Abstract
The management of patients with chest pain is a common and challenging clinical problem. Although most of these patients do not have a life-threatening condition, the clinician must distinguish between those who require urgent management of a serious problem such as acute coronary syndrome (ACS) and those with more benign entities who do not require admission. Although clinical judgment continues to be paramount in meeting this challenge, new diagnostic modalities have been developed to assist in risk stratification. These include markers of cardiac injury, risk scores, early stress testing, and noninvasive imaging of the heart. The basic clinical tools of history, physical examination, and electrocardiography are currently widely acknowledged to allow early identification of low-risk patients who have less than 5% probability of ACS. These patients are usually initially managed in the emergency department and transitioned to further outpatient evaluation or chest pain units. Multiple imaging strategies have been investigated to accelerate diagnosis and to provide further risk stratification of patients with no initial evidence of ACS. These include rest myocardial perfusion imaging, rest echocardiography, computed tomographic coronary angiography, and cardiac magnetic resonance imaging. All have very high negative predictive values for excluding ACS and have been successful in reducing unnecessary admissions for patients at low to intermediate risk of ACS. As patients with acute chest pain transition from the evaluation in the emergency department to other outpatient settings, it is important that all clinicians involved in the care of these patients understand the tools used for assessment and risk stratification.
ACC = American College of Cardiology; ACS = acute coronary syndrome; ADP = accelerated diagnostic protocol; AHA = American Heart Association; CAD = coronary artery disease; CHF = chronic heart failure; CMRI = cardiac magnetic resonance imaging; CPU = chest pain unit; CT = computed tomography; CTCA = computed tomographic coronary angiography; ECG = electrocardiography; ED = emergency department; ETT = exercise treadmill testing; MI = myocardial infarction; MPI = myocardial perfusion imaging; TIMI = Thrombolysis in Myocardial Infarction
Chest pain and symptoms consistent with myocardial ischemia are one of the most common reasons for emergency department (ED) evaluation, accounting for approximately 8% to 10% of the 119 million ED visits yearly.1 Chest pain is one of the few disease processes in which patients may initially appear to be well but in fact have an underlying life-threatening condition. Inadvertent discharge of patients with acute coronary syndrome (ACS) has been associated with a short-term mortality of 2%, as well as major risk of liability.2 Identifying patients with chest pain who are at risk of adverse events is important not only to ED physicians but also to all physicians who evaluate such patients. In one insurance industry—based study, the physician group most likely to be sued for missed myocardial infarction (MI) was family practitioners (32%), followed by general internists (22%) and ED physicians (15%).3
Myocardial infarction can be misdiagnosed for a number of reasons. Misinterpretation of findings on electrocardiography (ECG) occurs in 23% to 40% of misdiagnosed MIs.4-7 Younger age,4,6,7 physician inexperience,8 and atypical presentations4,6,7 are more common in these patients. An insurance claims—based study found that in 28% of cases no diagnostic study, not even ECG, was ordered.3 Therefore, standardizing the evaluation process is critical for identifying patients who initially appear to be low risk but who actually have ACS.
To meet this challenge, an increasing array of diagnostic modalities have been investigated during the past 2 decades, including new cardiac biomarkers, clinical risk scores, early stress testing, and noninvasive imaging of the myocardium and coronary arteries.9,10 These have been incorporated into various accelerated diagnostic protocols (ADPs) or chest pain unit (CPU) pathways to provide a rapid, cost-effective mechanism for evaluation.
INITIAL RISK STRATIFICATION
The goal of the initial evaluation of a patient who presents to an outpatient setting with potential ACS has changed from diagnosis to risk stratification. In many cases, the approach is similar for patients being evaluated in the office and the ED and should include a history, physical examination, and ECG. Patients should be classified into 1 of 4 categories11: (1) those with evidence of ST-segment elevation on initial ECG; (2) those without ST-segment elevation but who are at high risk on the basis of ECG findings, hemodynamic instability, or history; (3) those who have no objective evidence of ACS but have symptoms that warrant evaluation; and (4) those who have an obvious noncardiac cause for their symptoms.
Category 1 patients should be evaluated for immediate reperfusion therapy. Category 2 patients should be admitted to the hospital and, in the absence of contraindications, should receive antiplatelet and antithrombotic treatment. Patients with ongoing symptoms, persistent ECG changes, or hemodynamic instability should be evaluated for emergent coronary angiography. Further treatment of category 4 patients is based on the alternative diagnosis. Category 3, the low-risk chest pain cohort, is an important one because it accounts for most patients undergoing ED evaluation. Although no single variable (history, physical examination, ECG findings) can identify a patient at such low risk that additional evaluation is unnecessary, the combination of multiple clinical parameters can be used to better determine the initial evaluation process.
ELECTROCARDIOGRAPHY
Whether a patient presents to an office or an ED, the initial ECG is the easiest, simplest, most important tool for early risk stratification. Current recommendations indicate that it should be performed within 10 minutes of ED presentation11 and may best be considered one of the “vital signs” for patients with chest pain. All offices should have this capability as well as a mechanism to provide rapid interpretation. The presence of ST-segment elevation should prompt consideration for immediate reperfusion therapy. ST-segment depression is associated with a marked increase in risk of MI and ischemic complications.12,13 As little as 0.5 mm of ST-segment depression predicts increased risk; the greater the extent of depression, the higher the likelihood of MI and death.13 Although T-wave inversion is usually considered consistent with ischemia, risk is lower than with ST-segment depression.12 The presence of significant Q waves is consistent with prior MI; however, when evaluated independently of other findings, it is less predictive of adverse cardiac events than ST-segment depression or elevation. In the absence of ischemic symptoms, atrial fibrillation is associated with a low rate of MI and does not mandate evaluation for myocardial necrosis in the absence of other high-risk features.14 However, at presentation initial ECG findings in most patients do not show ischemia; in these cases, risk of MI and cardiac complications is low,15-17 such that in the absence of other high-risk findings, evaluation can occur in settings other than an intensive care unit, such as an ED or CPU.
Despite its importance, ECG has a number of limitations, including a relatively low diagnostic sensitivity for ACS, especially for unstable angina; ischemic changes are apparent at the time of presentation in only 20% to 30% of patients who have an acute MI.17,18 Conversely, 5% to 10% of patients with MI have normal findings on ECG at presentation.17,18 The sensitivity of ECG is affected by the anatomic location of the culprit vessel and is less likely to be diagnostic in patients with left circumflex lesions.19
Ongoing investigation has sought to identify ways to improve the diagnostic sensitivity of initial ECG. Serial assessment (every 15-30 minutes) should be performed routinely in patients with ongoing symptoms or ECG findings that are suggestive but not diagnostic of ischemia.11 With continuous ST-segment monitoring, an alternative mechanism, 12-lead ECG, is performed at prespecified time intervals, and an alarm sounds when significant ST-segment changes occur.20 However, the yield is low in low-risk patient populations.21
Other methods that have been evaluated include addition of posterior leads and multilead ECG devices. Results have been mixed for routine use of posterior leads22,23; yield is likely higher when used to differentiate the patient with anterior ST-segment depression who has ischemia alone from one who has acute posterior MI. The use of body mapping and multilead ECG devices can increase sensitivity for identifying patients who have posterior MI or left bundle branch block MI.24 However, the cost-effectiveness of routine use is unclear.
PATIENT HISTORY
Despite recent advances in newer diagnostic techniques, the history remains critically important in the initial evaluation of patients with chest pain. Because objective evidence of ACS is present in only a few patients, it is used to stratify them into higher- and lower-risk groups, allowing the appropriate level of additional diagnostic testing to be targeted.
Patients often do not consider their symptoms as “pain”; therefore, questions are better targeted at a description of their “discomfort.” The characteristics of the discomfort and the presence of associated symptoms are useful for risk stratification.8,25,26 Questioning should address symptom location, onset, character, severity, radiation, alleviating and exacerbating factors, time course, history of similar episodes, and associated symptoms, including diaphoresis, shortness of breath, and nausea or vomiting.
