Abstract
Influenza is an important contributor to population and individual morbidity and mortality. The current influenza pandemic with novel H1N1 has highlighted the need for health care professionals to better understand the processes involved in creating influenza vaccines, both for pandemic as well as for seasonal influenza. This review presents an overview of influenza-related topics to help meet this need and includes a discussion of the burden of disease, virology, epidemiology, viral surveillance, and vaccine strain selection. We then present an overview of influenza vaccine—related topics, including vaccine production, vaccine efficacy and effectiveness, influenza vaccine misperceptions, and populations that are recommended to receive vaccination. English-language articles in PubMed published between January 1, 1970, and October 7, 2009, were searched using key words human influenza, influenza vaccines, influenza A, and influenza B.
ACIP = Advisory Committee on Immunization Practices; CDC = Centers for Disease Control and Prevention; CI = confidence interval; COPD = chronic obstructive pulmonary disease; FDA = Food and Drug Administration; GBS = Guillain-Barré Syndrome; GISN = Global Influenza Surveillance Network; HA = hemagglutinin; HAI = hemagglutination inhibition; HIV = human immunodeficiency virus; LAIV = live-attenuated influenza vaccine; M = matrix; NA = neuraminidase; NI = NA inhibitor; NS = nonstructural protein; PB = polymerase basic; TIV = trivalent inactivated vaccine; WHO = World Health Organization
The current influenza A pandemic with novel H1N1 and the race to develop effective vaccines against it have increased the profile of influenza and influenza vaccination among the lay public and medical professional community, making them more likely to inquire about influenza strain surveillance and vaccine development. Furthermore, deaths attributed to novel H1N1 influenza infection have highlighted the substantial contribution of influenza infection to overall morbidity and mortality and the importance of vaccination against seasonal influenza.
Although seasonal influenza is the most common vaccine-preventable cause of death in the United States, influenza vaccination rates remain unacceptably low for all categories of people at highest risk.1-9 Both patient-related factors (eg, lack of awareness of need and concern over adverse effects) as well as physician-related factors (eg, failure of physician to recommend for it or recommendation by physician against it) contribute to poor vaccine uptake.10,11 Because misinformation and lack of physician recommendation are among the most common reasons why susceptible people do not receive vaccination, this review aims to provide a better understanding of seasonal influenza vaccines to a wide medical audience. A PubMed search for relevant English-language articles published between January 1, 1970, and October 7, 2009, was performed to find pertinent literature using the key words human influenza, influenza vaccines, influenza A, and influenza B.
BURDEN OF DISEASE
Much of the morbidity and mortality of seasonal influenza results from complications of influenza infection (eg, bacterial superinfections, exacerbations of underlying illnesses) rather than from primary influenza pneumonia itself.9,12-16 In the United States alone, influenza causes 200,000 excess hospitalizations each year.17-19 Furthermore, it appears that influenza-attributed mortality has increased over time in the United States, from 7000 to 32,000 annual deaths in the late 1970s to 36,000 to 72,000 annual deaths in the late 1990s.9 This increase in influenza-attributable mortality likely relates to host factors, such as the increasing numbers of elderly and immunocompromised persons, and viral factors, such as intrinsic virulence and the increasingly rapid global spread of new strains.
Influenza vaccination is the most effective means of protecting susceptible individuals and decreasing viral transmission within a community, prompting expansion of guidelines to recommend influenza vaccination for broader groups of people.1 The effectiveness of the vaccine is not 100% and is, in fact, least effective in those at highest risk, namely young children, the elderly, and the immunosuppressed.1 This has spurred research into new influenza vaccines and technologies, such as live-attenuated influenza vaccines (LAIVs), vaccine adjuvants, and other vaccine candidates still being developed, to protect those at greatest risk.20,21 Because of the immense clinical importance of this virus as well as the ever-changing nature of treatment and prevention of this disease, it is imperative for health care professionals to be well-versed in the topic of influenza vaccination so as to be able to identify those who could benefit from vaccination as well as to educate themselves and their patients.
VIROLOGY
Influenza is an enveloped, single-stranded, negative-sense RNA virus in the Orthomyxoviridae family of viruses. Influenza is divided into 3 major types: A, B, and C. Influenza A viruses infect a wide variety of animals, including humans, birds, pigs, horses, and many others, although the tropism of any particular influenza virus is generally highly adapted to a particular host. Influenza B viruses infect a smaller number of species, namely humans and seals, and are a substantial cause of annual influenza epidemics. Most human influenza infections are caused by influenza A or B; influenza C viruses, which infect humans and pigs, rarely account for human infections and epidemics.22
The influenza genome is segmented. Influenza A and B viruses have 8 segments in their genome, and influenza C viruses have 7. The major influenza types also differ in that influenza A and B viruses express hemagglutinin (HA) and neuraminidase (NA) as surface antigens, whereas influenza C viruses express an HA-esterase-fusion protein on their surface.22 Because influenza A and B are the cause of most epidemics and are the intended targets of seasonal influenza vaccination, the following discussion on virology will focus on these 2 virus types.
The 11 proteins of influenza A and B are encoded by 8 gene segments. Hemagglutinin and NA are expressed on the surface of the virus and are required for entry and exit, respectively, from the host cell (Figure 1).23 Influenza A viruses are subtyped on the basis of the major subtype of HA and NA expressed (eg, H3N2, H1N1). Matrix 2 (M2) protein is a transmembrane ion channel that acidifies the viral interior to allow for replication. Polymerase basic (PB) protein 1, PB protein 1-F2, PB protein 2, and polymerase acidic protein form influenza RNA polymerase. Like all RNA viruses, influenza RNA polymerase lacks a proofreading mechanism, resulting in frequent mutations in these genes and, consequently, a constantly changing antigenic appearance. Nucleoprotein combines with the 3 polymerase proteins to form ribonucleoprotein complexes that are transported to the nucleus by the M1 protein. Nonstructural proteins 1 and 2 are involved in expression of viral proteins and viral replication, respectively.22,24 Influenza viruses can undergo reassortment of these gene segments; ie, 2 or more influenza viruses infecting the same cell can exchange gene segments, thus creating a new hybrid virus with gene segments derived from the parent viruses.
FIGURE 1.
Structure of the influenza virus. The 8 gene segments are contained within a viral envelope with hemagglutinin (HA) and neuraminidase (NA) forming most of the antigenic determinants. The portion of the matrix 2 (M2) protein that is outside the viral envelope is antigenic. PA = polymerase acidic; PB = polymerase basic.
From Science,23 with permission.
MAJOR IMMUNOGENIC PROTEINS
Hemagglutinin is a glycopeptide expressed on the surface of influenza viruses that facilitates entry of the virus into host cells.25 It is so named because of its property of agglutinating red blood cells. The tropism of an influenza virus to a specific host is, in large part, mediated by HA.26,27 Titers of host antibodies directed against HA, as measured by a hemagglutination inhibition assay (HAI), represent most neutralizing antibodies directed against influenza and have correlated with protection from viral challenge.28,29 Across viruses affecting different host species, 16 major HA subtypes of influenza A have been identified.30
Neuraminidase is an influenza surface glycopeptide that is responsible for release of viral progeny from an infected cell.22 Nine major NA subtypes of influenza A have been identified. Unlike antibodies directed against HA, NA-specific antibodies alone are not sufficient to prevent infection but may be able to mitigate the severity and duration of disease.31-33 During the 1968 Hong Kong influenza pandemic, vaccine directed at an H2N2 virus had 54% efficacy against the pandemic H3N2 strain, suggesting partial protection conferred by antibodies directed against NA.34 Neuraminidase is also the target for the NA-inhibitor (NI) class of antivirals (eg, oseltamivir and zanamivir).
The M2 protein is a transmembrane proton channel protein responsible for acidifying the virion to allow replication.35 Although only a few of the antibodies generated after influenza infection are directed against M2, these antibodies have been shown to be protective in animal studies.36-40 Because M2 sequences have been highly conserved among disparate influenza strains during the past century, even pandemic strains, the M2 protein is being investigated as a target for a potential “universal” influenza vaccine.21,41-45 The M2 protein of influenza A is also the target of the adamantane class of antivirals (eg, amantadine and rimantadine).
ANTIVIRAL DRUGS
Two classes of antiviral medications are currently used to treat and prevent influenza infections, the adamantanes and NIs.46 The adamantanes amantadine and rimantadine act on the M2 protein of influenza A, although a single mutation at amino acid 31 in the matrix gene renders high-level resistance to these medications.47 The adamantanes are not effective against influenza B, and the development of wide-spread adamantane resistance in seasonal H3N2 (99%) and seasonal H1N1 (10%) strains during the 2008-2009 season has limited their utility.1 Two NIs are available for treatment of influenza A and B infections: oseltamivir (oral) and zanamivir (inhaled). Oseltamivir-resistant viruses have recently increased in circulation, especially among seasonal H1N1 strains (99%).48,49 Mutation at amino acid 274 is most commonly associated with NI resistance, but mutations at amino acids 292 and 294 have also conferred resistance.48 At this time, zanamivir-resistant viruses have not emerged, and experimental methods to generate zanami-vir-resistant influenza have not yielded viable viruses.46,50 Thus, oseltamivir-resistant viruses have remained sensitive to zanamivir.
When the strain type is unknown and both novel H1N1 and seasonal influenza are circulating, empiric treatment with either zanamivir alone or oseltamivir in combination with rimantadine should be used for infected patients at high risk of complications and for low-risk patients who present within 48 hours of symptom onset.46 Influenza B infections can be treated with oseltamivir or zanamivir. Influenza A (seasonal H1N1) infections can be treated with either rimantadine or zanamivir, and influenza A (H3N2) infections can be treated with oseltamivir or zanamivir. Early in the current influenza pandemic, most isolates of novel influenza H1N1 have been resistant to the adamantanes but have, with rare exception, remained susceptible to oseltamivir, and treatment with oseltamivir or zanamivir is recommended.51,52 Some concerns have been published regarding the development of antiviral resistance with monotherapy.53 In adults, the use of NIs has been shown to reduce influenza symptoms by approximately 1 day as well as influenza-related complications and hospitalizations.54-57 Studies of NIs in children have shown a reduction in influenza symptoms by approximately 1.25 days as well as a reduction in influenza complications and subsequent physician visits.58-61 Given the rapidly evolving nature of antiviral resistance, updated recommendations, such as those by the Infectious Diseases Society of America, the Centers for Disease Control and Prevention (CDC), and the American Academy of Pediatrics, should be consulted when deciding on treatment or chemoprophylaxis for patients.46,62
EPIDEMIOLOGY
Antigenic Evolution of Virus
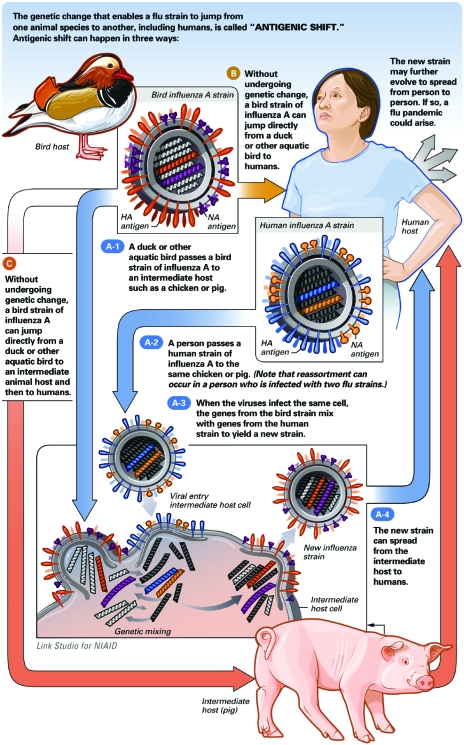
Circulating influenza viruses are constantly mutating. Minor antigenic changes due to random mutational events, called antigenic drift, are responsible for annual influenza epidemics. The changes to HA and NA that occur help the virus evade the immune response generated in a host population through prior infection or vaccination. Much less frequently, but with much more dire consequences, a circulating strain of influenza will develop with a new major antigenic type of HA or NA to which the population has no prior exposure (Figure 2). These antigenic shifts are responsible for influenza pandemics, characterized by infection so widespread as to cause societal disruption and global mortality often measured in the millions. Antigenic shifts can occur as a result of direct mutational changes of a zoonotic influenza to produce efficient human-to-human transmission. Such a mutational change likely occurred during the 1918 “Spanish Flu” pandemic; evidence suggests that a highly pathogenic avian influenza H1N1 virus acquired mutations to easily infect humans.64,65 Alternatively, antigenic shifts can occur by reassortment, whereby 2 influenza viruses of different host tropism infect a common host cell and lead to the creation of an influenza virus with the antigenic determinants of one influenza virus and the host tropism and pathogenicity of another. An example of this is the 1968 “Hong Kong Flu,” in which a human H3N2 influenza virus emerged after reassortment of a human H2N2 virus and an avian H3N2 virus, likely in a common swine host.66,67 The gene segments of the current novel H1N1 pandemic implicate reassortment of human, swine, and avian viruses as the source of this pandemic strain.68
FIGURE 2.
