Abstract
Previous studies from our laboratory have shown the neuroprotective potential of ketones after TBI in the juvenile brain. It is our premise that acutely after TBI, glucose may not be the optimum fuel and decreasing metabolism of glucose in the presence of an alternative substrate will improve cellular metabolism and recovery. The current study addresses whether TBI will induce age-related differences in the cerebral metabolic rates for glucose (CMRglc) after cortical controlled impact (CCI) and whether ketone metabolism will further decrease CMRglc after injury. Postnatal day 35 (PND35; n = 48) and PND70 (n = 42) rats were given either sham or CCI injury and placed on either a standard or a ketogenic (KG) diet. CMRglc studies using 14C-2deoxy-d-glucose autoradiography were conducted on days 1, 3, or 7 post-injury. PND35 and PND70 standard-fed CCI-injured rats exhibited no significant neocortical differences in CMRglc magnitude or time course compared to controls. Measurement of contusion volume also indicated no age differences in response to TBI. However, PND35 subcortical structures showed earlier metabolic recovery compared to controls than PND70. Ketosis induced by the KG diet was shown to affect CMRglc in an age-dependent manner after TBI. The presence of ketones after injury further reduced CMRglc in PND35 and normalized CMRglc in PND70 rats at 7 days bilaterally after injury. The changes in CMRglc seen in PND35 TBI rats on the KG diet were associated with decreased contusion volume. These results suggest that conditions of reduced glucose utilization and increased alternative substrate metabolism may be preferable acutely after TBI in the younger rat.
Key words: age, metabolism, pediatric brain injury, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is often accompanied by early increases in serum glucose, which has been associated with poor outcome in adult and pediatric head injury (Rovlias and Kotsou, 2000; Zauner et al., 1997; Cochran et al., 2003). While there has been heavy focus on the relationship between plasma glucose and outcome, few studies have addressed the consequent cerebral glucose changes. Microdialysis studies in TBI patients and animal models have shown that cerebral extracellular glucose (ECglc) concentrations decrease and extracellular lactate (EClac) increase after brain injury (Goodman et al., 1999; Alessandri et al., 2000), suggesting active metabolism of glucose early after injury.
Changes in cerebral glucose metabolism after TBI have been reported following adult fluid percussion injury (FPI) (Andersen and Marmarou, 1989, 1992; Yoshino et al., 1991, 1992; Kawamata et al., 1992; Richards et al., 2001; Chen et al., 2004) and human TBI (Bergsneider et al., 1997; O'Connell et al., 2005). In an experimental FPI study, the adult rat brain showed a transient elevation in cerebral metabolic rate for glucose (CMRglc; μmol/100 g/min) followed by a prolonged period of glucose metabolic depression returning to control levels within 10 days. While postnatal day (PND) 17 rats, who sustained a similar injury, showed an immediate increase in CMRglc as the adult, the suckling brain showed a faster recovery from the injury-induced glucose metabolic depression, returning to control levels within 3 days (Thomas et al., 2000).
In addition to age-related differences in the duration of CMRglc depression after FPI, there are injury type differences in cerebral glucose metabolism following injury to adult rats. The depressed CMRglc observed after FPI differs greatly from that observed after controlled cortical impact (CCI) injury in the adult rat (Sutton et al., 1994) in both regional distribution and duration. Following CCI injury, long-term depressed glucose metabolism is observed in the ipsilateral cortex, hippocampus, and thalamic nuclei beginning at day 1 and remains depressed at 10 days post-injury. Currently, age-related differences in CMRglc after CCI injury have not been addressed.
During this period of depressed glucose metabolism, there is an increased flux of glucose through the pentose phosphate pathway (Bartnik et al., 2005), free radical production, and activation of PARP via DNA damage (Hall, 1993). PARP-mediated DNA repair process can deplete cytosolic NAD+ pool and can inhibit GAPDH (a key enzyme in the glycolytic pathway). Under these conditions of impaired glycolytic metabolism, glucose may not be the most favorable energy substrate. Administration of ketones (Prins, 2008) during this period of glucose metabolic depression has resulted in age-related neuroprotection (Prins et al., 2005). PND35 and PND45 rats placed on a KG diet immediately after CCI injury showed a 58% and a 39% reduction, respectively, in cortical contusion volume at 7 days after injury (Prins et al., 2005). The greater presence of monocarboxylate transporters during development and the age-related induction of monocarboxylate transporter 2 (Prins and Giza, 2006) after CCI injury may explain why younger animals were able to utilize this alternative substrate more rapidly after the injury.
It is our premise that, acutely after TBI, the metabolic fate of glucose is altered and that providing an alternative substrate in the face of this injury-induced depression of glucose metabolism will improve cellular metabolism and recovery of function. The current study was designed to address two hypotheses: (1) that TBI will induce age-related differences in CMRglc after CCI injury and (2) that putting injured animals on an ketogenic diet will enhance the cerebral metabolism of ketones, which will further decrease the need for cerebral glycolysis, thereby reducing CMRglc acutely after injury in an age-dependent manner.
