Abstract
OBJECTIVE: To define age-adjusted incidence trends in multiple myeloma (MM) in a well-characterized population during a long period, given that some, but not all, studies have reported increasing MM incidence over time and that clinical experience from some centers suggests an increased incidence mainly in younger age groups.
PATIENTS AND METHODS: We identified all patients (N=773) with MM diagnosed in Malmö, Sweden, from January 1, 1950, through December 31, 2005. Using census data for the population of Malmö, we calculated age- and sex-specific incidence rates. Incidence rates were also calculated for 10-year birth cohorts. Analyses for trends were performed using the Poisson regression.
RESULTS: From 1950 through 2005, the average annual age-adjusted (European standard population) incidence rate remained stable (Poisson regression, P=.07 for men and P=.67 for women). Also, comparisons between 10-year birth cohorts (from 1870-1879 to 1970-1979) failed to detect any increase. Between 1950-1959 and 2000-2005, the median age at diagnosis of MM increased from 70 to 74 years, and the proportion of newly diagnosed patients aged 80 years or older increased from 16% to 31%.
CONCLUSION: Our finding of stable MM incidence rates for all age groups during the past 5 decades suggests that recent clinical observations of an increase of MM in the young may reflect an increased referral stream of younger patients with MM, which in turn might be a consequence of improved access to better MM therapies. Importantly, because of the aging population, the proportion of patients with MM aged 80 years or older doubled between 1950-1959 and 2000-2005.
Stable multiple myeloma (MM) incidence rates for all age groups during the past 5 decades suggest that recent clinical observations of an increase of MM in the young may reflect an increased referral stream of younger patients with MM, which in turn might be a consequence of improved access to better therapies for MM.
Multiple myeloma (MM), a malignant plasma cell disorder, constitutes about 1% of all reported neoplasms and 12% to 15% of hematologic malignancies.1 It is mainly a disease of the elderly and has a steep increase in incidence with advancing age.2 Reported incidence rates vary substantially worldwide, but to what extent this variation is real or can be explained by differences in access to health care, case ascertainment in central registries, and other factors is unclear. Indeed, worldwide incidence rates vary from 0.4 to 5 per 100,000 person-years, with rates being higher in Western than in Asian countries.3 Incidence rates are 1.5 times higher among men than among women and 2 times higher among African Americans than among white people.4
A steady increase in MM incidence and mortality over time has been reported in some studies from the United States and Europe.5-7 In a survey of cancer mortality in Europe between 1960 and 1999, Levi et al8 observed a steady increase in MM mortality but a tendency to level off in the last calendar years. Similarly, an increase in MM incidence from the mid-1950s through the 1960s and 1970s was observed in studies from the United States, Denmark, and Sweden.9-11 In addition, clinical experience from some centers has suggested an increased incidence mainly in younger age groups. In contrast, prior studies from the United States; Malmö, Sweden; and Switzerland of areas with high levels of case ascertainment and diagnostic accuracy (Rochester, MN, 1945-2002, n=165; Vaud, Switzerland, 1978-2001, n=674; Malmö, Sweden, 1970-1979, n=140) have failed to demonstrate an increased incidence of MM over time.2,12-14
The etiology of MM remains elusive.2 Several studies have investigated the hypothesis that repeated or chronic stimulation of the immune system may lead to MM15,16; however, results have generally been inconsistent. Also, evidence from multiply affected families, case-control studies, and population-based registry studies suggests a role for genetic factors in the causation of MM.17,18
Because temporal changes in MM incidence may indicate the introduction of environmental factors important in the etiology of the disease, it is crucial to elucidate any increase over time. Information from central cancer registries may lead to biased results due to variations over time in diagnostic procedures and criteria, reporting strategies to registries, and access to health care for all inhabitants. These limitations can be reduced by studying incidence rates in a defined population with unrestricted access to health care during the study period and reviewing patient records to guarantee unchanged diagnostic criteria. Malmö, Sweden, offers excellent opportunities to do this. In a prior study designed to evaluate incidence patterns between 1950 and 1979, we found no overall increase; however, there was a trend for an increase among men.13 In the current study, we have expanded and updated our previously assembled unique cohort by adding more than 25 years of data, making this the longest and largest study on incidence trends for MM in a well-characterized population. The aim of our study was to assess differences in MM incidence over time from 1950 to 2005.
