse Texas Red-conjugated IgM (1:200, Jackson ImmunoResearch Lab, Baltimore, MD), donkey anti-mouse FITC-conjugated IgM (1:200, Jackson ImmunoResearch Lab) or donkey anti-mouse Texas Red-conjugated IgG (1:200, Jackson ImmunoResearch Lab), combined with donkey anti-rabbit Cyκ™2-conjugated IgG (1:200, Jackson ImmunoResearch Lab) or donkey anti-rabbit Texas Red-conjugated IgG (1:200, Jackson ImmunoResearch Lab). Mouse and rabbit isotype controls (Zymed Lab) were used to confirm the specificity of the immunofluorescence labeling. The cultures were mounted with Gel/Mount aqueous mounting media (Biomeda Corp., Foster City, CA) containing Hoechst 33342, a nuclear dye (0.5 µM; Sigma). An Olympus BX60 Upright Microscope and an Olympus FV1000 Confocal Laser Scanning Biological Microscope (Olympus America Inc., Melville, NY) were used to capture representative images.
To quantify cells that express cell-specific markers and/or myelin inhibitors, randomly selected fields of each sample were photographed using a 20× objective. The percentage of cells labeled by a specific marker was determined by counting the total number of cells labeled with this marker, divided by the total number of Hoechst-labeled nuclei. For each staining, at least 500 cells per coverslip were counted from at least 3 randomly selected regions. Cell counts from different samples in each condition were then averaged and statistically analyzed as described below.
Western blot analysis
OPCs and differentiating oligodendrocytes at 0, 2, 4, 6 and 8 days in vitro after T3 induction were detached by accutase and sedimented. After the removal of supernatant, the cell pellet (2× 106 cells/150 µl lysis buffer) was lysed in lysis buffer (20 mM Tris–HCl, pH 7.4, 137 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF, Sigma], 1% Triton X-100, 1 mM sodium orthovanadate, 2 mM NaPPi, 25 mM β-glycerophosphate, and 10% glycerol and protease inhibitor cocktail III [1 µl/100 µl lysis buffer, Calbiochem, San Diego, CA]) by repetitive pipetting and sonication. The homogenate was centrifuged and the protein concentrations were quantified by Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Inc., Hercules, CA).
For Western blot analysis, equal amounts of protein from each sample were separated using 7.5% SDS-PAGE. After electrophoresis, the protein samples were transferred to a PVDF membrane (Bio-Rad Laboratories) overnight at 4 °C. The membrane was incubated in blocking buffer [Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST) and 5% milk powder], followed by rabbit polyclonal anti-Nogo-A (1:200, Santa Cruz), or anti-MAG (1:500, Zymed Laboratories) incubation. The membrane was then incubated in an ECL-conjugated anti-rabbit secondary antibody (1:12,000 and 1:5000) and visualized using the Chemiluminescence (ECL) plus detection system (Amersham Bioscience, Buckinghamshire, England). To quantify the protein, the expression bands of investigated molecules were scanned using a densitometer SI scanner (Molecular Dynamics/Amersham Biosciences; n = 5 rats/group) and analyzed using Image Quant v.5.1 (Amersham Biosciences). The house-keep protein β-actin was used as an internal control.
Adult OPC isolation
Adult OPCs were immunopanned with an O4 antibody from the adult spinal cord of Fischer rats. Briefly, the dissected spinal cords were minced into 1 mm3 pieces and incubated in HBSS containing 0.1% papain, 0.1% neutral protease, and 0.01% DNase for 30 min at 37 °C. The digestion was stopped by the addition of an equal volume of DMEM containing 10% fetal bovine serum. Tissues were dissociated by repeated trituration with fire-polished Pasteur pipettes and were filtered through a 70 µm nylon mesh. The cells were then incubated on an anti-RAN-2 antibody- (ATCC, Rockville, MA) coated dish for 30 min to deplete type-1 astrocytes and meningeal cells and then transferred to an O4 antibody-coated dish for 45 min to select for adult OPCs. The purified adult OPCs on the dish were removed with trypsin and cultured in DMEM/F12 medium containing N2 and B27 supplements, FGF2 (20 ng/ml), PDGF-aa (10 ng/ml), insulin (5 µg/ml), and BSA (0.1%). In all cases, an aliquot of cells was analyzed the next day to determine the efficiency of the immunopanning. Only those cell preparations in which >95% of the bound cells expressed A2B5 were used in the experiments. The results were confirmed by FACS analysis.
DRG preparation
DRGs were isolated from E15 Sprague-Dawley rat embryos (Charles River Laboratories) using a protocol modified from a previous study (Wood and Williams, 1984). Briefly, the DRGs were treated with 0.25% trypsin (Invitrogen) for 8 min at 37 °C to digest the ectoblast. Forty percent fetal bovine serum (FBS, Invitrogen) in L-15 was used to neutralize the effect of trypsin. The supernatant was removed after spinning for 5 min at 1500 rpm, and DRG explants were resuspended in 3 ml of 10% FBS/L-15 and spinning for 5 min at 1500 rpm. After washing twice, DRG explants were resuspended in 1 ml DRG-growth medium containing Neurobasal medium plus B27 (Invitrogen) supplemented with 200 mM/ml l-glutamine.
Neurite outgrowth on OPCs or adult oligodendrocytes
To test neurite outgrowth of DRG neurons on a monolayer of embryonic OPCs, we developed a “half-half seeding model” in which dissociated cells were seeded onto one half of the coverslip and DRGs were seeded onto the other half. Briefly, after dissociation, OPCs were resuspended with an OPC-growth medium at a density of 5× 104 cells/ml and seeded onto one half of a PLL/laminine-coated coverslip (250 µl/half coverslip) in a way that a sharp cell border was formed along the midline of the coverslip. On the following day, the medium was replaced with an OPC-growth medium (500 µl) containing 100 ng/ml nerve growth factor (NGF, Chemicon) that covered the entire coverslip. DRG explants (1–2 DRGs/coverslip) were then seeded onto the other half of the coverslip, approximately 0.5 mm from the cell border. The medium was changed every other day.
For adult oligodendrocytes co-culture with DRG explants, the resuspended adult OPCs (5× 104 cells/ml) were plated onto one half of the PLL/laminine-coated coverslips in an adult OPC-growth medium (DMEM/F12, 1×N2, 1×B27, 1×P/S, 20 ng/ml FGF-2, 10 ng/ml PDGF-aa, and 30 ng/ml Insulin). On the following day, the cultures were changed to a differentiation medium (DMEM/F12, 1×N2, 1×B27, 1×P/S, 30 ng/ml insulin) to induce adult OPC differentiation into mature oligodendrocytes. After 2 days of differentiation, 100 ng/ml NGF was added into the differentiation medium and DRG explants (1–2 DRGs/coverslip) were plated onto the coverslip 0.5 mm away from the differentiated oligodendrocyte border. The medium was changed every other day.
After 4–5 days in vitro, co-cultures were fixed with 4% PFA as described above and processed for immunofluorescent double-labeling. Neurite outgrowth was identified by a polyclonal anti-neurofilament antibody (NF-200, Sigma) double stained with either a monoclonal anti-A2B5 antibody for embryonic OPCs or an anti-MBP antibody for adult oligodendrocytes according to a method described above.
Quantification of neurite outgrowth
To assess neurite outgrowth from DRG neurons into monolayers of either embryonic OPCs or adult oligodendrocytes, we measured four indices, i.e., the length of axon fascicles, the area of axon fascicles, the number of cells that contacted axon fascicles, and the space between an axon fascicle and surrounding cell border. The length of axon fascicles that grew into the cell monolayer from its border to the fascicles tip was measured from images captured by a SPOT v 4.0.5 digital camera (Diagnostic Instruments, Sterling Heights, MI) with a standard ruler calibrated at 4× magnification. The lengths of 5 longest axonal fascicles from a single DRG were measured and averaged. The areas of the same fascicles measured above were measured and averaged by a Neurolucida System (MicroBrightField, Colchester, VT). The numbers of the cells (either OPCs or oligodendrocytes) contacting with each fascicle of a single DRG were counted and averaged using Image J (NIH, Bethesda, MD) at 20× magnification. The spaces between an axon fascicle and the surrounding cell border at both the fascicle mid-point and tip were measured from images captured by the SPOT v 4.0.5 digital camera with a standard ruler calibrated at 20× magnification.