Methods
Subjects
Male PND35 (131 ± 3.2 g) and PND70 (306 ± 3.1 g) Sprague-Dawley rats were given sham surgery or CCI injury and immediately placed on either a standard (Teklad no. 7013) or ketogenic (KG) diet (Bioserv no. F3666). Cerebral metabolic rates of glucose were determined using [14C]2-deoxy-D-glucose autoradiographic (2DG) method at 1, 3, or 7 days post-injury.
Animals were randomly assigned to sham or injury groups and diet groups as follows: PND35 sham (n = 6), PND35 sham KG (n = 6), PND35 CCI standard diet (n = 6 per 1-day, 3-day, 7-day timepoint), PND35 CCI KG diet (n = 6 per 1-day, 3-day, 7-day timepoint); PND70 sham (n = 6), PND70 CCI standard diet (n = 6 per 1-day, 3-day, 7-day timepoint), and PND70CCI KG diet (n = 6 per 1-day, 3-day, 7-day timepoint).
2DG autoradiography was performed in the sham animals at 24 h after surgery. Previous studies with 2DG autoradiography have shown that CMRglc are at normal levels 24 h after sham surgery for both neonatal and adult rats (Thomas et al., 2000; Yoshino et al., 1991). The effects of the hyperketonemia on CMRglc has been addressed in the literature in the normal adult brain; there has been shown to be no effect on CMRglc (Ruderman et al., 1974; Corddry et al., 1982; Cranet et al., 1985; Mans et al., 1987; Cherel et al., 1988; Al-Mudallal et al., 1995). In our experience as well, PND70 sham animals on KG diet for 24 h do not show any changes in CMRglc. Therefore, adult CCI KG-fed animals were compared to adult sham standard-fed animals to minimize the number of animals in the study. However, it is unclear whether PND35 sham rats on the KG diet would show changes in CMRglc; therefore, this group was included in the study.
CCI injury
As characterized previously (Prins et al., 2004), the developmental CCI injury model in the rat was used to generate a focal TBI to the left cortical hemisphere. Under isoflurane (1.5–2.0%/100%O2) applied by a gas mask, a midline incision was made and the skull was exposed. A 6-mm-diameter craniotomy was drilled over the left hemisphere under a microscope, centered at −4 mm anterior-posterior, 5 mm lateral relative to bregma. The bone flap was removed and the dura left intact in all animals. An electronically controlled pneumatic piston cylinder (Hydraulics Control, Inc., Emeryville, CA) was mounted onto a stereotaxic micromanipulator (Kopf Instruments, Tujunga, CA) to allow for precise localization of the impact center. The piston cylinder was angled 22° away from vertical to allow the flat (5-mm-diameter) impactor surface to make contact perpendicular to the brain's surface. The impactor tip compressed the brain 2 mm below the pial surface at 1.9 m/s. Following the injury, a small piece of gelfoam was placed over the craniotomy site to reduce bleeding and the wound sutured closed.
Diets
Animals were given free access to water and were maintained on either a standard rodent chow (Teklad no. 7013) or the KG diet (no. F3666; Bioserv, Frenchtown, NJ). Putting the animals on the KG diet results in an increase in blood concentrations of the ketone bodies ß-hydroxybutyrate (ßOHB) (Prins et al., 2005) and acetoacetate (Rho et al., 1999). The KG diet consists of 8.4% protein, 78.8% fat, 0.8% carbohydrates, and 5% fiber. The standard rodent chow contains 18.6% protein, 6.2% fat, 59.8% carbohydrates, and 4.5% fiber.
2DG autoradiography
Local cerebral metabolic rates for glucose (ICMRglc; μ mol/100 g/min) were measured by the 2DG method originally described by Sokoloff et al., 1977. Under isoflurane anesthesia (2 L/min), the femoral artery and vein were cannulated with a polyethylene tube (PE-50) on the day of study; each rat was restrained on a cardboard for at least 2 h to allow effects of anesthesia to diminish. Baseline blood-gas measurements were then taken (pH, pO2, pCO2; 1304 pH, Blood Gas Analyzer; Instrumentation Laboratory, Stockholm, Sweden) and 14C-2DG (150 m Ci/kg) was administered (i.v.) over 30 sec. Twelve timed arterial blood samples were collected in polyethylene-heparin lithium fluoride coated microcentrifuge tubes (Beckman Instruments, Fullerton, CA) during the 45-min experiment. At 45 min after 2DG injection, the rats were sacrificed with a lethal dose of sodium pentobarbital (100 mg/kg, i.v.), and the brains were removed and immediately frozen. Coronal sections (20 μm) were exposed to Kodak Biomax film with 14C-methylmethacrylate standards (Amersham, Arlington Heights, IL). Adjacent sections were processed for cresyl violet staining and examined under light-microscopy to determine the presence of overt histopathology. The optical densities were measured bilaterally in eight regions from autoradiographs using a computerized image analysis system (NIH Image). The regions of interest are illustrated in Figure 1 and were selected away from the injury core to avoid regions of blood-brain barrier disruption. Optical density values were obtained across at least three sections for each structure and averaged. The CMRglc was calculated using the equation originally described by Sokoloff et al. (1977).