How novel influenza strains are introduced into humans. HA = hemagglutinin; NA = neuraminidase. From National Institute of Allergy and Infectious Diseases (NIAID) Web site.63
The World Health Organization (WHO) developed the Global Influenza Surveillance Network (GISN) in 1947 to track the development and migration of influenza drift mutants for the purpose of vaccine strain selection.69 The network consists of 125 centers in 96 countries and continues to grow in order to broaden its reach and increase the depth of its global surveillance.70 The local centers collect samples of circulating viruses and send them to 1 of 4 WHO Collaborating Centers for Reference and Research on Influenza (located in Atlanta, United States; London, United Kingdom; Melbourne, Australia; and Tokyo, Japan).
Once received at one of the collaborating centers, HAI testing is performed on the virus using antisera generated against it and similar strains. Comparing the HAI titers of the virus with respect to different antisera allows the antigenic similarity of the virus to other strains to be determined.70 Hemagglutination inhibition assay data from the 4 collaborating centers are sent to the Centre for Pathogen Evolution at the University of Cambridge for antigenic mapping. Antigenic mapping allows for a quantitative method as well as a visual depiction of antigenic similarity of an influenza strain to other strains.71 The antigenic distance on the map between 2 strains is determined by the logarithm of the HAI of one virus strain using the antisera of the other strain. Data for the antigenic map for influenza A (H3N2) are available from the time of its emergence during the Hong Kong flu pandemic in 1968.71 After a new viral strain has been identified, it is named using the convention of influenza type, geographic origin, strain number, year of isolation, and viral subtype, as for example A/New Caledonia/20/1999 (H1N1), A/Wisconsin/67/2005 (H3N2), B/Malaysia/2506/2004, or A/Vietnam/1203/2004 (H5N1).
Sampling by the WHO GISN has been able to describe the antigenic evolution of circulating strains of influenza viruses throughout the world; however, it is important to emphasize the constant genetic evolution of influenza viruses even in small geographic areas during short periods. Because of the lack of proofreading of the influenza RNA polymerase, periods of increased influenza activity also mean periods of increased influenza genetic diversity. Although these drift mutants are often shown to be antigenically similar to the major circulating strain using conventional HAI methods, numerous subpopulations exist with slight antigenic variation. An Austrian study performed RNA sequencing and HAI assays on influenza viruses recovered from Fall 2002 through Spring 2005 and found that the minor cocirculating drift variants discovered by sequencing were genetically and antigenically similar to the major circulating strain of the following influenza season.72 This underscores the importance of the minor drift variants that emerge during influenza outbreaks and the need to continue to evolve our strategies for viral surveillance.
Global Migration Of Influenza Viruses
Several mechanisms have been postulated to explain how a particular influenza strain spreads across the world and becomes the dominant circulating strain.70 Some mechanisms, such as seasonal migration between hemispheres and emergence from a common geographic area, are considered major mechanisms and others, such as low-level persistence of virus circulating within particular locales, are considered minor.70 Seasonal migration and emergence from a common geographic area are supported by studies such as those by Nelson et al,73 in which phylogenetic analysis was performed on all influenza A (H3N2) strains recovered from New York State, Australia, and New Zealand in successive years. They found that strains circulating in Australia and New Zealand were related to strains previously circulating in New York State, and vice versa, suggesting migration of influenza strains between the northern and southern hemispheres during seasonal changes, migration of strains originating and propagating from a common area, or a combination of the two. A subsequent study by Russell et al74 performed antigenic and phylogenetic analysis on influenza strains recovered from around the world. By evaluating the chronological and geographic appearance of particular influenza strains, they found that most circulating influenza strains originate in East-Southeast Asia and then spread to Oceania, Europe, and North America and then to South America. Their data also suggest that influenza strains are able to be maintained in the “East-Southeast Asian circulation network” by migrating between countries in the region before spreading globally. Factors contributing to the maintenance of influenza strains within the East-Southeast Asian circulation network are temporal overlapping of influenza outbreaks and substantial travel between countries in the region.70 Studies demonstrating that minor cocirculating drift variants can become the major circulating strain in subsequent years72 support local persistence of viral strains as a mechanism contributing to the dominant circulating strain; however, further evidence suggests that this is only a minor mechanism.70,71,73-75 Although phylogenetic analysis of influenza A (H3N2) strains collected in New York State from 1997 to 2005 found substantial local genetic diversity generated within circulating influenza strains during periods of influenza activity as well as frequent reassortment between viruses, the major contributor to genetic diversity and subsequent dominant strains was found to be the introduction of new strains, likely through migration of human influenza viruses.74,75
VACCINE STRAIN SELECTION
How Are Vaccine Strains Selected?
The WHO meets twice annually to review surveillance data and make recommendations as to which strains should be contained in the following season's influenza vaccine; recommendations for the northern hemisphere are made in February, and recommendations for the southern hemisphere are made in September each year. Two influenza A strains (one H3N2 and one H1N1) and an influenza B strain are recommended for vaccine inclusion after analysis of WHO GISN data. Committee members involved in vaccine composition recommendations include representatives from the 4 Collaborating Centers for Reference and Research on Influenza as well as representatives of several key national laboratories.76
In the United States, the WHO recommendations are considered, but the final vaccine composition recommendations are made by an advisory committee to the Food and Drug Administration (FDA). Several factors are taken into consideration when making recommendations regarding influenza vaccine composition: (1) predictions of what viral strains are likely to cause the following season's influenza epidemics on the basis of worldwide surveillance data, (2) the antigenic similarity of a chosen vaccine strain to the predicted circulating strain, (3) the immunogenicity of a selected strain to develop adequate humoral immunity, and (4) the suitability of a viral strain for use in vaccine production (eg, does it grow to high viral titer in eggs?).77 The strain recommendations are based on data from the CDC, the US Department of Defense, and vaccine manufacturers. The FDA recommendations for vaccine composition are often identical to the WHO recommendations, but this is not always the case.
How Well Are Vaccine Strains Matched to Circulating Virus?
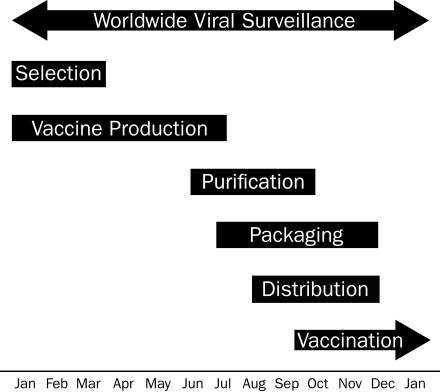
The rigorous process of vaccine candidate selection is important because the effectiveness of the vaccine can be reduced if the vaccine components are not well matched to the circulating strains.1,78-84 The process of vaccine production takes 6 to 8 months from the time of candidate strain recommendation to actual production and delivery (Figure 3), permitting time for substantial changes to the antigenic appearance of the predominant circulating strains. How well the influenza vaccine will work in a particular season can be predicted by the antigenic distance between the vaccine candidate and the circulating strain. Antigenic distance is determined using the HAI titer of the circulating strain in the presence of antisera to the vaccine candidate. An antigenic distance of 2 units (4-fold change in HAI titer) is considered to be a substantial antigenic difference and suggests a sub-optimal match between vaccine candidate and circulating strain.74 The pattern of antigenic changes in the predominant strain has been such that generally only minor changes occur from year to year, with relatively abrupt changes occurring sporadically.71 In most years, the vaccine candidates are antigenically very similar to the circulating strains even though antigenic drift had undoubtedly occurred in the circulating strains. An unanticipated large change in the antigenic appearance of circulating strains results in a poor match and a less effective influenza vaccine. For example, during the 2007-2008 influenza season, an A/Wisconsin/67/2005 (H3N2)—like virus was recommended for the vaccine but an A/Brisbane (H3N2)—like virus became the predominant circulating strain.85 In years when the vaccine candidates are not well matched to the circulating strains, enough antigenic similarity generally still exists to confer some benefit, especially in preventing severe outcomes.1,78,80,81,86
FIGURE 3.
Northern hemisphere influenza vaccine production time-line. Global viral surveillance occurs year-round, which informs the selection of the vaccine strains that occurs between January and March of each year. Vaccine production begins in January and continues through July. Purification and testing start in June and continue through October. Single-dose syringes and multidose vial lots are filled and packaged between July and December and are distributed between August and December for vaccination between late September and the end of the influenza season (often in spring).
VACCINE MANUFACTURE
After the 3 strains are selected for influenza vaccine inclusion by the FDA, the CDC provides reference viral seed strains to the FDA, which subsequently distributes these viruses to the vaccine manufacturers. For trivalent inactivated vaccine (TIV), the vaccine strains are grown separately in embryonated chicken eggs and then harvested. After being harvested, the viruses are inactivated with formalin, and the HA and NA subunits are released by disrupting the lipid envelope to produce split-virion vaccine. The 3 vaccine strains are then combined and packaged as single-dose syringes or multidose vials with 15 μg of each of the 3 subunits contained in each dose of vaccine.87 The amount of virus produced in each embryonated chicken egg is dependent on the growth characteristics of the reference virus strains. On average, approximately one egg is required to produce one dose of one vaccine strain.
Live-attenuated influenza vaccine is produced by reassortment of each of 3 reference strains with a cold-adapted vaccine strain such that the resultant viruses have the HA and NA genes of the reference strain and the 6 internal genes of the cold-adapted strain. The cold-adapted strain was originally created by passaging influenza A/Ann Arbor/6/1960 at successively lower temperatures until a mutated strain emerged that was cold adapted, temperature sensitive, and attenuated (grows at 25°C, not at core body temperatures, and does not produce systemic symptoms of influenza disease).20,88 The 3 reassortant cold-adapted influenza vaccine strains each season are grown in embryonated chicken eggs, harvested, and then combined to create LAIV doses.
