Abstract
OBJECTIVE: To examine the association between abnormal exercise electrocardiographic (E-ECG) test results and mortality (all-cause and that resulting from coronary heart disease [CHD] or cardiovascular disease [CVD]) in a large population of asymptomatic men with metabolic syndrome (MetS).
PATIENTS AND METHODS: A total of 9191 men (mean age, 46.9 years) met the criteria of having MetS. All completed a maximal E-ECG treadmill test (May 14, 1979, through April 9, 2001) and were without a previous CVD event or diabetes at baseline. Main outcomes were all-cause mortality, mortality due to CHD, and mortality due to CVD. Cox regression analysis was used to quantify the mortality risk according to E-ECG responses.
RESULTS: During a follow-up of 14 years, 633 deaths (242 CVD and 150 CHD) were identified. Mortality rates and hazard ratios (HRs) across E-ECG responses were the following: for all-cause mortality: HR, 1.36; 95% confidence interval (CI), 1.09-1.70 for equivocal responses and HR, 1.41; 95% CI, 1.12-1.77 for abnormal responses (Ptrend<.001); for mortality due to CVD: HR, 1.29; 95% CI, 0.88-1.88 for equivocal responses and HR, 2.04; 95% CI, 1.46-2.84 for abnormal responses (Ptrend<.001); and for mortality due to CHD: HR, 1.62; 95% CI, 1.02-2.56 for equivocal responses and HR, 2.45; 95% CI, 1.62-3.69 for abnormal responses (Ptrend<.001). A positive gradient for CHD, CVD, and all-cause mortality rates across E-ECG categories within 3, 4, or 5 MetS components was observed (P<.001 for all).
CONCLUSION: Among men with MetS, an abnormal E-ECG response was associated with higher risk of all-cause, CVD, and CHD mortality. These findings underscore the importance of E-ECG tests to identify men with MetS who are at risk of dying.
Among men with metabolic syndrome, an abnormal exercise electrocardiographic (E-ECG) response was associated with higher risk of all-cause, cardiovascular disease, and coronary heart disease mortality; these findings underscore the importance of E-ECG tests to identify men with metabolic syndrome who are at risk of dying.
ACLS = Aerobics Center Longitudinal Study; CHD = coronary heart disease; CI = confidence interval; CRF = cardiorespiratory fitness; CVD = cardiovascular disease; DM = diabetes mellitus; ECG = electrocardiography; E-ECG = exercise ECG; HR = hazard ratio; MET = metabolic equivalent; MetS = metabolic syndrome; NDI = National Death Index; SRI = stress-recovery index
Metabolic syndrome (MetS) is a clustering of cardiovascular disease (CVD) risk factors,1 including abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, and insulin resistance,2 that currently affects nearly 25% of Americans and is a growing concern because of increasing rates of obesity and hypertension.3 Because many of the components of MetS are associated with an increased risk of CVD and death, a noninvasive diagnosis of subclinical CVD in patients with MetS is important and may optimize secondary preventive interventions in this high-risk population.
We showed earlier that abnormal exercise electrocardiographic (E-ECG) results during maximal exercise testing was associated with an elevated risk of incident coronary heart disease (CHD), CVD, and all-cause mortality in 2854 men with diabetes mellitus (DM).4 Thus, although DM is considered a CHD risk equivalent, important additional information for risk stratification can be obtained from exercise testing. We also showed that exercise testing can be used to identify women with impaired fasting glucose, a predecessor to DM and MetS, who are at high risk of all-cause mortality.5 Callaham et al6 studied 1747 US veterans with DM and showed that exercise-induced ST-segment depression was associated with more CVD events during a mean follow-up of 2 years than was observed in participants without ST-segment depression. In a study of 45 patients with exercise-induced silent ischemia, Weiner et al7 reported that patients with DM had worse outcomes in terms of CVD events than persons without DM.