Statistical analysis
Data were expressed as mean ± standard deviation (S.D.). Two-way ANOVA was used to compare temporal expressions of different cell markers as well as myelin inhibitors at different developmental stages of oligodendrocyte lineage cells. A student t-test was used to compare neurite outgrowth of DRG neurons co-cultured with either embryonic OPCs or adult oligodendrocytes. Differences were considered significant if p<0.05.
Results
Differential expression patterns of Nogo-A and MAG in OPC differentiation in vitro
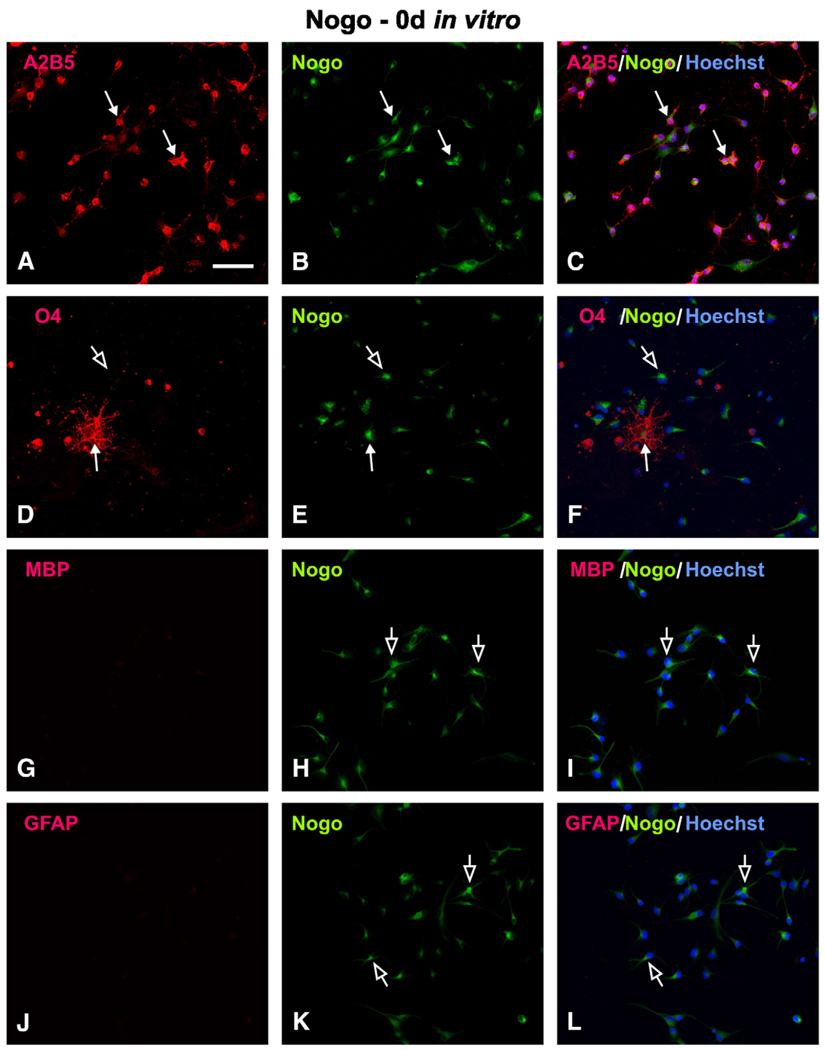
OPCs isolated from embryonic rats could be subsequently expanded for multiple passages in vitro, and exhibited bipolar or tripolar morphology. Immunofluorescent staining showed that >94% of these cells expressed A2B5 (data not shown), an OPC marker. In the presence of T3, the OPCs readily differentiated into O4-, O1-, RIP-, and MBP-positive cells along with morphological changes such as the formation of multipolar processes characteristic of mature oligodendrocytes. Using this in vitro OPC differentiation model, we first determined at which differentiation stage those OPCs expressed myelin inhibitors. Specifically, we examined localization of two myelin-inhibitors, Nogo-A and MAG, in cells that expressed oligodendrocyte lineage or astrocyte markers. Unexpectedly, OPCs at 0 day expressed Nogo-A (Fig. 1). At this stage, most of the cells were A2B5- and Nogo-A double-labeled (Figs. 1A–C, arrows), which composed >96% of the OPC population. At this stage, few, if any, O4- (Fig. 1D, arrow) and MBP-IR (Fig. 1G) oligodendrocytes or GFAP-IR astrocytes (Fig. 1J) were found; therefore, few colocalization of these markers and Nogo-A was identified (Figs. 1D–L).
Fig. 1.
Cellular localization of Nogo-A in OPCs at 0 day in vitro. (A–C) Most Nogo-A-IR (B, solid arrow) cells co-expressed A2B5 (A, solid arrow), which can be appreciated in the merged image (C, solid arrow). (D–F) Nogo-A (E, solid arrow) was also localized in a few O4+ cells (D, solid arrow), as can be seen in the merged image (F, solid arrow). Note that most OPCs were Nogo-A+ and O4− (D–F, open arrow). The presence of Nogo-A+ but MBP− (G–I, open arrow) or GFAP− (J–L, open arrow) cells in these panels further confirms the specificity of Nogo-A expression at the OPC stage. Scale Bar: 50 µm.
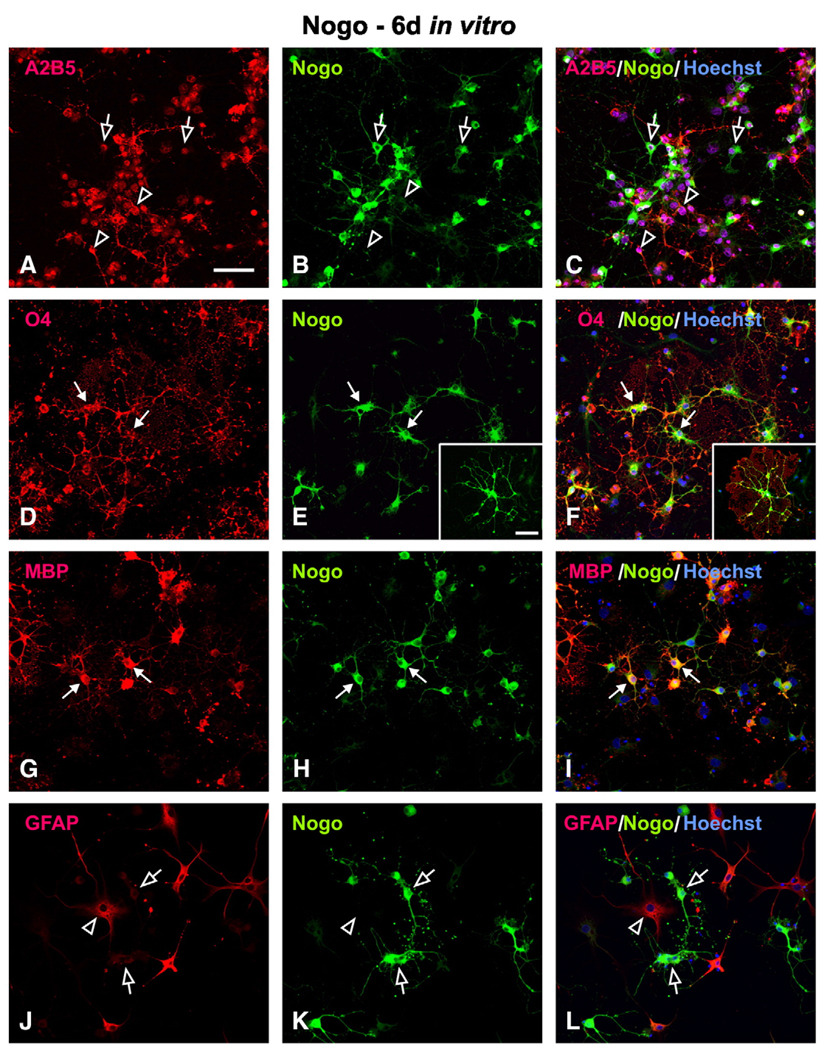
During the course of T3-induced oligodendrocyte lineage differentiation in vitro, a progressive increase in the expression of more mature oligodendrocyte markers was observed. In general, a sequential increase in O4-, O1-, RIP- and MBP-IR was seen. At 6 days in vitro, colocalization of Nogo-A and O4 (Figs. 2D–F) or MBP (Figs. 2G–I) was clearly seen. At this time point, as high as 92.1 % RIP-IR and 91.5% MBP-IR oligodendrocytes were Nogo-A-positive. Compared with the mild expression of Nogo-A at 0 day, the Nogo-A expression in the differentiated oligodendrocytes was more intense (insert in Fig. 2E). Since the number of A2B5-IR cells decreased over time, the percent of A2B5+/Nogo-A+ cells also decreased along with an increase in the percent of A2B5−/Nogo-A+ cells (Figs. 2A–C; open arrows). Some cells, however, remained A2B5+/Nogo-A− (Figs. 2A–C; open arrowheads, 31.8 %). These cells, however, are unlikely undifferentiated OPCs seen at early stages of development but rather differentiated type II astrocytes which expressed both A2B5 and GFAP (data not shown). As anticipated, Nogo-A was not localized in astrocytes expressing GFAP (Figs. 2J–L; open arrows).