Each lot of TIV and LAIV is evaluated by the FDA before delivery, which generally begins in September each year with most doses delivered by October. Highly publicized influenza vaccine shortages have occurred, underscoring the need to develop new production technologies as well as to work with manufacturers to ensure a stable vaccine production capacity.89 Previous shortages have resulted from contamination of embryonated eggs, underestimation of vaccine demand, reduction in numbers of vaccine manufacturers, and influenza outbreaks that occur earlier than anticipated, among others. Strategies for addressing some of these issues have been proposed.89 A more in-depth discussion of new technologies that may mitigate some of these problems is beyond the scope of this review but has been published elsewhere.21
INDICATIONS
The Advisory Committee on Immunization Practices (ACIP) of the CDC annually updates its recommendations for seasonal influenza vaccination. The current recommendations for seasonal influenza vaccination are summarized in Table 1 and those for the H1N1 influenza vaccination in Table 2.1 Trivalent inactivated vaccine and LAIV are contraindicated in persons with anaphylactic egg allergy, and the risks and benefits must be weighed with caution before administration of influenza vaccine to an individual with a history of Guillain-Barré Syndrome (GBS) developing within 6 weeks after influenza vaccine receipt; LAIV has similar contraindications and has been licensed by the FDA only for use in nonpregnant persons aged 2 to 49 years who have no risk factors for increased morbidity from influenza.1 Seasonal influenza dosing recommendations are summarized in Table 3.
TABLE 1.
Populations Recommended for Annual Seasonal Influenza Vaccination
TABLE 2.
Populations Recommended for 2009-2010 Pandemic H1N1 Influenza Vaccination
TABLE 3.
Seasonal Influenza Vaccine Dosinga
EFFICACY AND EFFECTIVENESS
Studies evaluating the degree of protection conferred by influenza vaccines generally relate their results in terms of efficacy or effectiveness; the distinction between the two terms is subtle but very important. Efficacy refers to the reduction in culture-confirmed influenza illness, whereas effectiveness refers to the reduction in other secondary end points, such as influenza-like illness, pneumonia, hospitalization, and mortality. The efficacy and effectiveness of an influenza vaccine are dependent on host factors such as age, underlying comorbid conditions, and immune competence, as well as vaccine factors such as the type of vaccine (TIV vs LAIV) and the degree of antigenic match between the vaccine strains and circulating strains. Serologic data suggest that the timing of vaccination is also important because protective antibody levels are generally not developed until 2 weeks after vaccination.92,93
Healthy Adults
In adults younger than 65 years who have no substantial medical comorbid conditions, influenza vaccination is highly effective and efficacious.1,94 During years when the influenza vaccine is well matched to the circulating strains, TIV is efficacious in preventing 70% to 90% of culture-confirmed influenza illness; efficacy is reduced to 50% to 80% or lower in years when the vaccine is not well matched.1,79,81,95-98 Meta-analyses have found the effectiveness of TIV against influenza-like illnesses to be 16% to 42%.96,99 The benefit of influenza vaccination of healthy adults is best demonstrated by the effectiveness of TIV in reducing hospitalization by 90%, upper respiratory infections by 25%, physician visits by 44%, and sick days off of work by 43%.80,98
In a year of poor antigenic match, a randomized study found that LAIV was still effective in reducing febrile upper respiratory illnesses by 17%, days of work missed by 16%, and physician visits by 17%.86 Studies directly comparing the efficacy of LAIV to TIV in healthy adults have not shown a clear advantage of one vaccine over the other in this population and at times have had conflicting results.1,20,81,94,97,98,100-103 A randomized trial in healthy volunteers during a year of poor vaccine match found an efficacy of 77% for TIV and of 57% for LAIV; however, the risk difference of 46% (95% confidence interval [CI], -44% to 82%) was not statistically significant.81 A population-based, cohort study of more than a million military personnel during 3 influenza seasons found a significant increase in effectiveness in clinically diagnosed pneumonia or influenza for those receiving TIV rather than LAIV (reduction in incidence, 19.8 per 1000 person-years; P<.001) in 1 year of the study.101 A subsequent retrospective cohort study of military personnel during 2 influenza seasons found conflicting results. Nonrecruits had greater protection from TIV than from LAIV, with an adjusted incidence rate ratio of 1.17 (95% CI, 1.14-1.20) during the first season and 1.33 (95% CI, 1.30-1.36) during the second season. However, Army and Air Force recruits had greater protection from LAIV than TIV; among LAIV vs TIV recipients, adjusted incidence rates of influenza-like illness were 22% to 51% lower during the first year of the study and 8% to 47% lower during the second year.100 These conflicting results may be secondary to confounding by differences in age, prior vaccination, and circulating antibody levels.
Older Adults
The burden of influenza morbidity and mortality is most evident in the elderly population, and people older than 65 years are least likely to mount a protective immunologic response to influenza vaccine.1,94 The degree of protection afforded by influenza vaccination in elderly people has recently been readdressed in the literature. A randomized trial of people older than 60 years who had no substantial medical comorbid conditions found that those receiving TIV had a 47% lower risk of developing clinical influenza infection and a 58% lower risk of having serologic evidence of influenza infection compared with those receiving placebo.104 However, cohort studies have found the vaccine to be substantially less effective in protecting elderly nursing home residents from respiratory infections; effectiveness rates range from 20% to 40% in years of good antigenic match, whereas no appreciable protection is afforded in years of poor antigenic match.1,105-108 Some have argued that the evidence of protection derived from these cohort studies was subject to selection bias.109,110 Jackson et al110 conducted a cohort study in which vaccinated elderly nursing home residents had a 39% lower relative risk of death during the period before influenza season, suggesting that healthier elderly people were more likely to be vaccinated. A subsequent cohort study of community-dwelling adults older than 65 years including more than 700,000 person-seasons of observation found a 27% reduction in risk of hospitalization due to pneumonia or influenza and a 48% reduction in risk of death among those receiving TIV.111 Analysis of data during periods outside of influenza season found no evidence of selection bias toward healthier people receiving vaccines. The limited data on the efficacy of LAIV in elderly people have not shown any benefit in this population, and it is currently not recommended for those aged 50 years and older.1,112,113
Children
Although children younger than 5 years account for fewer than 100 of the approximate 36,000 annual deaths in the United States attributable to seasonal influenza, young children have substantial morbidity related to the infection, with those younger than 5 years having a 0.6 to 1.5 per 1000 risk of hospitalization each year and those younger than 5 months having a 2.3 to 4.5 per 1000 risk.114 Influenza-infected children also shed virus in greater quantity and for longer duration than adults, permitting spread of infection to others, including immunocompromised and elderly people. Influenza vaccination is able to reduce viral shedding in children who become infected and thereby to reduce influenza transmission.115-119 As a result of immunologic naivety to influenza antigens, children younger than 9 years should receive 2 doses of influenza vaccine, 1 month apart, during the first year of vaccination to have adequate protection.1,120,121
Meta-analyses have found the efficacy against confirmed disease in healthy children to be 59% to 65% for TIV and 72% to 82% for LAIV; the efficacy increases with increasing age and decreases during years of poor antigenic match.94,95,122,123 Current data suggest that LAIV has superior efficacy to TIV in healthy children, at least in seasons in which the match between the circulating virus and the vaccine virus is poor.20 Randomized trials directly comparing the efficacy of TIV and LAIV have demonstrated an advantage to LAIV compared with TIV. A randomized trial by Ashkenazi et al124 of more than 2000 children found a 50% relative reduction in culture-confirmed influenza infection in those receiving LAIV vs those receiving TIV. Belshe et al125 found a similar 55% relative reduction in risk of laboratory-confirmed influenza in a randomized trial of more than 8000 children, and Fleming et al126 found a 35% relative reduction in risk of laboratory-confirmed disease among more than 2000 asthmatic children.124-126
Vaccination of children has also been shown to decrease the burden of disease in other children, household contacts, and community-dwelling elderly.127-131 A randomized trial found that influenza vaccination of children in day-care centers was able to reduce febrile respiratory illness by 42% among unvaccinated household contacts and more profoundly affected school-aged household contacts, with an 80% reduction in febrile respiratory illnesses and greater than 70% reduction in days of school missed as well as physician visits.132 Furthermore, a Russian study found that mass vaccination of children, with vaccine coverage of 57% of those aged 3 to 6 years and 72% of those aged 7 to 17 years, correlated with a 3.4-fold reduction of influenza-like illnesses among unvaccinated, community-dwelling elderly.131
SPECIAL POPULATIONS
Health Care Professionals
Health care professionals are in frequent contact with influenza and, because healthy adults often develop asymptomatic influenza infection, unvaccinated health care professionals can unknowingly spread the virus to the patients under their care.133,134 In settings of low health care professional influenza vaccination, influenza can be a substantial cause of nosocomial pneumonia and can lead to outbreaks with potentially devastating consequences in susceptible hosts.135-137 Among health care professionals at the University of Virginia Health System, an increase in influenza vaccination from 4% to 67% during the course of a decade corresponded with a reduction in influenza infection among health care professionals from 42% to 9% and a reduction in the proportion of nosocomial influenza cases from 32% to 3%.138 A study of long-term care facilities in Scotland that offer influenza vaccination for their health care professionals vs those that do not found a 40% reduction in all-cause mortality among residents of the care facilities in which vaccination was offered to health care professionals (with 51% vaccine receipt in intervention facilities compared with 5% in control facilities).139 For health care professionals in contact with highly immunocompromised patients, TIV is preferred over LAIV if available, but LAIV is preferred over not receiving vaccine at all.1 Despite recommendations for vaccination since 1981, influenza vaccine receipt among health care professionals is around 44%, prompting calls for, and implementation of, mandatory influenza vaccination for health care professionals.140-146 Legislation has been passed in Alabama, Arkansas, and Kentucky requiring influenza vaccination for health care professionals in long-term care facilities.147 Several institutions, such as Virginia Mason Clinic and the Barnes-Jewish Hospital System, have adopted mandatory health care professional influenza vaccination policies with superb results in uptake. Most recently, New York State joined California by passing legislation requiring health care professionals to receive influenza vaccine annually.147 Because of vaccine shortages, this will likely not be implemented until the 2010-2011 season. In early October 2009, the Infectious Diseases Society of America released their clinical standard that both seasonal and pandemic H1N1 influenza vaccines be mandatory for all health care professionals.148
Pregnant Women
Pregnant women are at increased risk of complications from influenza infection, including hospitalization for acute cardiopulmonary disease at a rate similar to that of people with chronic medical conditions, as well as fetal malformation.149-153 Furthermore, infants younger than 6 months are at increased risk of complications from infection and rely on maternal antibodies for protection. Vaccination with TIV has been shown to be safe and effective for pregnant women, prompting the CDC and the American College of Obstetricians and Gynecologists to recommend annual influenza vaccination for all women who will be pregnant during the influenza season.1,154 A randomized controlled trial found an efficacy of 36% in preventing laboratory-confirmed influenza disease in the pregnant mother and an efficacy of 63% in preventing laboratory-confirmed influenza in her infants up to the age of 6 months.155 Because it is a live vaccine, LAIV is not recommended in women known to be pregnant.1
Immunocompromised Hosts