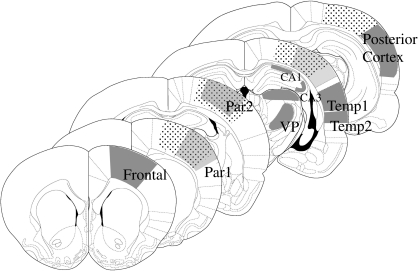
FIG. 1.
Diagram of coronal sections illustrating the cerebral metabolic rates for glucose (CMRglc) regions of interest measured. The region with small black dots indicates the cortical contusion impact site. Solid gray areas show the regions from which CMRglc values were obtained. Relative to bregma, frontal cortex (1.7 mm), parietal cortex area 1 (0.7 mm), CA1 and CA3 (−3.8 mm), VPM/VPL (−3.8 mm), temporal cortex (area1, −3.8 mm; area2, −3.8mm), and the posterior cortex (−5.3 mm).
The blood samples collected (20–50 μL) were immediately placed on ice, centrifuged, and the plasma assayed for 14C-activity using a scintillation counter (LS 5000 CE; Beckman Instruments). Remaining plasma was processed for glucose and lactate levels with the YSI 2700 Biochemistry Analyzer, and ketone concentrations were determined with Ketosite test cards (Stanbiochem, Boerne, TX) on a GDS STATSite Analyzer (Elkhart, IN). This analyzer measures the ß-hydroxybutyrate (ßOHB) content of the plasma, which reflects the conversion of the fats in the ketogenic diet to ketones.
Cortical contusion volume
Coronal sections (20, μm) were collected every 0.24 mm between +1.7 mm and −7.64 mm relative to bregma and were stained with cresyl violet. The cross-sectional area of the contralateral and ipsilateral cortex was determined by tracing the remaining intact cortical area. At 7 days post-injury with the current injury severity, the contusion cavity was formed and remained restricted to the ipsilateral cortex. Within the ipsilateral cortex, regions of dark-stained small cells (possibly gliosis) were not included in the remaining cortical tissue. It should be noted that the developing brain shows greater brain growth in the anterior-posterior plane with cerebral maturation. However, the PND35 rat brains fell within 88–98% of adult values across these parameters. For this reason, both aged groups were analyzed using the same methodology. NIH Image analysis program was used to measure the cortical areas and the hemispheric differences were calculated as the cross-sectional injury area. Subtraction of the left cortical hemisphere from the right eliminated the need to estimate true cortical surface within the cavitated tissue and has been previously used for contusion volume analysis (Sutton et al., 1993). The total lesion volume was calculated by integrating the area from each section with the distance between each section.
Statistical analysis
For each measured structure, the mean and standard deviation (SD) were determined in the injured and sham-operated groups. Mean CMRglc was compared for each time point after injury between experimental and control groups using t-test with Bonferroni adjustment to control for inflated alpha. A value of p < 0.05 was considered to be statistically significant. Physiological parameters were statistically analyzed with an unpaired t-test.
Results
Physiological parameters
There was no significant difference in the initial body weights between PND35 groups, except 7-day KG diets, and no difference between the PND70 rat groups (Table 1). Following CCI injury, PND35 rats lost an average of 1 g at 24 h, with subsequent gain of 19 g at 3 days and 54 g at 7 days. PND70 rats showed a similar trend with 10-g weight loss at 24 h, followed by 17- and 26-g weight gains at 3 and 7 days post-injury. Rats maintained on the KG diet showed greater weight loss across all time points. At 24 h, the initial weight loss in animals maintained on the KG diet was 5 g in PND35 and 18 g in PND70 rats. By 3 days, there was less weight loss in both age groups. The largest weight differences were seen after 7 days on the KG diet with −18 and −20 g, in PND35 and PND70 rats, respectively. Both the TBI-induced and KG-diet-induced weight losses are within the expected ranges given previous reports (Prins et al., 2005).
Table 1.