PATIENTS AND METHODS
Malmö is the third largest city in Sweden. Its population increased from 192,668 in 1950 to 271,271 in 2005. The medical needs of the population have been served by 1 main hospital throughout the study period and by 2 additional, smaller hospitals for geriatric and psychiatric patients during the first 3 decades. All patients with MM were seen at Malmö University Hospital, which is the only hospital in the area with an emergency unit and a laboratory unit that performs serum protein electrophoresis, where all such analyses were performed.
To ensure complete case ascertainment, we used multiple sources to identify patients. For the whole study period, the registries of discharge diagnosis from all 3 hospitals were searched to identify all reported patients with MM. From 1993, outpatient registries also were searched. Computerized diagnostic registration systems were available for all inpatient care from 1969 and for outpatient care visits from 1993. In 1958 the nationwide population-based Swedish Cancer Registry was established, and we used this registry in parallel.19 Furthermore, we identified all incident M proteins detected at the Department of Clinical Chemistry, Malmö University Hospital, between 1950 and 2005. Finally, local autopsy registries from the whole period were included to trace back information on incident cases of MM not reported elsewhere. In this study, only patients living in Malmö at the time of diagnosis were included.
All records of patients with a diagnosis of MM, plasmacytoma, or extramedullary plasmacytoma were carefully reviewed, including bone marrow examinations, serum and urine electrophoresis, and x-ray examinations, to verify the diagnosis of MM. The minimal criteria for the diagnosis were a monoclonal immunoglobulin in serum or urine (or both) and at least one of the following: (1) 10% or more plasma cells and atypical morphology in bone marrow smear, (2) lytic skeletal lesions or pathologic fractures, (3) hypercalcemia, or (4) renal failure (for 2-4, there should be no other explanation than the plasma cell dyscrasia). Cases without an observed M protein in serum or urine were accepted if they had histologic evidence of plasma cell proliferation and typical skeletal lesions or were diagnosed first on autopsy and no serum or urine protein electrophoresis was performed. Patients with solitary plasmacytoma were included only if they developed generalized myeloma. The number of patients with a revised diagnosis after the reviewing process was low (<5%), confirming a high diagnostic accuracy and completeness of the registries.
For each patient we obtained information about sex and year and age at MM diagnosis. Census data for the population of Malmö were used to calculate the age-specific and sex-specific incidence rates in 10-year and 4-year periods. Census data from the middle of each period were used. Age- and sex-specific incidence rates were also calculated for 10-year birth cohorts (1870-1879 to 1970-1979). For comparison of rates over time, the direct method of standardization by 5-year age groups to the European standard population was used. Analyses for trends were performed by the Poisson regression.
RESULTS
A total of 773 patients with MM (373 men and 400 women) were diagnosed in Malmö between January 1, 1950, and December 31, 2005. Among these, 13 cases (1.7%) were diagnosed first at autopsy. A previous diagnosis of monoclonal gammopathy of undetermined significance (MGUS) was known in 132 patients (17.1%). In the vast majority of the remaining 641 patients, serum electrophoresis was not performed before myeloma diagnosis or the results were not available.
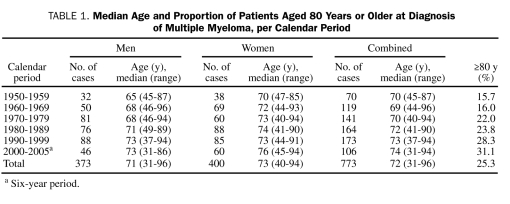
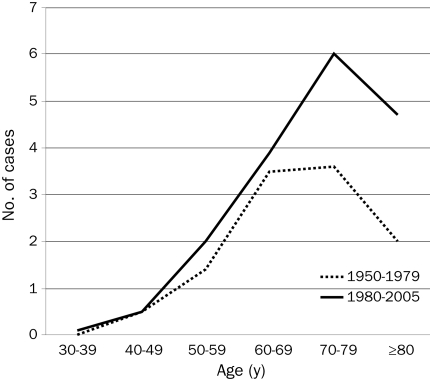
The median age and the proportion of patients aged 80 years or older at the time of diagnosis are shown in Table 1. The median age increased from 70 years in 1950-1959 to 74 years in 2000-2005, and the proportion of patients aged 80 years or older doubled from 16% to 31%. When the calendar periods 1950-1979 and 1980-2005 were compared, a major increase was noted in the number of cases diagnosed in older age groups (Figure 1).
