Abstract
The discussion of abnormal results of breast imaging and abnormal pathologic findings can be challenging for health care professionals and often is stressful for patients. Although most imaging findings and biopsy results are negative and do not infer a substantial increase in breast cancer risk, the subsequent conversation between the patient and her practitioner is more effective and informative with a thorough review of the pathologic results and an appreciation of the importance of radiologic-histologic concordance. This article provides insight into and understanding of breast imaging and biopsy techniques and of histologic findings as a means to timely and appropriate decision making and action by the patient and her health care professional.
ADH = atypical ductal hyperplasia; ALH = atypical lobular hyperplasia; BIRADS = Breast Imaging Reporting and Data System; MRI = magnetic resonance imaging
Among the many women who undergo annual mammography while receiving care from a primary care professional or a subspecialist, the occasional patient is faced with the discovery of suspect or abnormal findings and recommendations for a breast biopsy. The resulting conversation between the patient and her practitioner may result in concerns on the part of the patient that may be avoided with better comprehension of the results and appreciation of the importance of radiologic-pathologic concordance. This review provides insight into and understanding of breast imaging and biopsy techniques and of histologic findings as a means to prompt appropriate decision making and action by the patient and her practitioner.
BREAST IMAGING
Despite the recent controversy regarding the risks and benefits of mammographic screening,1 the American Cancer Society continues to recommend annual screening mammography for women beginning at age 40 years.2,3 Women should be informed of the potential harms and limitations of screening before testing.3 Standard craniocaudal and mediolateral oblique views of each breast are typically obtained. In special circumstances (eg, evaluation of large breasts and implants), additional screening images may be required.
Quality mammographic imaging by technologists and radiologists must meet standards set and regulated by the Mammography Quality Standards Act.4 Use of adequate breast compression allows for a smaller dose of radiation and spreads out the tissue better, resulting in fewer callbacks for overlapping structures and fewer motion artifacts that can limit test sensitivity. Improper breast positioning during mammography can lead to missed cancers because of tissue exclusion. Motion artifact can blur microcalcifications that might represent early-stage cancers.
Before 2000, mammography involved use of a flouorescent screen and film. Newer technology uses a digital recorder that detects x-rays as an electrical signal that is converted to digital data.5 Digital mammography has greater diagnostic accuracy than film screen mammography in premenopausal and perimenopausal women, women younger than 50 years, and women with dense breast tissue.6 Use of digital mammography in this group should be stongly considered.
About 10% of women have a recall from screening mammography for additional evaluation. Of these, 8% to 10% will undergo biopsy. Breast cancer will be diagnosed in approximately 4 of every 1000 women undergoing screening mammography.7
Diagnostic mammography is performed under the direct supervision of a radiologist when abnormalities such as a palpable breast mass, a focal area of breast pain, or nipple discharge are noted or when patients are recalled from screening mammography because of an abnormal or specific mammographic finding. Clinical information provided by the health care professional is critical to ensure proper examination. Diagnostic mammography may include spot compression or magnification views. On-site review and communication to the patient about the diagnostic work-up and findings serve to reduce patient anxiety.
The American College of Radiology Breast Imaging Reporting and Data System (BIRADS) lexicon was developed to standardize terms used to describe breast density and mammographic findings and to provide assessment and management recommendations.8
BIRADS category 0 specifies that the mammogram assessment was incomplete and that additional work-up (review of prior studies or additional imaging) is necessary. If additional imaging is needed after screening mammography, the radiology department or facility contacts the patient to arrange further imaging. BIRADS category 1 indicates negative breast imaging findings. BIRADS category 2 signifies benign changes and that no further evaluation is necessary. Patients who have BIRADS category 1 and 2 results are advised to continue routine (yearly) mammogram screening. BIRADS category 3 indicates a result that is probably benign (findings are malignant in <2% of cases), and follow-up at 6 months is recommended. Shorter intervals may be used in special circumstances, such as acute trauma or infection.9 BIRADS categories 4 and 5 findings are suspect, and biopsy is necessary to rule out a cancer diagnosis.