Symptoms that are described as pressure, tightness, squeezing, or indigestion or those that are similar to pri- or ACS events can be considered typical, identifying the patient at increased risk (although many of these patients will ultimately not be diagnosed as having ACS). Atypical symptoms, such as stabbing, pleuritic, and pinprick discomfort, are usually associated with a noncardiac etiology and place a patient at lower risk.25 Chest pains described as sharp should be further differentiated into those described as knife-like or pinprick (atypical) and those that are considered more typical after further clarification. Symptoms such as nausea and vomiting have been associated with increased risk.25,26 Symptoms that are relieved by rest or sublingual nitroglycerin should not be used as a diagnostic test for determining chest pain etiology in the acute setting because they are not predictive.27,28
Although traditional coronary artery disease (CAD) risk factors predict long-term risk of disease, they have limited value for identifying patients with ACS who present with acute symptoms29-32 and are outweighed in those patients by ECG, chest pain characteristics, history of CAD, and age. In multiple prior reports, none of the classic CAD risk factors have emerged as independent predictors of acute MI.29-32 Clinical decisions based on the absence of these may underestimate risk in patients who have few risk factors but have ACS, and therefore their absence alone should not be used to determine whether a patient warrants evaluation for ACS.11
Atypical presentations are more likely in elderly persons, in whom dyspnea may predominate over chest pain.33 Women are more likely than men to have atypical presentations.34,35 Despite anecdotal evidence, most previous studies have found no significant increase in the prevalence of unrecognized or atypical presenting MI in patients with diabetes.34,35
PHYSICAL EXAMINATION
Findings on physical examination are usually normal in most low-risk patients undergoing an ACS evaluation. However, certain findings can be useful for risk stratification and for determining symptom etiology.11 Important findings identifying high-risk patients include chronic heart failure (CHF) and hemodynamic instability (low blood pressure, elevated heart rate). Abnormal vital signs are recognized high-risk findings included in a number of scoring systems that stratify patients with ACS for subsequent adverse events. Current guidelines indicate that a new mitral regurgitation murmur identifies a high-risk patient; however, this is uncommon in typical practice.11 The presence of bruits, which usually indicate peripheral or cerebrovascular arterial disease, increases the risk of concomitant CAD. The examination should also target potential noncardiac causes for the patient's symptoms, such as prominent murmurs (endocarditis), friction rub (pericarditis), fever, abnormal lung sounds (pneumonia), and reproducible chest pain after palpation (musculoskeletal). In the latter case, there should be no other suggestive findings, and symptoms should be completely reproduced by palpation to be considered noncardiac.
BIOMARKERS
Current recommendations advise that all patients with suspected ACS should undergo serial cardiac biomarker sampling.11,36,37 If baseline data are negative, further sampling should be obtained 6 to 8 hours later depending on symptom onset. Creatine kinase and creatine kinase MB were the traditional markers for identifying patients with MI; however, because of their less than optimal sensitivity and specificity, current recommendations indicate troponin as the preferred biomarker. Troponin is considered the criterion standard cardiac biomarker for diagnosing MI because troponin I and troponin T are not detected in the blood of healthy persons. Troponin has many characteristics of an optimal diagnostic marker: it has superior sensitivity and specificity compared with other markers and helps identify patients with increased short- and long-term risk of cardiac events.11 Elevations identify patients who benefit selectively from aggressive treatment, such as antithrombotic38 and antiplatelet39 pharmacotherapy, as well as early coronary intervention.40 Therefore, it is recommended that every marker strategy include either troponin I or troponin T. Because many assays for troponin I exist, knowledge of a particular assay's characteristics and reference ranges is important for interpreting the results.41 An important consideration is that a number of other nonatherothrombotic conditions can result in myocardial damage (Table 1).42 Distinguishing troponin elevations related primarily to ACS from non—ACS-related disease is important but can be difficult. A serial rise and fall in troponin is more likely to be associated with an ACS etiology.37
TABLE 1.
Differential Diagnosis of Increased Troponin Level in Patients Without Acute Coronary Syndrome or Heart Failure
The time to diagnosis has been successfully reduced using marker combinations, shorter sampling time points, and quantification of serial changes in markers over the course of short time periods. However, current-generation troponin assays have improved sensitivity and accuracy at lower levels, allowing detection of minimal cardiac damage with higher reproducibility than earlier assays. These higher-sensitivity troponin assays can more accurately identify MI than traditional markers43-45; their use will likely obviate the need for other markers, such that a “troponin-only” marker strategy will become more common.
An important consideration is that more sensitive troponin assays have identified asymptomatic myocardial damage in patients who have underlying cardiovascular disease.46,47 If sampled in patients presenting to the ED for a reason other than ACS, these elevations could lead to diagnostic confusion. In most cases, however, levels tend to be low, and MI could likely be excluded through serial sampling, as already discussed.
Many biomarkers have been studied in patients with ACS in an effort to provide earlier diagnosis and improve short- and long-term prognosis. These have targeted different stages of ACS, such as thrombosis, plaque rupture, ischemia, and inflammation. In ACS trials, many of these markers have had added value for identifying patients at risk of cardiac events, particularly mortality37; however, few of these markers are commercially available and those that are have added little value when applied to broad, heterogeneous populations, such as those presenting to the ED with undifferentiated chest pain.48 These markers are used in evaluating patients with suspected CHF but are otherwise not regularly used in clinical practice (with the exception of brain-type natriuretic peptides). An elevated brain-type natriuretic peptide level provides powerful risk stratification across a broad spectrum of patients with ACS,49 although it lacks specificity for ACS, limiting its utility in patients with undifferentiated chest pain. Although increases are usually associated with impaired left ventricular systolic function, increases can be seen in a variety of cardiac conditions that result in increased left or right ventricular stretch, such as valvular abnormalities or hypertrophy.50 An important consideration is that values considered elevated and predictive of adverse events in patients with ACS are significantly lower than those seen in most patients with CHF. Another commercially available biomarker, high-sensitivity C-reactive protein, appears to have value for long-term prediction of cardiac events. However, in low-risk patients with chest pain, it is not useful for identifying ACS.51
CLINICAL RISK SCORES
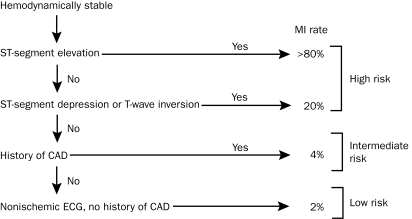
A recommended approach to risk stratification for patients with potential ACS is the application of scoring systems based on the history and initial clinical presentation. The simplest scheme relies on 1 set of cardiac markers, ECG findings, and history of CAD (Figure 1). If findings and history are unremarkable, the patient can be considered low risk, with a probability of MI of less than 5% to 6%.15 Some of the first validated chest pain algorithms were derived by Goldman and colleagues.15,30,31,52 These algorithms are based primarily on ECG findings and chest pain characteristics. The Goldman algorithm is useful in predicting the need for intensive care unit admission, development of cardiovascular complications, and outcomes; it can therefore be used to facilitate decisions regarding disposition, such as whether to admit to a cardiac care or observation unit.52 Because the risk of MI in lower-risk patients remains greater than 1%, it cannot identify patients who can be rapidly discharged from the ED without further evaluation.
FIGURE 1.
Risk of myocardial infarction based on presenting characteristics. CAD = coronary artery disease; ECG = electrocardiography; MI = myocardial infarction.
Data from N Engl J Med.15
Other risk stratification schemes of varying complexity have been derived from clinical trials.53-55 Likely the best known is the Thrombolysis in Myocardial Infarction (TIMI) risk score.55 This scoring system comprises 7 equally rated variables: cardiac biomarker elevation, ST-segment depression, older age (≥65 years), aspirin use within the past week, 2 or more episodes of chest pain in the past 24 hours, 3 or more of the standard CAD risk factors, and known coronary stenosis of 50% or more. An increase in score is associated with a stepwise increase in incidence of the combination of death, MI, and need for urgent revascularization. A modified TIMI risk score, in which elevated biomarkers or ischemic ECG changes are removed, has been studied in lower-risk patients, with mixed results.56,57 However, even in patients with the lowest TIMI scores (eg, 0-1), further evaluation is required because the event rate in this group is not negligible.57 Because these scores provide relatively similar prognostic information,58 no one score can be recommended over another.
Scoring systems have a number of limitations. Most were derived from ACS clinical trials that frequently excluded the highest- and lowest-risk patients.53,55 For example, neither renal insufficiency nor CHF, 2 variables associated with very high risk, is included in the TIMI risk score.55 Because variables were derived from case report forms and multivariate analysis, they are not always logical, and absolute reliance on them can result in an inaccurate estimation of risk. Rather than any specific scoring system, identification and recognition of the variables common to different scoring systems, such as evidence of hemodynamic instability, older age, CHF, renal dysfunction, ECG findings, and injury marker variables, are more important in the initial ED evaluation. Favorable scores identify lower patient risk and allow consideration for management in a CPU or outpatient setting.
