Abstract
OBJECTIVE: To identify and assess the clinical features and treatment response of light chain (AL) amyloidosis diagnosed in patients with previous diagnosis of multiple myeloma (MM).
PATIENTS AND METHODS: From a prospectively maintained database, we identified 47 patients seen between January 1, 1990, and August 31, 2008, with a diagnosis of AL amyloidosis that was made at least 6 months after MM diagnosis; these patients form the study group.
RESULTS: Among the 47 patients, 36 developed typical features, 3 had atypical features, and 8 had an incidental finding of amyloidosis. Amyloid deposits were demonstrated in bone marrow, subcutaneous fat aspirate, or organ biopsy in 24, 19, and 12 patients, respectively. One organ was involved in 29 patients (62%), whereas 11 patients (23%) had involvement in more than one organ. At diagnosis of AL amyloidosis, treatment was changed or started in 22 patients, whereas the same treatment was continued in 21 patients, and no treatment data were available for the rest. The best hematologic response included partial response or better in 11 patients (23%) and stable disease in 18 patients (38%). Improvement in an organ was seen in 3 of the 21 evaluable patients. The median overall survival from diagnosis of AL amyloidosis was 9.1 months (95% confidence interval, 4-14). Of the 6 patients still alive, 2 underwent peripheral blood stem cell transplant, and none had cardiac involvement or involvement in more than one organ.
CONCLUSION: Delayed onset of AL amyloidosis is rarely seen in patients with MM and requires a high index of suspicion for prompt diagnosis. Outcome of these patients is poor, especially in the presence of cardiac involvement.
Delayed onset of light chain (AL) amyloidosis is rarely seen in patients with multiple myeloma and requires a high index of suspicion for prompt diagnosis; outcome of these patients is poor, especially if they have cardiac involvement.
AL = light chain; BM = bone marrow; CI = confidence interval; CR = complete response; FA = fat aspirate; HDT = high-dose therapy; MM = multiple myeloma; OS = overall survival; PBSCT = peripheral blood stem cell transplant; PR = partial response; SD = stable disease; VGPR = very good partial response
Immunoglobulin light chain (AL) amyloidosis is a relatively uncommon disorder characterized by the deposition of monoclonal Ig light chains as insoluble and aggregated amyloid fibrils in various tissues, leading to progressive organ dysfunction and death. Both multiple myeloma (MM) and AL amyloidosis are incurable clonal plasma cell proliferative disorders; they are part of a group of disorders termed monoclonal gammopathies, which includes monoclonal gammopathy of undetermined significance and Waldenström macroglobulinemia, among others. Monoclonal gammopathy of undetermined significance, the most commonly diagnosed monoclonal gammopathy, affects more than 3% of the population older than 50 years1 and has the potential to progress to MM or AL amyloidosis, albeit at a low rate of 1% per year.2 Although AL amyloidosis and MM have distinct clinical presentations, patients may present with findings suggestive of both disorders simultaneously. In addition, patients may present with features of MM or AL amyloidosis and subsequently develop features suggestive of the other.
The “progression” of AL amyloidosis to MM or development of frank MM in a patient with AL amyloidosis is not seen commonly, probably because of the shorter survival of patients with AL amyloidosis. In one series of 1596 patients diagnosed as having AL amyloidosis during a period of 35 years, only 6 had progression to MM.3 In contrast, a diagnosis of AL amyloidosis is made more frequently in a patient with a preexisting diagnosis of MM. Some reports suggest that 10% to 15% of patients with MM may develop overt AL amyloidosis during the course of their disease, and up to 38% of patients with MM may have clinically occult AL amyloid deposits.3,4 We designed the current study to identify and describe the clinical features, diagnosis, treatment patterns and response, and outcome among patients who were diagnosed as having AL amyloidosis subsequent to a diagnosis of MM. We describe 47 patients with a histologic diagnosis of AL amyloidosis, made at least 6 months after the initial diagnosis of MM, thus excluding patients with symptomatic AL amyloidosis at the time of the diagnosis of MM.