The BIRADS lexicon also organizes breast density into 4 categories. Breast density is the subjective measure of the ratio of fibroglandular tissue to fatty tissue. Category 1 is almost entirely fat-replaced breast tissue, 2 is scattered fibroglandular densities (25%-50% glandular), 3 is heterogeneously dense (51%-75% glandular), and 4 is extremely dense (>75% glandular). The higher the density (ie, a predominance of fibroglandular tissue), the lower the sensitivity for cancer detection; mammographic sensitivity is 98% in fat-replaced breasts and 50% in dense breasts.10 Studies investigating the risk of breast cancer related to breast density, independent of other risk factors, found it to be 4 to 6 times greater in women with extremely dense breast tissue than in those with little to no breast density. This risk is in addition to the masking effect thought to be responsible for cancers detected within a year of imaging.10
Breast ultrasonography is used to detect correlates to palpable masses, distinguish cystic from solid masses, or determine the extent of disease in known or suspected cancers. It may be the initial step in the evaluation of a young woman (aged <30 years) with a clinical concern such as a palpable mass or nipple discharge.11
Magnetic resonance imaging (MRI) is an adjunct tool for evaluating breast disease. Indications for breast MRI include evaluation of the local extent of disease and screening of the contralateral breast in women with newly diagnosed breast cancer, breast evaluation in patients with metastatic axillary adenopathy and an unknown primary tumor, and evaluation of patients with suspected recurrent breast cancer. Magnetic resonance imaging may be helpful for monitoring breast cancer response to neoadjuvant chemotherapy, for evaluating inconclusive or indeterminate findings on mammography or ultrasonography that may require biopsy, or for evaluating the integrity of silicone implants. Magnetic resonance imaging has been used as a screening tool for patients determined to be at high risk of breast cancer (known BRCA gene mutation carrier, strong family history of breast or ovarian cancer) after a thorough risk assessment.12 Currently, there is no evidence for or against the use of breast MRI for increased breast density, histologic atypia, or lobular carcinoma in situ. Magnetic resonance imaging is not a substitute for mammography and should not be used to preclude biopsy of a suspect finding.
COMMON MAMMOGRAPHIC FINDINGS
Calcifications are small depositions of calcium ranging in size from a fraction of a millimeter to several millimeters. Those that do not appear to be classically benign are further evaluated with magnification views to assess morphologic features, size, and distribution because these descriptors have diagnostic indications.13
Breast masses are 3-dimensional lesions that, when noted on mammography, are described by shape, margin characteristics, and density. Circumscribed margins confer a less than 2% risk of malignancy. Masses with indistinct or spiculated margins have an associated cancer risk of 44% to 60% but could be indicative of high-risk lesions, such as atypical hyperplasia, as well as benign lesions.
Architectural distortion results from disruption of normal homogeneous breast parenchymal patterns. It can be found with malignancy or with the benign changes that present as a mass.
TISSUE SAMPLING
Needle and excisional biopsies may be undertaken in the evaluation of abnormal breast imaging findings. Needle biopsies are done percutaneously with local anesthesia and provide a minimally invasive evaluation of mammographic or clinical abnormalities.
Fine-needle aspiration may be done to sample abnormal lymph nodes or when core needle biopsy of the breast is not possible because of the position of the lesion or comorbid conditions such as chronic anticoagulation. A 25-gauge needle is used; false-negative results may result from small sample size and sampling errors.
Core needle biopsy is usually done with vacuum-assisted or automated biopsy devices that range in size from 14 to 9 gauge. Incorporation of larger-gauge needles to acquire more tissue samples has decreased the likelihood of false-negative or false-positive findings.14 With fine-needle aspiration or core needle biopsy, a radiographic marker is deposited at the biopsy site to identify the area for future follow-up or to guide surgical excision of nonpalpable lesions. Core needle biopsy can be performed with palpation, with ultrasonographic and MRI guidance, and stereotactically. Stereotactic breast biopsies use 15° spatially opposed mammographic images that allow accurate 3-dimensional lesion location. Magnetic resonance imaging—guided core needle biopsy is used if the lesion is mammographically or sonographically occult.
A limitation to successful breast biopsy is breast size. Small, thin breasts may not accommodate the needle used for stereotactic biopsy. Very large breasts can be challenging for ultrasonography-guided biopsy if a lesion is located deep in the breast. Lesions located near the chest wall can be difficult to access during stereotactic or MRI-guided biopsies.