DIFFERENTIAL DIAGNOSIS
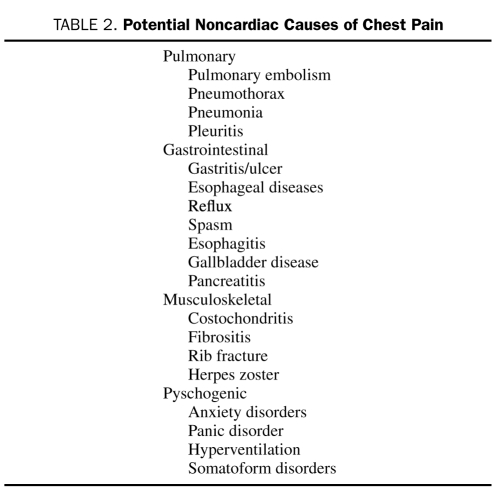
When patients present to the ED with chest pain, differentiating ischemic from nonischemic causes is difficult and frequently the major focus of the evaluation. Because morbidity is high if a cardiac etiology is not diagnosed early, the overlap of symptoms necessitates an initial diagnostic strategy that assumes symptoms are cardiac-related unless other causes are obviously apparent. However, a high awareness of the many other causes of chest pain is needed (Table 2) to guide the treatment of patients with more common, less serious disorders and to ensure that life-threatening noncardiac etiologies are not overlooked. Although the ED visit should be focused on identifying patients with life-threatening diseases, further evaluation on discharge from the ED often is warranted to determine the etiology of the symptoms, particularly if they recur.
TABLE 2.
Potential Noncardiac Causes of Chest Pain
Some of the more serious causes of chest pain that can be confused with cardiac ischemia include pericarditis, aortic dissection, and pulmonary embolism. Chest pain from pericarditis is more commonly pleuritic and positional but can also be a severe, steady retrosternal pain.10 The presence of a friction rub on examination is diagnostic of pericarditis; however, it is not always apparent at initial presentation. Instead, patients with pericarditis may present with diffuse ST-segment elevation, and therefore the overlap of symptoms and ECG findings can lead to misdiagnosis of acute MI, potentially resulting in the inadvertent administration of thrombolytic agents or emergent coronary angiography.59 In confusing cases, early echocardiography may be helpful by demonstrating the presence of a pericardial effusion or the presence or absence of wall motion abnormalities.
Aortic dissection is another cause of chest pain that requires urgent diagnosis because early medical and/or surgical intervention can reduce the high short-term mortality rate. Fortunately, the incidence of acute dissection is substantially lower than that of MI. Patients with aortic dissection typically describe a sudden onset of severe ripping or tearing pain, often with radiation to the back. Other diagnostic clues include pulse deficits, a substantial difference in right and left arm blood pressures, focal neurologic deficits, or pain that is unrelieved despite large doses of narcotics. ST-segment elevation associated with aortic dissection is uncommon (<5%-10% of patients with proximal aortic dissection),60 usually as a result of involvement of the coronary ostia or from hemopericardium, and thus leads to inappropriate treatment.
Other cardiac causes of chest pain that should be considered include aortic stenosis, pulmonary hypertension, and hypertrophic cardiomyopathy. Up to 20% of patients with typical chest pain have angiographically normal coronary arteries but impaired coronary vasodilator reserve. This is more commonly seen in women and has been attributed to abnormal microvascular circulation. Although the symptoms are associated with substantial morbidity, patients are at low risk of cardiac mortality.61
Pulmonary embolism is a potentially life-threatening cause of noncardiac chest pain. Typically, it is associated with dyspnea, tachypnea, and pleuritic chest pain. Electrocardiographic findings, such as evidence of right heart strain, anterior T-wave inversion, and right bundle branch block, may mimic ischemic changes; however, the most common ECG finding is sinus tachycardia alone.62 Troponin elevations, usually associated with significant right ventricular strain, identify a subset with a high mortality.63
Esophageal disease commonly causes chest pain that is difficult to distinguish from cardiac chest pain. In a study of 910 patients who underwent esophageal motility testing after cardiac disease was excluded, 28% of the patients had esophageal dysmotility, and an additional 21% had baseline manometric abnormalities that were thought to suggest esophageal pain.64 These results and others65 suggest that esophageal disorders are a frequent etiology of chest pain in patients in whom ACS has been excluded.
The prevalence of psychiatric disorders in patients presenting to the ED with chest pain is relatively uncommon. In one study, 35% of the 229 patients screened met Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition criteria for panic disorder (18%) and/or depression (23%); however, only 6% were identified by ED physicians as having a psychosocial component to their chest pain syndromes.66 Formal screening of all patients will identify a higher prevalence of psychiatric diagnoses.
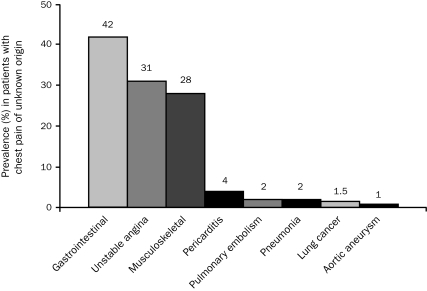
The frequency of noncardiac causes of chest pain in ED patients has been described in a number of studies. Fruergaard et al67 performed one of the few comprehensive studies that attempted to determine the etiology of chest pain. In contrast to most studies that performed selective testing after exclusion of ACS, all 148 patients without a clear-cut diagnosis in the study by Fruergaard et al underwent multiple tests designed to identify the etiology of the symptoms (Figure 2). Although most (77%) had 1 diagnosis, 21% had 2, and 3 patients had 3 diagnoses, a finding consistent with other studies that have found a frequent overlap in potential causes of chest pain.
FIGURE 2.
Prevalence of diagnoses in patients with chest pain of unknown origin.
Adapted from Eur Heart J,67 with permission from Oxford University Press.
ADDITIONAL INITIAL DIAGNOSTIC TESTING
Current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines recommend that all patients with suspected ACS undergo ECG and cardiac biomarker testing. Recommendations from the American College of Radiology assigned a value of 9 (most appropriate) for chest radiographic evaluation of patients with acute chest pain who have a low probability of disease.68 Of the 5000 ED-obtained chest radiographs reviewed in one large study, including 629 obtained for a primary symptom of chest pain, findings on 25% were serious enough to affect clinical decision making.69 However, only limited data are available assessing the value of chest radiography in patients previously defined as low risk on the basis of history and physical examination findings.
CHEST PAIN UNITS AND ADPs
Chest pain units were developed as a mechanism for the rapid assessment and exclusion of ACS in low-risk patients in a cost-effective manner that avoids routine admission and prolonged hospital stays. These units provide an integrated, protocol-driven approach to further stratify low-risk patients by short-term observation and serial assessment of clinical variables, ECG findings, and cardiac biomarker levels.9,11,15,70 They are usually directed by an emergency physician, but their successful implementation requires close coordination with cardiology and other (eg, hospitalist, radiologist, nuclear medicine specialist, nursing) personnel involved in the patient's management.70 These units allow the rapid acquisition of diagnostic and prognostic information and provide an intermediary step in the transition from the ED to either an outpatient or inpatient setting.
If at any point findings on tests (serial ECG, biomarkers) are positive, the patient is admitted to the hospital for further evaluation. If findings are negative, the patient typically undergoes subsequent provocative testing. Standardizing the approach to evaluating low-risk patients reduces both testing variability and costs.
EXERCISE TREADMILL TESTING
Standard exercise treadmill testing (ETT) is a cornerstone of risk stratification in CPUs.11,71 Its advantages include relatively modest cost, availability, ease of performance, and its ability to provide important prognostic information. Criteria for selecting this test are the patient's ability to exercise and normal findings on a baseline ECG that allows interpretation of exercise-induced ST-segment alterations. If findings on ECG are not interpretable, the addition of cardiac imaging is required and may incorporate pharmacologic stress, depending on the patient's ability to exercise.
The efficacy and safety of incorporating ETT into current CPU protocols has been confirmed by many studies of patients who underwent ETT after 12 or fewer hours of negative observation.72-77 A Science Advisory of the AHA concluded that symptom-limited ETT after 8 to 12 hours of evaluation in low- to intermediate-risk patients is safe.78 This strategy has a very high negative predictive value in patients in the CPU for short- and long-term adverse events. Although the positive predictive value is low, positive ETT results are uncommon, and the number of unnecessary admissions is therefore reduced, decreasing costs. An abbreviated protocol has been used successfully in selected low-risk patients. Either no or less than a full set of serial cardiac biomarkers were obtained before ETT72-76; if the markers were negative, ETT was performed. With this strategy, no adverse events had occurred at 30 days.72,76 Although imaging (either echocardiography or myocardial perfusion imaging [MPI]) is often added to ETT in low- to intermediate-risk patients, current ACC/AHA guidelines for stress testing as well as for management of non—ST-segment elevation ACS recommend ETT without imaging in patients who can exercise and do not have substantial baseline ECG changes that preclude interpretation.11,79
An important variable that adds predictive power is functional capacity. Failure to achieve more than 3 metabolic equivalents is associated with increased risk.80 In contrast, patients who achieve more than 10 metabolic equivalents and have negative findings on stress ECG have a very low incidence of ischemia on MPI.81 Risk stratification can be further enhanced by integrating multiple ETT variables into scores, such as the Duke treadmill score.82 If patients do not reach 85% of age-predicted maximum heart rate and ECG reveals no evidence of ischemia, the test is considered nondiagnostic and further assessment, such as with stress imaging, may be considered.