Currently, no known studies have evaluated the association between abnormal E-ECG responses and all-cause, CHD, and CVD mortality risk in men with MetS. Although sparse, some studies have examined the association between E-ECG responses and CHD risk in men with components of MetS. Ekelund et al8 reported that positive findings on E-ECG was an independent predictor of CVD events in men with hypercholesterolemia. Laukkanen et al9 reported that exercise-induced ischemia was associated with a higher risk of adverse outcomes in persons at high risk of CHD. Bigi et al10 suggested that the stress-recovery index (SRI) predicts all-cause mortality in persons with hypertension.
Therefore, our study primarily aimed to evaluate the association between abnormal E-ECG test results and mortality (all-cause and that due to CHD or CVD) in a large population of asymptomatic men with MetS. We showed earlier that a maximal E-ECG test performed in asymptomatic men free of CVD can predict future risk of CHD death,11 and that an abnormal test result was a more powerful predictor of risk in those with DM than those without the diagnosis.4 The current study will expand this earlier report and focus on men with MetS.
PATIENTS AND METHODS
The current analysis included 9191 men, aged 20 to 80 years, from the Aerobics Center Longitudinal Study (ACLS) who had complete data for cardiorespiratory fitness (CRF) and MetS. The sample was restricted to men with no personal history of myocardial infarction or stroke, and at least 1 year of follow-up data at the time of the baseline examination. Other inclusion criteria for the current analyses required participants to have MetS and to have achieved at least 85% of their age-predicted maximal heart rate on the maximal E-ECG treadmill test at baseline. Participants with DM at baseline, defined as a fasting plasma glucose concentration of at least 126 mg/dL (to convert to mmol/L, multiply by 0.0555), a history of physician diagnosis of the disease, or insulin use, were excluded from the study. Most men were white and from middle or upper socioeconomic strata. All participants attended the Cooper Clinic in Dallas, TX, for clinical evaluations between May 14, 1979, and April 9, 2001, and gave informed consent to participate in the clinical examination and subsequent follow-up study. The Cooper Institute Institutional Review Board reviewed and approved the study protocol annually.
Metabolic Syndrome
Baseline examinations were performed after patients had been fasting for at least 12 hours and included a thorough preventive medical evaluation by a physician and a wide variety of clinical measurements. Waist circumference was measured at the level of the umbilicus with a plastic anthropometric tape. Systolic and diastolic blood pressures were recorded at rest, as the first and fifth Korotkoff sounds, using standard auscultation methods. Concentrations of total and high-density lipoprotein cholesterol, triglycerides, and glucose were measured using automated bioassays in accordance with the Centers for Disease Control and Prevention Lipid Standardization Program. MetS was defined according to the criteria established by the American Heart Association and the National Heart, Lung, and Blood Institute.12
Participants were classified as having MetS if they had 3 or more of the following 5 risk factors: (1) abdominal obesity (waist girth >102 cm); (2) high level of triglycerides (≥150 mg/dL [to convert to mmol/L, multiply by 0.0113]); (3) low level of high-density lipoprotein cholesterol (<40 mg/dL [to convert to mmol/L, multiply by 0.0259]); (4) high blood pressure (≥130/85 mm Hg); and (5) elevated fasting glucose (≥100 mg/dL). As in our previous report,13 history of physician-diagnosed hypertension was used to identify persons with an abnormal blood pressure.
Exercise Test
A maximal symptom-limited E-ECG treadmill test was performed using a modified Balke protocol.14 The treadmill test began with the patient walking 88 m/min (3.3 mph) at 0% grade. At the end of the first minute, elevation was increased to 2% and thereafter increased 1% per minute until the 25th minute. Beyond 25 minutes, elevation remained constant while speed was increased each minute by 5.4 m/min (0.2 mph) until exhaustion. The test end point was volitional exhaustion or termination by the physician for medical reasons. During E-ECG testing, 12-lead electrocardiography (ECG) was monitored continuously. Exercise duration on this protocol is highly correlated with measured maximal oxygen uptake (r=0.92).15 Maximal metabolic equivalents (METs; 1 MET = 3.5 mL oxygen uptake/kg-1 per minute-1) were estimated from the final treadmill speed and grade. We used our previously published age-specific distribution of treadmill durations from the overall ACLS population to define CRF as low (lowest 20%), moderate (middle 40%), or high (upper 40%); we did so to maintain consistency with our previous reports and because low CRF, defined in this way, is an independent predictor of mortality16,17 and morbidity.18 Assignment to a CRF category was based on age norms of treadmill performance rather than on an absolute fitness standard. The respective cut points for total treadmill time and METs in the low, moderate, and high CRF groups were described in detail in a previous report.18 Heart rate recovery was defined as the heart rate decline during the first 5 minutes after the completion of the maximal E-ECG test (heart ratemax — heart rate5 min of recovery). Heart rate reserve is calculated as 220 — age — resting heart rate.