Fig. 2.
Cellular localization of Nogo-A in mature oligodendrocytes at 6 days in vitro. (A–C) Co-localization of A2B5+ and Nogo-A can be found in a sub-population of cells at this stage (open arrow). Many other cells, however, expressed A2B5 but not Nogo-A (open arrowhead). (D–I) Nogo-A was co-localized in O4-positive (D–F; solid arrow) and MBP-positive (G–I; solid arrow) oligodendrocytes. Note that Nogo-A-IR was found not only in the cytoplasm but also in the fine processes of mature oligodendrocytes (insets in E, F). (J–L) Nogo-A (open arrow) was not co-localized in GFAP-positive astrocytes (arrow head). Scale Bar: 50 µm.
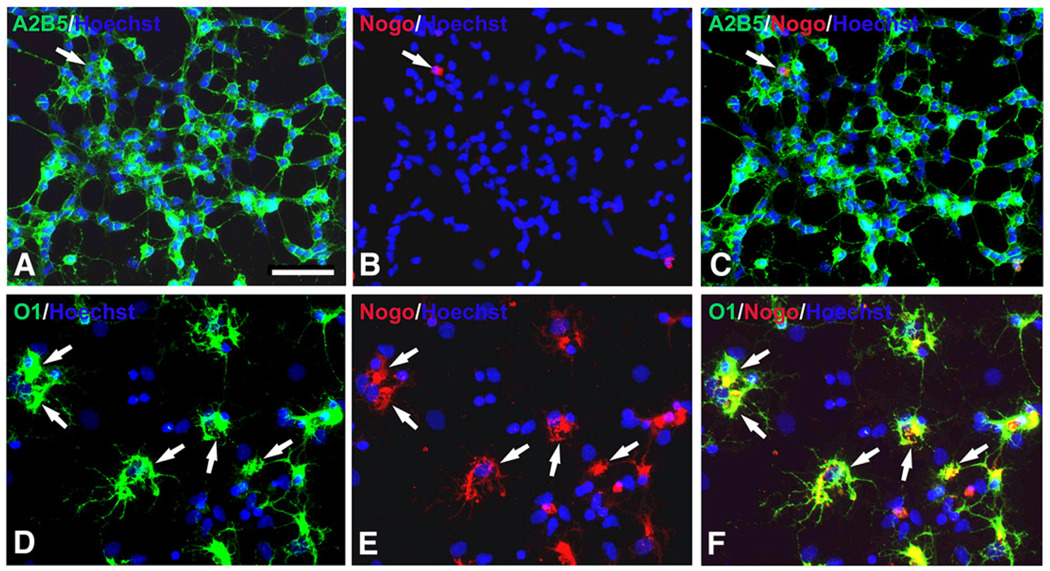
Results described above used Nogo-A antibody against an intracellular domain. However, Nogo-A contains both intracellular and extracellular domains (GrandPre et al., 2000). To determine whether the extracellular domain was also expressed at different developmental stages of oligodendrocytes, we checked expression of Nogo-A extracellular domain in OPCs and differentiated oligodendrocytes. Our result showed that the extracellular domain of Nogo-A was not expressed by OPCs but expressed by almost all of differentiated O1+ oligodendrocytes (Fig. 3).
Fig. 3.
Expression of extracellular domain of Nogo-A in OPCs and O1+ oligodendrocytes in vitro. (A–C) Few immunoreactivity of extracellular domain of Nogo-A was found in A2B5+ OPCs (arrow). (D–F) Extracellular domain of Nogo-A was almost completely co-localized in O1-positive oligodendrocytes (arrows). Scale Bar: 50 µm.