Failure of ETT to reproduce the chest pain prompting the ED visit reduces the likelihood of ACS. Although the sensitivity of ETT to detect CAD is lower than that of other imaging techniques,79 the additional information obtained by other techniques may not be cost-effective in a low-risk population.83,84
The cost-effectiveness of ADPs that include ETT has been demonstrated in comparisons of this strategy with regular care. In one study, ETT was performed after a 12-hour observation period in 317 patients with negative findings on cardiac marker testing and serial ECG. The negative predictive value was 98%, with a cost saving of $567 per patient compared with patients admitted for usual care.74 In another study, a rapid protocol was associated with reduced length of stay (11 vs 23 hours) and cost ($624 less per patient) compared with usual care. Further study of an ADP compared with usual care revealed no difference in cardiac events in the 2 groups after 6 months, but the cost of care and cardiac procedures were 61% higher in patients receiving regular care.85
OUTPATIENT STRESS TESTING
Ideally, ADPs would be available at all times; however, this is not feasible for many institutions. An alternative strategy, recognized by ACC/AHA guidelines,11 approves outpatient ETT in selected low-risk patients with chest pain, provided they meet the following criteria: (1) no further ischemic chest discomfort, (2) normal or nonischemic findings on initial and follow-up ECG, and (3) normal cardiac biomarker measurements. Observational data have found this strategy to be safe, with no adverse cardiac events during the interval between hospital discharge and outpatient ETT.86 When the preferred strategy of predischarge testing is unavailable (eg, nights, weekends), the very low short-term likelihood of a cardiac event supports the use of outpatient ETT, which appears to be a safe alternative to prolonged admission. The utility of outpatient ETT is predicated on performance of the test within 72 hours (24 hours is preferable), reliability of the patient to follow up for the test, and close communication between the CPU physician and the patient's personal physician.
ACUTE CARDIAC IMAGING
In some institutions, additional diagnostic imaging is used to rapidly stratify the risk of patients before completing serial marker sampling and provocative testing. These include acute rest MPI, computed tomographic coronary angiography (CTCA), echocardiography, and cardiac magnetic resonance imaging. The advantage of adding early imaging (MPI, magnetic resonance imaging, echocardiography) is that abnormal perfusion and wall motion occur within seconds of ischemia onset, so that MI can be identified before the detection of cardiac biomarkers in the blood-stream. Ischemia, for which no current biomarker exists, can likewise be detected.
ACUTE MPI
Acute rest MPI using technetium agents has been shown to accurately identify low- and high-risk patients who present with chest pain.87 A perfusion defect indicates acute ischemia, acute infarction, or old infarction. Patients can be injected while they are experiencing symptoms and undergo delayed imaging after stabilization. The images obtained provide a “snapshot” of myocardial perfusion at the time of injection. Normal perfusion is associated with very low clinical risk, allowing patients to be discharged with further outpatient routine stress testing, if indicated, to detect underlying CAD.87 In addition, wall motion and thickening are simultaneously assessed, allowing differentiation of perfusion defects resulting from artifacts or soft tissue attenuation from those occurring as a result of ischemia.88 Left ventricular ejection fraction is also obtained, providing quantitative determination of systolic function.
In one of the few randomized trials performed to assess optimal diagnostic strategies in the ED, the value of rest MPI was demonstrated in a prospective, multicenter trial of 2475 patients who presented to the ED with chest pain and nonischemic findings on ECG.89 Patients were randomized to receive usual care with or without the addition of rest MPI. Sensitivity of the 2 strategies was similar (96% and 97%, respectively). Despite the addition of a potentially expensive technology, patients in the rest MPI arm had a significantly lower hospitalization rate, resulting in an estimated cost savings of $70 per patient. Cost reductions may also occur through more appropriate selection of diagnostic testing, with lower rates of coronary angiography in low-risk patients.90 On the basis of its high sensitivity and negative predictive value, rest MPI has a class 1 indication in current guidelines for evaluating patients with nonischemic findings on ECG.87
Acute rest MPI has several limitations. A perfusion defect can indicate a new or old infarct. Recognition of MI requires assessment with cardiac biomarkers. To differentiate prior infarction from acute ischemia, follow-up imaging during a pain-free state is required. Resolution of a perfusion defect indicates that the initial defect was secondary to acute ischemia; if the defect remains unchanged, prior MI would be the more likely etiology. Sensitivity of MPI is dependent on the total ischemic area; small areas of myocardium at risk (3%-5% of the left ventricle) may not be detected. Therefore, rest MPI is optimally used in conjunction with cardiac biomarker measurement, which offers complementary information. Because it can quantitate the ischemic area, rest MPI may be a more optimal means of assessing ischemic risk than cardiac injury markers alone. The availability of MPI for off-hour imaging is a potential logistical issue. In one study, however, patients presenting from midnight to 6 am were injected with technetium Tc 99m sestamibi during that interval, with imaging performed after 6 am; no difference in diagnostic accuracy between delayed and immediate imaging was found.91 Finally, the use of rest imaging does not eliminate the need for stress testing in all patients, and therefore subsequent outpatient evaluation needs to be coordinated.
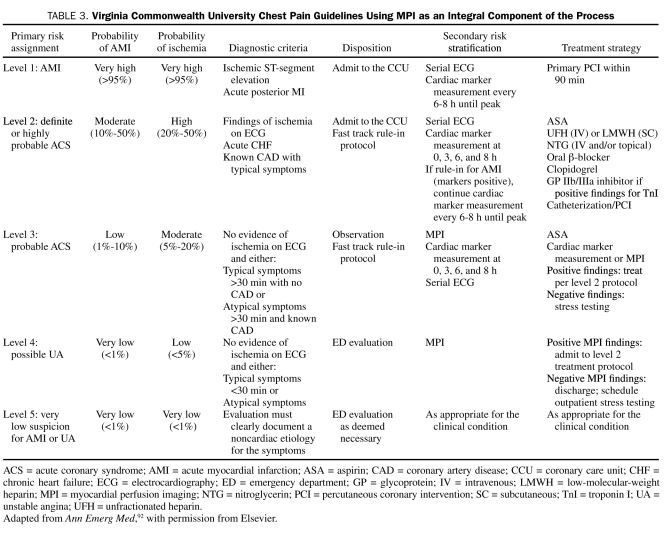
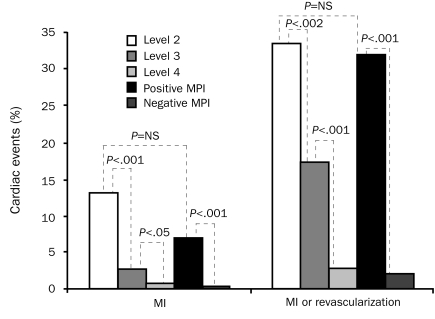
The optimal use of rest MPI is in conjunction with a standardized risk stratification protocol (Table 3). In this protocol, patients are stratified according to the heretofore mentioned ACC/AHA guidelines. Low-risk patients are further stratified into level 3 patients, who are admitted for observation, and level 4 patients, who are discharged and scheduled for outpatient stress testing if acute MPI findings are negative. If MPI findings are positive, both types of patients are admitted, and care is advanced to the level provided patients with ACS.92 In one study, patients who had positive MPI findings had event rates similar to patients initially considered at high risk of cardiac events (level 2 patients) (Figure 3).
TABLE 3.
Virginia Commonwealth University Chest Pain Guidelines Using MPI as an Integral Component of the Process
FIGURE 3.
Use of acute myocardial perfusion imaging (MPI) in a risk stratification scheme. Risk of cardiac events, either myocardial infarction (MI) or the combination of MI and revascularization, increased as risk level increased from level 4 to 2. Patients who had positive findings on MPI had an event rate similar to level 2 patients (high-risk acute coronary syndrome). NS = not significant.
Adapted from Ann Emerg Med,92 with permission from Elsevier.
COMPUTED TOMOGRAPHIC CORONARY ANGIOGRAPHY
Compared with other imaging techniques, CTCA provides anatomic rather than functional information regarding coronary patency. Application of computed tomography (CT) to coronary artery imaging has become feasible with the advent of multislice CT. The acquisition of CT data is synchronized to the surface ECG and collected for 10 to 20 seconds while patients hold their breath during injection of contrast medium. As with the validation of other tests, most studies have examined the diagnostic accuracy in moderate- to high-risk patients who are undergoing coronary angiography for clinical indications. Accuracy has been high, with a consistently high specificity and a very high negative predictive value for exclusion of substantial CAD, although positive predictive value has not been as high.93
Studies evaluating the utility of CTCA in a population of low-risk patients in the ED are limited. Of 368 patients with acute chest pain who underwent CTCA in a recent study, 31 (8%) had ACS.94 The sensitivity and negative predictive value of coronary CTCA for ACS were 100% for absence of CAD and 77% and 98%, respectively, for significant stenosis. Only 1 ACS occurred in the absence of calcified plaque. Of patients with acute chest pain and low to intermediate likelihood of ACS, 50% were free of CAD by CT and had no ACS.95 Other studies of CTCA in the ED setting are consistent with these short-term findings.96,97 However, in many cases, patients evaluated were at very low risk and likely would have done well without imaging.