PATIENTS AND METHODS
A computerized database search of the prospectively maintained dysproteinemia database was performed to identify all patients with a diagnosis of MM as well as a diagnosis of AL amyloidosis seen at Mayo Clinic. From among 4319 patients with a diagnosis of MM with at least 6 months of follow-up and seen at Mayo Clinic between January 1, 1990, and August 31, 2008, we identified 50 patients in whom the diagnosis of AL amyloidosis followed the diagnosis of MM by at least 6 months. Three patients without histologic proof of AL amyloidosis were excluded, and the remaining 47 patients form the basis of this study.
The diagnosis of MM was made using conventional criteria.5 The diagnosis of AL amyloidosis required the presence of amyloid in tissues (fat aspirate [FA], bone marrow [BM], or organ biopsy) by Congo red stain (which produces a pathognomonic apple green birefringence under polarized light). The organ involvement and the best response to treatment (organ and hematologic) for AL amyloidosis were assessed as per the consensus criteria reported in the 10th International Symposium on Amyloid and Amyloidosis.6 The best organ response for nerve, soft tissue, and pulmonary involvement was assessed clinically. The hematologic status of MM (response to previous treatment) at the time of the diagnosis of AL amyloidosis was assessed as per the criteria listed by the International Myeloma Working Group.7 All patients provided written informed consent for use of their medical records. The current study was approved by the Mayo Clinic Institutional Review Board in accordance with federal regulations and the Declaration of Helsinki.
Continuous variables were summarized by calculating the median and range, and nominal variables were summarized as proportions. The overall survival (OS) was defined as the time between diagnosis and death or the last follow-up with the patient and was estimated by the Kaplan-Meier method.
RESULTS
Of the 47 patients with a diagnosis of biopsy-proven AL amyloidosis, at least 6 months after the initial diagnosis of MM, 29 (62%) were men. The heavy chain class of MM was IgG, IgA, IgM, and IgD in 16 patients (34%), 12 patients (26%), 1 patient (2%), and 1 patient (2%), respectively, whereas 17 patients (36%) had a light chain MM. The light chain was λ and κ in 32 patients (68%) and 15 patients (32%), respectively. All patients were followed up until December 31, 2008, or until death. The median age at the time of diagnosis of MM was 63 years (range, 42-82 years), and the median age at the time of diagnosis of AL amyloidosis was 68 years (range, 45-86 years). The mean and median time to the diagnosis of AL amyloidosis after the diagnosis of MM was 52 months and 42 months (range, 8-134 months), respectively. The median follow-up for the 6 patients still alive at last follow-up is 38 months from the diagnosis of AL amyloidosis, and 41 patients (87%) have died. The study patients had a median OS of 68.5 months (95% confidence interval [CI], 53-86) from the diagnosis of MM and 9.1 months (95% CI, 4-14) from the diagnosis of AL amyloidosis. The median OS of 4319 MM patients with at least 6 months of follow-up who were seen during a similar period was 54 months (95% CI, 52-55).
Diagnosis of Amyloidosis
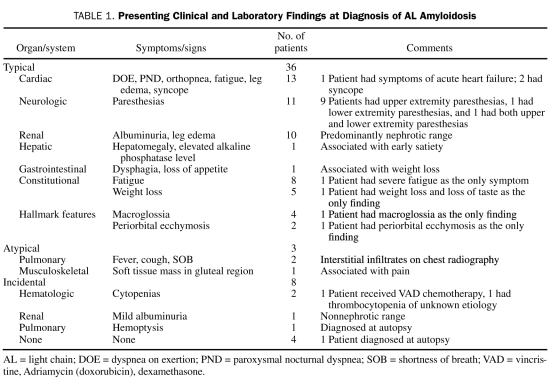
Among the 47 patients, 36 (77%) had developed the typical features suggestive of AL amyloidosis, whereas 3 (6%) had developed atypical features, and 8 (17%) had an incidental finding of AL amyloidosis. The presenting clinical and laboratory findings are detailed in Table 1.
TABLE 1.
Presenting Clinical and Laboratory Findings at Diagnosis of AL Amyloidosis
AL amyloidosis was diagnosed by BM biopsy or subcutaneous FA in 24 patients (51%) and 19 patients (40%), respectively. Both BM biopsy and FA were positive in 11 patients (23%). A carpal tunnel tissue biopsy was positive in 6 patients (13%), and 1 patient had a positive biopsy from a gluteal mass. The diagnosis of AL amyloidosis was established by demonstrating amyloid in various target organs in 12 patients (26%) (lung [5], kidney [4], heart [1], small bowel [1], and liver [1]). Echocardiographic findings suggestive of amyloid cardiomyopathy, such as thickened ventricular wall and/or interventricular septum, and diastolic dysfunction were observed in 15 patients (32%); corresponding restrictive changes were revealed by cardiac catheterization in 1 patient. The median ejection fraction was 47%, and the median interventricular septal thickness was 15 mm at the time of cardiac involvement.