When high-risk lesions are identified radiographically and percutaneous biopsy cannot be performed, patients should be referred for surgical excisional biopsy after localization of the area of suspicion. Localization by wire, dye, or radioactive seeds enhances accuracy and surgical precision at the time of excision. Any imaging method that allows lesion visualization (mammography, ultrasonography, or MRI) may be used.15
Correlation of histologic and imaging findings is essential for providing appropriate recommendations regarding risk management. Occasionally, pathologic findings may not be concordant with the imaging findings noted by the radiologist or surgeon. Communication among the ordering physician, the radiologist, the surgeon, and the pathologist is critical for ensuring that the correct area, whether clinically or radiographically detected, is appropriately queried and assessed.
In a radiographically detected lesion (either a mass or an area of calcifications) in which benignity is strongly favored (ie, the characteristics of the lesion indicate a <2% chance of malignancy), a short-term radiographic follow-up is a reasonable option. This would include repeated imaging in 6 months and bilateral imaging 6 months later. If the lesion is thought to be stable, a 1-year diagnostic follow-up is recommended. After 2 years of bilateral diagnostic mammography or other appropriate imaging, the patient should return to annual mammographic screening.
COMMON HISTOLOGIC FINDINGS ON BREAST BIOPSY
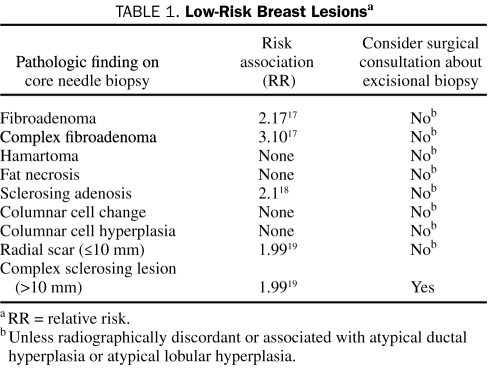
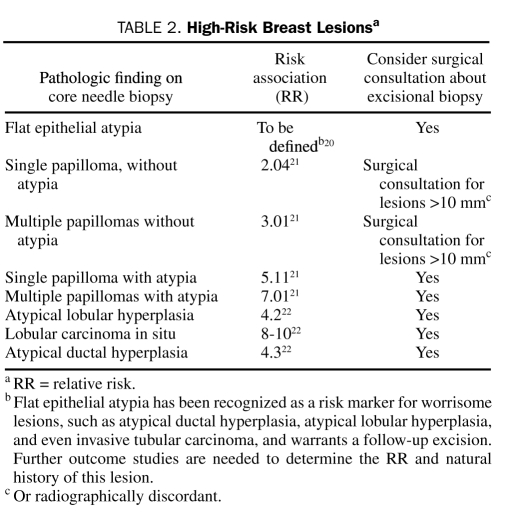
Although benign breast disease has been recognized as an important risk factor for breast cancer,16 the risk varies with the histologic lesion (Tables 1 and 2). The following descriptions should assist in making recommendations regarding breast cancer risk and benign biopsy findings.
TABLE 1.
Low-Risk Breast Lesionsa
TABLE 2.
High-Risk Breast Lesionsa
Fibroadenomas are common breast tumors that occur at any age, although the peak incidence is during the second and third decades of life. Management varies depending on the patient's age at the time of diagnosis. In women older than 35 years, resection of fibroadenomas is advised23 because the associated finding is in situ carcinoma in 2% of patients and the risk of invasive breast cancer is increased.17 A large proportion of fibroadenomas in women younger than 20 years spontaneously resolve.24
Fibroadenomas associated with specific histologic features (cysts ≥3 mm, sclerosing adenosis, epithelial calcifications, or papillary apocrine changes) are classified as complex fibroadenomas and have a relative risk of 2 to 4 for subsequent breast carcinoma.17 Rapid growth in a mass previously diagnosed as fibroadenoma should raise suspicion of stromal or epithelial transformation and should prompt surgical excision.
Fibroepithelial lesions have various histologic appearances and behavior from benign to malignant.25 Lesions that have overlapping histologic features with phyllodes tumor are reported as cellular fibroepithelial lesions. Excision is recommended in these cases to avoid underdiagnosis of a phyllodes tumor. Diagnostic difficulties arise in distinguishing cellular fibroadenoma from benign phyllodes tumor; thus, phyllodes tumor is further subclassified into benign, borderline, and malignant on the basis of semiquantitative criteria.25
In general, fibroadenoma occurs in young women (aged <30 years), and phyllodes tumor occurs most commonly in women older than 45 years. Both fibroadenoma and phyllodes tumor can range in size from a few millimeters to several centimeters.