Patients with impaired immunity due to conditions such as human immunodeficiency virus (HIV) infection, malignancy, and organ transplant or those who require immunosuppressant medications are at increased risk of developing complications from influenza infection.156 Before the era of highly active antiretroviral therapy, patients with AIDS had an increased risk of death from respiratory infection during influenza season compared with controls (0.94-1.26 per 1000 person-years vs 0.009-0.01 per 1000 person-years for controls).157 Highly active antiretroviral therapy has reduced cardiopulmonary hospitalization rates by 53% and deaths by 77%, but patients with chronic HIV infection are still at higher risk of complications from influenza infection than the general population.158 Vaccination of HIV patients with TIV has been found to be safe and does not affect CD4 count or progression to AIDS or death; accounts of low-level and transient “blips” in viral load after vaccination have not been associated with any observable consequences.159 Vaccination with TIV has also been shown to be efficacious: a prospective cohort study of HIV patients found a reduction of laboratory-confirmed influenza infection from 21.2% to 6.1% among vaccinated patients.160 A trial randomizing 102 HIV-positive patients to receive TIV or placebo found a 20% absolute risk reduction in symptomatic respiratory infection and a 100% reduction in laboratory-confirmed influenza infection.161 At this time, LAIV is not recommended for use in HIV patients, although some studies have found LAIV to be safe in HIV-infected adults and children, especially in those with high CD4 counts.1,162-164
Cancer patients have high rates of influenza as a result of the immunologic changes from the underlying malignancy, chemotherapy, or radiation.165,166 The case fatality rate can be as high as 33% in those at highest risk, such as those with hematologic malignancies undergoing chemotherapy.167 Efficacy studies are not available in this population; however, antibody studies suggest that cancer patients are able to mount an immune response to TIV, especially when it is given between rounds of chemotherapy, although the response is lower than that of the general population.156,168-174 Given the substantial consequences of infection, the safety of TIV, and the likely benefit from immunization, ACIP has recommended that this population receive annual influenza vaccination.1
Solid organ transplant recipients, especially lung transplant recipients, are at increased risk of influenza infection and its complications, including precipitation of allograft rejection.175-179 Effectiveness and efficacy data are also lacking in this population; however, many studies have shown that solid organ transplant recipients are able to mount an immunologic response to vaccination, albeit one that is less than that expected from the general population.156,180-191
Vaccination with TIV is also recommended for those receiving long-term immunosuppressive therapy (eg, long-term systemic corticosteroid therapy).1 Data regarding its efficacy and effectiveness in patients taking immunosuppressants are difficult to generalize because the studies are conducted on the basis of the underlying condition requiring the immunosuppressant rather than the immunosuppressant itself. Nonetheless, conditions requiring long-term corticosteroid therapy are associated with an increased risk of influenza infection and complications, and TIV has been shown to be safe and immunogenic in this population.1,156
Chronic Pulmonary Diseases
Influenza is known to precipitate chronic obstructive pulmonary disease (COPD) exacerbations and accounts for 7.5% of hospital admissions for COPD.192,193 Influenza vaccination with TIV was shown to reduce hospitalization for pneumonia and influenza by 48% and mortality by 30% in elderly patients with COPD.194 A large Taiwanese study found influenza vaccination to be associated with a 45% reduction in mortality in patients with COPD.195 Annual vaccination is recommended for patients with COPD, but only with TIV at this time. A study randomizing more than 2000 patients with COPD to receive TIV and LAIV or TIV and placebo found LAIV to be safe in this population.1,112
Asthmatic children and adults also have a large burden of influenza disease, which can result in hospitalization and death.57,196,197 Vaccination with TIV has been found to be safe in this population, specifically with regard to the concern for the development of acute asthma exacerbation after vaccination, and is recommended annually for those with asthma, although no clear evidence supports its efficacy.1,198-200 Because its safety in this population is uncertain, LAIV is not currently recommended for use in people with asthma; a randomized trial found an increase in clinically relevant wheezing among those younger than 24 months in the 24 hours after receiving LAIV, regardless of whether they had asthma.125
Chronic Heart Disease
The exacerbation of underlying cardiac comorbid conditions is a substantial contributor to overall influenza hospitalization and death.9,17 A study of nearly 35,000 people who had died of acute myocardial infarction or chronic ischemic heart disease found a 30% increased odds of death from acute myocardial infarction and a 10% increased odds of death from chronic ischemic heart disease during the times of peak influenza season compared with other times of the year.201 Influenza vaccination with TIV has been found to be beneficial in this population and is recommended by the ACIP.1 A cohort study of more than 1300 community-dwelling elderly adults with coronary artery disease or congestive heart failure found a 37% reduction in adjusted risk of winter mortality among those receiving influenza vaccine, reducing the attributable risk by 8.2 deaths per 1000 person-winters.13
COMMON VACCINE MISPERCEPTIONS AND FEARS
Misperceptions regarding influenza vaccine, most often concerning adverse effects and efficacy, are common reasons why people recommended to receive vaccine forgo immunization.202-204 As already discussed, influenza vaccination has clear efficacy in reducing the burden of disease. It is common to hear that people did not receive influenza vaccination because they or someone they know “got the flu” from prior vaccination. The reason for this misperception becomes clear when one takes into account that influenza vaccination is given during the late fall and winter when rhinoviruses and other respiratory viruses are in high circulation. Because TIV used in the United States is a subunit vaccine containing only inactivated antigenic determinants without any viral genetic material or even a complete set of viral particles, it is biologically impossible for the vaccine to cause infection. Study after study has confirmed that adults receiving TIV are no more likely than those receiving placebo to have upper respiratory illnesses after vaccination; in fact, the only consistent difference between the 2 groups in adults is that those receiving TIV are more likely to have soreness at the injection site.1,98,205-207 Similar results have been seen with LAIV, in which mild and transient rhinorrhea and low-grade fever were more common in LAIV recipients than in those receiving placebo.20,86,125,208,209
The rate of GBS of 1 attributed case per 100,000 doses after vaccination for swine flu in 1976-1977 has raised concern regarding influenza vaccination among those with a history of GBS.210-212 Subsequent studies of seasonal influenza vaccine have found little to no increase in the risk of GBS after vaccination compared with baseline.213 The absolute risk of GBS after seasonal influenza vaccination is very small, with studies unable to exclude an attributable risk as high as 1 case per 1,000,000 doses.214-218 However, the risk of GBS in people with prior GBS is greater than that in the general population and, as a precaution, the ACIP has recommended that people who are not at high risk of complications from influenza infection but who have had GBS develop within 6 weeks after influenza vaccination forgo influenza vaccination and consider antiviral prophylaxis instead.1 For people who have a history of GBS after influenza vaccination and are at high risk of influenza complications, the risks and the benefits of influenza vaccination should be discussed in depth, especially because the risk of GBS after influenza infection is greater than the risk after influenza vaccination.214-219
The misperception of thimerosal being linked to neurodevelopmental disorders has been widely publicized in the lay press. Thimerosal is used as a preservative in multidose vials of TIV but is not present in single-dose vials of TIV or in LAIV. Concerns of a link between thimerosal and neurodevelopmental disorders have been exhaustively studied and conclusively refuted.220-224 In fact, the initial publication in Lancet that first raised the issue225 was discovered to have been founded on falsified data created by an investigator who was under the employ of an attorney representing a family of a child with autism226; that paper has been retracted by 10 of the 12 original authors.227
Some in the lay public have endorsed the notion of children attaining immunity through natural infection by wild-type viruses rather than through vaccination. Often forgotten is the immense morbidity and mortality in children caused by natural infections that have been nearly eliminated through vaccination.6 The case for vaccination is especially important for influenza because children have high annual attack rates with substantial morbidity, are integral to the transmission of virus to other highly susceptible hosts, and are likely not protected by prior infection because of the virus's constant antigenic changes.114,127-131
Others have expressed concern that children are receiving too many vaccinations and that the number of antigens to which they are exposed through immunization may overwhelm or weaken the immune system. The immune systems of infants, children, and adults are able to respond to a vast number of antigens, and it has been estimated that infants have the capacity to respond to 10,000 vaccines at one time.228 Furthermore, although the number of vaccinations required for children has increased since 1960, the total number of antigens in the recommended childhood series has dramatically decreased from approximately 3200 in 1960 to approximately 125 in 2000.228 This has been accomplished through newer vaccine formulations, such as acellular pertussis vaccine and split virion influenza vaccine, that have limited the vaccine components to the most protective antigenic determinants. Children have shown the ability to respond to multiple simultaneous vaccinations as they would if the vaccinations were given independently, negating the theoretical basis of alternative vaccination schedules that have gained recent popularity.228,229 The idea that vaccines weaken the immune system has been repeatedly disproven as vaccinated children are not more susceptible to subsequent infections from other pathogens than children who did not receive vaccine.228,230-232
Because children have substantial morbidity from influenza infection, spread the infection to others, and receive considerable protection from vaccination, it is imperative that unfounded fears do not become barriers to vaccination against influenza or other preventable diseases.233
FUTURE DIRECTIONS
The current novel H1N1 influenza pandemic, as well as the reduced efficacy of seasonal influenza vaccines in those populations most at risk, has highlighted the need for continued development of new vaccines. New vaccine technologies, such as DNA vaccines, virus-like particles, and vectored vaccines, as well as new adjuvant technologies, hold promise to create influenza vaccines conferring better protection. Research into highly conserved influenza antigens, such as the M2 protein, may result in a universal influenza vaccine, one that is even able to confer protection against pandemic viruses. Furthermore, investigation into mammalian cell culture technology promises to eliminate the requirement for growth of vaccine virus in eggs, thereby substantially reducing the time from strain identification to vaccine production. Details of these and other future directions of vaccine development are beyond the scope of this review but have been reviewed elsewhere.21
CONCLUSION
Influenza remains a major contributor to morbidity and mortality in the United States and worldwide both directly and indirectly through exacerbation of underlying medical comorbid conditions. The development of vaccines against seasonal and pandemic influenza requires careful global surveillance as well as in-depth knowledge of virology, epidemiology, and immunology. Vaccination remains the most effective way to protect vulnerable populations from disease. It is important for health care professionals to understand the basics of influenza vaccines, including the facts regarding their efficacy and adverse effects, to better inform their patients so that those at highest risk can be protected. The current influenza pandemic with novel H1N1 has raised the profile of influenza and influenza vaccines. We must capture this opportunity to educate the lay public and health care professionals about the benefits of seasonal influenza vaccination and to answer questions and correct misconceptions.
REFERENCES
- 1.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009 [published correction appears in MMWR Recomm Rep. 2009;58(32):896-897] MMWR Recomm Rep. 2009;58(RR-8):1-52 [PubMed] [Google Scholar]
- 2.Cho BH, Kolasa MS, Messonnier ML. Influenza vaccination coverage rate among high-risk children during the 2002-2003 influenza season. Am J Infect Control. 2008;36(8):582-587 [DOI] [PubMed] [Google Scholar]
- 3.Loulergue P, Mir O, Alexandre J, Ropert S, Goldwasser F, Launay O. Low influenza vaccination rate among patients receiving chemotherapy for cancer [letter]. Ann Oncol. 2008;19(9):1658 [DOI] [PubMed] [Google Scholar]
- 4.Nakamura MM, Lee GM. Influenza vaccination in adolescents with high-risk conditions. Pediatrics 2008;122(5):920-928 [DOI] [PubMed] [Google Scholar]
- 5.Nowalk MP, Lin CJ, Zimmerman RK, et al. Self-reported influenza vaccination rates among health care workers in a large health system. Am J Infect Control. 2008;36(8):574-581 [DOI] [PubMed] [Google Scholar]
- 6.Roush SW, Murphy TV, Preventable Disease Table Working Group Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 2007;298(18):2155-2163 [DOI] [PubMed] [Google Scholar]
- 7.Shah SI, Turcotte F, Meng HD. Influenza vaccination rates of expectant parents with neonatal intensive care admission. J Matern Fetal Neonatal Med. 2008;21(10):752-757 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics National Vital Statistics System. LCWK3 Deaths, percent of total deaths, and death rates for the 15 leading causes of death in selected age groups, by race and sex: United States, 2005. http://www.cdc.gov/nchs/data/dvs/LCWK3_2005.pdf. http://www.cdc.gov/nchs/data/dvs/LCWK3_2005.pdf Accessed December 11, 2009.