TABLE 1.
Median Age and Proportion of Patients Aged 80 Years or Older at Diagnosis of Multiple Myeloma, per Calendar Period
FIGURE 1.
Average number of cases of multiple myeloma diagnosed per year, by age at diagnosis and calendar period (1950-1979 vs 1980-2005).
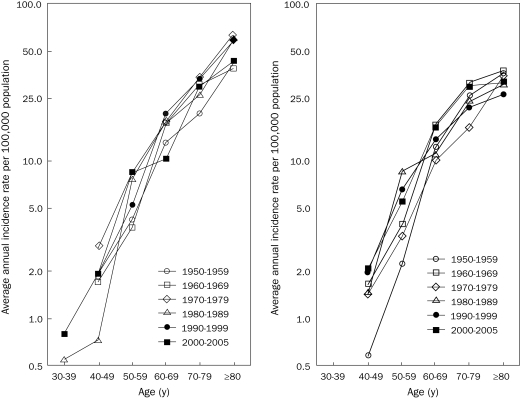
The total number of cases and the average annual age-adjusted incidence of MM per 100,000 person-years are shown for each 10-year period in Table 2. Although the number of new cases diagnosed per year increased during the study period, the age-adjusted incidence did not change significantly (Poisson regression, P=.07 for men and P=.67 for women). Figure 2 shows the average age-specific incidence of MM per 100,000 person-years in men and women for each 10-year period. The incidence rates increased with age in both men and women, even in the highest age groups, and were consistently higher in men. There was no increased incidence over time in any age group.
TABLE 2.
Average Annual Crude and Age-Adjusted Incidence Rates of Multiple Myeloma in Malmö, Sweden, 1950-2005, by Sex
FIGURE 2.
Annual incidence rates of multiple myeloma per 100,000 population in Malmö, Sweden, 1950-2005, by age and sex (left, men; right, women).
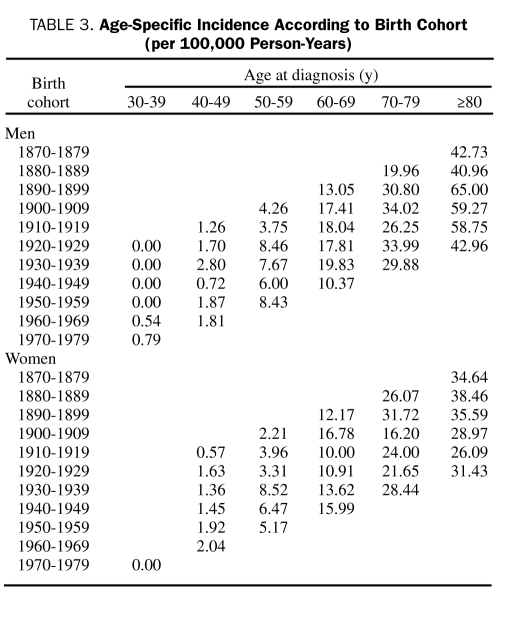
Age- and sex-specific incidence of MM per birth cohort is shown in Table 3. There was no trend to increased incidence over time.
TABLE 3.
Age-Specific Incidence According to Birth Cohort (per 100,000 Person-Years)
DISCUSSION
Two main observations emerged from the current study. First, the average annual age-adjusted incidence of MM remained stable during the whole study period. The number of MM cases diagnosed per year more than doubled from the first to the last calendar period, which is explained entirely by the changing age distribution of the population. Second, the median age at diagnosis increased from 70 to 74 years, and the proportion of very old (≥80 years at diagnosis) increased from 16% to 31%. We think that our results reflect the true incidence of MM in Malmö during the whole length of the study for several reasons. The population of Malmö has been stable and has had unrestricted access to Malmö's only university hospital, where all cases of MM are diagnosed and managed. Also, there has been a long-standing interest in MM in Malmö under the influence of Dr Jan Waldenström, chief of the Department of Medicine at the Malmö University Hospital between 1950 and 1972. In addition, by using multiple parallel approaches, we maximized the ascertainment of MM cases. Furthermore, all medical records (inpatient and outpatient) were carefully reviewed to ensure that uniform diagnostic criteria were applied.