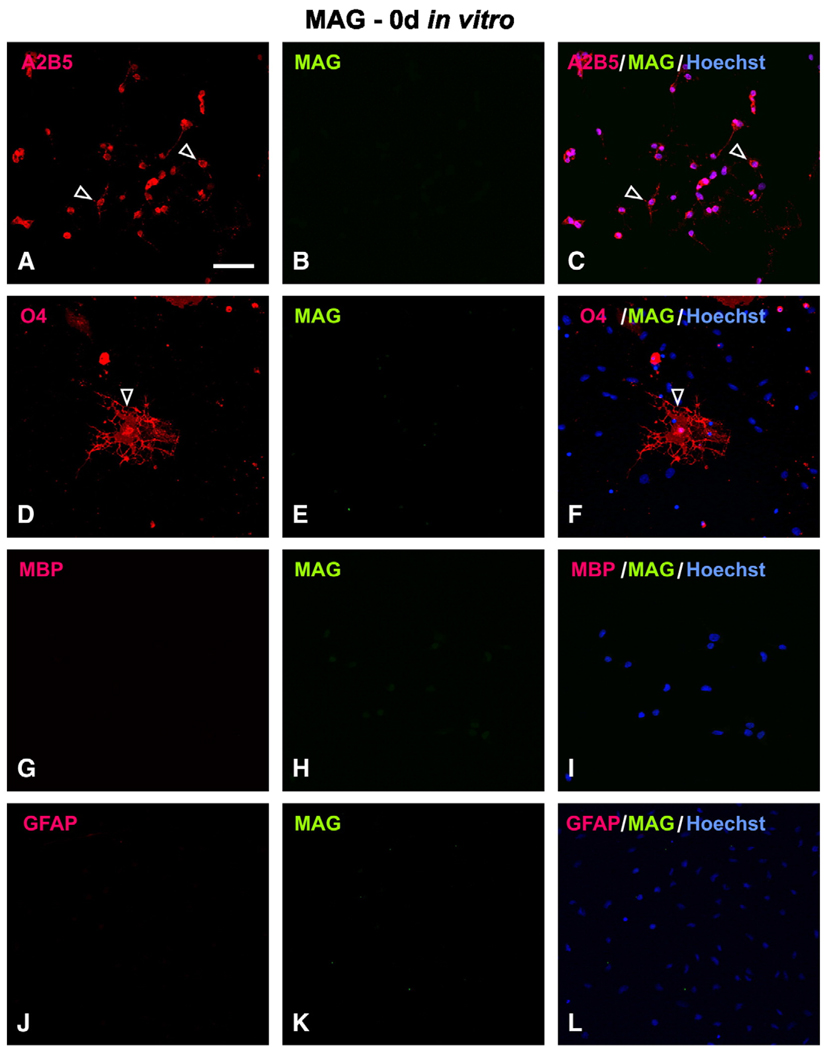
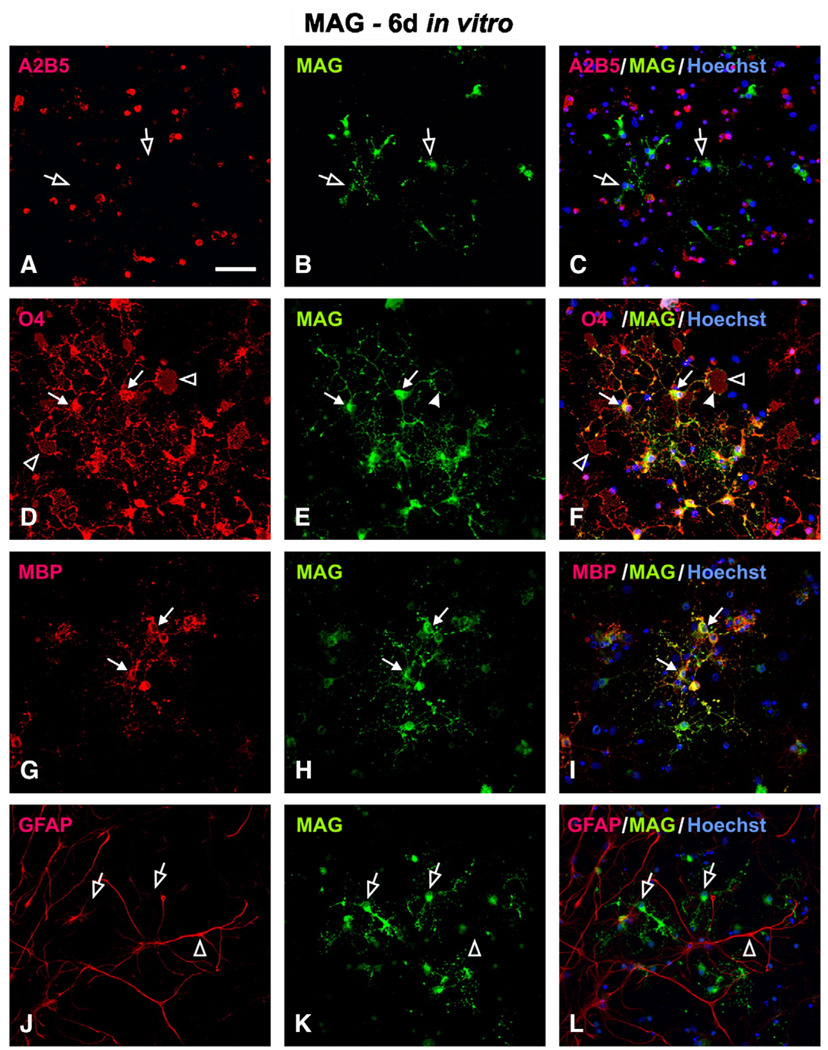
In contrast to the early expression of Nogo-A in OPCs at 0 day in vitro, MAG was not expressed in the same OPC population. Immunofluorescent double-labeling showed that cells immunoreactive for A2B5 and O4 (Figs. 4A, D; arrowheads) were MAG-negative (Figs. 4B, E). In fact, the entire cell population at 0 day was MAG-negative (Fig. 4, middle column). After T3 induction, MAG-IR was found increased in cells expressing oligodendrocyte markers. For example, at 6 days in vitro, colocalization of MAG was found in cells that immunoreactive for O4 (Figs. 5D–F), O1 (not shown), RIP (not shown) and MBP (Figs. 5G–I). The mature oligodendrocytes typically contained several primary processes from which many secondary and tertiary processes are emanated (Fig. 5D). In addition, they form membrane layer structure (Fig. 5D; open arrowheads) which has the potential to wrap axons. MAG-IR was punctuatedly distributed in the cytoplasm, along primary and secondary processes, and on the surface of the membrane (Figs. 5E, F; arrowheads). Interestingly, colocalization of MAG was not found in A2B5-IR cells at this time point (Figs. 5A–C), consistent with the earlier finding that MAG was not expressed in OPCs at 0 day. Not surprisingly, MAG was not colocalized in astrocytes that expressed GFAP (Figs. 5J–L).
Fig. 4.
Lack of MAG expression in OPCs at 0 day in vitro. MAG immunoreactivity (IR) was not found in A2B5+ (A–C; open arrowhead) nor O4+ cells (D–F; open arrowhead). At this early developmental stage, neither MBP+ (G–I) nor GFAP+ (J–L) cells were detected. Scale Bar: 50 µm.
Fig. 5.
Cellular localization of MAG in mature oligodendrocytes at 6 day in vitro. (A–C) MAG-positive cells did not co-express A2B5 (open arrow). Co-localization of MAG was found in mature oligodendrocytes that expressed O4 (D–F, solid arrow) or MBP (G–I, solid arrow). In these cells, MAG-IR was distributed either in the cytoplasm, along the processes, or forming puncta (E, F, solid arrowhead) on the surface of membrane structures (D, F, open arrowhead). (J–L) MAG-IR (open arrow) was not found in GFAP-positive astrocytes (open arrowhead). Scale Bar: 50 µm.
Quantification of Nogo-A and MAG expression in OPC differentiation in vitro
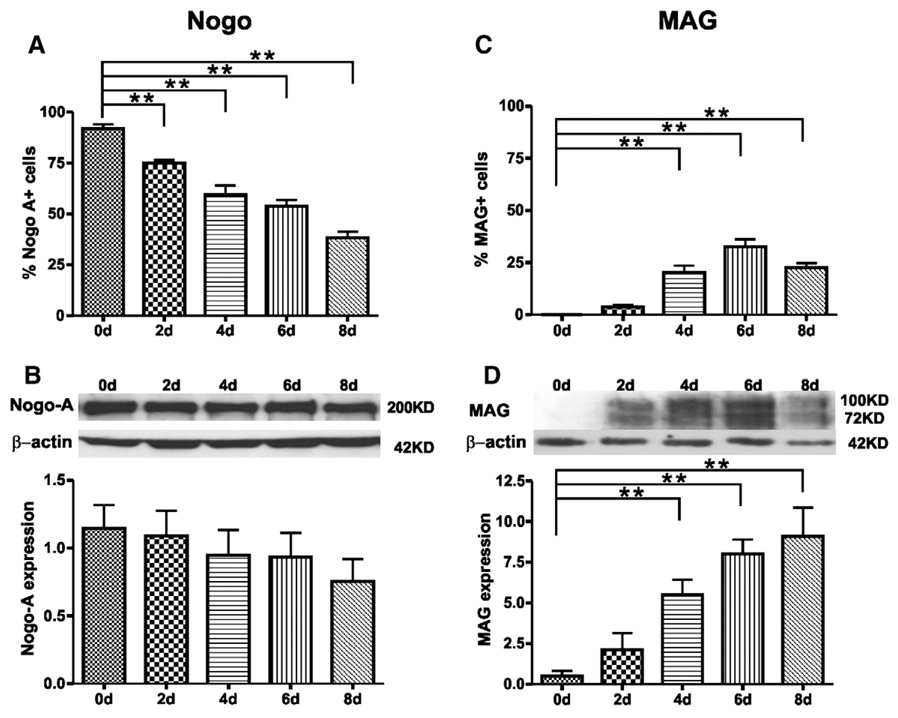
To quantify the expression profiles of Nogo-A and MAG, we counted the number and estimated the percent of Nogo-A- or MAG-IR cells in cultures at 0, 2, 4, 6, and 8 days after T3 induction (Fig. 6). At 0 day in vitro, >91% cells were Nogo-A-IR. The percentage of Nogo-A-IR cells gradually decreased over time and the difference in the percent of Nogo-A-IR cells between 0 day and 2 (p = 0.03), 4 (p<0.001), 6 (p<0.001), or 8 days (p<0.001) was statistically significant (Fig. 6A). The decrease in the percent of Nogo-A-IR cells in vitro was likely due to a steady increase in the number of GFAP-IR astrocytes. In contrast to Nogo-A, MAG-IR cells were not found at 0 day. The percentage of MAG-IR cells gradually increased over time and the difference in the percent of MAG-IR cells between 0 day and 4 (p<0.001), 6 (p<0.001), or 8 days (p<0.001) was statistically significant (Fig. 6C).