In a randomized trial of low-risk patients with possible ACS, CTCA was compared to standard care, which included an 8-hour rule-out protocol to exclude MI, followed by stress MPI (Figure 4). 96 Overall diagnostic accuracy was similar, with low event rates in each arm. Cost as well as time to diagnosis and discharge (15.0 vs 3.4 hours) was lower in the CTCA arm.75,96 Of the 9 patients who underwent coronary angiography in the CTCA arm, 8 had severe disease, 6 of whom underwent revascularization. In the stress MPI arm, 3 patients underwent coronary angiography, one of whom had severe disease. However, this trial also demonstrated the potential limitations associated with a CTCA strategy. Approximately 25% of patients evaluated were ineligible because of underlying arrhythmias, conditions preventing β-blocker administration, and renal insufficiency. Of patients who did have CTCA, 25% required further diagnostic testing with stress MPI because of equivocal CTCA findings. Although CTCA holds promise for diagnosing patients as having ACS, these data indicate that larger, multicenter studies are required before this technology can be considered widely applicable.
FIGURE 4.
Schematic of patient distribution based on whether patients were randomized to acute computed tomographic coronary angiography (CTCA) or standard of care, which uses stress myocardial perfusion imaging (MPI) as the preferred risk stratification method.
Adapted from J Am Coll Cardiol,96 with permission from Elsevier.
Computed tomographic coronary angiography has the potential to provide a more comprehensive examination of patients with chest pain97,98 and to exclude other life-threatening etiologies, such as pulmonary embolism and aortic dissection (“triple rule-out”). Although technically feasible, such scans are more challenging, requiring larger volumes of contrast medium and longer scan times and resulting in increased breath-holding times and more radiation.97 Although small-scale studies have suggested that the overall image quality is high,97,98 large-scale clinical studies have not been performed.
Computed tomographic coronary angiography has limitations. As already described, up to 25% of patients may not be candidates for this technique because of obesity, intolerance to β-blockade, arrhythmia, allergies to contrast medium, or renal insufficiency.96 Image interpretation is more difficult in the setting of coronary calcification and in patients with prior stent placement.99 Increased use of CTCA has led to recognition of the potential long-term risk of radiation exposure,100 both with CTCA as well as other imaging techniques.101 The average radiation dose of a 64-slice CTCA varies widely depending on sex, body size, imaging protocol, and the type of CT. Although total body radiation dose is similar to that of rest/stress MPI protocols, the effective dose to breast and lung tissue is roughly 3-fold higher, increasing the estimated lifetime attributive risk of malignancies to these tissues, particularly in younger women.102 This increased risk has resulted in renewed emphasis on protocols designed to reduce radiation exposure and on the design of newer scanners. Cost analyses have not always included the potential cost of downstream imaging and diagnostic testing required to follow up incidental findings, which can be common,103 particularly pulmonary nodules that may require repeated follow-up lung CT. The diagnostic accuracy of CTCA is dependent on the experience of those interpreting the images, an important consideration given that current diagnostic performance estimates are derived primarily from studies done in centers with expertise in this area. Because of these issues, CTCA may be most useful for evaluating lower-risk patients who have nondiagnostic test results by other modalities or patients with repeated ED visits, in whom the benefit of coronary angiography is likely to be low. Larger multicenter trials that are currently under way should provide additional information on patient populations in which CTCA would be appropriate and provide data on the diagnostic accuracy and cost-effectiveness of this imaging technique compared with traditional evaluation tools.
ECHOCARDIOGRAPHY
On the basis of its high degree of reliability for assessing cardiac wall motion, echocardiography has been used for diagnosis and risk assessment in patients presenting to the ED with symptoms suggesting ACS for more than 30 years. Regional wall motion abnormalities induced by ischemia are detected by echocardiography almost immediately after its onset and precede ECG alterations and symptoms.104 Factors that determine the diagnostic accuracy of rest echocardiography to detect ACS include infarct size and timing of the study in relation to symptoms. More than 20% of the transmural thickness of the myocardium must be affected for a wall motion abnormality to be detected.105 These factors likely contribute to the wide variability in negative (57%-98%) and positive (31%-100%) predictive values of resting echocardiography for MI found in 9 studies that included almost 1000 patients.106 As with rest MPI, echocardiography cannot be used to determine the age of an abnormality. Probably the greatest limitation to its widespread adoption is the logistical difficulty of supplying highly skilled personnel for around-the-clock image acquisition and interpretation.
Echocardiographic contrast agents can be added to improve image quality and endocardial border detection, decreasing the number of segments that are not visible.107 In addition to improving left ventricular opacification, echocardiographic contrast agents can be used to quantitate myocardial perfusion. Decreased uptake is consistent with suboptimal perfusion.108 In a large study, contrast echocardiography increased detection and prediction of major cardiac events compared with usual care and significantly increased prediction of long-term events.109 One important limitation of most echocardiographic contrast perfusion studies is that most have been limited to single centers; additional multicenter validation studies will be necessary before widespread adoption.
CARDIAC MAGNETIC RESONANCE IMAGING
Cardiac magnetic resonance imaging (CMRI) has superior image quality to most other noninvasive imaging, allowing assessment of perfusion, function, and valvular abnormalities during a single imaging session; however, imaging of coronary arteries remains inferior to CTCA.110 In patients with potential ACS, CMRI may provide “one-stop shopping.” If findings on initial rest imaging are negative, stress CMRI with adenosine could be performed immediately, eliminating the need for later testing. A number of relatively small single-center studies have evaluated the efficacy of acute rest CMRI for ED management of patients with chest pain. In a prospective study of 161 patients, sensitivity and specificity for identification of ACS were 84% and 85%, respectively.111 Adding T2-weighted imaging to assess myocardial edema increased the detection of ACS to 93%.112 Adding stress CMRI to the evaluation, another study found a sensitivity and specificity for identification of CAD of 96% and 83%, respectively, using adenosine stress perfusion and late gadolinium enhancement.113 An important limitation to using CMRI in the setting of acute chest pain is the logistical difficulty of providing imaging on a routine basis. Thus, this method is likely to be used only in centers capable of supplying the personnel to perform and interpret testing.
TRANSITION INTO THE OUTPATIENT SETTING
The chest pain evaluation may represent a potential “teachable moment,” as it may be the first and only contact with a physician for a number of years, particularly for the younger patient who is relatively healthy. Therefore, in patients in whom ACS has been excluded, focusing on primary prevention and addressing risk factors are important. Many patients who have undergone a CPU evaluation will have had lipids sampled; therefore, early follow-up with a primary care physician for subsequent evaluation and treatment is valuable, particularly if treatment is not initiated at the time of discharge. Although blood pressures may not be representative of baseline pressures due to stress, markedly elevated blood pressures are unlikely to normalize in the long term and therefore early follow-up (within 1-2 weeks) is important for reassessment. Instructions on smoking cessation are clearly important as well.
Patients discharged home from the ED often have had varying degrees of evaluation depending on the treating physician's clinical impression of risk of CAD. Patients with negative CPU evaluations, including a diagnostic study, usually have a noncardiac etiology for their symptoms.15 Further evaluation for alternative sources of chest pain may be warranted, particularly in patients who have recurrent symptoms, and should be directed by their primary care physician. Identification of the origin and management of symptoms may improve quality of life, diminish diagnostic uncertainty, and prevent unnecessary returns to the ED.
OUTPATIENT OFFICE MANAGEMENT
Patients who call the office reporting active chest pain, including those with atypical symptoms, should be directed immediately to an ED that can initiate reperfusion therapy. Although patients with CAD are more likely to be seen in cardiology offices, such patients are increasingly being seen by their primary care physician. Because patients may develop symptoms at the time of or shortly after arrival, all offices should have automatic external defibrillators and be capable of performing immediate resuscitation in the event of a cardiac arrest; rapid ECG should also be available. In large offices with a large number of patients with CAD, it may also be prudent to offer cardiac biomarker measurement, and point-of-care devices are making this increasingly feasible.
More frequently, patients present after having developed symptoms during the past few days but are without active symptoms in the preceding 24 to 48 hours. In this scenario, as for the ED evaluation, ECG should be performed. If findings on ECG are abnormal or suggestive of acute ischemia, the patient should be referred immediately to an ED, preferably by emergency medical services.