Equivocal E-ECG responses were those with an ST-segment depression between 0.5 and 1.0 mm in amplitude and lasting at least 0.08 seconds.14 Abnormal E-ECG responses, detected by the supervising physician, included rhythm and conduction disturbances and ischemic ST-T wave abnormalities, as described in detail elsewhere.11,14 Previously, to establish the percentage of abnormal test results due to the major criteria of ST-segment depression or elevation of 1 mm or more lasting 0.08 seconds or more from the J-point, 357 abnormal test responses, from 1436 abnormal test results identified in the overall database, were randomly selected and reanalyzed blindly by a group of 3 physicians. We found that 90% of these randomly selected abnormal test responses were abnormal because of ST-segment depression or elevation with or without chest pain, and the specificity of the testing was about 90%.11
Covariates
Information on smoking habits and parental history of CVD were obtained from a standardized questionnaire. Resting ECG procedures followed a standard manual of operations. Body mass index was calculated as the weight in kilograms divided by height in meters squared.
Mortality Surveillance
Follow-up continued until participants died or until December 31, 2003, for survivors. Deaths were identified from the National Death Index (NDI). The underlying cause of death was determined from the NDI report or by a nosologist's review of official death certificates obtained from the Department of Vital Records in the decedent's state of residence. The NDI has established validity for use in cohort studies, with a sensitivity of 96% and specificity of 100%.19 Before 1999, mortality was defined by the International Classification of Diseases, Ninth Revision (CVD, codes 390 to 449.9; CHD, codes 410 to 414), and from 1999-2003, by the Tenth Revision (CVD, codes I00 to I78; CHD, codes I20 to I25). The mean ± SD follow-up time was 13.9±6.4 years (range, 1.0-24.6 years). We computed man-years of follow-up as the sum of follow-up time among decedents and survivors.
Statistical Analyses
Baseline characteristics of the population were examined by E-ECG category and by vital status. Cox proportional hazards regression was used to estimate the adjusted hazard ratio (HR), 95% confidence interval (CI), and death rates (per 10,000 man-years) between E-ECG groups and time to development of death from all causes, CVD, or CHD. Risk factors included baseline measures: age (in years), examination year, smoking status (current smoker or not), body mass index (kg/m2), abnormal resting ECG responses (present or not), family history of CVD (present or not), heart rate reserve, and treadmill time duration (minutes). Tests of linear trends across risk factor categories were computed using integer scoring (values 1, 2, and 3) for the 3 categories.