Physiological Parameters and Changes in Plasma Substrates (mM) with Age, Injury, and Diet
| Group | Initial weight (g) | Blood pH | PCO2 | PO2 | Glucose (mM) | Lactate (mM) | ßOHB (mM) |
|---|---|---|---|---|---|---|---|
| PND35 standard | |||||||
| Sham | 129 ± 1.9 | 7.43 ± 0.01 | 39 ± 1.7 | 94 ± 6.1 | 10.2 ± 0.44 | 1.1 ± 0.1 | 0.14 ± 0.05 |
| 1 day | 148 ± 12.2 | 7.42 ± 0.017 | 37 ± 0.8 | 95 ± 1.5 | 9.34 ± 0.4 | 0.86 ± 0.06* | 0.32 ± 0.05 |
| 3 days | 123 ± 5.2 | 7.43 ± 0.01 | 38 ± 0.2 | 91 ± 3.2 | 10.5 ± 0.45 | 0.99 ± 0.08 | 0.45 ± 0.09 |
| 7 days | 132 ± 3.6 | 7.41 ± 0.01 | 39 ± 1.6 | 88 ± 3.4 | 10.4 ± 0.39 | 0.91 ± 0.05 | 0.17 ± 0.04 |
| PND35 KG diet | |||||||
| Sham | 142 ± 14.2 | — | — | — | 7.6 ± 0.6* | 0.8 ± 0.04* | 1.8 ± 0.1* |
| 1 day | 127 ± 6.0 | 7.38 ± 0.01* | 37 ± 1.0 | 90 ± 1.1 | 7.2 ± 0.42* | 0.8 ± 0.03* | 1.68 ± 0.23* |
| 3 days | 139 ± 7.6 | 7.41 ± 0.02 | 36 ± 1.1 | 89 ± 3.3 | 8.6 ± 0.56* | 0.92 ± 0.11 | 1.67 ± 0.1* |
| 7 days | 113 ± 6.9 | 7.41 ± 0.00 | 34 ± 1.4 | 97 ± 1.8 | 8.45 ± 1.0 | 1.04 ± 0.14 | 1.88 ± 0.09* |
| PND70 standard | |||||||
| Sham | 305 ± 6.6 | 7.41 ± 0.01 | 42 ± 0.7* | 91 ± 3.4 | 10.36 ± 1.0 | 0.90 ± 0.2 | 0.29 ± 0.16 |
| 1 day | 305 ± 7.4 | 7.42 ± 0.01 | 38 ± 0.8 | 91 ± 2.8 | 9.24 ± 0.6 | 0.69 ± 0.1* | 0.36 ± 0.07 |
| 3 days | 298 ± 10.2 | 7.41 ± 0.01 | 41 ± 1.4 | 88 ± 3.8 | 10.69 ± 0.6 | 0.66 ± 0.04* | 0.22 ± 0.03 |
| 7 days | 321 ± 8.8 | 7.41 ± 0.01 | 40 ± 1.4 | 91 ± 2.3 | 9.19 ± 0.4 | 0.66 ± 0.06* | 0.31 ± 0.09 |
| PND70 KG diet | |||||||
| Sham | |||||||
| 1 day | 298 ± 5.7 | 7.42 ± 0.007 | 39 ± 1.5 | 85 ± 5.4 | 6.62 ± 0.29* | 0.68 ± 0.05* | 1.55 ± 0.07* |
| 3 days | 297 ± 10.1 | 7.41 ± 0.01 | 35 ± 1.1* | 89 ± 3.7 | 9.38 ± 0.61 | 0.75 ± 0.05* | 1.30 ± 0.13* |
| 7 days | 312 ± 6.7 | 7.42 ± 0.01 | 36 ± 0.8* | 86 ± 2.1 | 9.19 ± 1.1 | 1.04 ± 0.29 | 1.16 ± 0.26* |
p < 0.05, relative to age-matched standard diet sham.
Blood pH, pCO2, and pO2 values were not significantly different among standard-fed animals (Table 1). The only exception was the PND70 sham animals, which showed slightly higher pCO2 values. PCO2 was decreased in the PND70 rats at 3 and 7 days after injury on the KG diet, as was the pH in the injured PND35 1-day KG group.
Plasma levels of glucose, lactate, and ß-hydroxybutyrate (ßOHB) were measured at 1, 3, and 7 days after TBI on both the standard and KG diet (Table 1). Both PND35 and PND70 animals maintained on the standard diet showed no significant change in plasma glucose after injury. PND35 rats showed a significant (p < 0.05) decrease in plasma lactate at 1 day, while PND70 rats showed decreased plasma lactate at all days measured after injury. Plasma ketone levels were not significantly different after injury or between age groups among the standard-fed animals. PND35 and PND70 animals on the KG diet showed a significant (p < 0.05) increase in plasma ßOHB and a significant (p < 0.05) decrease in plasma glucose and lactate. The increase in plasma ketones was greater among PND35 animals at all time points after injury, including sham controls.
CMRglc in PND35 and PND70 sham standard-fed animals
The average cortical rates of glucose metabolism obtained from PND35 and PND70 sham animals were within the previously reported ranges (Yoshino et al., 1991; Thomas et al, 2000; Nehlig et al., 1988). There was no significant difference in the CMRglc of the cortex, thalamus, or hippocampus between PND35 and PND70 rats, which is consistent with previously published developmental profile of glucose metabolism across these age groups (Nehlig et al., 1988).
CMRglc in PND35 injured standard-fed
Following CCI injury in PND35 rats, ipsilateral cortical, hippocampal, and thalamic structures showed a significant decrease in CMRglc at day 1, reaching its lowest levels at day 3 after injury (Table 2, Fig. 2). While ipsilateral cortical structures remained significantly depressed at 7 days, hippocampal and thalamic structures exhibited sham levels of CMRglc by 7 days after injury. The contralateral structures showed no significant change in CMRglc, except the temporal and posterior cortex at day 3 after injury.