Incidence rates increased with advancing age in both men and women, even in the oldest age groups, for all periods, which indicates a high level of case ascertainment also in the elderly. For men aged 80 years or older, the incidence rates increased to more than 40 per 100,000 person-years. Increasing incidence rates may be caused by new environmental factors that confer an increased risk of myeloma. Our observation of stable incidence rates does not support this hypothesis. Also, comparisons between 10-year birth cohorts ranging from 1870-1879 to 1970-1979 detected no increase.
A number of studies have reported increasing MM incidence over time. A dramatic increase of MM incidence was reported from the United States, England and Wales, and other countries in the 1950s to the 1970s.5-7 In studies extending to later calendar periods, a tendency of MM incidence to level off in recent years was observed. For instance, in Denmark the reported annual incidence in men increased from 1.3 per 100,000 person-years in 1943 to 3.3 in 1962 with no further increase in the following 2 decades.10 In a study of patients reported to the Swedish Cancer Registry between 1973 and 2003, the age-adjusted incidence increased in both sexes from 1973-1979 to 1980-1986 but then remained stable.11 Similarly, in a survey of cancer mortality in Europe from 1960 to 1999, Levi et al8 reported a slow but steady increase in MM mortality with a tendency to level off in recent calendar years. In a comparison of time trends in MM incidence between 1973 and 1997 based on Cancer Incidence in Five Continents, Hirabayashi and Katanoda20 observed a marked increase in African Americans in the United States and a modest increase in some but not all European registries. Several studies have reported a preferential increase in incidence and mortality rates in older age groups. In England and Wales, mortality rates for patients between 70 and 74 years of age increased between 1970-1980 and 1981-1985 while the rates remained stable for younger age groups.21 In 4 geographic areas in the United States (Connecticut; Atlanta, GA; San Francisco-Oakland, CA; and Detroit, MI), the age-adjusted incidence of MM increased from 1.5 to 3.8 per 100,000 person-years in men and from 1.1 to 2.7 per 100,000 person-years in women between 1947-1950 and 1969-1971, primarily because of increases in older age groups.22 No significantly increased incidence was observed between 1969-1971 and 1983-1984. In a study based on the Swedish Cancer Registry, a significant increase in incidence rates was observed only in men older than 70 years of age.23
In contrast, in the current study we found stable incidence rates throughout the whole period 1950-2005 and no evidence of increasing rates in any age group. Our findings are in accordance with 2 recent reports. In a study from Olmsted County, MN, of 165 patients with MM diagnosed between 1945 and 2001, Kyle et al2 found no increase in age-adjusted incidence. Their study, like ours, was based on data from a defined population with high access to health care and a careful registration system, and it reports increasing age-specific incidence rates with advancing age also in the very old. The incidence rate for the whole period was 4.3 per 100,000 person-years (adjusted to 2000 US population), and the age-specific incidence for patients aged 80 years or older was 34.9 per 100,000 person-years, which is similar to our results. In a study from the canton of Vaud, Switzerland, Levi et al14 compared the age-adjusted incidence of MM in 1978-1982 and 1998-2001 and found no evidence of an increase (men, 3.6 vs 2.4, and women, 1.9 vs 2.1, per 100,000 person-years adjusted to world standard population).
We think that the increase in the incidence and mortality of MM previously reported may, at least to a large extent, be explained by improved case ascertainment, especially in the elderly. In studies of stable populations with access to comprehensive medical care and subject to uniform diagnostic criteria, such as in Malmö and Olmsted County,24 no increase was observed in any age group. In a previous report from Malmö during the period 1950-1979, we found a slight but significant increase in men; however, this observation was not confirmed in our current extended analysis. Most likely this was a chance observation and emphasizes the importance of a long period in studies of trend over time. Regarding the clinical experience from larger MM clinics (sometimes mentioned during talks at scientific meetings) suggesting that incidence might be increased among younger age groups, we found no evidence of an increased incidence of MM in the young. Although it remains to be proven, we have speculated that such clinical observations might reflect an increased referral stream of younger patients with MM, which in turn might be a consequence of improved access to better MM therapies.