Cumulative hazard plots grouped by exposures suggested no appreciable violations of the proportional hazards assumption. We excluded events that occurred during the first year of follow-up to reduce potential confounding caused by procedure-related deaths and potential influence of undetected subclinical disease at baseline. We also examined the association within number of MetS components (3, 4, and 5). Finally, we examined the joint effects of E-ECG responses and CRF on mortality. For this analysis, we created 6 ECG-fitness combination categories. Because of the small number of CHD deaths in participants with high CRF (6 deaths for group with equivocal E-ECG findings and 6 deaths for group with abnormal E-ECG findings), we used an alternative CRF grouping (fit vs unfit) to analyze the joint effects of E-ECG responses and CRF on mortality. We compared the effect of each combination of E-ECG responses and CRF status (normal-unfit, equivocal-unfit, abnormal-unfit, normal-fit, equivocal-fit, abnormal-fit) with the referent group (abnormal and unfit). All P values were calculated assuming 2-sided alternative hypotheses; P values <.05 were taken to indicate statistically significant comparisons. All analyses were performed using SAS statistical software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
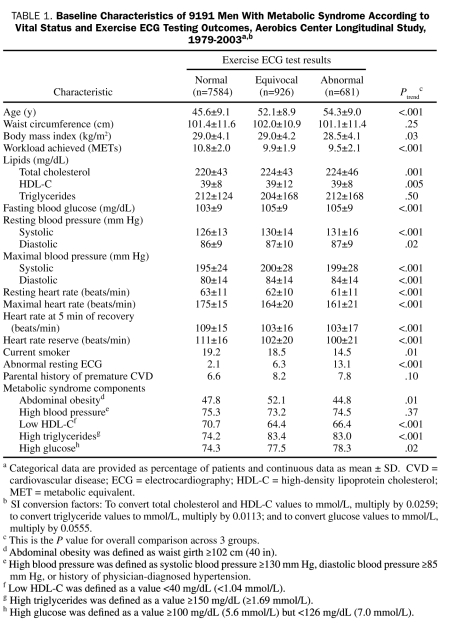
At baseline, the mean ± SD age of all study participants was 46.9±9.5 years; the mean ± SD treadmill test duration, 15.8±4.3 minutes; and the mean ± SD maximal METs, 10.6±2.0. The prevalence of unfit was 23.2%. The characteristics of the participants at baseline are summarized in Table 1. In 127,663 man-years of follow-up, 633 deaths were identified, 242 (38%) of which were due to CVD and 150 (24%) to CHD.
TABLE 1.
Baseline Characteristics of 9191 Men With Metabolic Syndrome According to Vital Status and Exercise ECG Testing Outcomes, Aerobics Center Longitudinal Study, 1979-2003a,b
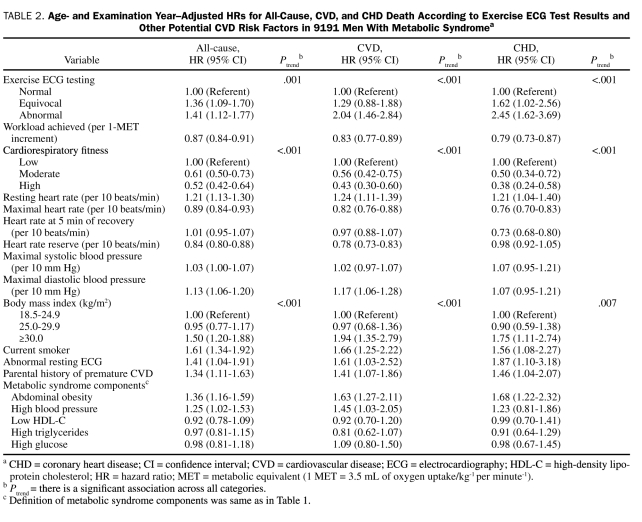
We examined age- and examination year—adjusted relative risk for mortality from all causes, CVD, and CHD (Table 2). Exercise-ECG test results were a strong predictor of CHD mortality risk (HR, 1.62; 95% CI, 1.02-2.56 for equivocal E-ECG test results and HR, 2.45; 95% CI, 1.62-3.69 for abnormal E-ECG test results; Ptrend<.001). The other predictors of CHD death were abnormal resting ECG results (HR, 1.87; 95% CI, 1.10-3.18), obesity (HR, 1.75; 95% CI, 1.11-2.74), abdominal obesity (HR, 1.68; 95% CI, 1.22-2.32), current smoker (HR, 1.56; 95% CI, 1.08-2.27), and parental history of CVD (HR, 1.46; 95% CI, 1.04-2.07). The HR for CHD mortality was 0.79 (95% CI, 0.73-0.87) for each 1-MET increment of achieved workload and was 0.50 (95% CI, 0.34-0.72) for the moderate CRF group and 0.38 (95% CI, 0.24-0.58) for the high CRF group vs the low CRF group. Patterns and magnitude of the association between exposure variables and all-cause and CVD mortality risk were similar to those for CHD (Table 2).
TABLE 2.