The main concern about phyllodes tumor is its high propensity for local recurrence; therefore, excision is always recommended with a margin of clearance of at least 10 mm.25 Mastectomy should be considered for large lesions and for malignant phyllodes tumor, although metastases rarely occur even in malignant phyllodes tumor.
Hamartomas are well-circumscribed lesions that occur at a median age of 40 years. Their reported incidence is 0.1% to 0.7%,26 and they present as painless, unilateral, mobile masses ranging in size from 20 mm to 50 mm. Mammographically, hamartomas appear as well-circumscribed masses. Given their distinct appearance on imaging, biopsy is not required. They are not associated with an increased risk of breast cancer.
Fat necrosis represents 2.75% of all benign breast lesions; its incidence is 0.6%.27 The etiologic factors include trauma, radiotherapy, warfarin anticoagulation, breast infection, and invasive breast procedures. In most cases, fat necrosis is clinically occult; however, it can present on clinical examination as a solitary irregular mass or multiple smooth, round, firm nodules. It may be associated with inflammatory skin changes, nipple retraction, and lymphadenopathy occasionally mimicking carcinoma.27 The mammographic and sonographic findings of fat necrosis are occasionally indistinguishable from those of carcinoma,28 and therefore biopsy may be necessary. Fat necrosis confers no increased risk of breast cancer.
Sclerosing adenosis is a benign form of fibrocystic change. The mammographic appearance may include a discrete mass or focal architectural distortion29 and involve calcifications that appear similar to those seen in carcinoma. A percutaneous image-guided biopsy is mandatory for histologic diagnosis.
The cause of sclerosing adenosis is unknown, but it decreases in incidence after menopause and is speculated to be an abnormal pattern of glandular regression or involution after lactation.27 Sclerosing adenosis is associated with a 1.5 to 2.0 relative risk of invasive breast carcinoma independent of its association with other proliferative lesions of the breast. Excisional biopsy is not indicated for pure sclerosing adenosis.
Columnar cell lesions include the spectrum of columnar cell change, columnar cell hyperplasia, or flat epithelial atypia. These lesions are defined as the replacement of normal ductal epithelial cells lining the terminal ductal lobular unit with 3 to 5 layers of columnar cells that display mild to moderate cytologic atypia. Flat epithelial hyperplasia has been recognized as a risk marker for worrisome lesions, such as atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), and invasive tubular carcinoma, and warrants a follow-up excision. However, further outcome studies to assess and define the relative risk of breast cancer and the natural history of this lesion are ongoing.20,30 Radiographically, these lesions are associated with pleomorphic microcalcifications that tend to prompt a biopsy given their suspect appearance. Atypical lobular hyperplasia and ADH may occur in association with columnar cell lesions (ALH 5.0% and ADH 3.5% in patients with columnar cell lesions compared with 1.9% ALH and 1.4% ADH in patients without columnar cell lesions).31 Lesions associated with atypia require a surgical consultation to determine the role of excisional biopsy for excluding malignancy.
Radial scars and complex sclerosing lesions of the breast are benign and have an incidence of 0.03% to 0.09%.32 Radial scars can be up to 10 mm, whereas complex sclerosing lesions are more than 10 mm. Radiographically, they occasionally resemble invasive ductal carcinoma and thus prompt core needle biopsy. Small foci of radial scars may be detected incidentally at the time of core needle biopsy performed because of other radiographic findings.32
A study performed at Mayo Clinic evaluated the breast cancer risk in women with radial scars from a cohort of 9262 patients with benign breast disease.19 Radial scars were found to impart a relative risk of 1.99 (95% confidence interval, 1.49-2.61). The key point of the study was that radial scars do not confer an increased risk over that of other proliferative lesions, with or without atypia. Therefore, excision of these lesions is not advised unless associated with atypia.