Pattern of Organ Involvement
Zero, 1, 2, and 3 or more organs were involved in 6 (13%), 31 (66%), 7 (15%), and 1 patient, respectively, at the time of initial diagnosis of AL amyloidosis. Of these patients, 3 developed involvement in one other organ, whereas 1 patient had another 2 organs involved during follow-up. Overall, involvement in 1, 2, and 3 or more organs occurred in 29 (62%), 9 (19%), and 2 (4%) patients, respectively. The heart and the kidney were involved in 16 patients (34%) and 12 patients (26%), respectively. Isolated involvement of the heart or kidney occurred in 6 patients (13%) and 7 patients (15%), respectively. Neurologic symptoms were observed in 11 patients (23%), including 8 patients with carpal tunnel syndrome. Pulmonary involvement occurred in 5 patients (11%), including 2 patients in whom the diagnosis was made at autopsy. The involvement of other organs was as follows: soft tissue (7), gastrointestinal (2), and liver (1). Among the 11 patients with involvement in more than one organ, 10 had cardiac involvement, and 5 had renal involvement, along with other organs. Five patients had no evidence of involvement of an organ at initial diagnosis or at follow-up. Among these 5 patients, 4 had coincidental amyloid deposits found on BM biopsy, which was performed either to evaluate a response to therapy or to rule out concomitant myelodysplastic syndrome, whereas 1 patient experienced fatigue and weight loss and underwent BM biopsy to evaluate for AL amyloidosis. Finally, involvement of an organ could not be ascertained in 2 patients who developed paresthesias while receiving thalidomide therapy.
Hematologic Status at Diagnosis of Amyloidosis
The hematologic status of MM at the time of the histologic diagnosis of AL amyloidosis could be determined in 40 (85%) of the 47 patients. Twenty-two patients (47%) had stable disease (SD), 12 (26%) had progressive disease, 6 (13%) had a partial response (PR) or better (PR, 3; very good partial response [VGPR], 3), and 7 (15%) had a non-evaluable hematologic status at the time of diagnosis of AL amyloidosis. Among patients with an evaluable hematologic status and involvement of a single organ from AL amyloidosis, 14 had SD, 3 had PR or better (PR, 1; VGPR, 2), and 8 had progressive disease. Among patients with an evaluable hematologic status who had involvement in more than one organ, 5 patients had SD, 1 patient had PR or better, and 2 had progressive disease. Among patients who had biochemically progressive MM at the time of AL amyloidosis diagnosis, 8 patients had single organ involvement (3 with kidney involvement, 2 each with heart and nerve involvement, and 1 with soft tissue involvement), 2 had no organ involvement, and 2 had involvement in more than one organ. Because of its recent availability in the early 2000s, only 15 patients underwent a serum free light chain assessment at the time of AL amyloidosis diagnosis, and all but 1 patient had an abnormal free light chain ratio (<0.26 and >1.65).
Treatment
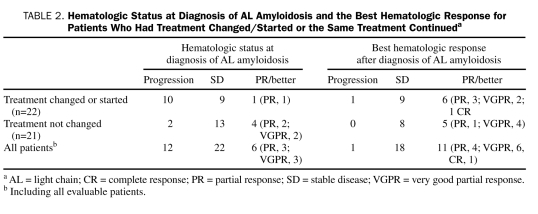
Patients in our study group received a median of 2 treatments for MM before the diagnosis of AL amyloidosis. If treatment of MM was changed within 2 months of the diagnosis of AL amyloidosis, the treatment change was assumed to be secondary to AL amyloidosis. As such, 8 patients (17%) had a change in treatment within this period. Fourteen patients (30%) were not receiving any therapy and had a new treatment initiated within days to weeks of the diagnosis of AL amyloidosis (range, 0-134 days). The treatment was not changed or changed after 2 months from diagnosis of AL amyloidosis in 21 patients (45%). The hematologic status at AL amyloidosis diagnosis and the best hematologic response for the 2 groups are detailed in Table 2. Among the remaining 4 patients (9%), 2 were diagnosed at autopsy, and 2 were lost to follow-up and had no treatment documentation. Overall, 12 patients (26%) had received high-dose therapy (HDT) with peripheral blood stem cell transplant (PBSCT): before the diagnosis of AL amyloidosis in 7 patients, after the diagnosis of AL amyloidosis in 3 patients, and once before and once after the diagnosis of AL amyloidosis in 2 patients.