Age- and Examination Year—Adjusted HRs for All-Cause, CVD, and CHD Death According to Exercise ECG Test Results and Other Potential CVD Risk Factors in 9191 Men With Metabolic Syndromea
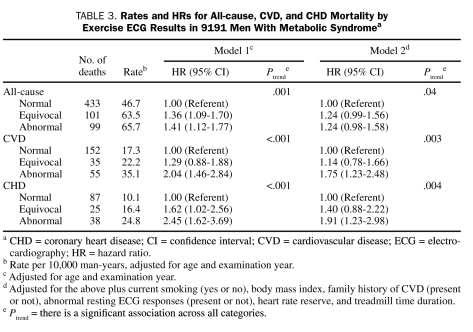
An inverse gradient (Ptrend<.001) of CHD, CVD, and all-cause mortality rates was observed across E-ECG groups (Table 3). After adjustment for risk factors, men with equivocal and abnormal E-ECG test results had a 40% and 91% higher risk of death from CHD (Ptrend=.004), a 14% and 75% higher risk of death from CVD (Ptrend=.003), and a 24% and 24% higher risk of all-cause death (Ptrend=.04) than did men with normal E-ECG test results, respectively.
TABLE 3.
Rates and HRs for All-cause, CVD, and CHD Mortality by Exercise ECG Results in 9191 Men With Metabolic Syndromea
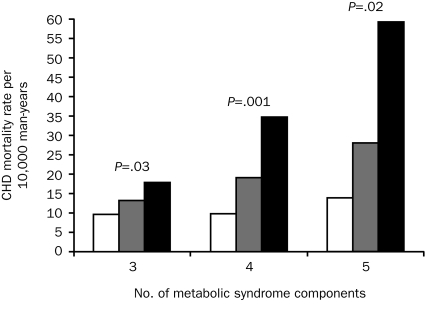
We next examined whether the number of MetS components modified the association between E-ECG testing and CHD mortality (Figure 1). We computed CHD mortality rates by E-ECG test results grouped on the number of MetS components at baseline. As expected, patients with more components and an abnormal E-ECG test result had higher CHD mortality. We observed positive gradients for CHD mortality rates across E-ECG categories within 3, 4, or 5 components (Ptrend<.05, respectively). All-cause and CVD mortality followed a similar pattern (data not shown).
FIGURE 1.
Age- and examination year—adjusted coronary heart disease (CHD) mortality rates (per 10,000 man-years) by exercise electrocardiographic (E-ECG) results and number of metabolic syndrome components (3, 4, and 5) in 9191 men with metabolic syndrome. Normal, equivocal, and abnormal E-ECG test results are indicated by white, gray, and black bars, respectively. The P values are for a test of linear trend across E-ECG groups. The number of men (number of CHD deaths) in the normal, equivocal, and abnormal E-ECG groups were 4949 (57), 549 (13), and 414 (18) in those with 3 components; 2058 (21), 286 (8), and 215 (16) in those with 4 components; and 577 (9), 91 (4), and 52 (4) in those with 5 components, respectively.
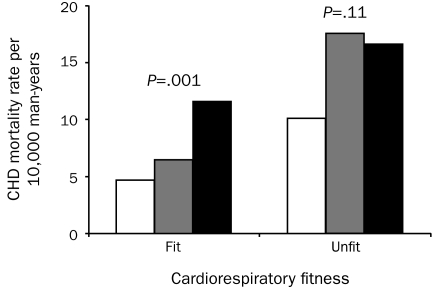
Finally, we analyzed the joint associations of E-ECG responses and CRF with CHD mortality. Figure 2 shows the age- and examination year—adjusted death rates for CHD mortality across E-ECG response groups (normal, equivocal, and abnormal) and CRF categories (unfit/fit), which resulted in 6 E-ECG—CRF groups. The death rate in unfit men with abnormal E-ECG responses was the highest among the 6 combination groups. The adjusted death rate per 10,000 man-years was inversely related to E-ECG responses in the fit group (Ptrend=.001) but not in the unfit group (Ptrend =.11) and was inversely related to CRF in the normal (P=.002) and equivocal (P=.03) E-ECG response groups but not in the abnormal E-ECG response group (P=.28). Cardiovascular disease and all-cause mortality followed a similar pattern (data not shown).
FIGURE 2.