Table 2.
Rates of Glucose Metabolism following CCI Injury among Standard Fed PND35 and PND70 Rats
| |
PND35 |
PND70 |
||||||
|---|---|---|---|---|---|---|---|---|
| Area | Sham | 1 day | 3 days | 7 days | Sham | 1 day | 3 days | 7 days |
| Frontal | ||||||||
| Ipsi | 87.4 ± 6.3 | 50.7 ± 9.6** | 47.5 ± 3.4** | 65.8 ± 7.52* | 91.2 ± 6.1 | 50.7 ± 8.9** | 45.4 ± 2.5** | 59.1 ± 6.3** |
| Contra | 90.0 ± 6.0 | 91.8 ± 6.9 | 82.9 ± 5.8 | 92.4 ± 5.9 | 92.2 ± 5.5 | 88.9 ± 6.1 | 86.7 ± 3.1 | 83.9 ± 3.2 |
| Parietal 1 | ||||||||
| Ipsi | 87.5 ± 6.9 | 43.1 ± 9.8** | 45.8 ± 3.6** | 64.0 ± 5.6* | 92.4 ± 5.6 | 34.0 ± 8.8** | 50.3 ± 5.9** | 56.9 ± 6.3** |
| Contra | 88.9 ± 5.6 | 91.2 ± 6.4 | 81.3 ± 6.6 | 90.1 ± 6.3 | 95.1 ± 5.8 | 90.3 ± 6.2 | 85.6 ± 3.4 | 80.7 ± 2.7 |
| Post cortex | ||||||||
| Ipsi | 96.9 ± 8.2 | 53.7 ± 6.2** | 46.1 ± 4.3** | 63.0 ± 7.3* | 95.0 ± 9.2 | 49.3 ± 8.2** | 56.5 ± 7.1** | 62.3 ± 7.5** |
| Contra | 106.1 ± 6.8 | 98.0 ± 9.6 | 85.9 ± 5.7* | 102.5 ± 7.7 | 102.7 ± 6.9 | 102.0 ± 5.0 | 101.6 ± 7.1 | 95.6 ± 5.9 |
| Temporal 1 | ||||||||
| Ipsi | 92.8 ± 6.2 | 42.0 ± 5.9** | 51.2 ± 3.7** | 69.2 ± 4.8** | 99.1 ± 7.0 | 42.3 ± 6.2** | 50.7 ± 6.3** | 69.8 ± 7.5* |
| Contra | 90.6 ± 6.9 | 90.2 ± 10.2 | 84.4 ± 4.7* | 95.3 ± 7.9 | 95.6 ± 7.1 | 97.2 ± 4.3 | 84.0 ± 3.9 | 85.5 ± 2.3 |
| Temporal 2 | ||||||||
| Ipsi | 89.1 ± 5.2 | 55.6 ± 6.0** | 47.0 ± 4.9** | 58.3 ± 5.1** | 98.9 ± 6.9 | 54.6 ± 6.4** | 52.1 ± 7.9** | 67.9 ± 1.8* |
| Contra | 96.5 ± 2.2 | 89.4 ± 8.9 | 86.1 ± 3.2* | 91.6 ± 8.9 | 101.0 ± 8.6 | 97.0 ± 8.0 | 93.0 ± 6.1 | 85.1 ± 5.6 |
| CA1 | ||||||||
| Ipsi | 58.3 ± 4.7 | 45.7 ± 4.1 | 39.3 ± 2.7* | 54.8 ± 7.8 | 54.6 ± 3.0 | 39.9 ± 4.7* | 33.3 ± 4.9* | 50.0 ± 4.5 |
| Contra | 56.3 ± 5.7 | 52.7 ± 6.3 | 43.2 ± 4.2 | 54.2 ± 7.3 | 54.9 ± 4.7 | 53.0 ± 5.0 | 41.3 ± 2.4* | 47.0 ± 4.6 |
| CA3 | ||||||||
| Ipsi | 62.5 ± 5.0 | 45.5 ± 3.9* | 44.9 ± 1.9* | 55.8 ± 8.2 | 62.1 ± 3.7 | 36.8 ± 5.1** | 35.0 ± 2.3** | 50.9 ± 3.5* |
| Contra | 59.3 ± 5.2 | 57.0 ± 4.7 | 50.6 ± 4.1 | 58.4 ± 7.0 | 59.6 ± 4.2 | 58.7 ± 4.6 | 50.6 ± 2.1 | 53.3 ± 3.7 |
| VPM/VPL | ||||||||
| Ipsi | 73.8 ± 5.0 | 53.8 ± 5.7* | 48.1 ± 3.3** | 69.8 ± 9.7 | 72.8 ± 4.0 | 47.7 ± 5.0** | 42.2 ± 1.7** | 61.4 ± 3.1* |
| Contra | 77.9 ± 4.2 | 88.6 ± 7.4 | 71.6 ± 4.4 | 81.5 ± 5.7 | 80.2 ± 3.8 | 85.9 ± 5.7 | 72.6 ± 4.3 | 76.1 ± 4.5 |
denotes value of p < 0.05 relative to age matched shams; **denotes values of p < 0.01 relative to age matched shams.