- 9.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289(2):179-186 [DOI] [PubMed] [Google Scholar]
- 10.Colley E. Influenza vaccination in adults with a long-term condition. Community Pract. 2008;81(4):25-28 [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Influenza vaccination and self-reported reasons for not receiving influenza vaccination among Medicare beneficiaries aged ≥65 years—United States, 1991-2002. MMWR Morb Mortal Wkly Rep. 2004;53(43):1012-1015 [PubMed] [Google Scholar]
- 12.Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. 2008;130(3):304-309 [DOI] [PubMed] [Google Scholar]
- 13.de Diego C, Vila-Córcoles A, Ochoa O, et al. EPIVAC Study Group Effects of annual influenza vaccination on winter mortality in elderly people with chronic heart disease. Eur Heart J. 2009;30(2):209-216 [DOI] [PubMed] [Google Scholar]
- 14.Wat D, Gelder C, Hibbitts S, et al. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7(4):320-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchinson AF, Ghimire AK, Thompson MA, et al. A community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPD. Respir Med. 2007;101(12):2472-2481 [DOI] [PubMed] [Google Scholar]
- 16.Griffin MR, Coffey CS, Neuzil KM, Mitchel EF, Jr, Wright PF, Edwards KM. Winter viruses: influenza- and respiratory syncytial virus—related morbidity in chronic lung disease. Arch Intern Med. 2002;162(11):1229-1236 [DOI] [PubMed] [Google Scholar]
- 17.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292(11):1333-1340 [DOI] [PubMed] [Google Scholar]
- 18.Grijalva CG, Craig AS, Dupont WD, et al. Estimating influenza hospitalizations among children. Emerg Infect Dis. 2006;12(1):103-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullooly JP, Bridges CB, Thompson WW, et al. Influenza- and RSV-associated hospitalizations among adults. Vaccine 2007;25(5):846-855 [DOI] [PubMed] [Google Scholar]
- 20.Tosh PK, Boyce TG, Poland GA. Flu myths: dispelling the myths associated with live attenuated influenza vaccine. Mayo Clin Proc. 2008;83(1):77-84 [DOI] [PubMed] [Google Scholar]
- 21.Tosh PK, Poland GA. Emerging vaccines for influenza. Expert Opin Emerg Drugs 2008;13(1):21-40 [DOI] [PubMed] [Google Scholar]
- 22.Lamb RA, Krug RM. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, eds. Fields Virology Vol 1 4th ed.Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1487-1531 [Google Scholar]
- 23.Kaiser J. A one-size-fits-all flu vaccine [published corrections appear in Science. 2006;312(5779):1472 and 2006;312(5776):999]?Science 2006;312(5772):380-382 http://www.sciencemag.org/cgi/reprint/312/5772/380.pdf Accessed November 30, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Cheung TK, Poon LL. Biology of influenza A virus. Ann N Y Acad Sci 2007April;1102:1-25 [DOI] [PubMed] [Google Scholar]
- 25.Steinhauer DA, Wharton SA. Structure and function of the haemagglutinin. In: Nicholson KG, Webster RG, Hay AJ, eds. Textbook of Influenza Oxford, UK: Blackwell Science; 1998:54-64 [Google Scholar]
- 26.Vines A, Wells K, Matrosovich M, Castrucci MR, Ito T, Kawaoka Y. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J Virol. 1998;72(9):7626-7631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 1994;205(1):17-23 [DOI] [PubMed] [Google Scholar]
- 28.Staudt LM, Gerhard W. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin, I: significant variation in repertoire expression between individual mice. J Exp Med. 1983;157(2):687-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24(1):157-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56(1):152-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers DC, Kilbourne ED, Johansson BE. Neuraminidase-specific antibody responses to inactivated influenza virus vaccine in young and elderly adults. Clin Diagn Lab Immunol. 1996;3(5):511-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilbourne ED, Laver WG, Schulman JL, Webster RG. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol 1968;2(4):281-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy BR, Kasel JA, Chanock RM. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med. 1972;286(25):1329-1332 [DOI] [PubMed] [Google Scholar]
- 34.Eickhoff TC, Meiklejohn G. Protection against Hong Kong influneza by adjuvant vaccine containing A2-Ann Arbor-67. Bull World Health Organ 1969;41(3):562-563 [PMC free article] [PubMed] [Google Scholar]
- 35.Betakova T. M2 protein-a proton channel of influenza A virus. Curr Pharm Des. 2007;13(31):3231-3235 [DOI] [PubMed] [Google Scholar]
- 36.Wu F, Huang JH, Yuan XY, Huang WS, Chen YH. Characterization of immunity induced by M2e of influenza virus. Vaccine 2007;25(52):8868-8873 [DOI] [PubMed] [Google Scholar]
- 37.Gabbard J, Velappan N, Di Niro R, et al. A humanized anti-M2 scFv shows protective in vitro activity against influenza. Protein Eng Des Sel. 2009;22(3):189-198 [DOI] [PubMed] [Google Scholar]
- 38.Black RA, Rota PA, Gorodkova N, Klenk HD, Kendal AP. Antibody response to the M2 protein of influenza A virus expressed in insect cells. J Gen Virol. 1993;74(pt 1):143-146 [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Li H, Chen YH. N-terminus of M2 protein could induce antibodies with inhibitory activity against influenza virus replication. FEMS Immunol Med Microbiol. 2003;35(2):141-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Zou P, Chen YH. Monoclonal antibodies recognizing EVETPIRN epitope of influenza A virus M2 protein could protect mice from lethal influenza A virus challenge. Immunol Lett. 2004;93(2-3):131-136 [DOI] [PubMed] [Google Scholar]
- 41.Fiers W, De Filette M, Birkett A, Neirynck S, Min Jou W. A “universal” human influenza A vaccine. Virus Res. 2004;103(1-2):173-176 [DOI] [PubMed] [Google Scholar]
- 42.Denis J, Acosta-Ramirez E, Zhao Y, et al. Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform. Vaccine 2008;26(27-28):3395-3403 [DOI] [PubMed] [Google Scholar]
- 43.De Filette M, Martens W, Roose K, et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;283(17):11382-11387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huleatt JW, Nakaar V, Desai P, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 2008;26(2):201-214 [DOI] [PubMed] [Google Scholar]
- 45.Tompkins SM, Zhao ZS, Lo CY, et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis. 2007;13(3):426-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional out-break management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(8):1003-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang JW, Ngai KL, Wong JC, Lam WY, Chan PK. Emergence of adamantane-resistant influenza A(H3N2) viruses in Hong Kong between 1997 and 2006. J Med Virol 2008;80(5):895-901 [DOI] [PubMed] [Google Scholar]
- 48.Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med. 2009;360(10):953-956 [DOI] [PubMed] [Google Scholar]
- 49.Dharan NJ, Gubareva LV, Meyer JJ, et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 2009;301(10):1034-1041 [DOI] [PubMed] [Google Scholar]
- 50.Zürcher T, Yates PJ, Daly J, et al. Mutations conferring zanamivir resistance in human influenza virus N2 neuraminidases compromise virus fitness and are not stably maintained in vitro. J Antimicrob Chemother 2006;58(4):723-732 [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention (CDC) Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(32):893-896 [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention (CDC) Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 Season. http://www.cdc.gov/h1n1flu/recommendations.htm. http://www.cdc.gov/h1n1flu/recommendations.htm Accessed December 11, 2009.
- 53.Poland GA, Jacobson RM, Ovsyannikova IG. Influenza virus resistance to antiviral agents: a plea for rational use. Clin Infect Dis. 2009;48(9):1254-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jefferson TO, Demicheli V, Di Pietrantonj C, Jones M, Rivetti D. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev. 2006;3:CD001265 [DOI] [PubMed] [Google Scholar]
- 55.Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163(14):1667-1672 [DOI] [PubMed] [Google Scholar]
- 56.Nordstrom BL, Sung I, Suter P, Szneke P. Risk of pneumonia and other complications of influenza-like illness in patients treated with oseltamivir. Curr Med Res Opin. 2005;21(5):761-768 [DOI] [PubMed] [Google Scholar]
- 57.Jefferson T, Demicheli V, Rivetti D, Jones M, Di Pietrantonj C, Rivetti A. Antivirals for influenza in healthy adults: systematic review [published correction appears in Lancet. 2006;367(9528)2060] Lancet 2006;367(9507):303-313 [DOI] [PubMed] [Google Scholar]
- 58.Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children [published correction appears in Pediatr Infect Dis J. 2001;20(4):421] Pediatr Infect Dis J. 2001;20(2):127-133 [DOI] [PubMed] [Google Scholar]
- 59.Barr CE, Schulman K, Iacuzio D, Bradley JS. Effect of oseltamivir on the risk of pneumonia and use of health care services in children with clinically diagnosed influenza. Curr Med Res Opin 2007;23(3):523-531 [DOI] [PubMed] [Google Scholar]
- 60.Mäkelä MJ, Pauksens K, Rostila T, et al. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J Infect. 2000;40(1):42-48 [DOI] [PubMed] [Google Scholar]
- 61.Monto AS, Fleming DM, Henry D, et al. Efficacy and safety of the neuraminidase inhibitor zanamivirin in the treatment of influenza A and B virus infections. J Infect Dis. 1999;180(2):254-261 [DOI] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC) CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008-09 influenza season. Health Alert Network Web site December19, 2008. http://www2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279 Accessed December 11, 2009
- 63.US Department of Health and Human Services. National Institutes of Health Flu (influenza). National Institute of Allergy and Infectious Diseases Web site. http://www3.niaid.nih.gov/topics/Flu/Research/basic/AntigenicShiftIllustration.htm. http://www3.niaid.nih.gov/topics/Flu/Research/basic/AntigenicShiftIllustration.htm Accessed January 22, 2010.
- 64.Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150(1):86-112 [PMC free article] [PubMed] [Google Scholar]
- 65.Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Characterization of the 1918 influenza virus polymerase genes. Nature 2005;437(7060):889-893 [DOI] [PubMed] [Google Scholar]
- 66.Bean WJ, Schell M, Katz J, et al. Evolution of the H3 influenza virus hemagglutinin from human and nonhuman hosts. J Virol 1992;66(2):1129-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawaoka Y, Krauss S, Webster RG. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol 1989;63(11):4603-4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a novel swine-origin influenza A (H1N1) virus in humans [published correction appears in N Engl J Med. 2009;361(1):102] N Engl J Med. 2009;360(25):2605-2615 [DOI] [PubMed] [Google Scholar]
- 69.Cox NJ, Brammer TL, Regnery HL. Influenza: global surveillance for epidemic and pandemic variants. Eur J Epidemiol. 1994;10(4):467-470 [DOI] [PubMed] [Google Scholar]
- 70.Russell CA, Jones TC, Barr IG, et al. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine 2008;26(suppl 4):D31-D34 [DOI] [PubMed] [Google Scholar]
- 71.Smith DJ, Lapedes AS, de Jong JC, et al. Mapping the antigenic and genetic evolution of influenza virus. Science 2004;305(5682):371-376 [DOI] [PubMed] [Google Scholar]
- 72.Redlberger M, Aberle SW, Heinz FX, Popow-Kraupp T. Dynamics of antigenic and genetic changes in the hemagglutinins of influenza A/H3N2 viruses of three consecutive seasons (2002/2003 to 2004/2005) in Austria. Vaccine 2007;25(32):6061-6069 [DOI] [PubMed] [Google Scholar]
- 73.Nelson MI, Simonsen L, Viboud C, Miller MA, Holmes EC. Phylogenetic analysis reveals the global migration of seasonal influenza A viruses. PLoS Pathog. 2007;3(9):1220-1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008;320(5874):340-346 [DOI] [PubMed] [Google Scholar]
- 75.Nelson MI, Simonsen L, Viboud C, et al. Stochastic processes are key determinants of short-term evolution in influenza A virus. PLoS Pathog. 2006;2(12):e125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.World Health Organization Pandemic (H1N1) 2009. http://www.who.int/csr/disease/swineflu/en/index.html. http://www.who.int/csr/disease/swineflu/en/index.html Accessed December 11, 2009.