Age- and examination year—adjusted coronary heart disease (CHD) mortality rates per 10,000 man-years according to exercise electrocardiographic (E-ECG) responses and cardiorespiratory fitness categories (fit and unfit). Normal, equivocal, and abnormal E-ECG test results are indicated by white, gray, and black bars, respectively. The P values are for a test of linear trend across E-ECG groups. The number of men (number of CHD deaths) in the normal, equivocal, and abnormal ECG groups were 5887 (59), 688 (15), and 484 (24) in fit men; and 1697 (28), 238 (10), and 197 (14) in unfit men.
DISCUSSION
In this prospective study of men with MetS, we observed that E-ECG test results were a strong predictor of CHD, CVD, and all-cause mortality risk, and these findings were seen in categories created by the number of MetS components. For example, there were strong direct trends for CHD mortality (P<.05 for all categories) in men with 3, 4, or 5 MetS components. There was also a direct gradient of CHD, CVD, and all-cause mortality rates across E-ECG groups. After adjustment for potential risk factors, men with equivocal and abnormal E-ECG test results had a 40% and 91% higher risk of CHD death, a 14% and 75% higher risk of CVD death, and a 24% and 24% higher risk of all-cause death when compared with men with normal E-ECG tests.
Our results concerning the positive association between abnormal E-ECG results and a higher risk of CHD mortality are in unison with other population-based studies of asymptomatic men with other CVD risk factors.9,20-22 The Framingham Heart Study-Offspring found that ST-segment depression provided additional prognostic information in age- and Framingham risk score—adjusted models in men, particularly among those in the highest-risk group (10-year predicted CHD risk of ≥20%).20 This study evaluated 3043 asymptomatic men and women, who underwent a symptom-limited E-ECG test and were followed up for 18.2 years.20 Aijaz et al23 conducted maximal symptom-limited E-ECG stress tests, using the Bruce protocol. They examined 10,897 middle-aged men and women and found that poor exercise capacity and limited heart rate recovery were associated with higher risk of mortality in both persons with and without CVD.23 They also observed an association between a higher risk of mortality and abnormal E-ECG test results in the group free of CVD.23 The association was stronger with a greater number of abnormal E-ECG results. Persons having 3 E-ECG abnormalities had a risk that was equivalent to the risk in individuals with CVD who had normal E-ECG results.23 In another study, a 3-fold higher risk of CHD among patients with 1 or more risk factors and 2 or more abnormal features on exercise testing was found by Bruce et al.21 Similar to the findings from Bruce et al, Rywik et al22 reported that abnormal E-ECG responses (≥1-mm horizontal or down-sloping ST-segment depression) were associated with a doubling of risk of CHD events.
Few studies have reported on the efficacy of E-ECG stress testing for its ability to predict CHD in persons with MetS or the components of MetS. A double-blinded, placebo-controlled clinical trial was conducted by Ekelund et al8 in an effort to determine if positive findings on E-ECG are predictive of CVD events in hypercholesterolemic men. They observed 3806 asymptomatic, hypercholesterolemic men for an average of 7.4 years. They reported that the risk of CHD mortality in association with a positive E-ECG test was 5.7- and 4.9-fold higher in the cholestyramine and placebo groups, respectively.8 Their results suggested that E-ECG responses are a valuable and independent predictor of CHD mortality risk in men with hypercholesterolemia. Bigi et al10 evaluated the importance of E-ECG for predicting mortality in hypertensive individuals with angina. They observed 460 hypertensive individuals with known or suspected coronary artery disease. They used the SRI, based on the comparative analysis of ST-segment depression in the heart rate domain during exercise and recovery ECG phases, as the outcome measure for the E-ECG. They found that male sex (HR, 1.53; 95% CI, 1.01-2.34), peak double product (HR, 0.70; 95% CI, 0.54-0.90), and SRI (HR, 0.69; 95% CI, 0.59-0.81) were independent predictors of mortality. Their results suggest that the SRI predicts all-cause mortality in hypertensive individuals with angina and may potentially provide important prognostic information in replacement of other clinical and standard exercise testing data.10 Laukkanen et al9 showed results that exercise-induced silent ischemia was associated with adverse outcomes in persons who were at high risk of developing CHD because of smoking, hypercholesterolemia, or hypertension. This observation was made by using a maximal symptom-limited E-ECG stress test in 1769 middle-aged men.9 In a previous article, our group also showed the usefulness of E-ECG for predicting CHD in patients with DM. We observed 2854 men with DM who completed a maximal E-ECG test and were without a previous CVD event at baseline. Our results suggested that equivocal and abnormal E-ECG responses were associated with higher risk of all-cause, CVD, and CHD mortality.4
In previous studies, exercise testing has been shown to be an important predictor of survival.4,24 Prakash et al24 found that abnormal E-ECG test results were significantly more common in those who died during the follow-up, and exercise capacity (<5 METs) was independently and significantly associated with all-cause mortality. We also found that men with higher CRF levels had higher survival rates when compared with unfit men and that abnormal E-ECG test results were associated with an increased risk of all-cause mortality.4,25,26 Our current results further strengthen the prognostic power of E-ECG testing in men with MetS.