Fig. 6.
Quantification of Nogo-A and MAG expression during oligodendrocyte differentiation in vitro. (A, C) The percentage of cells that expressed either Nogo-A (A) or MAG (C) (n = 5 per time point; **, p<0.01 vs. 0 day). (B, D) Western blot analysis further confirmed the expression patterns of Nogo-A (B) and MAG (D). The top panel in B and D shows a representative time course of Nogo-A or MAG expression. The bottom panel in B and D shows compiled results in a bar graph for each time point (n = 5 per time point;**, p<0.01 vs. 0 day). Note that MAG expression was not detected at 0 day by either cell counts (C) orWestern blot analysis (D). Its expression, however, increased significantly at later time points (4, 6 and 8 days).
The expression profiles of Nogo-A and MAG were further confirmed using Western blot analysis. For Nogo-A, a 200 kDa band was identified representing its full length (Fig. 6B). Nogo-A expression was detected abundantly at the OPCs stage (0 day) and throughout the entire time points examined. The expression of Nogo-A was slightly decreased at later time points but the difference was not statistically significant (Fig. 6B). The difference was likely due to an increase in the number of astrocytes that was Nogo-A negative. In contrast to Nogo-A, the expression of MAG, at molecular weights of 72 kDa and 100 kDa, was not detected at 0 day. Such an expression, however, was steadily increased over time (Fig. 6D). MAG expression was significantly increased at 4 (10.94-fold, p = 0.008), 6 (16.00-fold, p<0.001) and 8 days (18.16-fold, p<0.001) post-T3 induction in vitro. Thus, the temporal expression profiles of both Nogo-A and MAG in Western blot analysis matched well with that of immunofluorescence staining results.
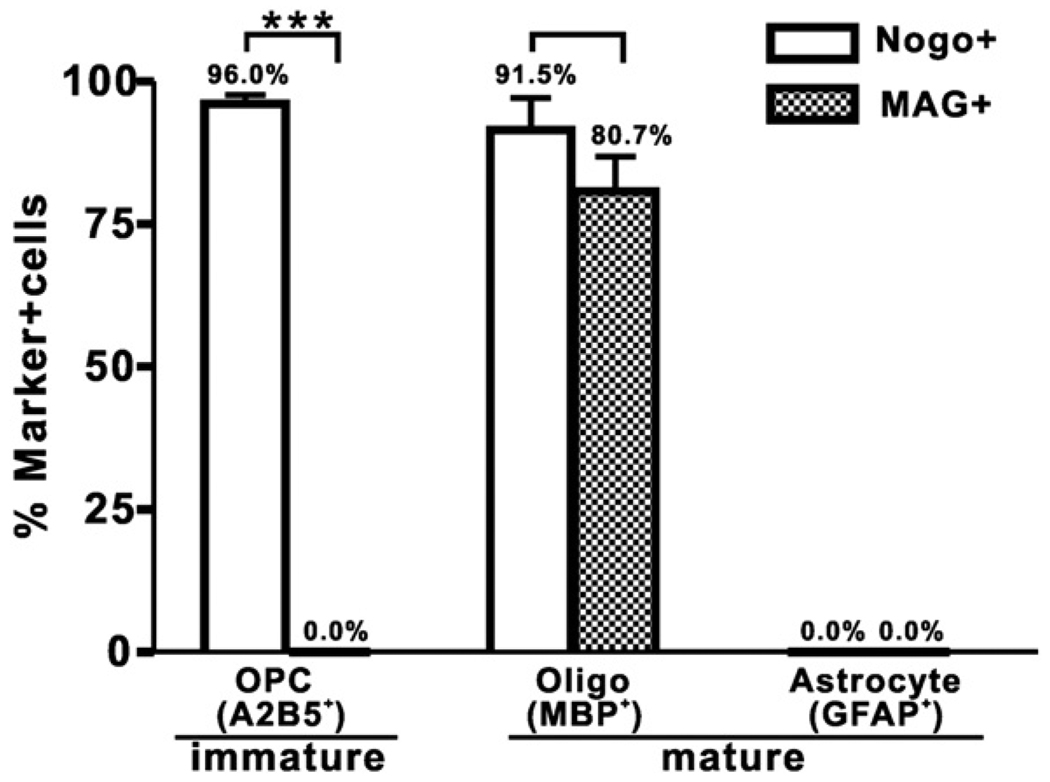
The difference in expression profiles between the two myelin inhibitors at OPC (0 day) and mature oligodendrocyte (6 days) stages in vitro is illustrated in Fig. 6. At the OPC stage, cells expressed only Nogo-A (96.0%) but not MAG (0.0%) whereas at the mature oligodendrocyte stage cells expressed both Nogo-A (91.5%) and MAG (80.7%). As anticipated, neither Nogo-A nor MAG was expressed in cells expressing GFAP (Fig. 7).
Fig. 7.
Comparison between Nogo-A and MAG expressions in OPCs and OPC-derived oligodendrocytes and astrocytes. OPCs expressed Nogo-A (96% of A2B5+-OPC) but not MAG (p<0.001). In contrast, mature oligodendrocytes expressed both Nogo-A (91.5% of MBP+-Oligo) and MAG (80.7% of MBP+-Oligo) and there was no statistically significant difference between the two. As anticipated, mature astrocytes expressed neither Nogo-A nor MAG.