- 77.Weir JP, Director, Division of Viral Products Influenza vaccine production Presented at: National Influenza Vaccine Summit; Atlanta, GA; January24, 2006 [Google Scholar]
- 78.Belongia EA, Kieke BA, Donahue JG, et al. Marshfield Influenza Study Group Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 Season to the 2006-2007 Season. J Infect Dis. 2009;199(2):159-167 [DOI] [PubMed] [Google Scholar]
- 79.Bridges CB, Thompson WW, Meltzer MI, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA 2000;284(13):1655-1663 [DOI] [PubMed] [Google Scholar]
- 80.Herrera GA, Iwane MK, Cortese M, et al. Influenza vaccine effectiveness among 50-64-year-old persons during a season of poor antigenic match between vaccine and circulating influenza virus strains: Colorado, United States, 2003-2004 [published correction appears in Vaccine. 2008;26(32)4101-4103] Vaccine 2007;25(1):154-160 [DOI] [PubMed] [Google Scholar]
- 81.Ohmit SE, Victor JC, Rotthoff JR, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355(24):2513-2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pyhälä R, Haanpää M, Kleemola M, Tervahauta R, Visakorpi R, Kinnunen L. Acceptable protective efficacy of influenza vaccination in young military conscripts under circumstances of incomplete antigenic and genetic match. Vaccine 2001;19(23-24):3253-3260 [DOI] [PubMed] [Google Scholar]
- 83.Shuler CM, Iwamoto M, Bridges CB, et al. Vaccine effectiveness against medically attended, laboratory-confirmed influenza among children aged 6 to 59 months, 2003-2004. Pediatrics 2007;119(3):e587-e595 [DOI] [PubMed] [Google Scholar]
- 84.Strickler JK, Hawksworth AW, Myers C, Irvine M, Ryan MA, Russell KL. Influenza vaccine effectiveness among US military basic trainees, 2005-06 season. Emerg Infect Dis. 2007;13(4):617-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Centers for Disease Control and Prevention Flu season summary (September 30, 2007-May 17, 2008) http://www.cdc.gov/flu/weekly/weeklyarchives2007-2008/07-08summary.htm. http://www.cdc.gov/flu/weekly/weeklyarchives2007-2008/07-08summary.htm Accessed January 27, 2010.
- 86.Nichol KL, Mendelman PM, Mallon KP, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 1999;282(2):137-144 [DOI] [PubMed] [Google Scholar]
- 87.Orenstein WA, Schaffner W. Lessons learned: role of influenza vaccine production, distribution, supply, and demand—what it means for the provider. Am J Med. 2008;121(7)(suppl 2):S22-S27 [DOI] [PubMed] [Google Scholar]
- 88.Maassab HF. Biologic and immunologic characteristics of cold-adapted influenza virus. J Immunol. 1969;102(3):728-732 [PubMed] [Google Scholar]
- 89.Poland GA. Pharmacology, vaccinomics, and the second golden age of vaccinology [editorial]. Clin Pharmacol Ther. 2007;82(6):623-626 [DOI] [PubMed] [Google Scholar]
- 90.National Center for Immunization and Respiratory Diseases. Centers for Disease Control and Prevention (CDC) Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-10):1-8 [PubMed] [Google Scholar]
- 91.Centers for Disease Control and Prevention (CDC) Centers for Disease Control and Prevention Web site. http://www.cdc.gov/FLU/freeresources/2009-10/pdf/dosagechart.pdf. http://www.cdc.gov/FLU/freeresources/2009-10/pdf/dosagechart.pdf Accessed January 27, 2010.
- 92.Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171(1):198-203 [DOI] [PubMed] [Google Scholar]
- 93.Lawson F, Baker V, Au D, McElhaney JE. Standing orders for influenza vaccination increased vaccination rates in inpatient settings compared with community rates. J Gerontol A Biol Sci Med Sci 2000;55(9):M522-M526 [DOI] [PubMed] [Google Scholar]
- 94.Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine 2008;26(suppl 4):D17-D22 [DOI] [PubMed] [Google Scholar]
- 95.Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008;(2):CD004879 [DOI] [PubMed] [Google Scholar]
- 96.Villari P, Manzoli L, Boccia A. Methodological quality of studies and patient age as major sources of variation in efficacy estimates of influenza vaccination in healthy adults: a meta-analysis. Vaccine 2004;22(25-26):3475-3486 [DOI] [PubMed] [Google Scholar]
- 97.Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine 2000;18(11-12):957-1030 [DOI] [PubMed] [Google Scholar]
- 98.Nichol KL, Lind A, Margolis KL, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med. 1995;333(14):889-893 [DOI] [PubMed] [Google Scholar]
- 99.Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007;(2):CD001269 [DOI] [PubMed] [Google Scholar]
- 100.Eick AA, Wang Z, Hughes H, Ford SM, Tobler SK. Comparison of the trivalent live attenuated vs. inactivated influenza vaccines among U.S. military service members. Vaccine 2009;27(27):3568-3575 [DOI] [PubMed] [Google Scholar]
- 101.Wang Z, Tobler S, Roayaei J, Eick A. Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. JAMA 2009;301(9):945-953 [DOI] [PubMed] [Google Scholar]
- 102.Edwards KM, Dupont WD, Westrich MK, Plummer WD, Jr, Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994;169(1):68-76 [DOI] [PubMed] [Google Scholar]
- 103.Treanor JJ, Kotloff K, Betts RF, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 1999;18(9-10):899-906 [DOI] [PubMed] [Google Scholar]
- 104.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals: a randomized double-blind placebo-controlled trial. JAMA 1994;272(21):1661-1665 [PubMed] [Google Scholar]
- 105.Coles FB, Balzano GJ, Morse DL. An outbreak of influenza A (H3N2) in a well immunized nursing home population. J Am Geriatr Soc. 1992;40(6):589-592 [DOI] [PubMed] [Google Scholar]
- 106.Libow LS, Neufeld RR, Olson E, Breuer B, Starer P. Sequential out-break of influenza A and B in a nursing home: efficacy of vaccine and amantadine. J Am Geriatr Soc. 1996;44(10):1153-1157 [DOI] [PubMed] [Google Scholar]
- 107.Monto AS, Hornbuckle K, Ohmit SE. Influenza vaccine effectiveness among elderly nursing home residents: a cohort study. Am J Epidemiol. 2001;154(2):155-160 [DOI] [PubMed] [Google Scholar]
- 108.Ohmit SE, Arden NH, Monto AS. Effectiveness of inactivated influenza vaccine among nursing home residents during an influenza type A (H3N2) epidemic. J Am Geriatr Soc. 1999;47(2):165-171 [DOI] [PubMed] [Google Scholar]
- 109.Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, Baxter R. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol. 2009;170(5):650-656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35(2):337-344 [DOI] [PubMed] [Google Scholar]
- 111.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373-1381 [DOI] [PubMed] [Google Scholar]
- 112.Gorse GJ, O'Connor TZ, Young SL, et al. Efficacy trial of live, cold-adapted and inactivated influenza virus vaccines in older adults with chronic obstructive pulmonary disease: a VA cooperative study. Vaccine 2003;21(17-18):2133-2144 [DOI] [PubMed] [Google Scholar]
- 113.Targonski PV, Poland GA. Intranasal cold-adapted influenza virus vaccine combined with inactivated influenza virus vaccines: an extra boost for the elderly? Drugs Aging 2004;21(6):349-359 [DOI] [PubMed] [Google Scholar]
- 114.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355(1):31-40 [DOI] [PubMed] [Google Scholar]
- 115.Murphy BR, Coelingh K. Principles underlying the development and use of live attenuated cold-adapted influenza A and B virus vaccines. Viral Immunol. 2002;15(2):295-323 [DOI] [PubMed] [Google Scholar]
- 116.Talbot TR, Crocker DD, Peters J, et al. Duration of virus shedding after trivalent intranasal live attenuated influenza vaccination in adults. Infect Control Hosp Epidemiol. 2005;26(5):494-500 [DOI] [PubMed] [Google Scholar]
- 117.Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work, and secondary illness in families. Arch Pediatr Adolesc Med. 2002;156(10):986-991 [DOI] [PubMed] [Google Scholar]
- 118.Monto AS, Davenport FM, Napier JA, Francis T., Jr Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis. 1970;122(1):16-25 [DOI] [PubMed] [Google Scholar]
- 119.Weycker D, Edelsberg J, Halloran ME, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine 2005;23(10):1284-1293 [DOI] [PubMed] [Google Scholar]
- 120.Neuzil KM, Jackson LA, Nelson J, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccinenaive 5-8-year-old children. J Infect Dis. 2006;194(8):1032-1039 [DOI] [PubMed] [Google Scholar]
- 121.Walter EB, Neuzil KM, Zhu Y, et al. Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming? Pediatrics 2006;118(3):e570-e578 [DOI] [PubMed] [Google Scholar]
- 122.Manzoli L, Schioppa F, Boccia A, Villari P. The efficacy of influenza vaccine for healthy children: a meta-analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr Infect Dis J. 2007;26(2):97-106 [DOI] [PubMed] [Google Scholar]
- 123.Negri E, Colombo C, Giordano L, Groth N, Apolone G, La Vecchia C. Influenza vaccine in healthy children: a meta-analysis. Vaccine 2005;23(22):2851-2861 [DOI] [PubMed] [Google Scholar]
- 124.Ashkenazi S, Vertruyen A, Aristegui J, et al. CAIV-T Study Group Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25(10):870-879 [DOI] [PubMed] [Google Scholar]
- 125.Belshe RB, Edwards KM, Vesikari T, et al. CAIV-T Comparative Efficacy Study Group Live attenuated versus inactivated influenza vaccine in infants and young children [published correction appears in N Engl J Med. 2007;356(12):1283] N Engl J Med. 2007;356(7):685-696 [DOI] [PubMed] [Google Scholar]
- 126.Fleming DM, Crovari P, Wahn U, et al. CAIV-T Asthma Study Group Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J 2006;25(10):860-869 [DOI] [PubMed] [Google Scholar]
- 127.Esposito S, Marchisio P, Cavagna R, et al. Effectiveness of influenza vaccination of children with recurrent respiratory tract infections in reducing respiratory-related morbidity within the households. Vaccine 2003;21(23):3162-3168 [DOI] [PubMed] [Google Scholar]
- 128.Piedra PA, Gaglani MJ, Kozinetz CA, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine 2005;23(13):1540-1548 [DOI] [PubMed] [Google Scholar]
- 129.King JC, Jr, Stoddard JJ, Gaglani MJ, et al. Effectiveness of school-based influenza vaccination. N Engl J Med. 2006;355(24):2523-2532 [DOI] [PubMed] [Google Scholar]
- 130.Davis MM, King JC, Jr, Moag L, Cummings G, Magder LS. County-wide school-based influenza immunization: direct and indirect impact on student absenteeism. Pediatrics 2008;122(1):e260-e265 [DOI] [PubMed] [Google Scholar]
- 131.Ghendon YZ, Kaira AN, Elshina GA. The effect of mass influenza immunization in children on the morbidity of the unvaccinated elderly. Epidemiol Infect. 2006;134(1):71-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hurwitz ES, Haber M, Chang A, et al. Effectiveness of influenza vaccination of day care children in reducing influenza-related morbidity among household contacts. JAMA 2000;284(13):1677-1682 [DOI] [PubMed] [Google Scholar]
- 133.Dash GP, Fauerbach L, Pfeiffer J, et al. 2004 APIC Immunization Practices Working Group APIC position paper: improving health care worker influenza immunization rates. Am J Infect Control. 2004;32(3):123-125 [DOI] [PubMed] [Google Scholar]
- 134.Joint Commission on Accreditation of Healthcare Organizations Providing a safer environment for health care personnel and patients through influenza vaccination: strategies from research and practice, 2009. http://www.jointcommission.org/NR/rdonlyres/814E02F2-1E1C-4D76-9043-DBB3E-12A205A/0/Flu_Monograph.pdf. http://www.jointcommission.org/NR/rdonlyres/814E02F2-1E1C-4D76-9043-DBB3E-12A205A/0/Flu_Monograph.pdf Accessed January 4, 2010.