Strengths of the current study include an extensive baseline examination for detecting subclinical disease, a long follow-up period (mean, 13.9 years), a broad age range (20-80 years), the use of measured risk factors, a variety of mortality end points, and finally, an assessment of the prognostic value of an E-ECG stress test. All stress tests conducted were maximal tests, and all ECGs were 12-lead and continually monitored for 10 minutes after the conclusion of each test.4,11,14 The use of this extensive monitoring ensured that we would capture most, if not all, abnormalities that may have occurred.
One limitation of the current study was a population that was male and predominantly white, well-educated, and middle- to upper-class. Having a participant group with such a homogeneous demographic profile limits the ability to generalize the results of our study. However, this should not affect its internal validity. No strong reason exists to assume that the benefits of E-ECG testing would be any less in women or other ethnic groups. In addition, parallel analyses in women and men from our previous studies have shown that the inverse gradient of mortality across levels of CRF is similar for the 2 sexes.16,17,27 This study also did not have information regarding medication usage, dietary habits, glycated hemoglobin levels, or the presence of end-organ damage. Mortality data were obtained primarily from the NDI, which has established validity. However, the possibility of misclassification of CVD and CHD deaths still exists. Any potential issues of death certificate analyses are not relevant for all-cause mortality because of the consistency of results for all-cause, CVD, and CHD mortality. These results are reassuring, and therefore we do not think these issues will cause a major misinterpretation of our data. Future studies are needed to confirm our findings.
CONCLUSION
Abnormal E-ECG responses were associated with higher risk of all-cause, CVD, and CHD mortality among men with MetS. Assessment of E-ECG using maximal stress testing provides important prognostic information independent of CRF level and traditional CHD risk factors. These findings underscore the importance of E-ECG tests as a determinant of CVD outcomes. We encourage health care professionals to consider the potential prognostic value of E-ECG to further enhance risk stratification in this high-risk group of men.
Acknowledgments
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and we thank the staff at the Cooper Institute for data entry and data management.
Footnotes
The Aerobics Center Longitudinal Study was supported by National Institutes of Health grants AG06945 and HL62508.
