To the Editor: Smoldering multiple myeloma (SMM) is a premalignant plasma cell proliferative disorder defined by a serum M protein (SMP) of 3 g/dL or higher and/or 10% or greater of bone marrow plasma cells (BMPC), without end-organ damage.1 The clinical course and prognosis of 276 patients diagnosed as having SMM between 1970 and 1995 have been reported.2 At initial diagnosis, a risk stratification model divided patients into group 1 (BMPC ≥10%, SMP ≥3 g/dL), group 2 (BMPC ≥10%, SMP <3 g/dL), and group 3 (BMPC <10%, SMP ≥3 g/dL), which resulted in a median time to progression of 2, 8, and 19 years, respectively. The study identified BMPC percent and the SMP level at diagnosis as the 2 independent risk factors for progression.2
The study analyzed various baseline factors with respect to progression of SMM, but plasma cell labeling index (PCLI) was not evaluated because it was not routinely performed until the slide-based immunofluorescence method3 became available in 1984. The PCLI is a powerful and independent predictor of survival in patients with newly diagnosed multiple myeloma because it provides a measure of the proliferative rate of the malignant BMPC.4 Additionally, the incorporation of PCLI into the international staging system of multiple myeloma provides prognostic value for stage 1 and 2 patients.5
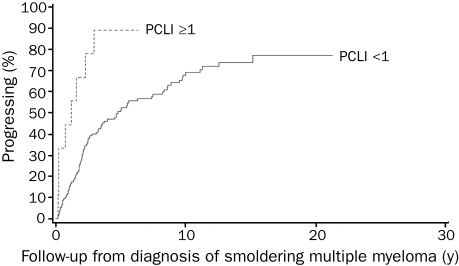
We conducted this study to determine if the baseline PCLI would be a risk factor for progression of SMM. Of the 175 patients diagnosed as having SMM after January 1, 1984, 161 (92%) had PCLI at diagnosis, and these patients form the study group. The progression rates were estimated using the Kaplan-Meier method. Among the 68 patients in group 1, progression occurred in 44 of 63 with PCLI lower than 1 and 4 of 5 with PCLI of 1 or greater. The 2- and 5-year rates of progression were 40.4% (95% confidence interval [CI], 26.4%-51.7%) and 68.4% (95% CI, 52.8%-78.9%) (PCLI <1) compared with 60.0% (95% CI, 0%-86.3%) and 80.0% (95% CI, 0%-96.3%) (PCLI ≥1) (P=.4). Among the 83 patients in group 2, progression occurred in 42 of 79 with PCLI lower than 1 and all 4 patients with PCLI of 1 or greater. The 2- and 5-year rates of progression were 21.2% (95% CI, 11.0%-30.1%) and 43.1% (95% CI, 29.7%-54.0%) (PCLI <1) compared with 75.0% (95% CI, 0%-95.4%) and 100% (PCLI ≥1) (P<.001). No analysis could be performed for group 3 because all 10 patients had PCLI lower than 1. Overall, the median time to progression was 1.2 years (PCLI ≥1) and 2.6 years (PCLI <1) (P<.001) (Figure). At 1 year after diagnosis of SMM, progression occurred more than twice as frequently in patients with PCLI of 1 or greater (50%) than in those with PCLI lower than 1 (23%).
FIGURE.
Kaplan-Meier curves for progression of smoldering multiple myeloma based on plasma cell labeling index (PCLI) ≥1 and PCLI<1; P<.001.
In conclusion, SMM patients with BMPC of 10% or greater and PCLI of 1 or greater had higher rates of progression, but because of a small number of patients with PCLI of 1 or greater, a statistical significance occurred only in group 2. Our study demonstrates the usefulness of PCLI as a risk factor for progression of SMM, especially among those with BMPC of 10% or greater and SMP lower than 3 g/dL. Along with other risk factors, it can be used to further stratify SMM patients as a guide in the design of clinical trials and follow-up and to ensure timely institution of effective therapy before organ impairment develops. The main limitation of PCLI is that the test is not widely available. Other methods to estimate the proliferative rate, such as flow cytometric assessment of the percentage of S-phase plasma cells or gene expression profiling, may be equally good alternatives and should be studied.
References
- 1.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749-757 [PubMed] [Google Scholar]
- 2.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590 [DOI] [PubMed] [Google Scholar]
- 3.Greipp PR, Kumar S. Plasma cell labeling index. Methods Mol Med. 2005;113:25-35 [DOI] [PubMed] [Google Scholar]
- 4.Greipp PR, Lust JA, O'Fallon WM, Katzmann JA, Witzig TE, Kyle RA. Plasma cell labeling index and beta 2-microglobulin predict survival independent of thymidine kinase and C-reactive protein in multiple myeloma. Blood 1993;81(12):3382-3387 [PubMed] [Google Scholar]
- 5.Kapoor P, Snozek C, Colby CL, et al. Incorporation of the plasma cell labeling index into the international staging system of multiple myeloma: impact on prognostic value [abstract 8591]. J Clin Oncol. 2008;26(May20suppl):15S [Google Scholar]