OPCs expressing Nogo-A but not MAG were less inhibitory to neurite outgrowth than mature oligodendrocytes expressing both Nogo-A and MAG
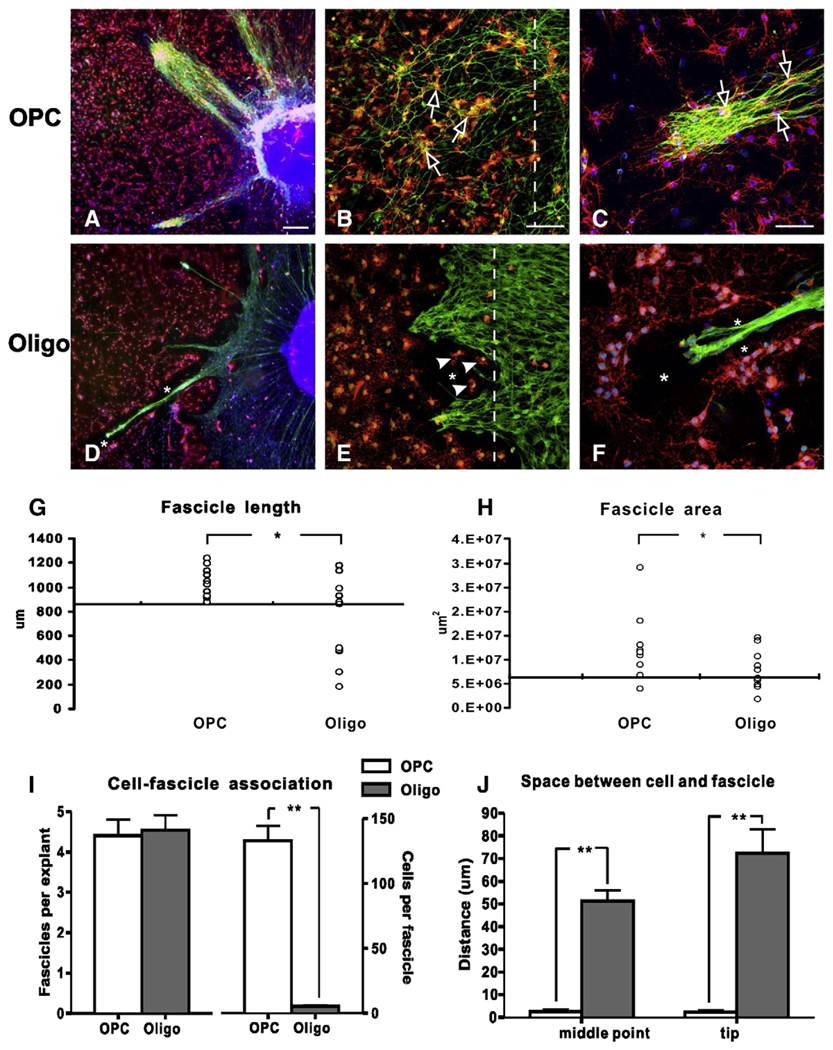
Since we observed differential expression profiles of the two myelin inhibitors between OPCs and mature oligodendrocytes, our next experiment was to determine whether these two cell populations act differently in terms of neurite growth-inhibition. Since the purity of mature oligodendrocytes induced from embryonic OPCs decreased over time due to the increased proliferation of astrocytes in vitro, we used mature oligodendrocytes isolated from the adult rat spinal cord. These adult oligodendrocytes are highly pure (>90%) and expressed mature oligodendrocyte markers MBP as well as the two myelin inhibitors Nogo-A and MAG. We then used the “half-half seeding model” to examine neurite outgrowth from DRG neurons across the margin of seeded cell substrate. On a PLL/laminin coated surface of the DRG-seeded side, neurites grew vigorously radiating from the DRG explants (data not shown). When encountering the border of cell monolayer, both OPCs and oligodendrocytes showed inhibitory properties in that less neurites penetrated into the cell monolayer. However, OPCs were more permissive to neurite outgrowth than the mature oligodendrocytes. In the OPC-DRG co-culture, thick bundles of neurite fascicles penetrated into the OPC-monolayer (Fig. 8A). At the cell border, many single, unfasciculated neurites grew into the OPC-monolayer through direct contact on cell substrate (Fig. 8B). In fact, both fasciculated (Fig. 8C) and unfasciculated (Fig. 8B) fibers penetrated into the OPC monolayer and contacted directly on the surface of OPCs. In contrast, mature oligodendrocytes were largely inhibitory to neurite outgrowth. Only thin bundles of neurites penetrated into the mature oligodendrocyte monolayer and grew for shorter distances (Fig. 8D). Strikingly, no contact was found between neurite bundles and mature oligodendrocytes. In areas where only a few oligodendrocytes were present (Fig. 8E; arrowheads), neurite outgrowth was not observed. In fact, an acellular gap or space was found consistently between growing neurites and mature oligodendrocytes, either surrounding the neurite fascicles (Figs. 8D, F) or along the border between oligodendrocytes and neurites (Fig. 8E).
Fig. 8.
Comparison of DRG neurite outgrowth on the OPCs or mature oligodendrocytes at 3 days in vitro. (A–C) Representative photomicrographs show DRG neurites (NF+, green) growing into the OPC (A2B5+, red) monolayer in thick bundles (A) or scattered fibers (B). Note that these neurites grew in close proximity to the OPCs and contacted directly on the surface of the OPCs (B, C, open arrows). At higher magnification (C), the growing neurites were found to contact directly on the surface of the OPCs (open arrow). (D–F) Although DRG neurite bundles (NF+, green) penetrated into the mature oligodendrocyte monolayer (MBP+, red), they were thin and grew for a shorter distance. Notably, an acellular space (asterisk) between growing neurites and mature oligodendrocytes was observed. Interestingly, the presence of only a few oligodendrocytes (E, arrowhead) at the border effectively blocked the growth of DRG neurites. At higher magnification (F), the lack of growth cone-like structures at the tip of neurites, the creation of an acellular space surrounding the neurite fascicle (asterisks), and the lack of direct contact between the growing neurites and mature oligodendrocytes are clearly seen. Cultures in A, C, D and F were counterstained with Hoechst 33342, a fluorescent nuclear dye. Dashed lines in B and E indicate the seeding border of OPCs and oligodendrocytes, respectively. Measurements of fascicle length (G) and area (H) showed that DRG neurites grew significantly farther into the OPC monolayer (p = 0.011) and occupied more area (p = 0.043) compared to that of the oligodendrocytes. (I) Although the number of fascicles that penetrated into the cell monolayer between the two cell types was similar (left panel), significantly fewer oligodendrocytes contacted the growing neurites compared to that of the OPCs (right panel, **, p<0.001). (J) Less space was found between OPCs and growing neurites either at the midpoint (left panel) or the tip of the fascicles (right panel) compared to the mature oligodendrocytes (**, p<0.001). Scale bars, A, D: 400 µm; B, E: 100 µm, C, F: 50 µm.
Quantitative data showed that the lengths of neurite fascicles were significantly longer when grown on the OPC substrate than the oligodendrocyte substrate (Fig. 8G; 1042 ± 123 µm vs. 717 ± 342 µm, p = 0.011). Additionally, in 9 of 10 OPC-seeded cultures, the lengths of neurites exceeded the median length (877 µm) of neurites grown on the oligodendrocyte-seeded cultures. Similarly, neurite fascicles occupied more surface area in the OPC-seeded group than the oligodendrocyte-seeded group. The fascicle areas in 9 of 10 OPC cases exceeded the median (6.2 mm2) of the oligodendrocyte cases and the difference was statistically significant (Fig. 8H; p = 0.043). In both groups, the number of fascicles that grew into the cell-seeded area was similar (Fig. 8I, left). However, 25 times more OPCs contacted DRG neurites than oligodendrocytes (Fig. 8I, right; 133 ± 37 vs. 5 ± 3 per fascicle, p<0.001). Finally, there was significantly less space between cells and fascicles in the OPC-seeded group than the oligodendrocyte-seeded group, both at the fascicle midpoint (2.4 ± 3.9 µm vs. 51.1 ± 16.5 µm, p<0.001) and the tip (2.2 ± 2.9 µm vs. 72.2 ± 35.3 µm, p<0.001). These results collectively suggest that OPCs expressing Nogo-A but not MAG are more permissive to neurite growth than mature oligodendrocytes expressing both Nogo-A and MAG.
Discussion
A major finding of this study is that OPCs expressing Nogo-A (intracellular domain) but not MAG are significantly more permissive to neurite outgrowth than mature oligodendrocytes expressing both. Furthermore, the membrane-associated extracellular domain of Nogo-A was not expressed by OPCs but expressed by almost all O1+ oligodendrocytes. It is very possible that the lack of expression of the extracellular domain of Nogo-A and MAG on OPCs may account for the permissiveness of these cells to neurite outgrowth compared to mature oligodendrocytes that express both molecules. These results suggest that OPC transplantation may be a feasible strategy to promote axonal regeneration, in addition to its known action on remyelination, in the damaged CNS.