Intraductal papillomas are discrete, benign papillary tumors that may be solitary or multiple, arise in a single duct or several, and frequently arise in contiguous areas. Solitary papillomas, most common in patients in the sixth decade of life, are usually central (periareolar) in location. Although patients often present with symptoms such as nipple discharge or a palpable subareolar mass, solitary papillomas may be mammographically detected, clinically silent lesions. Multiple papillomas, most often found in women in their 40s or 50s, are usually peripheral, asymptomatic lesions identified on mammography.21
Studies indicate a higher risk of subsequent cancer in women with multiple rather than solitary papillomas.21 The relative risk for development of breast cancer is 2-fold for patients with a solitary papilloma and 3-fold for patients with multiple papillomas without atypia. Multiple papillomas with atypia have the greatest likelihood for development of cancer, with a relative risk of 7.01.21
If the radiographic findings correlate with the histologic findings and there is no evidence of atypia, a diagnosis of solitary papilloma on core needle biopsy is not an indication for excision, although this recommendation is controversial.33,34 If the radiographic finding is not concordant or if atypia is present, wide excision of the lesion (margins of at least 10 mm), subsequent biannual clinical breast examinations, and annual imaging are advised.35
Lobular neoplasia includes the entities ALH and lobular carcinoma in situ, which are often found incidentally at biopsy.36,37 Both these conditions are multifocal in the ipsilateral breast in up to 50% of patients and are present in the contralateral breast in up to one-third of patients.37 Lobular neoplasia confers a subsequent risk of invasive carcinoma (either ductal or lobular) in either breast; the relative risk is 4 to 5 for ALH and 8 to 10 for lobular carcinoma in situ.22 Studies have shown that lobular neoplasia may be a precursor lesion, because most subsequent breast occurrences are in the ipsilateral breast.36
Because of the uncertainty regarding the biologic behavior of lobular neoplasia, controversies continue with regard to its management. The current standard for lobular neoplasia detected on core needle biopsy is careful continued clinical and radiologic follow-up with or without antiestrogenic hormonal therapy.37 Surgical excision is indicated in the event of radiologic-pathologic discordance, in the presence of ductal features in association with lobular proliferation (pleomorphism), or if an associated biologically aggressive lesion requires surgical excision.37
Atypical ductal hyperplasia represents 10% of radiographically detected lesions,38 in which it is usually associated with calcifications. Atypical ductal hyperplasia confers a relative risk of 4 to 5 for subsequent breast cancer. Because it is a known precursor lesion, surgical excision is recommended because there is a 15% possibility that invasive or in situ carcinoma may be associated with this finding.25 Severe ADH involving 3 or more foci at the time of core needle biopsy is a strong predictor of a more significant lesion at the time of surgical excision (39%) than ADH detected in 1 or 2 foci (7%).39
SUMMARY
The management of radiographically detected benign breast disease depends strongly on histologic evaluation and distinction between benign and atypical changes to best advise patients of their risk of future development of breast malignancy. Concordance between imaging and pathologic findings must be well established before a discussion with the patient. The information provided to patients, along with subsequent advice regarding breast cancer surveillance or intervention based on the histologic findings and their position on the spectrum from normal to severely abnormal, is part of the management strategy designed to assist them in making the best decisions about reducing the risk of breast cancer.
Supplementary Material
Acknowledgments
The authors acknowledge Sandhya Pruthi, MD, for her assistance in the development of this manuscript.
On completion of this article, you should be able to (1) describe the different imaging methods available for breast examination and identify the different indications for performing them, (2) discuss common mammographic findings, and (3) describe histologic findings detected on breast biopsies, their risk associations with breast cancer, and the current specific management of detected breast lesions.
CME Questions About Breast Imaging
-
Which one of the following is not a purpose of breast ultrasonography?
Screening tool for women starting at age 40 years
Evaluation of a young woman (<30 years of age) with a palpable mass
Distinguishing cystic from solid breast masses
Evaluation of an older woman who had recent mammography and a new clinical finding
Determination of the extent of disease in known or suspected cancer
-
Which one of the following is not described in the mammographic report?
Calcifications (size and distribution)
Ratio of dense fibroglandular tissue to fatty tissue
Masses
Architectural distortion
Consistency of the mass (cystic vs solid)
-
In which one of the following clinical scenarios can magnetic resonance imaging be used?
A substitute for mammography
Screening tool for patients at high risk of breast cancer
Patients with a histologic diagnosis of atypia or lobular carcinoma in situ
New patient for whom there is clinical suspicion of a palpable breast mass
Patient with a palpable axillary lymph node with a newly diagnosed breast cancer
-
Which one of the following is not a feature of a radial scar?
Mimics breast cancer radiographically
Confers a relative risk of 1.99 for development of breast cancer
Has an incidence of 0.03% to 0.09% in population-based screening programs
Median size of lesion is 13 mm
Considered a precursor lesion to breast cancer
-
Which one of the following is not a feature of intraductal papilloma?