REFERENCES
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109(3):433-438 [DOI] [PubMed] [Google Scholar]
- 2.Blaha MJ, Bansal S, Rouf R, Golden SH, Blumenthal RS, Defilippis AP. A practical “ABCDE” approach to the metabolic syndrome. Mayo Clin Proc. 2008;83(8):932-941 [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287(3):356-359 [DOI] [PubMed] [Google Scholar]
- 4.Lyerly GW, Sui X, Church TS, Lavie CJ, Hand GA, Blair SN. Maximal exercise electrocardiography responses and coronary heart disease mortality among men with diabetes mellitus. Circulation 2008;117(21):2734-2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyerly GW, Sui X, Lavie CJ, Church TS, Hand GA, Blair SN. The association between cardiorespiratory fitness and risk of all-cause mortality among women with impaired fasting glucose or undiagnosed diabetes mellitus. Mayo Clin Proc. 2009;84(9):780-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaham PR, Froelicher VF, Klein J, Risch M, Dubach P, Friis R. Exercise-induced silent ischemia: age, diabetes mellitus, previous myocardial infarction and prognosis. J Am Coll Cardiol. 1989;14(5):1175-1180 [DOI] [PubMed] [Google Scholar]
- 7.Weiner DA, Ryan TJ, Parsons L, et al. Significance of silent myocardial ischemia during exercise testing in patients with diabetes mellitus: a report from the Coronary Artery Surgery Study (CASS) Registry. Am J Cardiol. 1991;68(8):729-734 [DOI] [PubMed] [Google Scholar]
- 8.Ekelund LG, Suchindran CM, McMahon RP, et al. Coronary heart disease morbidity and mortality in hypercholesterolemic men predicted from an exercise test: the Lipid Research Clinics Coronary Primary Prevention Trial. J Am Coll Cardiol. 1989;14(3):556-563 [DOI] [PubMed] [Google Scholar]
- 9.Laukkanen JA, Kurl S, Lakka TA, et al. Exercise-induced silent myocardial ischemia and coronary morbidity and mortality in middle-aged men. J Am Coll Cardiol. 2001;38(1):72-79 [DOI] [PubMed] [Google Scholar]
- 10.Bigi R, Cortigiani L, Gregori D, De Chiara B, Parodi O, Fiorentini C. Exercise versus recovery electrocardiography for predicting outcome in hypertensive patients with chest pain. J Hypertens. 2004;22(11):2193-2199 [DOI] [PubMed] [Google Scholar]
- 11.Gibbons LW, Mitchell TL, Wei M, Blair SN, Cooper KH. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol. 2000;86(1):53-58 [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome; an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13(6):322-327 [PubMed] [Google Scholar]
- 13.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation 2005;112(4):505-512 [DOI] [PubMed] [Google Scholar]
- 14.Gibbons L, Blair SN, Kohl HW, Cooper K. The safety of maximal exercise testing. Circulation 1989;80(4):846-852 [DOI] [PubMed] [Google Scholar]
- 15.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92(1):39-46 [DOI] [PubMed] [Google Scholar]
- 16.Blair SN, Kampert JB, Kohl HW, III, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996;276(3):205-210 [PubMed] [Google Scholar]
- 17.Kampert JB, Blair SN, Barlow CE, Kohl HW., III Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol. 1996;6(5):452-457 [DOI] [PubMed] [Google Scholar]
- 18.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165(12):1413-1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837-839 [DOI] [PubMed] [Google Scholar]
- 20.Balady GJ, Larson MG, Vasan RS, Leip EP, O'Donnell CJ, Levy D. Usefulness of exercise testing in the prediction of coronary disease risk among asymptomatic persons as a function of the Framingham risk score. Circulation 2004;110(14):1920-1925 [DOI] [PubMed] [Google Scholar]
- 21.Bruce RA, DeRouen TA, Hossack KF. Value of maximal exercise tests in risk assessment of primary coronary heart disease events in healthy men: five years' experience of the Seattle heart watch study. Am J Cardiol. 1980;46(3):371-378 [DOI] [PubMed] [Google Scholar]
- 22.Rywik TM, O'Connor FC, Gittings NS, Wright JG, Khan AA, Fleg JL. Role of nondiagnostic exercise-induced ST-segment abnormalities in predicting future coronary events in asymptomatic volunteers. Circulation 2002;106(22):2787-2792 [DOI] [PubMed] [Google Scholar]
- 23.Aijaz B, Babuin L, Squires RW, et al. Long-term mortality with multiple treadmill exercise test abnormalities: comparison between patients with and without cardiovascular disease. Am Heart J. 2008;156(4):783-789 [DOI] [PubMed] [Google Scholar]
- 24.Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001;120(3):1003-1013 [DOI] [PubMed] [Google Scholar]
- 25.Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary coronary prevention. Mayo Clin Proc. 2009;84(4):373-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavie CJ, Milani RV. Cardiac rehabilitation and exercise training programs in metabolic syndrome and diabetes. J Cardiopulm Rehabil. 2005;25(2):59-66 [DOI] [PubMed] [Google Scholar]
- 27.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004;27(1):83-88 [DOI] [PubMed] [Google Scholar]