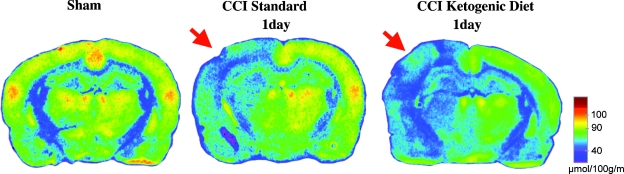
FIG. 2.
Representative colorized autoradiographs showing changes in cerebral metabolic rates for glucose (CMRglc) after controlled cortical impact (CCI) injury among standard-fed and ketogenic-fed postnatal day 35 (PND35) animals 1 day after injury. Arrow indicates ipsilateral hemisphere.
CMRglc in PND70 injured standard-fed
Following CCI injury in PND70 rats, ipsilateral cortical, hippocampal, and thalamic structures showed a significant decrease in CMRglc on days 1 and 3 (Table 2). The magnitude of the CMRglc depression was similar between PND35 (1–11%) and PND70 (2–14%) rats after CCI injury on the standard diet. In contrast to the PND35 CCI rats, where thalamic and hippocampal structures showed an earlier return of metabolic rates, only PND70 hippocampal structures attained sham levels of glucose metabolic rates by 7 days (Table 2). The PND70 ipsilateral cortex and thalamus remained depressed at day 7 after injury. The contralateral structures showed no significant change in CMRglc after injury relative to sham, with the exception of the CA1 region of the dorsal hippocampus, which remained depressed at 3 days post-injury.
CMRglc in PND35 injured KG-fed
PND35 sham animals maintained on the KG diet did not show a significant difference in CMRglc compared to standard-fed shams in any structure (Table 3). However, injured PND35 rats maintained on the KG diet showed significant decreases in all ipsilateral structures at 1, 3, and 7 days post-injury relative to KG-fed sham, and the magnitude of the CMRglc depression was on average 13% greater compared to standard-fed CCI-injured animals (Table 3, Fig. 2). The contralateral cortical, hippocampal, and thalamic structures in PND35 rats also showed a pronounced decrease in CMRglc at post-injury days 1 and 7. The magnitude of this depression was on average 8.9% greater than that of the ipsilateral regions.
Table 3.
Rates of Glucose Metabolism following CCI Injury Among Ketogenic Fed PND35 and PND70 Rats
| |
PND35 |
PND70 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area | Sham KG | 1 day | 3 days | 7 days | 1 day | 3 days | 7 days | |||
| Frontal cortex | ||||||||||
| Ipsi | 93.5 ± 9.8 | 38.9 ± 4.7** | 52.6 ± 3.8** | 38.3 ± 6.1** | 41.4 ± 5.4** | 43.7 ± 5.2** | 78.9 ± 8.2 | |||
| Contra | 92.8 ± 9.7 | 68.7 ± 4.0* | 81.4 ± 5.4 | 48.8 ± 6.7 | 82.3 ± 9.8 | 82.4 ± 8.7 | 103.1 ± 11.7 | |||
| Parietal 1 | ||||||||||
| Ipsi | 94.1 ± 9.1 | 33.9 ± 2.4** | 55.3 ± 6.3* | 40.0 ± 5.6* | 28.4 ± 3.3** | 43.3 ± 4.5** | 79.9 ± 8.7 | |||
| Contra | 93.6 ± 10.1 | 68.4 ± 3.4* | 80.0 ± 5.8 | 49.4 ± 6.2* | 83.3 ± 10.3 | 81.4 ± 9.0 | 102.0 ± 9.4 | |||
| Post cortex | ||||||||||
| Ipsi | 102 ± 9.9 | 35.0 ± 2.1** | 49.7 ± 3.3** | 38.4 ± 5.8** | 36.9 ± 2.9** | 44.0 ± 5.6** | 81.4 ± 7.4 | |||
| Contra | 101 ± 9.1 | 71.3 ± 2.0* | 90.1 ± 7.8 | 49.7 ± 7.7** | 93.6 ± 9.7 | 89.7 ± 9.0 | 116.0 ± 12.1 | |||
| Temporal 1 | ||||||||||
| Ipsi | 88.1 ± 7.9 | 28.5 ± 2.3** | 52.2 ± 3.7** | 41.0 ± 5.4** | 35.3 ± 3.6** | 47.8 ± 8.4** | 84.0 ± 9.4 | |||
| Contra | 87.9 ± 8.5 | 65.9 ± 3.4* | 77.4 ± 5.8 | 49.6 ± 6.6** | 84.0 ± 9.0 | 83.2 ± 8.4 | 109.0 ± 14.4 | |||
| Temporal 2 | ||||||||||