- 135.Engels O, Goldman N, Doyen M, Duyse M, Van Beers D, Vergison A. Reduction of the nosocomial influenza A burden in a paediatric hospital by immunization Paper presented at: 15th European Congress of Clinical Microbiology and Infectious Diseases; April2-5, 2005; Copenhagen, Denmark [Google Scholar]
- 136.Goldstein AO, Kincade JE, Gamble G, Bearman RS. Policies and practices for improving influenza immunization rates among healthcare workers. Infect Control Hosp Epidemiol. 2004;25(11):908-911 [DOI] [PubMed] [Google Scholar]
- 137.Maltezou HC, Drancourt M. Nosocomial influenza in children. J Hosp Infect. 2003;55(2):83-91 [DOI] [PubMed] [Google Scholar]
- 138.Salgado CD, Giannetta ET, Hayden FG, Farr BM. Preventing nosocomial influenza by improving the vaccine acceptance rate of clinicians. Infect Control Hosp Epidemiol. 2004;25(11):923-928 [DOI] [PubMed] [Google Scholar]
- 139.Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomised controlled trial. Lancet 2000;355(9198):93-97 [DOI] [PubMed] [Google Scholar]
- 140.Poland GA. Health care workers and influenza vaccine: first do no harm, then do the right thing. J Am Pharm Assoc (2003) 2004;44(5):637-638 [DOI] [PubMed] [Google Scholar]
- 141.Poland GA, Tosh P, Jacobson RM. Requiring influenza vaccination for health care workers: seven truths we must accept. Vaccine 2005;23(17-18):2251-2255 [DOI] [PubMed] [Google Scholar]
- 142.Tosh PK, Jacobson RM, Poland GA. Mandatory influenza vaccination for health care workers—a timely step forward. Md Med. 2006;7(1):21-23 [PubMed] [Google Scholar]
- 143.Tilburt JC, Mueller PS, Ottenberg AL, Poland GA, Koenig BA. Facing the challenges of influenza in healthcare settings: the ethical rationale for mandatory seasonal influenza vaccination and its implications for future pandemics. Vaccine 2008;26(suppl 4):D27-D30 [DOI] [PubMed] [Google Scholar]
- 144.Tucker SJ, Poland GA, Jacobson RM. Requiring influenza vaccination for health care workers. Am J Nurs. 2008;108(2):32-34 [DOI] [PubMed] [Google Scholar]
- 145.Poland GA, Ofstead CL, Tucker SJ, Beebe TJ. Receptivity to mandatory influenza vaccination policies for healthcare workers among registered nurses working on inpatient units. Infect Control Hosp Epidemiol. 2008;29(2):170-173 [DOI] [PubMed] [Google Scholar]
- 146.Ofstead CL, Tucker SJ, Beebe TJ, Poland GA. Influenza vaccination among registered nurses: information receipt, knowledge, and decision-making at an institution with a multifaceted educational program. Infect Control Hosp Epidemiol. 2008;29(2):99-106 [DOI] [PubMed] [Google Scholar]
- 147.National Influenza Vaccine Summit. Prevent influenza now! Immunization Action Coalition Web site. http://www.preventinfluenza.org/profs_workers.asp. http://www.preventinfluenza.org/profs_workers.asp Accessed December 18, 2009.
- 148.Infectious Diseases Society of America IDSA policy on mandatory immunization of health care workers against seasonal and 2009 H1N1 influenza. http://www.idsociety.org/redirector.aspx?id=15413. http://www.idsociety.org/redirector.aspx?id=15413 Accessed December 18, 2009.
- 149.Hartert TV, Neuzil KM, Shintani AK, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol. 2003;189(6):1705-1712 [DOI] [PubMed] [Google Scholar]
- 150.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342(4):232-239 [DOI] [PubMed] [Google Scholar]
- 151.Lindsay L, Jackson LA, Savitz DA, et al. Community influenza activity and risk of acute influenza-like illness episodes among healthy unvaccinated pregnant and postpartum women. Am J Epidemiol. 2006;163(9):838-848 [DOI] [PubMed] [Google Scholar]
- 152.Munoz FM. Influenza virus infection in infancy and early childhood. Paediatr Respir Rev. 2003;4(2):99-104 [PubMed] [Google Scholar]
- 153.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148(11):1094-1102 [DOI] [PubMed] [Google Scholar]
- 154.ACOG Committee on Obstetric Practice ACOG committee opinion number 305, November 2004: Influenza vaccination and treatment during pregnancy. Obstet Gynecol. 2004;104(5, pt 1):1125-1126 [PubMed] [Google Scholar]
- 155.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555-1564 [DOI] [PubMed] [Google Scholar]
- 156.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9(8):493-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med. 2001;161(3):441-446 [DOI] [PubMed] [Google Scholar]
- 158.Neuzil KM, Coffey CS, Mitchel EF, Jr, Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr. 2003;34(3):304-307 [DOI] [PubMed] [Google Scholar]
- 159.Sullivan PS, Hanson DL, Dworkin MS, Jones JL, Ward JW, Adult and Adolescent Spectrum of HIV Disease Investigators Effect of influenza vaccination on disease progression among HIV-infected persons. AIDS 2000;14(17):2781-2785 [DOI] [PubMed] [Google Scholar]
- 160.Yamanaka H, Teruya K, Tanaka M, et al. Efficacy and immunologic responses to influenza vaccine in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;39(2):167-173 [PubMed] [Google Scholar]
- 161.Tasker SA, Treanor JJ, Paxton WB, Wallace MR. Efficacy of influenza vaccination in HIV-infected persons: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131(6):430-433 [DOI] [PubMed] [Google Scholar]
- 162.King JC, Jr, Fast PE, Zangwill KM, et al. HIV Influenza Study Group Safety, vaccine virus shedding and immunogenicity of trivalent, cold-adapted, live attenuated influenza vaccine administered to human immunodeficiency virus-infected and noninfected children. Pediatr Infect Dis J. 2001;20(12):1124-1131 [DOI] [PubMed] [Google Scholar]
- 163.King JC, Jr, Treanor J, Fast PE, et al. Comparison of the safety, vaccine virus shedding, and immunogenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults. J Infect Dis. 2000;181(2):725-728 [DOI] [PubMed] [Google Scholar]
- 164.Levin MJ, Song LY, Fenton T, et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine 2008;26(33):4210-4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schepetiuk S, Papanaoum K, Qiao M. Spread of influenza A virus infection in hospitalised patients with cancer [letter]. Aust N Z J Med. 1998;28(4):475-476 [DOI] [PubMed] [Google Scholar]
- 166.Yousuf HM, Englund J, Couch R, et al. Influenza among hospitalized adults with leukemia. Clin Infect Dis. 1997;24(6):1095-1099 [DOI] [PubMed] [Google Scholar]
- 167.Couch RB, Englund JA, Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997;102(3A):2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brydak LB, Machala M, Centkowski P, Warzocha K, Bilinski P. Humoral response to hemagglutinin components of influenza vaccine in patients with non-Hodgkin malignant lymphoma. Vaccine 2006;24(44-46):6620-6623 [DOI] [PubMed] [Google Scholar]
- 169.Feery BJ, Sullivan JR, Hurley TH, Evered MG. Immunization with influenza vaccine in patients with haematological malignant disease. Med J Aust. 1977;1(9):292-294 [DOI] [PubMed] [Google Scholar]
- 170.Lo W, Whimbey E, Elting L, Couch R, Cabanillas F, Bodey G. Antibody response to a two-dose influenza vaccine regimen in adult lymphoma patients on chemotherapy. Eur J Clin Microbiol Infect Dis. 1993;12(10):778-782 [DOI] [PubMed] [Google Scholar]
- 171.Mazza JJ, Yale SH, Arrowood JR, et al. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005;3(4):214-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ortbals DW, Liebhaber H, Presant CA, Van Amburg AL, III, Lee JY. Influenza immunization of adult patients with malignant diseases. Ann Intern Med. 1977;87(5):552-557 [DOI] [PubMed] [Google Scholar]
- 173.Shildt RA, Luedke DW, Kasai G, El-Beheri S, Laham MN. Antibody response to influenza immunization in adult patients with malignant disease. Cancer 1979;44(5):1629-1635 [DOI] [PubMed] [Google Scholar]
- 174.Stiver HG, Weinerman BH. Impaired serum antibody response to inactivated influenza A and B vaccine in cancer patients. Can Med Assoc J. 1978;119(7):733-735, 738 [PMC free article] [PubMed] [Google Scholar]
- 175.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. J Heart Lung Transplant. 2002;21(5):559-566 [DOI] [PubMed] [Google Scholar]
- 176.Briggs JD, Timbury MC, Paton AM, Bell PR. Viral infection and renal transplant rejection. BMJ 1972;4(5839):520-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest 2001;119(4):1277-1280 [DOI] [PubMed] [Google Scholar]
- 178.Keane WR, Helderman JH, Luby J, Gailiunas P, Hull AR, Kokko JP. Epidemic renal transplant rejection associated with influenza A victoria. Proc Clin Dial Transplant Forum 1978; November18-20:8232-8236 [PubMed] [Google Scholar]
- 179.Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant. 2002;2(3):287-291 [DOI] [PubMed] [Google Scholar]
- 180.Admon D, Engelhard D, Strauss N, Goldman N, Zakay-Rones Z. Antibody response to influenza immunization in patients after heart transplantation. Vaccine 1997;15(14):1518-1522 [DOI] [PubMed] [Google Scholar]
- 181.Birdwell KA, Ikizler MR, Sannella EC, et al. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am J Kidney Dis. 2009;54(1):112-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Blumberg EA, Albano C, Pruett T, et al. The immunogenicity of influenza virus vaccine in solid organ transplant recipients. Clin Infect Dis. 1996;22(2):295-302 [DOI] [PubMed] [Google Scholar]
- 183.Cavdar C, Sayan M, Sifil A, et al. The comparison of antibody response to influenza vaccination in continuous ambulatory peritoneal dialysis, hemodialysis and renal transplantation patients. Scand J Urol Nephrol. 2003;37(1):71-76 [DOI] [PubMed] [Google Scholar]
- 184.Duchini A, Hendry RM, Nyberg LM, Viernes ME, Pockros PJ. Immune response to influenza vaccine in adult liver transplant recipients. Liver Transpl. 2001;7(4):311-313 [DOI] [PubMed] [Google Scholar]
- 185.Hayney MS, Welter DL, Francois M, Reynolds AM, Love RB. Influenza vaccine antibody responses in lung transplant recipients. Prog Transplant. 2004;14(4):346-351 [DOI] [PubMed] [Google Scholar]