Although the age-adjusted incidence was stable, the absolute number of patients with MM and the age distribution changed significantly from 1950-1959 to 2000-2005. Indeed, the proportion of very old (>80 years) patients with MM almost doubled during these years. A high median age at diagnosis also has been reported in some studies.23,25 This presents a challenge to the medical profession and stresses the need for new potent, but less toxic, therapies suitable for older patients. Although survival may have improved in recent years, MM is still considered an incurable disease with an average overall survival of 3 to 4 years.11 The growing number of elderly patients with MM, who have a limited life expectancy due to advanced age, is often not included in clinical trials.26 In this patient group, it is important to apply treatment strategies that emphasize improvement of quality of life and limit adverse effects.
Strengths of our study include its long observation period, a well-defined population with access to comprehensive medical care during the study period, efforts to achieve a complete case ascertainment, and review of all cases to ensure uniform and up-to-date diagnostic criteria. To our knowledge, it is the largest study reported that fulfills these criteria. It also has several limitations. We cannot totally exclude that improvement of diagnostic methods during the long study period can influence the results. Even if the population of Malmö has been stable, we have no data on the number of patients in different age groups moving out of and into Malmö during the study period. The conclusions may be valid only for white populations and not necessarily applicable to other ethnic groups or countries. Also, the number of cases is low in the current study compared with studies based on national registries.
CONCLUSION
We report a stable age-adjusted incidence of MM throughout 56 years of follow-up but a highly changing age distribution with a doubling of the absolute number of patients with MM diagnosed at 80 years of age or older. Because environmental and occupational factors were not studied in this population, we could not evaluate such risk factors in relation to incidence during the study period. However, our observation of stable incidence rates over a long period indirectly suggests that, during the past 5 decades, there is no evidence of major changes with regard to environmental or occupational factors that play a role in the causation of MM. On the basis of our results, we have speculated that recent clinical observations of an increase of MM in the young may reflect an increased referral stream of younger patients. Our findings further stress the need for new effective MM drugs with a more favorable toxicity profile to improve survival further, particularly in the elderly.
Acknowledgments
The authors wish to thank Jan Åke Nilsson, PhD, for help with the statistical analyses.
Footnotes
This research was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute in the United States and by grants from the Foundation for Research on Blood Diseases in Malmö, Sweden.
REFERENCES
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer Statistics 2000. CA Cancer J Clin. 2000;50:7-33_ [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ., III Incidence of multiple myeloma in Olmsted County, Minnesota: trend over 6 decades. Cancer 2004;101:2667-2674 [DOI] [PubMed] [Google Scholar]
- 3.Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia 2009;23(10):1691-1697 Epub 2009 Jul 9 [DOI] [PubMed] [Google Scholar]
- 4.Lewis DR, Pottern LM, Brown LM, et al. Multiple myeloma among blacks and whites in the United States: the role of chronic antigenic stimulation. Cancer Causes Control. 1994;5:529-539 [DOI] [PubMed] [Google Scholar]
- 5.Blattner WA, Blair A, Mason TJ. Multiple myeloma in the United States, 1950-1975. Cancer 1981;48:2547-2554 [DOI] [PubMed] [Google Scholar]
- 6.Devesa SS, Silverman DT. Cancer incidence and mortality trends in the United States:1935-74. J Natl Cancer Inst. 1978;60:545-571 [DOI] [PubMed] [Google Scholar]