| Ipsi | 105 ± 10.5 | 37.5 ± 2.5** | 49.1 ± 4.3** | 38.3 ± 5.4** | 45.1 ± 5.8** | 42.5 ± 5.1** | 78.2 ± 7.4 | |||
| Contra | 106 ± 9.0 | 65.3 ± 4.6** | 81.6 ± 7.7 | 48.9 ± 6.3** | 88.3 ± 10.1 | 82.1 ± 8.2 | 108.6 ± 11.2 | |||
| CA1 | ||||||||||
| Ipsi | 61.1 ± 5.7 | 38.9 ± 1.0* | 46.5 ± 5.6 | 36.4 ± 5.6* | 37.2 ± 5.2* | 36.3 ± 7.4 | 63.7 ± 8.7 | |||
| Contra | 59.2 ± 5.8 | 41.7 ± 2.4 | 50.2 ± 5.9 | 28.9 ± 5.5* | 45.4 ± 7.0 | 47.3 ± 7.7 | 58.5 ± 4.7 | |||
| CA3 | ||||||||||
| Ipsi | 75.8 ± 7.5 | 38.7 ± 1.4* | 52.6 ± 6.5 | 34.8 ± 5.0** | 34.5 ± 4.8** | 34.3 ± 8.0* | 69.3 ± 12.6 | |||
| Contra | 73.8 ± 6.4 | 47.9 ± 2.1 | 59.1 ± 6.9 | 32.3 ± 5.7* | 51.1 ± 7.5 | 53.1 ± 8.2 | 65.8 ± 6.9 | |||
| VPM/VPL | ||||||||||
| Ipsi | 89.4 ± 7.7 | 36.8 ± 3.8** | 51.6 ± 3.7* | 42.0 ± 6.0** | 40.9 ± 4.1** | 45.2 ± 5.5** | 86.7 ± 14.5 | |||
| Contra | 89.4 ± 8.1 | 61.8 ± 3.5* | 73.2 ± 7.3 | 43.8 ± 7.0** | 79.5 ± 9.8 | 73.0 ± 7.2 | 99.5 ± 15.8 | |||
Gray blocks indicate significant (p < 0.05) difference relative to standard fed, same age, region, and timepoint (Table 2).
CMRglc in PND70 injured KG-fed
Previous studies have shown that sham adult rats (PND65-90) maintained on the KG diet do not show changes in CMRglc relative to standard-fed rats (Cherel et al., 1985; Corddry et al., 1982; Al-Mudallal et al., 1995). For this reason, PND70 injured KG-fed animals were compared to PND70 sham standard-fed. PND70 injured rats maintained on the KG diet showed a significant decrease in CMRglc within the cerebral cortical, hippocampal, and thalamus at 1 and 3 days after injury relative to sham. The CMRglc for all ipsilateral structures returned to sham levels at 7 days (Table 3, Fig. 3). PND70 injured KG-fed rats showed no significant changes in contralateral cortical, hippocampal, or thalamic CMRglc at any time point relative to injured standard-fed animals.
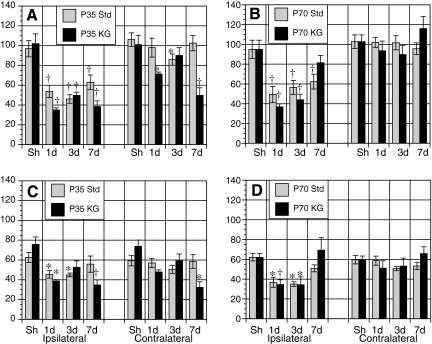
FIG. 3.
Changes in cerebral metabolic rates for glucose (CMRglc) within the posterior cortex (A,B) and CA3 (C,D) with time after controlled cortical impact (CCI) among postnatal day 35 (PND35) standard-fed and ketogenic-fed animals, and PND70 standard-fed and ketogenic-fed rats. †p < 0.01 and *p < 0.05 relative to age-matched sham.
Contusion volume
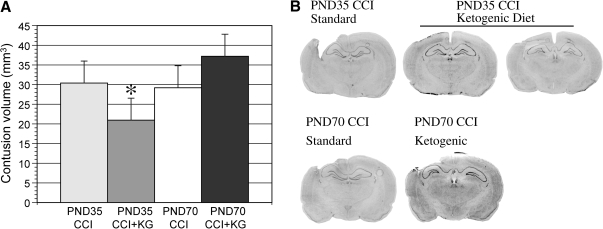
The CCI injury produced an evolving contusion, restricted to the cerebral cortex, which averaged 30.4 ± 3.9mm3 in PND35 and 29.2 ± 4.9 mm3 in PND70 rats on the standard diet (Fig. 4). There was no statistical difference in the contusion volume between the injured standard-fed age groups. The KG diet did show a significant 31% reduction in contusion volume among PND35 rats and a non-significant increase in volume in PND70 rats. The contralateral hemisphere at 7 days showed no morphological evidence of contrecoup injury.
FIG. 4.
Changes in cortical contusion volumes (mm3) between postnatal day 35 (PND35) and PND70 injured rats on standard or ketogenic (KG) diet at 7 days post-injury (A). Representative histological sections showing the extent of cortical preservation among PND35 injured ketogenic-fed animals (B). *p < 0.05 relative to age-matched standard-fed animals.