TABLE 2.
Hematologic Status at Diagnosis of AL Amyloidosis and the Best Hematologic Response for Patients Who Had Treatment Changed/Started or the Same Treatment Continueda
Response and Outcome
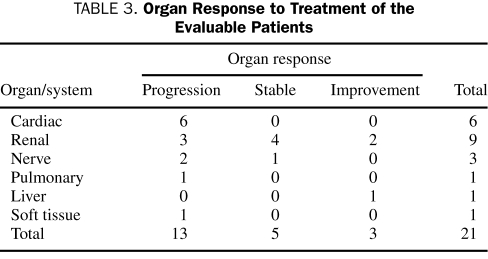
Organ Response. An organ response to treatment(s) was evaluable in 21 (45%) of the 40 patients with an organ involvement from AL amyloidosis. Overall, progression, stable organ, and organ improvement (excluding patients with surgical correction of carpal tunnel syndrome) occurred in 13 (28%), 5 (11%) and 3 (6%) patients, respectively (Table 3). Another 7 patients had surgical correction of carpal tunnel syndrome with subsequent improvement. An organ response could not be assessed in 12 patients (26%). Of these, 5 patients were lost to follow-up and died within a few months (range, 2-9 months) after the diagnosis of AL amyloidosis, 3 patients died less than 1 month after the diagnosis of AL amyloidosis, 2 patients were diagnosed at autopsy, and 2 patients had no documentation for follow-up tests to determine an organ response. Among patients with involvement of more than one organ and an evaluable organ response, 5 had progression, 1 had stable organ, and 1 had improvement. Among patients with subsequent progression of their organ disease, 8 had previously undergone a treatment change or had a new treatment initiated, whereas 5 continued taking the same treatment at the time of the diagnosis of AL amyloidosis.
TABLE 3.
Organ Response to Treatment of the Evaluable Patients
Hematologic Response. A hematologic response to any treatment of AL amyloidosis was ascertained in 30 (64%) of the 47 patients. Overall, 18 patients (38%) had SD, 11 (23%) had PR or better (PR, 4; VGPR, 6; complete response [CR], 1), and 1 had progression. Among the 13 patients with progressive organ disease, no patient had PR or better, whereas 9 patients had SD as the best hematologic response. In contrast, 2 of the 4 patients with a stable organ and 2 of the 3 patients with an organ improvement had PR or better.
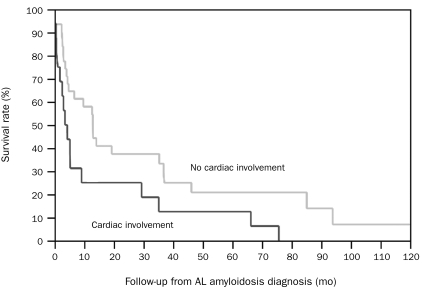
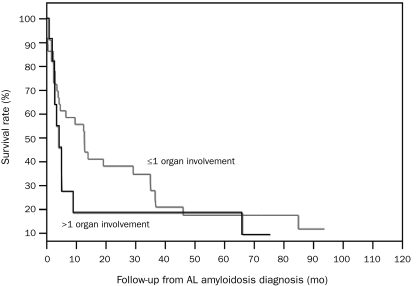
Outcome. Six patients (13%) were alive at the time of the last follow-up. None of the patients alive had cardiac involvement or involvement in more than one organ. The median OS for patients with cardiac and no cardiac involvement was 4.3 months (95% CI, 1-9) and 13 months (95% CI, 4-37) (P=.03), respectively (Figure 1); the median OS for patients with involvement in more than one organ and involvement in one organ or less was 4.3 months (95% CI, 2-9) and 13 months (95% CI, 4-35) (P=.15), respectively (Figure 2). Of the 6 patients alive, 5 had initially presented with typical symptoms of AL amyloidosis, 4 of whom developed kidney involvement and one of whom developed soft tissue (macroglossia) involvement; another patient (with an underlying diagnosis of MM and light chain deposition disease) in whom amyloid deposits were discovered incidentally on BM biopsy had no development of any organ involvement from AL amyloidosis. Four patients received HDT with PBSCT, including 2 with kidney involvement who received it as the first therapy after the diagnosis of AL amyloidosis; 1 patient with soft tissue involvement who received it a few months before the diagnosis of AL amyloidosis; and 1 patient with kidney involvement who received it a few years before the diagnosis of AL amyloidosis. Among the 11 patients with involvemen of more than one organ from AL amyloidosis, only 2 underwent a PBSCT after the diagnosis of AL amyloidosis, no patient achieved PR or better (to any treatment), and all patients had died at last follow-up. In contrast, among the 5 patients without organ involvement, 2 underwent PBSCT after the diagnosis of AL amyloidosis, 3 achieved VGPR (to any treatment), and 3 died of progression of MM or another disease.