To determine the time course of myelin inhibitor expression, it is essential to establish a reliable in vitro model of oligodendrocyte differentiation. Using a modified immunopanning method with A2B5 antibodies (Mayer-Proschel et al., 1997; Mujtaba et al.,1999; Cao et al., 2005), we were able to isolate highly purified OPCs from the embryonic rat spinal cord. Upon T3 induction, OPCs underwent sequential differentiation changes and acquired O4-, O1-, RIP-, and MBP-immunoreactivity over time, similar to those described by Rao and colleagues (Rao and Mayer-Proschel, 1997; Rao et al., 1998; Lee et al., 2000). This allowed the studies of temporal expression of myelin inhibitors in vitro.
OPCs expressed only intracellular domain of Nogo-A whereas mature oligodendrocytes expressed both intracellular and extracellular domains
If OPCs are permissive to neurite outgrowth, one anticipates that these cells would not express any of the known myelin inhibitors. In our initial attempt, Nogo-A was found to express in OPCs as well as mature oligodendrocytes, consistent with results obtained from the P2 rat brain reported by Strittmatter and colleagues (Wang et al., 2002c). However, Nogo-A contains both intracellular and extracellular domains (GrandPre et al., 2000). The antibodywe used in the first attempt only identifies the intracellular domain. Thus, we further examined the expression of the extracellular domain of Nogo-A by OPCs and differentiated oligodendrocytes using an antibody specifically against the extracellular domain of Nogo-A. Our result showed that the extracellular domain of Nogo-A was not expressed by OPCs but expressed by almost all differentiated O1+ oligodendrocytes. The confined distribution of Nogo-A within the cytoplasm of OPCs may account for the less inhibitory nature of OPCs to axonal growth even it is expressed at the OPC stage.
Notably, Nogo-A was expressed exclusively in oligodendrocyte lineage cells and was not expressed when cells differentiated into astrocytes. Although the majority of cells at the OPC stage expressed both A2B5 and Nogo-A, cells expressed either A2B5 or Nogo-A alone were also found. The presence of A2B5-positive but Nogo-A-negative cells indicates that these cells may be precursors of astrocytes that also express A2B5. The finding that Nogo-A is expressed in OPCs raises the possibility that its expression may occur prior to the OPC stage. Such a question remains to be investigated. Although Nogo-A is expressed in both OPCs and mature oligodendrocytes, the expression patterns are somewhat different. In OPCs only intracellular domain of Nogo-A was expressed whereas both intracellular and extracellular domains were expressed in mature oligodendrocytes. The lack of extracellular domain of Nogo-A expression, along with the lack of MAG expression in OPCs, suggests that these myelin-associated inhibitory molecules are not expressed on the surface of OPCs therefore less inhibitory to contact-mediated neurite outgrowth than their adult counterparts. Nonetheless, since neurite outgrowth into the OPC substrate was less extensive than into the acellular region of our coculture model, the presence of intracellular domain of Nogo-A in OPCs may still account for some inhibitory properties of these cells on neurite outgrowth. Alternatively, other inhibitory molecules that expressed by OPCs may play a role in neurite growth inhibition in this model.
MAG is not expressed in OPCs but is increasingly expressed during the course of oligodendrocyte lineage development
In contrast to Nogo-A, MAG was not expressed in OPCs but gradually increased its expression over time and reached full expression in mature oligodendrocytes. Although the percentage of MAG-IR cells increased, it remained less than 50% of the total cell population in vitro. This was most likely due to the increase of astrocytes that expressed neither Nogo-A nor MAG. When astrocytes were excluded from the counting, the percentage of cells that express MAG increased from 0% in OPCs to 80.7% in mature oligodendrocytes.
OPCs expressing intracellular Nogo-A but not MAG are more permissive to neurite outgrowth than mature oligodendrocytes
Since we observed differential expression of Nogo-A and MAG between OPCs and mature oligodendrocytes, we assessed whether these two oligodendrocyte lineage populations were different in neurite growth inhibition. Remarkably, we found that OPCs were significantly more permissive to neurite outgrowth than mature oligodendrocytes. Thicker fascicles and longer growth distances were found only in DRG neurites grown on OPCs compared to those on oligodendrocytes. More importantly, neurite-cell contact was found only in DRG-OPC co-cultures. In fact, an acellular space was consistently found between growing neurites and mature oligodendrocytes. Thus, in contrast to the well-characterized inhibitory effect of Nogo-A in mature oligodendrocytes (Chen et al., 2000; GrandPre et al., 2000; Prinjha et al., 2000; Domeniconi et al., 2002; Wang et al., 2002a; Domeniconi and Filbin, 2005), the inhibitory property of Nogo-A in OPCs during development, particularly when expressed intracellularly, is less effective, consistent with previous data showing that Nogo-A did not inhibit embryonic hippocampal neuronal outgrowth in vivo (Mingorance et al., 2004) and that the expression of Nogo-A and NgR in pre-E13 chick spinal cords was not sufficient to inhibit regeneration in vivo (O'Neill et al., 2004).
The increased neurite outgrowth on OPCs, in light of current literature, could best be explained by either the unique distribution of Nogo-A protein or their lack of MAG expression. Nogo-A exerts its inhibitory effects through two domains, i.e. a 66-amino-acid extracellular loop (Nogo-66) and an amino-terminal (Fournier and Strittmatter, 2001). These inhibitory domains could be expressed on the membrane surface or released after CNS injuries to inhibit axonal extension (McGee and Strittmatter, 2003; Spencer et al., 2003). In our case, it is possible that Nogo-A-IR was localized intracellularly at the OPC stage and, therefore, had less effect on neurite growth-inhibition. Indeed, intracellular expression of Nogo-A in OPCs has been implicated and the protein was found in association with endoplasmic reticulum and Golgi membranes (Oertle et al., 2003). The combined intracellular expression of Nogo-A and lack of MAG expression in OPCs may make these cells more permissive than mature oligodendrocytes to axonal growth. The fact that OPCs are more permissive than mature oligodendrocytes in terms of neurite outgrowth has raised a possibility that OPC transplantation may be used as an attractive repair strategy to promote the regeneration and myelination of damaged axons in the injured CNS.
Mature oligodendrocytes expressing both Nogo-A and MAG are non-permissive to neurite outgrowth
We also demonstrated that mature oligodendrocytes were nonpermissive to neurite outgrowth of DRG neurons. The presence of a consistent acellular space between growing neurites and mature oligodendrocytes indicates that DRG neurites not only were repelled by inhibitory molecules on the surface of mature oligodendrocytes but also soluble substances secreted by these cells. One possible candidate could be a soluble domain of MAG. MAG can be proteolyzed near its transmembrane domain by a calcium-activated protease and the soluble proteolytic product dMAG was found in the cerebrospinal fluid (CSF) in vivo (Stebbins et al., 1997). A second candidate could be the soluble Nogo-66 which is expressed on the surface of the cell membrane or released to induce growth cone collapse or neurite growth inhibition (Fournier et al., 2001; McGee and Strittmatter, 2003; Spencer et al., 2003).
Effects of oligodendrocyte lineage cells on the fasciculation of growing neurites
An interesting finding in the present study was that, while growing into the OPC or oligodendrocyte substrate, neurites formed bundles or fascicles. This was similar to what happens during development, when axons elongate in large tracts or fascicles (Vactor, 1998), extensively segregated by a matrix of fine processes and surrounded by glial sleeves (Fraher, 1997; O'Brien et al., 1998). Our result indicates that molecules associated with OPCs may provide directional guidance by channeling the growth of fasciculated axons and preventing aberrant sprouting along their pathways. Other molecules, such as neural cell adhesion molecule (NCAM) (Vactor, 1998) or ephrin-A5 may also play a role in axon fasciculation (Winslow et al., 1995).