Can present as an area of architectural distortion on mammography
Histologically, can be solitary or multiple
Confers a relative risk of 2- to 3-fold for development of breast cancer
Can present as nipple discharge
Should be excised surgically
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
Because the Concise Review for Clinicians contributions are now a CME activity, the answers to the questions will no longer be published in the print journal. For CME credit and the answers, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.US Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726 [DOI] [PubMed] [Google Scholar]
- 2.American Medical Association Report 16 of the Council on Scientific Affairs (A-99) Full Text: mammographic screening for asymptomatic women Published June1999. www.ama-assn.org/ama/no-index/about-ama/13541.shtml Accessed February 3, 2010
- 3.Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA 2010;303(2):162-163 [DOI] [PubMed] [Google Scholar]
- 4.Odle TG. MQSA update. Radiol Technol. 2003;74(3):202-220 [PubMed] [Google Scholar]
- 5.Lewin JM, D'Orsi CJ, Hendrick RE, et al. Clinical comparison of fullfield digital mammography and screen-film mammography for detection of breast cancer. AJR Am J Roentgenol. 2002;179(3):671-677 [DOI] [PubMed] [Google Scholar]
- 6.Pisano ED, Hendrick RE, Yaffe MJ, et al. DMIST Investigators Group Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology 2008;246(2):376-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morton MJ, Whaley DH, Brandt KR, Amrami KK. Screening mammograms: interpretation with computer-aided detection: prospective evaluation. Radiology 2006;239(2):375-383 [DOI] [PubMed] [Google Scholar]
- 8.Liberman L, Menell JH. Breast imaging reporting and data system (BIRADS). Radiol Clin North Am 2002;40(3):409-430 [DOI] [PubMed] [Google Scholar]
- 9.Leung JW, Sickles EA. The probably benign assessment. Radiol Clin North Am 2007;45(5):773-789 [DOI] [PubMed] [Google Scholar]
- 10.Dandolu V, Hernandez E. Mammographic breast density. N Engl J Med. 2007;356(18):1885-1887 [DOI] [PubMed] [Google Scholar]
- 11.Pruthi S. Detection and evaluation of a palpable breast mass. Mayo Clin Proc. 2001;76(6):641-647 [DOI] [PubMed] [Google Scholar]
- 12.Saslow D, Boetes C, Burke W, et al. American Cancer Society Breast Cancer Advisory Group American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography [published correction appears in CA Cancer J Clin. 2007;57(3):185] CA Cancer J Clin. 2007;57(2):75-89 [DOI] [PubMed] [Google Scholar]
- 13.Tse GM, Tan PH, Pang AL, Tang AP, Cheung HS. Calcification in breast lesions: pathologists' perspective. J Clin Pathol. 2008;61(2):145-151 [DOI] [PubMed] [Google Scholar]
- 14.Zuiani C, Londero V, Bestagno A, Puglisi F, Di Loreto C, Bazzocchi M. Proliferative high-risk lesions of the breast: contribution and limits of US-guided core biopsy. Radiol Med. 2005;110(5-6):589-602 [PubMed] [Google Scholar]
- 15.Hazard HW, Hansen NM. Image-guided procedures for breast masses. Adv Surg. 2007;41:257-272 [DOI] [PubMed] [Google Scholar]
- 16.Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229-237 [DOI] [PubMed] [Google Scholar]
- 17.Dupont WD, Page DL, Parl FF, et al. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331(1):10-15 [DOI] [PubMed] [Google Scholar]
- 18.Jensen RA, Page DL, Dupont WD, Rogers LW. Invasive breast cancer risk in women with sclerosing adenosis. Cancer 1989;64(10):1977-1983 [DOI] [PubMed] [Google Scholar]
- 19.Berg JC, Visscher DW, Vierkant RA, et al. Breast cancer risk in women with radial scars in benign breast biopsies. Breast Cancer Res Treat 2008;108(2):167-174 [DOI] [PubMed] [Google Scholar]
- 20.Kunju LP, Kleer CG. Significance of flat epithelial atypia on mammotome core needle biopsy: should it be excised? Hum Pathol. 2007;38(1):35-41 [DOI] [PubMed] [Google Scholar]