FIGURE 1.
Kaplan-Meier survival curves for patients with cardiac involvement and no cardiac involvement; P=.03. AL = light chain.
FIGURE 2.
Kaplan-Meier survival curves for patients with >1 organ and ≤1 organ involvement; P=.15. AL = light chain.
All patients with nerve involvement have died. Seven patients had no development in any other organ and died of progression of MM and its complications or of another disease; 1 patient developed symptomatic cardiac involvement, whereas cardiac involvement was strongly suspected before death in 3 patients, but no documented echocardiographic evidence was available.
Among the 8 patients with an incidental discovery of AL amyloidosis, only 1 developed symptomatic cardiac involvement (9 years after the diagnosis of MM) and died. Of the remaining, 1 patient is still alive, 3 patients have died of MM and its complications, 1 patient died of infection, and in 2 patients diagnosis was made at autopsy.
DISCUSSION
The presence of coexistent AL amyloidosis is a well-recognized phenomenon in MM and may be more prevalent than was previously recognized. Some of the previous studies retrospectively examined tissue specimens to identify patients with coexistent amyloidosis and MM. In the current study, we specifically wanted to analyze a group of patients in whom the diagnosis of AL amyloidosis was made at least 6 months after their diagnosis of MM, to better understand the presentation and treatment responses. The light chain isotype ratio of κ to λ in our study group (>1:2) is more similar to that observed in primary AL amyloidosis (1:3) than in MM (2:1). We also observed a greater than 2-fold prevalence of light chain MM in our study group compared with the general MM population (36% vs 15%, respectively), thereby supporting the hypothesis that patients with light chain MM and/or λ light chain isotype MM may be at a higher risk of developing AL amyloidosis during their disease course.
Of our study patients, 77% presented with clinical and laboratory features commonly associated with AL amyloidosis. However, the remaining 23% of patients either developed atypical features of AL amyloidosis or had an incidental discovery of amyloid deposits during evaluation of other diseases. The findings of nephrotic range albuminuria, peripheral edema, infiltrative cardiomyopathy, hepatomegaly, paresthesias (carpal tunnel syndrome), severe fatigue, orthostatic hypotension, and syncope, as well as the highly characteristic findings of macroglossia and periorbital ecchymosis, raised the suspicion of the possibility of coexistent AL amyloidosis in patients with MM. The heart, kidney, and nerves were the most commonly involved organs. Interestingly, a personal history of carpal tunnel syndrome or its surgical correction was obtained in 5 patients. The presenting features and the organ involvement of our study group are similar to those observed in patients with AL amyloidosis that presents as the primary disease.8-12
The most important prognostic factor in patients with AL amyloidosis is the presence of cardiac involvement, with a median OS of approximately 1.1 years after diagnosis of heart involvement and 0.75 years after onset of heart failure.13 The median OS of patients with cardiac amyloidosis is shorter than that for patients without heart involvement.12 Although isolated heart involvement is not common in AL amyloidosis,13 6 of the 16 patients in our study had isolated cardiac involvement. As expected, our patients with cardiac involvement had poor outcomes; in fact, all 3 of the patients who died within a few days of the diagnosis of AL amyloidosis had cardiac involvement, and all 6 of the patients with cardiac amyloidosis and an evaluable organ response had progression of heart disease. Only 1 patient with cardiac involvement (treated with melphalan and prednisone) achieved PR or better (VGPR) as the best hematologic response. None of the patients with cardiac involvement (isolated or multiorgan) are still alive. In contrast, among patients with kidney involvement, 2 had an organ improvement and 3 achieved PR or better (PR, 2; CR, 1); 4 patients were alive at last follow up, 2 of whom had received HDT with PBSCT after the diagnosis of AL amyloidosis, indicating a better response and survival in these patients, as observed in a prior study.14
The recognition of coexistent AL amyloidosis with MM is critical and helps to determine prognosis and influence treatment. In a recent series, the presence of amyloid deposits in patients with MM was found to be an independent high-risk prognostic factor, regardless of the presence or absence of AL amyloidosis symptoms at the time of diagnosis.15 In another study, patients with coexisting AL amyloidosis and MM were found to have a poorer prognosis, with a median survival of 1.1 years vs 2.9 years in patients without AL amyloidosis.11 However, the median OS (from the diagnosis of MM) of our study population was not significantly different from the median OS (54 months) of all patients seen with a diagnosis of MM with at least 6 months of follow-up during a similar period.