Additional myelin-associated molecules exist and may contribute to axon growth inhibition
While the present study has focused on the expressional pattern and inhibitory property of the two myelin inhibitors Nogo-A and MAG, the presence of other myelin-associated molecules should also be noted that requires further examination. The observation that neurite outgrowth into the OPC substrate was less extensive than that into the acellular region in our study suggests the existence of other OPC- or myelin-associated inhibitors. So far, at least half a dozen of myelin-derived inhibitors have been identified (Yiu and He, 2006). Three of them, i.e. Nogo-A, MAG and OMgp, are thought to be the most potent inhibitors of axonal regeneration. All three inhibitors are highly expressed by CNS oligodendrocytes, whereas in the PNS only a low level of MAG, but not Nogo-A and OMgp, was expressed by Schwann cells (Trapp, 1990; Huang et al., 2005; Yiu and He, 2006). Notably, the three inhibitors, while sharing no homology, can bind to a single neuronal receptor, NgR1, which together with co-receptors p75NTR or TROY, and LINGO-1, mediates the inhibitory effect (Yiu and He, 2006). Moreover, MAG can also bind to NgR2 (Venkatesh et al., 2005). Besides classical myelin inhibitors mentioned above, several repulsive axon guidance molecules may also serve as myelin-derived axon growth inhibitors such as Ephrin-B3 (Kullander et al., 2001), Sema4D (Moreau-Fauvarque et al., 2003), Sema5A (Goldberg et al., 2004), Netrin-1 (Manitt et al., 2006) and RGM (Hata et al., 2006). Since there are many inhibitors of neurite growth and complicated signal molecules in their downstream, it is arduous but important to clarify the complexity which they are responsible for regenerative failure. Thus, with a thorough understanding of the role of myelin inhibitors in regeneration failure, it will be possible for us to develop effective therapeutic strategies for repair of CNS injuries.
In summary, the present study shows that Nogo-A is constitutively expressed throughout oligodendrogliogenesis whereas MAG is expressed only at later stages of oligodendrocyte maturation. More importantly, OPCs expressing Nogo-A but not MAG are significantly more permissive to neurite outgrowth than mature oligodendrocytes that express both. Given that OPCs are CNS derived, capable of forming CNS myelin, and more efficient inmyelination than Schwann cells, the use of OPCs to promote growth of damaged axons and subsequently remyelination may represent an attractive strategy for the repair of CNS injuries.
Acknowledgments
This work was supported by NIH NINDS (NS36350, NS52290, NS50243, RR15576), the Kentucky Spinal Cord and Head Injury Research Trust (#4–16), the Daniel Heumann Fund for Spinal Cord Research, The Indiana Spinal Cord and Brain Injury Research Funds and Mari Hulman George Endowment, The 973 Project (2003CB515302), Shanghai Science and Technology Developing Foundation (00JC14021), and Shanghai Educational Committee Technology Foundation (99ZD08). The authors are grateful to Dr. Gaby U. Enzmann, Naikui Liu and Mr. George Harding for excellent technical assistance, to Ms. Darlene Burke for statistical analysis and to Mr. William Lee Tistworth for critical reading of the manuscript.
References
- Bamber NI, Li H, Lu X, Oudega M, Aebischer P, Xu XM. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur. J. Neurosci. 2001;13:257–268. [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J. Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau CH, Shum DKY, Xu XM. Combinatorial strategies towards spinal cord repair: enhancement of the Schwann cell bridge and modification of the graft-host interface. Preclinica. 2004;2:409–415. [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ R, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. J. Neurol. Sci. 2005;233:43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang KC, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin MT. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Strittmatter SM. Repulsive factors and axonal regeneration in the CNS. Curr. Opin. Neurobiol. 2001;11:89–94. doi: 10.1016/s0959-4388(00)00178-1. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fraher JP. Axon-glial relationships in early CNS-PNS transitional zone development: an ultrastructural study. J. Neurocytol. 1997;26:41–52. doi: 10.1023/a:1018511425126. [DOI] [PubMed] [Google Scholar]
- Gianino S, Stein SA, Li H, Lu XB, Biesiada E, Ulas J, Xu XM. Postnatal growth of corticospinal axons in the spinal cord of developing mice. Dev. Brain Res. 1999;112:189–204. doi: 10.1016/s0165-3806(98)00168-0. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, Barres BA. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J. Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S, Mueller BK, Yamashita T. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J. Cell. Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR, III, Colman DR. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- Kottis V, Thibault P, Mikol D, Xiao ZC, Zhang R, Dergham P, Braun PE. Oligodendrocyte-myelin glycoprotein (OMpg) is an inhibitor of neurite outgrowth. J. Neurochem. 2002;82:1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30:105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Manitt C, Wang D, Kennedy TE, Howland DR. Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. J. Neurosci. Res. 2006;84:1808–1820. doi: 10.1002/jnr.21070. [DOI] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Mingorance A, Fontana X, Sole M, Burgaya F, Urena JM, Teng FY, Tang BL, Hunt D, Anderson PN, Bethea JR, Schwab ME, Soriano E, del Rio JA. Regulation of Nogo and Nogo receptor during the development of the entorhinohippocampal pathway and after adult hippocampal lesions. Mol. Cell. Neurosci. 2004;26:34–49. doi: 10.1016/j.mcn.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, Dusart I, Chedotal A. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujtaba T, Piper DR, Kalyani A, Groves AK, Lucero MT, Rao MS. Lineage-restricted neural precursors can be isolated from both the mouse neural tube and cultured ES cells. Dev. Biol. 1999;214:113–127. doi: 10.1006/dbio.1999.9418. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- O'Brien D, Dockery P, McDermott K, Fraher JP. The ventral motoneurone axon bundle in the CNS—a cordone system? J. Neurocytol. 1998;27:247–258. doi: 10.1023/a:1006932931160. [DOI] [PubMed] [Google Scholar]
- O'Neill P, Whalley K, Ferretti P. Nogo and Nogo-66 receptor in human and chick: implications for development and regeneration. Dev. Dyn. 2004;231:109–121. doi: 10.1002/dvdy.20116. [DOI] [PubMed] [Google Scholar]
- Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brosamle C, Kaupmann K, Vallon R, Schwab ME. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J. Neurotrauma. 2006;23:453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay S, Webster HD. The Fine Structure of the Nervous System: The Neuron and Supporting Cells. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev. Biol. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J. Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T, Domeniconi M, Cao Z, Filbin MT. New roles for old proteins in adult CNS axonal regeneration. Curr. Opin. Neurobiol. 2003;13:133–139. doi: 10.1016/s0959-4388(03)00012-6. [DOI] [PubMed] [Google Scholar]
- Stebbins JW, Jaffe H, Fales HM, Moller JR. Determination of a native proteolytic site in myelin-associated glycoprotein. Biochemistry. 1997;36:2221–2226. doi: 10.1021/bi962385x. [DOI] [PubMed] [Google Scholar]
- Trapp BD. Myelin-associated glycoprotein. Location and potential functions. Ann. N. Y. Acad. Sci. 1990;605:29–43. doi: 10.1111/j.1749-6632.1990.tb42378.x. [DOI] [PubMed] [Google Scholar]
- Vactor DV. Adhesion and signaling in axonal fasciculation. Curr. Opin. Neurobiol. 1998;8:80–86. doi: 10.1016/s0959-4388(98)80011-1. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J. Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002a;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002b;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J. Neurosci. 2002c;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JW, Moran P, Valverde J, Shih A, Yuan JQ, Wong SC, Tsai SP, Goddard A, Henzel WJ, Hefti F, et al. Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation. Neuron. 1995;14:973–981. doi: 10.1016/0896-6273(95)90335-6. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75 (NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Wood PM, Williams AK. Oligodendrocyte proliferation and CNS myelination in cultures containing dissociated embryonic neuroglia and dorsal root ganglion neurons. Dev. Brain Res. 1984;12:225–241. doi: 10.1016/0165-3806(84)90045-2. [DOI] [PubMed] [Google Scholar]
- Xu XM, Chen A, Guenard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]