The initial diagnostic approach for those without ongoing symptoms or objective evidence of ACS is similar to that for the ED evaluation. The history should be focused on the type of chest pain and precipitating and relieving factors, among other variables. Subsequent diagnostic evaluation is based primarily on the initial risk stratification afforded by the history and findings on physical examination and ECG.
In addition to determining the trajectory of subsequent diagnostic evaluation before the patient leaves the office, initial therapy should be considered. Most patients should begin taking aspirin and be prescribed sublingual nitroglycerin (with instructions on how to use it). Patients should also be informed what to do if they develop more chest pain while awaiting diagnostic evaluation.
CONCLUSION
The initial evaluation of the patient with acute chest pain should be prompt and include ECG, measurement of a set of initial cardiac markers, and history taking and physical examination that focus on hemodynamic variables and evidence of systolic dysfunction. In those in whom the risk is moderate to high on the basis of the preceding criteria, further evaluation in an inpatient setting (either step-down or intensive care unit) is usually required. For most patients in whom none of these factors indicates high risk, a short-term observation protocol with serial marker sampling and subsequent provocative testing to exclude both infarction and ischemia usually follow. The choice of stress testing depends on the patient's ability to exercise and the interpretability of findings on ECG. Some institutions have succeeded in shortening the observation period by using newer imaging techniques that can assess function, perfusion, and/or coronary anatomy, excluding both infarction and ischemia with a high negative predictive value. The choice of imaging tool will be highly dependent on institutional availability and expertise, with some newer techniques usually limited to high-volume centers.
Supplementary Material
On completion of this article, you should be able to (1) recognize common causes of troponin elevations not related to acute coronary syndrome, (2) identify characteristics associated with high and low risk of ischemic complications in patients with possible myocardial infarction, and (3) describe the advantages and disadvantages of imaging tests (rest myocardial perfusion imaging, computed tomographic coronary angiography, and cardiac magnetic resonance imaging) for the early diagnosis and risk stratification of low-risk patients with chest pain.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Pitts SR, Niska RW, Xu J, Burt CW, US Dept of Health and Human Services National hospital ambulatory medical care survey: 2006 emergency department summary. National Health Statistics Reports Web site. http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf Published August 6, 2008. Accessed January 19, 2010. [PubMed]
- 2.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170 [DOI] [PubMed] [Google Scholar]
- 3.Physician Insurers Association of America Acute Myocardial Infarction Study Rockville, MD: Physician Insurers Association of America; 1996:1 [Google Scholar]
- 4.Lee TH, Rouan GW, Weisberg MC, et al. Clincial characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60(4):219-224 [DOI] [PubMed] [Google Scholar]
- 5.McCarthy BD, Beshansky JR, D'Agostino RB, et al. Missed diagnoses of acute myocardial infarction in the emergency department: results from a multicenter study. Ann Emerg Med. 1993;22(3):579-582 [DOI] [PubMed] [Google Scholar]
- 6.Pelberg AL. Missed myocardial infarction in the emergency room. Qual Assur Util Rev. 1989;4(2):39-42 [DOI] [PubMed] [Google Scholar]
- 7.Rusnak RA, Stair TO, Hansen KN, et al. Litigation against the emergency physician: common features in cases of missed myocardial infarction. Ann Emerg Med. 1989;18(10):1029-1034 [DOI] [PubMed] [Google Scholar]
- 8.Ting HH, Lee TH, Soukup JR, et al. Impact of physician experience on triage of emergency room patients with acute chest pain at three teaching hospitals. Am J Med. 1991;91(4):401-407 [DOI] [PubMed] [Google Scholar]
- 9.Amsterdam EA, Kirk JD, eds. Chest pain units. Cardiol Clin. 2005November;23(4):401-630 [DOI] [PubMed] [Google Scholar]
- 10.Cannon CP, Lee TH. Approach to the patient with chest pain. In: Libby P, Bonow RO, Mann DL, Zipes DP, eds. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine 8th ed.Philadelphia, PA: Saunders; 2008:1195-1205 [Google Scholar]
- 11.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) [published correction appears in J Am Coll Cardiol. 2008;51(9):974] J Am Coll Cardiol. 2007;50(7):e1-e157 [DOI] [PubMed] [Google Scholar]
- 12.Savonitto S, Ardissino D, Granger CB, et al. Prognostic value of the admission electrocardiogram in acute coronary syndromes. JAMA 1999;281(8):707-713 [DOI] [PubMed] [Google Scholar]
- 13.Kaul P, Newby LK, Fu Y, et al. Troponin T and quantitative ST-segment depression offer complementary prognostic information in the risk stratification of acute coronary syndrome patients. J Am Coll Cardiol. 2003;41(3):371-380 [DOI] [PubMed] [Google Scholar]
- 14.Zimetbaum PJ, Josephson ME, McDonald MJ, et al. Incidence and predictors of myocardial infarction among patients with atrial fibrillation. J Am Coll Cardiol. 2000;36(4):1223-1227 [DOI] [PubMed] [Google Scholar]
- 15.Lee TH, Goldman L. Evaluation of the patient with acute chest pain. N Engl J Med. 2000;342(16):1187-1195 [DOI] [PubMed] [Google Scholar]
- 16.Slater DK, Hlatky MA, Mark DB, Harrell FE, Jr, Pryor DB, Califf RM. Outcome in suspected acute myocardial infarction with normal minimally abnormal admission electrocardiographic findings. Am J Cardiol. 1987;60(10):766-770 [DOI] [PubMed] [Google Scholar]
- 17.Forest RS, Shofer FS, Sease KL, et al. Assessment of the standardized reporting guidelines ECG classification system: the presenting ECG predicts 30-day outcomes. Ann Emerg Med. 2004;44(3):206-212 [DOI] [PubMed] [Google Scholar]
- 18.Kontos MC, Roberts BD, Jesse RL, et al. Utility of the presenting electrocardiogram to predict mortality when troponin level is used to diagnose myocardial infarction. Am J Emerg Med. 2009;27(2):146-152 [DOI] [PubMed] [Google Scholar]
- 19.Huey BL, Beller GA, Kaiser DL, et al. A comprehensive analysis of myocardial infarction due to left circumflex artery occlusion: comparison with infarction due to right coronary artery and left anterior descending artery occlusion. J Am Coll Cardiol. 1988;12(5):1156-1166 [DOI] [PubMed] [Google Scholar]
- 20.Gibler WB, Sayre MR, Levy RC, et al. Serial 12-lead electrocardiographic monitoring in patients presenting to the emergency department with chest pain. J ElectroCardiol. 1993;26(suppl):238-243 [PubMed] [Google Scholar]
- 21.Fesmire FM, Hughes AD, Fody EP, et al. The Erlanger chest pain evaluation protocol: a one-year experience with serial 12-lead ECG monitoring, two-hour delta serum marker measurements, and selective nuclear stress testing to identify and exclude acute coronary syndromes. Ann Emerg Med. 2002;40(6):584-594 [DOI] [PubMed] [Google Scholar]
- 22.Zalenski RJ, Cooke D, Rydman R, Sloan EP, Murphy DG. Assessing the diagnostic value of an ECG containing leads V4R, V8, and V9: the 15-lead ECG. Ann Emerg Med. 1993;22(5):786-793 [DOI] [PubMed] [Google Scholar]
- 23.Zalenski RJ, Rydman RJ, Sloan EP, et al. Value of posterior and right ventricular leads in comparison to the standard 12-lead electrocardiogram in evaluation of ST-segment elevation in suspected acute myocardial infarction. Am J Cardiol. 1997;79(12):1579-1585 [DOI] [PubMed] [Google Scholar]
- 24.Hoekstra JW, O'Neill BJ, Pride YB, et al. Acute detection of ST-elevation myocardial infarction missed on standard 12-lead ECG with a novel 80-lead real-time digital body surface map: primary results from the multicenter OCCULT MI trial. Ann Emerg Med. 2009;54(6):779-788.e1 [DOI] [PubMed] [Google Scholar]
- 25.Lee TH, Cook EF, Weisberg M, Sargent RK, Wilson C, Goldman L. Acute chest pain in the emergency room: identification and examination of low-risk patients. Arch Intern Med. 1985;145(1):65-69 [PubMed] [Google Scholar]
- 26.Grijseels EW, Deckers JW, Hoest AW, et al. Implementation of a pre-hospital decision rule in general practice: triage patients with suspected myocardial infarction. Eur Heart J. 1996;17(1):89-95 [DOI] [PubMed] [Google Scholar]
- 27.Henrikson CA, Howell EE, Bush DE, et al. Chest pain relief by nitroglycerin does not predict active coronary artery disease. Ann Intern Med. 2003;139(12):979-986 [DOI] [PubMed] [Google Scholar]