Desikan et al4 reported that the presence of clinically occult AL amyloidosis does not alter the outcome of MM patients treated with single or tandem PBSCT. A similar intense therapeutic approach in MM patients with clinically occult amyloid has been suggested by some authors,15,16 but not by others.17 In our study, all 3 of the patients with an organ improvement and 2 of the 6 patients alive at the time of last follow-up had received HDT with PBSCT after the diagnosis of AL amyloidosis. However, overt involvement of the heart and kidney further compromises the performance status of this subset of patients with underlying MM. The presence of cardiac amyloidosis and multisystem organ involvement (≥2) is inversely related to survival10 and has been identified as a prognostic factor in patients with AL amyloidosis who are treated with HDT with PBSCT,18,19 resulting in high transplant-related mortality rates.16,20 Therefore, patient selection based on organ involvement is critical in identifying those who may be considered transplant eligible. Accordingly, patients in our group with multiorgan involvement were less likely to proceed to PBSCT and had a worse outcome compared with patients with no symptomatic organ involvement, who were more likely to receive PBSCT.
Although the size of our study group is small, our study highlights the strong association between hematologic response, organ response, and outcome.12 We observed that a greater number of patients with improvement of an organ had achieved a better hematologic response compared with patients with a stable or worsening organ disease. This highlights the fact that effective treatment of the underlying plasma cell clone suppresses the production of the amyloidogenic monoclonal light chain and induces improved organ function over time. However, the hematologic status at the time of the diagnosis of AL amyloidosis also provided a useful measure of the disease activity when symptoms developed. Although 26% of our patients had disease progression, 47% had SD, and 13% had PR or better, indicating the continued presence of clonal plasma cells and active deposition of amyloid. However, none of the patients had CR at diagnosis of AL amyloidosis or from MM at any time before the diagnosis of AL amyloidosis. Even the poor hematologic response rates after the diagnosis of AL amyloidosis indicate the refractory nature of the underlying plasma cell clone in these patients who received a median of 2 prior treatments before the diagnosis of AL amyloidosis. As expected, a greater number of patients with progression had a treatment change or a new treatment initiated at the time of the diagnosis of AL amyloidosis. However, the hematologic response and the organ response did not differ significantly between the 2 groups, except for the patients who received HDT with PBSCT around the time when AL amyloidosis was diagnosed.
CONCLUSION
A high degree of suspicion should be maintained and prompt investigations of patients with MM who develop findings suspicious for AL amyloidosis. Although the heart and kidney are most commonly involved in AL amyloidosis, manifestation of carpal tunnel syndrome is a cue for the development of AL amyloidosis in patients with MM, and a biopsy of the tissue specimen followed by Congo red staining should be performed. Early recognition and timely institution of effective therapy may help to suppress the abnormal plasma cell clone, with the hope of preserving or improving organ function. Prompt recognition of cardiac involvement is particularly important because such involvement adversely relates to OS and transplant-related mortality. Recognition of AL amyloidosis is important in MM patients undergoing PBSCT, who will benefit from melphalan dose adjustment. However, PBSCT may not always be feasible in this “fragile” group of patients; the combination of melphalan and dexamethasone21 can be used. Because of the paucity of published reports, current information is limited for this group of MM patients with subsequent development of AL amyloidosis. Further studies are needed to understand the prognosis and optimal management of these patients.