- 21.Lewis JT, Hartmann LC, Vierkant RA, et al. An analysis of breast cancer risk in women with single, multiple, and atypical papilloma. Am J Surg Pathol. 2006;30(6):665-672 [DOI] [PubMed] [Google Scholar]
- 22.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast: a long-term follow-up study. Cancer 1985;55(11):2698-2708 [DOI] [PubMed] [Google Scholar]
- 23.Kuijper A, Mommers EC, van der Wall E, van Diest PJ. Histopathology of fibroadenoma of the breast. Am J Clin Pathol. 2001;115(5):736-742 [DOI] [PubMed] [Google Scholar]
- 24.Cant PJ, Madden MV, Coleman MG, Dent DM. Non-operative management of breast masses diagnosed as fibroadenoma. Br J Surg. 1995;82(6):792-794 [DOI] [PubMed] [Google Scholar]
- 25.Putti TC, Pinder SE, Elston CW, Lee AH, Ellis IO. Breast pathology practice: most common problems in a consultation service. Histopathology 2005;47(5):445-457 [DOI] [PubMed] [Google Scholar]
- 26.Tse GM, Law BK, Ma TK, et al. Hamartoma of the breast: a clinicopathological review. J Clin Pathol. 2002;55(12):951-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast: a review. Breast 2006;15(3):313-318 [DOI] [PubMed] [Google Scholar]
- 28.Bilgen IG, Ustun EE, Memis A. Fat necrosis of the breast: clinical, mammographic and sonographic features. Eur J Radiol. 2001;39(2):92-99 [DOI] [PubMed] [Google Scholar]
- 29.Pojchamarnwiputh S, Muttarak M, Na-Chiangmai W, Chaiwun B. Benign breast lesions mimicking carcinoma at mammography. Singapore Med J. 2007;48(10):958-968 [PubMed] [Google Scholar]
- 30.Schnitt SJ. The diagnosis and management of pre-invasive breast disease: flat epithelial atypia: classification, pathologic features and clinical significance. Breast Cancer Res. 2003;5(5):263-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulos FI, Dupont WD, Simpson JF, et al. Histologic associations and long-term cancer risk in columnar cell lesions of the breast: a retrospective cohort and a nested case-control study. Cancer 2008;113(9):2415-2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doyle EM, Banville N, Quinn CM, et al. Radial scars/complex sclerosing lesions and malignancy in a screening programme: incidence and histological features revisited. Histopathology 2007;50(5):607-614 [DOI] [PubMed] [Google Scholar]
- 33.Rizzo M, Lund MJ, Oprea G, Schniederjan M, Wood WC, Mosunjac M. Surgical follow-up and clinical presentation of 142 breast papillary lesions diagnosed by ultrasound-guided core-needle biopsy. Ann Surg Oncol. 2008;15(4):1040-1047 [DOI] [PubMed] [Google Scholar]
- 34.Worsham MJ, Raju U, Lu M, Kapke A, Cheng J, Wolman SR. Multiplicity of benign breast lesions is a risk factor for progression to breast cancer. Clin Cancer Res. 2007;13(18 Pt 1):5474-5479 [DOI] [PubMed] [Google Scholar]
- 35.Harjit K, Willsher PC, Bennett M, Jackson LR, Metcalf C, Saunders CM. Multiple papillomas of the breast: is current management adequate? Breast 2006;15(6):777-781 [DOI] [PubMed] [Google Scholar]
- 36.Page DL, Schuyler PA, Dupont WD, Jensen RA, Plummer WD, Jr, Simpson JF. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study [published correction appears in Lancet. 2003;361(9373):1994] Lancet 2003;361(9352):125-129 [DOI] [PubMed] [Google Scholar]
- 37.Nagi CS, O'Donnell JE, Tismenetsky M, Bleiweiss IJ, Jaffer SM. Lobular neoplasia on core needle biopsy does not require excision. Cancer 2008;112(10):2152-2158 [DOI] [PubMed] [Google Scholar]
- 38.Simpson JF. Update on atypical epithelial hyperplasia and ductal carcinoma in situ. Pathology 2009;41(1):36-39 [DOI] [PubMed] [Google Scholar]
- 39.Wagoner MJ, Laronga C, Acs G. Extent and histologic pattern of atypical ductal hyperplasia present on core needle biopsy specimens of the breast can predict ductal carcinoma in situ in subsequent excision. Am J Clin Pathol. 2009;131(1):112-121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.