- 186.Kimball P, Verbeke S, Tolman D. Influenza vaccination among heart transplant recipients. Transplant Proc. 2001;33(1-2):1785-1786 [DOI] [PubMed] [Google Scholar]
- 187.Kumar SS, Ventura AK, VanderWerf B. Influenza vaccination in renal transplant recipients. JAMA 1978;239(9):840-842 [PubMed] [Google Scholar]
- 188.Mauch TJ, Crouch NA, Freese DK, Braunlin EA, Dunn DL, Kashtan CE. Antibody response of pediatric solid organ transplant recipients to immunization against influenza virus. J Pediatr. 1995;127(6):957-960 [DOI] [PubMed] [Google Scholar]
- 189.Mazzone PJ, Mossad SB, Mawhorter SD, Mehta AC, Schilz RJ, Maurer JR. The humoral immune response to influenza vaccination in lung transplant patients. Eur Respir J. 2001;18(6):971-976 [DOI] [PubMed] [Google Scholar]
- 190.Scharpé J, Evenepoel P, Maes B, et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant. 2008;8(2):332-337 [DOI] [PubMed] [Google Scholar]
- 191.Soesman NM, Rimmelzwaan GF, Nieuwkoop NJ, et al. Efficacy of influenza vaccination in adult liver transplant recipients. J Med Virol. 2000;61(1):85-93 [PubMed] [Google Scholar]
- 192.Hovden AO, Cox RJ, Haaheim LR. Influenza: the virus and prophylaxis with inactivated influenza vaccine in “at risk” groups, including COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2(3):229-240 [PMC free article] [PubMed] [Google Scholar]
- 193.Yap FH, Ho PL, Lam KF, Chan PK, Cheng YH, Peiris JS. Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong Kong. J Med Virol. 2004;73(4):617-623 [DOI] [PubMed] [Google Scholar]
- 194.Nichol KL, Baken L, Nelson A. Relation between influenza vaccination and outpatient visits, hospitalization, and mortality in elderly persons with chronic lung disease. Ann Intern Med. 1999;130(5):397-403 [DOI] [PubMed] [Google Scholar]
- 195.Wang CS, Wang ST, Lai CT, Lin LJ, Chou P. Impact of influenza vaccination on major cause-specific mortality. Vaccine 2007;25(7):1196-1203 [DOI] [PubMed] [Google Scholar]
- 196.Minor TE, Dick EC, Baker JW, Ouellette JJ, Cohen M, Reed CE. Rhinovirus and influenza type A infections as precipitants of asthma. Am Rev Respir Dis. 1976;113(2):149-153 [DOI] [PubMed] [Google Scholar]
- 197.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993;307(6910):982-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.American Lung Association Asthma Clinical Research Centers The safety of inactivated influenza vaccine in adults and children with asthma. N Engl J Med. 2001;345(21):1529-1536 [DOI] [PubMed] [Google Scholar]
- 199.Kmiecik T, Arnoux S, Kobryn A, Gorski P. Influenza vaccination in adults with asthma: safety of an inactivated trivalent influenza vaccine. J Asthma 2007;44(10):817-822 [DOI] [PubMed] [Google Scholar]
- 200.Kramarz P, Destefano F, Gargiullo PM, et al. Does influenza vaccination prevent asthma exacerbations in children? J Pediatr. 2001;138(3):306-310 [DOI] [PubMed] [Google Scholar]
- 201.Madjid M, Miller CC, Zarubaev VV, et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J. 2007;28(10):1205-1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bautista D, Vila B, Uso R, Tellez M, Zanon V. Predisposing, reinforcing, and enabling factors influencing influenza vaccination acceptance among healthcare workers. Infect Control Hosp Epidemiol. 2006;27(1):73-77 [DOI] [PubMed] [Google Scholar]
- 203.Canning HS, Phillips J, Allsup S. Health care worker beliefs about influenza vaccine and reasons for non-vaccination—a cross-sectional survey. J Clin Nurs. 2005;14(8):922-925 [DOI] [PubMed] [Google Scholar]
- 204.Weingarten S, Riedinger M, Bolton LB, Miles P, Ault M. Barriers to influenza vaccine acceptance: a survey of physicians and nurses. Am J Infect Control. 1989;17(4):202-207 [DOI] [PubMed] [Google Scholar]
- 205.Lester RT, McGeer A, Tomlinson G, Detsky AS. Use of, effectiveness of, and attitudes regarding influenza vaccine among house staff. Infect Control Hosp Epidemiol. 2003;24(11):839-844 [DOI] [PubMed] [Google Scholar]
- 206.Margolis KL, Nichol KL, Poland GA, Pluhar RE. Frequency of adverse reactions to influenza vaccine in the elderly: a randomized, placebo-controlled trial [published correction appears in JAMA. 1991;265(21):2810] JAMA 1990;264(9):1139-1141 [PubMed] [Google Scholar]
- 207.Wilde JA, McMillan JA, Serwint J, Butta J, O'Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals: a randomized trial. JAMA 1999;281(10):908-913 [DOI] [PubMed] [Google Scholar]
- 208.Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;338(20):1405-1412 [DOI] [PubMed] [Google Scholar]
- 209.Jackson LA, Holmes SJ, Mendelman PM, Huggins L, Cho I, Rhorer J. Safety of a trivalent live attenuated intranasal influenza vaccine, FluMist, administered in addition to parenteral trivalent inactivated influenza vaccine to seniors with chronic medical conditions. Vaccine 1999;17(15-16):1905-1909 [DOI] [PubMed] [Google Scholar]
- 210.Wood JM. Developing vaccines against pandemic influenza. Philos Trans R Soc Lond B Biol Sci 2001;356(1416):1953-1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Marks JS, Halpin TJ. Guillain-Barré syndrome in recipients of A/New Jersey influenza vaccine. JAMA 1980;243(24):2490-2494 [PubMed] [Google Scholar]
- 212.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976-1977. Am J Epidemiol. 1979;110(2):105-123 [DOI] [PubMed] [Google Scholar]
- 213.Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf. 2009;32(4):309-323 [DOI] [PubMed] [Google Scholar]
- 214.Haber P, DeStefano F, Angulo FJ, et al. Guillain-Barré syndrome following influenza vaccination. JAMA 2004;292(20):2478-2481 [DOI] [PubMed] [Google Scholar]
- 215.Juurlink DN, Stukel TA, Kwong J, et al. Guillain-Barré syndrome after influenza vaccination in adults: a population-based study. Arch Intern Med. 2006;166(20):2217-2221 [DOI] [PubMed] [Google Scholar]
- 216.Kaplan JE, Katona P, Hurwitz ES, Schonberger LB. Guillain-Barré syndrome in the United States, 1979-1980 and 1980-1981: lack of an association with influenza vaccination. JAMA 1982;248(6):698-700 [PubMed] [Google Scholar]
- 217.Souayah N, Nasar A, Suri MF, Qureshi AI. Guillain-Barre syndrome after vaccination in United States: a report from the CDC/FDA Vaccine Adverse Event Reporting System. Vaccine 2007;25(29):5253-5255 [DOI] [PubMed] [Google Scholar]
- 218.Souayah N, Nasar A, Suri MF, Qureshi AI. Guillain-Barré syndrome after vaccination in United States: data from the Centers for Disease Control and Prevention/Food and Drug Administration Vaccine Adverse Event Reporting System (1990-2005). J Clin Neuromuscul Dis. 2009;11(1):1-6 [DOI] [PubMed] [Google Scholar]
- 219.Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis. 2009;48(1):48-56 [DOI] [PubMed] [Google Scholar]
- 220.Centers for Disease Control and Prevention (CDC) Summary of the joint statement on thimerosal in vaccines. American Academy of Family Physicians, American Academy of Pediatrics, Advisory Committee on Immunization Practices, Public Health Service. MMWR Morb Mortal Wkly Rep. 2000;49(27):622, 631 [PubMed] [Google Scholar]
- 221.Parker SK, Schwartz B, Todd J, Pickering LK. Thimerosal-containing vaccines and autistic spectrum disorder: a critical review of published original data. Pediatrics 2004;114(3):793-804 [DOI] [PubMed] [Google Scholar]
- 222.Thompson WW, Price C, Goodson B, et al. Vaccine Safety Datalink Team Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med. 2007;357(13):1281-1292 [DOI] [PubMed] [Google Scholar]
- 223.Tozzi AE, Bisiacchi P, Tarantino V, et al. Neuropsychological performance 10 years after immunization in infancy with thimerosal-containing vaccines. Pediatrics 2009;123(2):475-482 [DOI] [PubMed] [Google Scholar]
- 224.Verstraeten T, Davis RL, DeStefano F, et al. Safety of thimerosal-containing vaccines: a two-phased study of computerized health maintenance organization databases. Pediatrics 2003;112(5):1039-1048 [PubMed] [Google Scholar]
- 225.Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998;351(9103):637-641 [DOI] [PubMed] [Google Scholar]
- 226.Deer B. Hidden records show MMR truth: a Sunday Times investigation has found altered data was behind the decade-long scare over vaccinations. [published online ahead of print Accessed January 4, 2010] The Sunday Times 2009;(February):8 http://www.timesonline.co.uk/tol/life_and_style/health/article5683643.ece Accessed January 22, 2010
- 227.Murch SH, Anthony A, Casson DH, et al. Retraction of an interpretation. Lancet 2004;363(9411):750 [DOI] [PubMed] [Google Scholar]
- 228.Offit PA, Quarles J, Gerber MA, et al. Addressing parents' concerns: do multiple vaccines overwhelm or weaken the infant's immune system? Pediatrics 2002;109(1):124-129 [DOI] [PubMed] [Google Scholar]
- 229.Offit PA, Moser CA. The problem with Dr Bob's alternative vaccine schedule. Pediatrics 2009;123(1):e164-e169 [DOI] [PubMed] [Google Scholar]
- 230.Black SB, Cherry JD, Shinefield HR, Fireman B, Christenson P, Lampert D. Apparent decreased risk of invasive bacterial disease after heterologous childhood immunization. Am J Dis Child 1991;145(7):746-749 [PubMed] [Google Scholar]
- 231.Davidson M, Letson GW, Ward JI, et al. DTP immunization and susceptibility to infectious diseases: is there a relationship? Am J Dis Child 1991;145(7):750-754 [PubMed] [Google Scholar]
- 232.Storsaeter J, Olin P, Renemar B, et al. Mortality and morbidity from invasive bacterial infections during a clinical trial of acellular pertussis vaccines in Sweden. Pediatr Infect Dis J. 1988;7(9):637-645 [DOI] [PubMed] [Google Scholar]
- 233.Poland GA, Jacobson RM. Understanding those who do not understand: a brief review of the anti-vaccine movement. Vaccine 2001;19(17-19):2440-2445 [DOI] [PubMed] [Google Scholar]