Acknowledgments
We thank Ms Cindy Johnson and Mr Colin Colby for their assistance with data collection for this study.
Footnotes
This study was supported in part by research grant CA 62242 from the National Cancer Institute.
REFERENCES
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362-1369 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564-569 [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Gertz MA, Kyle RA. Primary systemic amyloidosis with delayed progression to multiple myeloma. Cancer 1998;82(8):1501-1505 [PubMed] [Google Scholar]
- 4.Desikan KR, Dhodapkar MV, Hough A, et al. Incidence and impact of light chain associated (AL) amyloidosis on the prognosis of patients with multiple myeloma treated with autologous transplantation. Leuk Lymphoma 1997;27(3-4):315-319 [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA. Diagnostic criteria of multiple myeloma. Hematol Oncol Clin North Am 1992;6(2):347-358 [PubMed] [Google Scholar]
- 6.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79(4):319-328 [DOI] [PubMed] [Google Scholar]
- 7.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749-757 [PubMed] [Google Scholar]
- 8.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32(1):45-59 [PubMed] [Google Scholar]
- 9.Dispenzieri A, Merlini G, Comenzo RL. Amyloidosis 2008 BMT Tandem Meetings (February 13-17, San Diego). Biol Blood Marrow Transplant. 2008;14(suppl 1):6-11 [DOI] [PubMed] [Google Scholar]
- 10.Palladini G, Kyle RA, Larson DR, Therneau TM, Merlini G, Gertz MA. Multicentre versus single centre approach to rare diseases: the model of systemic light chain amyloidosis. Amyloid 2005;12(2):120-126 [DOI] [PubMed] [Google Scholar]
- 11.Abraham RS, Geyer SM, Price-Troska TL, et al. Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL). Blood 2003;101(10):3801-3808 [DOI] [PubMed] [Google Scholar]
- 12.Obici L, Perfetti V, Palladini G, Moratti R, Merlini G. Clinical aspects of systemic amyloid diseases. Biochim Biophys Acta 2005;1753(1):11-22 [DOI] [PubMed] [Google Scholar]
- 13.Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 1998;91(2):141-157 [DOI] [PubMed] [Google Scholar]
- 14.Leung N, Dispenzieri A, Lacy MQ, et al. Severity of baseline proteinuria predicts renal response in immunoglobulin light chain-associated amyloidosis after autologous stem cell transplantation. Clin J Am Soc Nephrol. 2007;2(3):440-444 [DOI] [PubMed] [Google Scholar]
- 15.Vela-Ojeda J, García-Ruiz Esparza MA, Padilla-González Y, et al. Multiple myeloma-associated amyloidosis is an independent high-risk prognostic factor. Ann Hematol. 2009;88(1):59-66 [DOI] [PubMed] [Google Scholar]
- 16.Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7-15 [DOI] [PubMed] [Google Scholar]
- 17.Guidelines on the diagnosis and management of AL amyloidosis. Br J Haematol. 2004;125(6):681-700 [DOI] [PubMed] [Google Scholar]
- 18.Comenzo RL, Vosburgh E, Falk RH, et al. Dose-intensive melphalan with blood stem-cell support for the treatment of AL (amyloid light-chain) amyloidosis: survival and responses in 25 patients. Blood 1998;91(10):3662-3670 [PubMed] [Google Scholar]
- 19.Moreau P, Leblond V, Bourquelot P, et al. Prognostic factors for survival and response after high-dose therapy and autologous stem cell transplantation in systemic AL amyloidosis: a report on 21 patients. Br J Haematol. 1998;101(4):766-769 [DOI] [PubMed] [Google Scholar]
- 20.Comenzo RL, Gertz MA. Autologous stem cell transplantation for primary systemic amyloidosis. Blood 2002;99(12):4276-4282 [DOI] [PubMed] [Google Scholar]
- 21.Palladini G, Perfetti V, Obici L, et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood 2004;103(8):2936-2938 [DOI] [PubMed] [Google Scholar]