- 7.Velez R, Beral V, Cuzick J. Increasing trends of multiple myeloma mortality in England and Wales:1950-79: are the changes real? J Natl Cancer Inst. 1982;69:387-392 [PubMed] [Google Scholar]
- 8.Levi F, Lucchini F, Negri E, Boyle P, La Vecchia C. Cancer mortality in Europe, 1995-1999, and an overview of trends since 1960 [published correction appears in Int J Cancer. 2004;111(6):981] Int J Cancer 2002;110:155-169 [DOI] [PubMed] [Google Scholar]
- 9.Miller BA, Ries LA, Hankey BF, et al., eds. SEER Cancer Statistics Review: 1973-1990 Bethesda, MD: National Cancer Institute, 1993 [Google Scholar]
- 10.Hansen NE, Karle H, Olsen JH. Trends in the incidence of multiple myeloma in Denmark 1943-1982: a study of 5500 patients. Eur J Haematol. 1989;42:72-76 [DOI] [PubMed] [Google Scholar]
- 11.Kristinsson SY, Landgren O, Dickman PW, Rangert Derolf Å, Björkholm M. Patterns of survival in Multiple Myeloma: a population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25:1993-1999 [DOI] [PubMed] [Google Scholar]
- 12.Kyle RA, Beard CM, O‘Fallon WM, Kurland LT. Incidence of multiple myeloma in Olmsted County, Minnesota 1978 through 1990, with a review of the trend since 1945. J Clin Oncol. 1994;12:1577-1583 [DOI] [PubMed] [Google Scholar]
- 13.Turesson I, Zettervall H, Cuzick J, Waldenström JF, Velez R. Comparison of trends in the incidence of multiple myeloma in Malmö, Sweden, and other countries, 1950-1979. N Engl J Med. 1984;310:421-424 [DOI] [PubMed] [Google Scholar]
- 14.Levi F, La Vecchia C. Trends in multiple myeloma [letter]. Int J Cancer 1990;46:755-756 [DOI] [PubMed] [Google Scholar]
- 15.Kristinsson SY, Björkholm M, Goldin LR, et al. Patterns of hematologic malignancies and solid tumors among 37,838 first-degree relatives of 13,896 patients with multiple myeloma in Sweden. Int J Cancer 2009;125(9):2147-2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landgren O, Rapkin JS, Mellemkjaer L, Gridley G, Goldin LR, Engels E. Respiratory tract infections in the pathway to multiple myeloma: a population-based study in Scandinavia. Haematologica 2006;91:1697-1700 [PubMed] [Google Scholar]
- 17.Landgren O, Kristinsson SY, Goldin LR, et al. Risk of plasma cell and lymphoproliferative disorders among 14621 first-degree relatives of 4458 patients with monoclonal gammopathy of undetermined significance in Sweden. Blood 2009;114(4):791-795 Epub 2009 Jan 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogmundsdóttir HM, Haraldsdóttir V, Johannesson GM, et al. Familiality of benign and malignant paraproteinemias: a population-based cancer-registry study of multiple myeloma families. Haematologica 2005;90:66-71 [PubMed] [Google Scholar]
- 19.Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol. 1984;23:305-313 [DOI] [PubMed] [Google Scholar]
- 20.Hirabayashi Y, Katanoda K. Comparison of trends in multiple myeloma incidence (1973-1997) in East Asia, Europe and United States, from Cancer Incidence in Five Continents, Vols IV-VIII. Jpn J Clin Oncol. 2008;38:720-721 [DOI] [PubMed] [Google Scholar]
- 21.Cuzick J. International time trends for multiple myeloma. Ann N Y Acad Sci 1990;609:205-214 [DOI] [PubMed] [Google Scholar]
- 22.Devesa SS, Silverman DT, Young JL, Jr, et al. Cancer incidence and mortality trends among whites in the United States, 1947-84. J Natl Cancer Inst. 1987;79(4):701-770 [PubMed] [Google Scholar]
- 23.Altieri A, Chen B, Bermejo JL, Castro F, Hemminki K. Familial risks and temporal incidence trends of multiple myeloma. Eur J Cancer 2006;42:1661-1670 [DOI] [PubMed] [Google Scholar]
- 24.Levi F, Te VC, Randimbison L, La Vecchia C. Incidence of multiple myeloma in Olmsted County, Minnesota [letter]. Cancer 2005;104(2):442 [DOI] [PubMed] [Google Scholar]
- 25.Phekoo KJ, Schey SA, Richards MA, et al. A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol. 2004;127:299-304 [DOI] [PubMed] [Google Scholar]
- 26.Wislöff F, Andersen P, Andersson TR, et al. Has the incidence of multiple myeloma in old age been underestimated? Eur J Haematol. 1991;47:333-337 [DOI] [PubMed] [Google Scholar]