- 28.Diercks DB, Boghos E, Guzman H, Amsterdam EA, Kirk JD. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005;45(6):581-585 [DOI] [PubMed] [Google Scholar]
- 29.Selker HP, Griffith JL, D'Agostino RB. A tool for judging coronary care unit admission appropriateness, valid for both real-time and retrospective use: a time-insensitive predictive instrument (TIPI) for acute cardiac ischemia: a multicenter study. Med Care 1991;29(7):610-627 [DOI] [PubMed] [Google Scholar]
- 30.Goldman L, Weinberg M, Weisberg M, et al. A computer-derived protocol to aid in the diagnosis of emergency room patients with acute chest pain. N Engl J Med. 1982;307(10):588-596 [DOI] [PubMed] [Google Scholar]
- 31.Goldman L, Cook EF, Brand DA, et al. A computer protocol to predict myocardial infarction in emergency department patients with chest pain. N Engl J Med. 1988;318(13):797-803 [DOI] [PubMed] [Google Scholar]
- 32.Tierney WM, Fitzgerald J, McHenry R, et al. Physicians' estimates of the probability of myocardial infarction in emergency room patients with chest pain. Med Decis Making 1986;6(1):12-17 [DOI] [PubMed] [Google Scholar]
- 33.Bayer AJ, Chadha JS, Farag RR, Pathy MS. Changing presentation of myocardial infarction with increasing old age. J Am Geriatr Soc. 1986;34(4):263-266 [DOI] [PubMed] [Google Scholar]
- 34.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. N Engl J Med. 1984;311(18):1144-1147 [DOI] [PubMed] [Google Scholar]
- 35.Sigurdsson E, Thorgeirsson G, Sigvaldason H, et al. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. Ann Intern Med. 1995;122(2):96-102 [DOI] [PubMed] [Google Scholar]
- 36.Fesmire FM, Decker WW, Diercks DB, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Non-ST-Segment Elevation Acute Coronary Syndromes Clinical policy: critical issues in the evaluation and management of adult patients with non-ST-segment elevation acute coronary syndromes. Ann Emerg Med. 2006;48(3):270-301 [DOI] [PubMed] [Google Scholar]
- 37.Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007;115(13):e356-e375 [DOI] [PubMed] [Google Scholar]
- 38.Morrow DA, Antman EM, Tanasijevic M, et al. Cardiac troponin I for stratification of early outcomes and the efficacy of enoxaparin in unstable angina: a TIMI 11B substudy. J Am Coll Cardiol. 2000;36(6):1812-1817 [DOI] [PubMed] [Google Scholar]
- 39.Newby LK, Ohman EM, Christenson RH, et al. Benefit of glycoprotein IIb/IIIa inhibition in patients with acute coronary syndromes and troponin T-positive status: the paragon-B troponin T substudy. Circulation 2001June19;103(24):2891-2896 [DOI] [PubMed] [Google Scholar]
- 40.Morrow DA, Cannon CP, Rifai N, et al. TACTICS-TIMI 18 Investigators Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction: results from a randomized trial. JAMA 2001;286(19):2405-2412 [DOI] [PubMed] [Google Scholar]
- 41.Apple FS, Jesse RL, Newby LK, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Circulation 2007;115(13):e352-e355 [DOI] [PubMed] [Google Scholar]
- 42.Kelley WE, Januzzi JL, Christenson RH. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clin Chem. 2009;55(12):2098-2112 Epub 2009 Oct 8 [DOI] [PubMed] [Google Scholar]
- 43.Eggers KM, Nordenskjold A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J. 2004;148(4):574-581 [DOI] [PubMed] [Google Scholar]
- 44.Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868-877 [DOI] [PubMed] [Google Scholar]
- 45.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858-867 [DOI] [PubMed] [Google Scholar]
- 46.Eggers KM, Lagerqvist B, Oldgren J, Venge P, Wallentin L, Lindahl B. Pathophysiologic mechanisms of persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome. Am Heart J. 2008;156(3):588-594 [DOI] [PubMed] [Google Scholar]
- 47.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation 2006;113(16):1958-1965 [DOI] [PubMed] [Google Scholar]
- 48.Apple FS, Smith SW, Pearce LA, Murakami MM. Assessment of the multiple-biomarker approach for diagnosis of myocardial infarction in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2009;55(1):93-100 [DOI] [PubMed] [Google Scholar]
- 49.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345(14):1014-1021 [DOI] [PubMed] [Google Scholar]
- 50.Troughton RW, Prior DL, Pereira JJ, et al. Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function. J Am Coll Cardiol. 2004;43(3):416-422 [DOI] [PubMed] [Google Scholar]
- 51.Diercks D, Kirk JD, Turnipseed S, Amsterdam E. Value of high sensitivity C reactive protein in low risk chest pain observation unit patients [abstract]. Acad Emerg Med. 2009;18(suppl 1):S118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldman L, Cook EF, Johnson PA. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med. 1996;334(23):1498-1504 [DOI] [PubMed] [Google Scholar]
- 53.Boersma E, Pieper KS, Steyerberg EW, et al. The PURSUIT Investigators predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation: results from an international trial of 9461 patients. Circulation 2000;101(22):2557-2567 [DOI] [PubMed] [Google Scholar]
- 54.Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345-2353 [DOI] [PubMed] [Google Scholar]
- 55.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284(7):835-842 [DOI] [PubMed] [Google Scholar]
- 56.Pollack CV, Jr, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13(1):13-18 [DOI] [PubMed] [Google Scholar]
- 57.Chase M, Robey JL, Zogby KE, et al. Prospective validation of the Thrombolysis in Myocardial Infarction Risk Score in the emergency department chest pain population. Ann Emerg Med. 2006;48(3):252-259 [DOI] [PubMed] [Google Scholar]
- 58.de Araújo Gonçalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J. 2005;26(9):865-872 [DOI] [PubMed] [Google Scholar]
- 59.Salisbury AC, Olalla-Gomez C, Rihal CS, et al. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clin Proc. 2009;84(1):11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283(7):897-903 [DOI] [PubMed] [Google Scholar]
- 61.Ockene IS, Shay MJ, Alpert JS, et al. Unexplained chest pain in patients with normal coronary arteriograms. N Engl J Med. 1980;303(22):1249-1252 [DOI] [PubMed] [Google Scholar]
- 62.Sreeram N, Cheriex EC, Smeets JL, et al. Value of the 12-lead electrocardiogram at hospital admission in the diagnosis of pulmonary embolism. Am J Cardiol. 1994;73(4):298-303 [DOI] [PubMed] [Google Scholar]
- 63.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 2007;116(4):427-433 [DOI] [PubMed] [Google Scholar]
- 64.Goyal RK. Changing focus on unexplained esophageal chest pain. Ann Intern Med. 1996;124(11):1008-1011 [DOI] [PubMed] [Google Scholar]
- 65.Hewson EG, Sinclair JW, Dalton CB, et al. Twenty-four-hour esophageal pH monitoring: the most useful test for evaluating noncardiac chest pain. Am J Med. 1991;90(5):576-583 [PubMed] [Google Scholar]
- 66.Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8(5):231-235 [DOI] [PubMed] [Google Scholar]
- 67.Fruergaard P, Launbjerg J, Hesse B, et al. The diagnoses of patients admitted with acute chest pain but without myocardial infarction. Eur Heart J. 1996;17(7):1028-1034 [DOI] [PubMed] [Google Scholar]
- 68.Stanford W, Levin DC, Bettmann MA, et al. Acute chest pain—no ECG evidence of myocardial ischemia/infarction. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000;215(suppl):79-84 [PubMed] [Google Scholar]
- 69.Buenger RE. Five thousand acute care/emergency department chest radiographs: comparison of requisitions with radiographic findings. J Emerg Med. 1988;6(3):197-202 [DOI] [PubMed] [Google Scholar]
- 70.Graff L, Joseph T, Andelman R, et al. American College of Emergency Physicians Information Paper: chest pain units in emergency departments—a report from the Short-Term Observation Services Section. Am J Cardiol. 1995;76(14):1036-1039 [DOI] [PubMed] [Google Scholar]
- 71.Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Exercise testing in chest pain units: rationale, implementation, and results. Cardiol Clin. 2005;23(4):503-516 [DOI] [PubMed] [Google Scholar]
- 72.Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Immediate exercise testing to evaluate low-risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol. 2002;40(2):251-260 [DOI] [PubMed] [Google Scholar]
- 73.Gibler WB, Runyon JP, Levy RC, et al. A rapid diagnostic and treatment center for patients with chest pain in the emergency department. Ann Emerg Med. 1995;25(1):1-8 [DOI] [PubMed] [Google Scholar]
- 74.Gomez MA, Anderson JL, Karagounis LA, Muhlestein JB, Mooers FB. An emergency department-based protocol for rapidly ruling out myocardial ischemia reduces hospital time and expense: results of a randomized study (ROMIO). J Am Coll Cardiol. 1996;28(1):25-33 [DOI] [PubMed] [Google Scholar]
- 75.Zalenski RJ, McCarren M, Roberts R, et al. An evaluation of a chest pain diagnostic protocol to exclude acute cardiac ischemia in the emergency department. Arch Intern Med. 1997;157(10):1085-1091 [PubMed] [Google Scholar]
- 76.Kirk JD, Turnipseed S, Lewis WR, Amsterdam EA. Evaluation of chest pain in low-risk patients presenting to the emergency department: the role of immediate exercise testing. Ann Emerg Med. 1998;32(1):1-7 [DOI] [PubMed] [Google Scholar]
- 77.Diercks DB, Gibler WB, Liu T, Sayre MR, Storrow AB. Identification of patients at risk by graded exercise testing in an emergency department chest pain center. Am J Cardiol. 2000;86(3):289-292 [DOI] [PubMed] [Google Scholar]
- 78.Stein RA, Chaitman BR, Balady GJ, et al. Safety and utility of exercise testing in emergency room chest pain centers: an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation 2000;102(12):1463-1467 [DOI] [PubMed] [Google Scholar]
- 79.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guidelines update for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 2002;40(8):1531-1540 [DOI] [PubMed] [Google Scholar]
- 80.Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006;114(19):2070-2082 [DOI] [PubMed] [Google Scholar]
- 81.Bourque JM, Holland BH, Watson DD, Beller GA. Achieving an exercise workload of > or = 10 metabolic equivalents predicts a very low risk of inducible ischemia: does myocardial perfusion imaging have a role? J Am Coll Cardiol. 2009;54(6):538-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mark DB, Shaw L, Harrell FE, Jr, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325(12):849-853 [DOI] [PubMed] [Google Scholar]
- 83.Ladenheim ML, Kotler TS, Pollock BH, et al. Incremental prognostic power of clinical history, exercise electrocardiography and myocardial perfusion scintigraphy in suspected coronary artery disease. Am J Cardiol. 1987;59(4):270-277 [DOI] [PubMed] [Google Scholar]
- 84.Hachamovitch R, Berman DS, Kiat H, et al. Value of stress myocardial perfusion single photon emission computer tomography in patients with normal resting electrocardiograms: an evaluation of incremental prognostic value and cost-effectiveness. Circulation 2002;105(7):823-829 [DOI] [PubMed] [Google Scholar]
- 85.Farkouh ME, Smars PA, Reeder GS, et al. A clinical trial of a chest-pain observation unit for patients with unstable angina. N Engl J Med. 1998;339(26):1882-1888 [DOI] [PubMed] [Google Scholar]
- 86.Meyer MC, Mooney RP, Sekera AK. A critical pathway for patients with acute chest pain and low risk for short-term adverse cardiac events: role of outpatient stress testing. Ann Emerg Med. 2006;47(5):435.e1-435.e3 [DOI] [PubMed] [Google Scholar]
- 87.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation 2003;108(11):1404-1418 [DOI] [PubMed] [Google Scholar]
- 88.Kontos MC, Haney A, Jesse RL, Ornato JP, Jesse RL, Tatum JL. Value of simultaneous functional assessment in association with acute rest perfusion imaging for predicting short- and long-term outcomes in emergency department patients with chest pain. J Nucl Cardiol. 2008;15(6):774-782 [DOI] [PubMed] [Google Scholar]
- 89.Udelson JE, Beshansky JR, Ballin DS, et al. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial [published correction appears in JAMA. 2003;289(2):178] JAMA 2002;288(21):2693-2700 [DOI] [PubMed] [Google Scholar]
- 90.Kontos MC, Schmidt KL, McCue M, et al. A comprehensive strategy for the evaluation and triage of the chest pain patient: a cost comparison study. J Nucl Cardiol. 2003;10(3):284-290 [DOI] [PubMed] [Google Scholar]
- 91.Schaeffer MW, Brennan TD, Hughes JA, Gibler WB, Gerson MC. Resting radionuclide myocardial perfusion imaging in a chest pain center including an overnight delayed image acquisition protocol. J Nucl Med Technol. 2007;35(4):242-245 [DOI] [PubMed] [Google Scholar]
- 92.Tatum JL, Jesse RL, Kontos MC, et al. Comprehensive strategy for the evaluation and triage of the chest pain patient. Ann Emerg Med. 1997;29(1):116-123 [DOI] [PubMed] [Google Scholar]
- 93.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135-2144 [DOI] [PubMed] [Google Scholar]
- 94.Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53(18):1642-1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rubinshtein R, Halon DA, Gaspar T, et al. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation 2007;115(13):1762-1768 [DOI] [PubMed] [Google Scholar]
- 96.Goldstein JA, Gallagher MJ, O'Neill WW, Ross MA, O'Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49(8):863-871 [DOI] [PubMed] [Google Scholar]
- 97.Vrachliotis TG, Bis KG, Haidary A, et al. Atypical chest pain: coronary, aortic, and pulmonary vasculature enhancement at biphasic single-injection 64-section CT angiography. Radiology 2007;243(2):368-376 [DOI] [PubMed] [Google Scholar]
- 98.Rahmani N, Jeudy J, White CS. Triple rule-out and dedicated coronary artery CTA: comparison of coronary artery image quality. Acad Radiol. 2009;16(5):604-609 [DOI] [PubMed] [Google Scholar]
- 99.Hendel RC. Is computed tomography coronary angiography the most accurate and effective noninvasive imaging tool to evaluate patients with acute chest pain in the emergency department? CT coronary angiography is the most accurate and effective noninvasive imaging tool for evaluating patients presenting with chest pain to the emergency department: antagonist viewpoint. Circ Cardiovasc Imaging 2009;2(3):264-275 [DOI] [PubMed] [Google Scholar]
- 100.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007;116(11):1290-1305 [DOI] [PubMed] [Google Scholar]
- 101.Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298(3):317-323 [DOI] [PubMed] [Google Scholar]
- 103.Lehman SJ, Abbara S, Cury RC, et al. Significance of cardiac computed tomography incidental findings in acute chest pain. Am J Med. 2009;122(6):543-549 [DOI] [PubMed] [Google Scholar]
- 104.Hauser AM, Gangadharan V, Ramos RG, et al. Sequence of mechanical, electrocardiographic and clinical effects of repeated coronary artery occlusion in human beings: echocardiographic observations during coronary angioplasty. J Am Coll Cardiol. 1985;5(2, pt 1):193-197 [DOI] [PubMed] [Google Scholar]
- 105.Lieberman AN, Weiss JL, Jugdutt BI, et al. Two-dimensional echocardiography and infarct size: relationship of regional wall motion and thickening to the extent of myocardial infarction in the dog. Circulation 1981;63(4):739-746 [DOI] [PubMed] [Google Scholar]
- 106.Lewis WR. Echocardiography in the evaluation of patients in chest pain units. Cardiol Clin. 2005;23(4):531-539, vii [DOI] [PubMed] [Google Scholar]
- 107.Cohen JL, Cheirif J, Segar DS, et al. Improved left ventricular endocardial border delineation and opacification with OPTISON (FS069), a new echocardiographic contrast agent: results of a phase III multicenter trial. J Am Coll Cardiol. 1998;32(3):746-752 [DOI] [PubMed] [Google Scholar]
- 108.Kaul S, Senior R, Dittrich H, et al. Detection of coronary artery disease with myocardial contrast echocardiography: comparison with Tc-sestamibi single-photon emission computed tomography. Circulation 1997;96(3):785-792 [PubMed] [Google Scholar]
- 109.Tong KL, Kaul S, Wang XQ, et al. Myocardial contrast echocardiography versus Thrombolysis In Myocardial Infarction score in patients presenting to the emergency department with chest pain and a nondiagnostic electrocardiogram. J Am Coll Cardiol. 2005;46(5):920-927 [DOI] [PubMed] [Google Scholar]
- 110.Pouleur AC, le Polain de Waroux JB, Kefer J, Pasquet A, Vanoverschelde JL, Gerber BL. Direct comparison of whole-heart navigator-gated magnetic resonance coronary angiography and 40- and 64-slice multidetector row computed tomography to detect the coronary artery stenosis in patients scheduled for conventional coronary angiography. Circ Cardiovasc Imaging 2008;1(2):114-121 [DOI] [PubMed] [Google Scholar]
- 111.Kwong RY, Schussheim AE, Rekhraj S, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation 2003;107(4):531-537 [DOI] [PubMed] [Google Scholar]
- 112.Cury RC, Shash K, Nagurney JT, et al. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation 2008;118(8):837-844 [DOI] [PubMed] [Google Scholar]
- 113.Plein S, Greenwood JP, Ridgway JP, Cranny G, Ball SG, Sivananthan MU. Assessment of non-ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;44(11):2173-2181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.