Discussion
Changes in substrate availability after TBI
The question of whether head-injured patients show changes in the blood chemistry as a result of TBI is difficult to determine given that blood glucose is aggressively managed within a specific range in human patients to prevent hyperglycemia (Vespa et al., 2006). Results from the current study show that PND35 rats maintained on standard chow had little change in blood concentrations of glucose or lactate. The non-significant trend of elevated plasma ßOHB may be mild ketosis from loss of appetite acutely after injury. PND70 rats on standard chow showed no changes in glucose or ßOHB, but did show significant decrease in plasma lactate. The decrease in plasma lactate may reflect cerebral and/or systemic use of lactate as an alternative fuel in this age group (Chen et al., 2000a,b). While both age groups show a significant increase in plasma ketones when maintained on the KG diet, PND35 rats show sustained decreases in blood glucose compared to PND70 rats. This difference may be related to the magnitude of ketosis achieved by each age group.
Age differences in CMRglc after TBI
Both PND35 and PND70 rats maintained on standard diet showed acute decreases in CMRglc of similar magnitude, which was sustained within ipsilateral cortical areas. While PND35 rats showed return of sham-level metabolic rates of glucose by post-injury day 7 within the hippocampus and thalamus, PND70 rats exhibited metabolic recovery in only the CA3 region of the hippocampus. These finding suggest that the CCI injury induced at the severity reported in this paper, produced cortically comparable metabolic responses in both age groups within the injured hemisphere. In contrast to our lateral FP injury which results in primarily ipsilateral cortical changes in CMRglc (Yoshino et al., 1991; Thomas et al., 2000), the current CCI injury produced significant contralateral changes. PND35 showed a decrease in CMRglc at 3 days in the temporal cortex and posterior cortex. This pattern re-emphasizes the fact that the contralateral hemisphere cannot serve as a “non-injured” control in this injury model.
The reason for the prolonged decrease in CMRglc after TBI remains as yet unknown. There are numerous possible mechanisms that may explain this depression including disruption of mitochondrial metabolism by calcium accumulation (Fineman et al., 1993; Osteen et al., 2001), ionic flux disruptions (Katayama et al., 1990), reduced cerebral blood flow (Cherian et al., 2004), or lactic acid accumulation (Kawamata et al., 1995). Another consideration is that following the initial surge in neurotransmitter release and ionic fluctuations the brain activity may become quiescent and therefore require less substrate. Several studies have shown decreased ability of the brain to show stimulation-evoked increases in CMRglc as early as 4hrs and as long as 2 months post-injury (Dietrich et al., 1994; Passineau et al., 2000). This quiescent state may be a mechanism to reduce secondary injury from activation, which has been observed after direct cortical stimulation (Ip et al., 2003).
Finally, glucose metabolic pathways may be altered after injury, directly impacting cerebral glucose uptake. 2DG autoradiography is a measure of glucose uptake, but it does not reveal how glucose is processed past hexokinase. 13C-glucose studies have shown that, during this period of depressed CMRglc, there is an increase in unmetabolized glucose, a decrease in 13C-labeled lactate and increase glucose metabolism through the pentose phosphate pathway (Bartnik et al., 2005, 2007). The decrease in oxidation of glucose and increase shunting of glucose towards NADPH synthesis suggests altered fates for glucose after TBI.
Age differences in CMRglc after TBI with ketones
How does the presence of ketones alter cerebral metabolism of glucose in the normal brain? In the normal adult rodent, ketosis induced by fasting, infusion, or diet has no affect on glucose uptake. A 30% reduction in CMRglc was reported only in a PND20 rat infused with ßOHB (Miller et al., 1986). Thus, in the rodent, cerebral fuel interaction of glucose and ketones appears to be age-dependent. In contrast, pigs and humans all show decreased CMRglc in response to ketosis (Kammula, 1976; Redies et al., 1989; Hasselbalch et al., 1994, 1996). While the decrease in CMRglc/glucose uptake during ketosis can reflect the shifting cerebral metabolism towards ketones, the lack of a decrease in glucose uptake does not necessary mean the opposite. Cerebral metabolism of ketones may not affect glucose uptake at some age groups, species or conditions, but may shift glucose from its normal oxidative fate towards another biochemical fate (pentose phosphate pathway, methylglyoxyl pathway). This is what is believed to happen in the adult rodent brain during ketosis. In the current study, PND35 sham rats on the KG diet showed no change in CMRglc, which is consistent with the adult rodent response reported in the literature and may indicate decreased oxidation of glucose, but no change in glucose uptake.
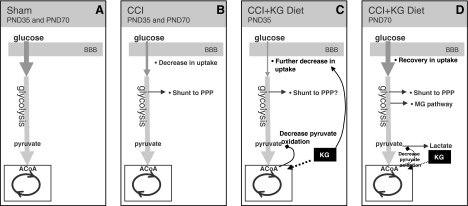
Injury-induced decreases in CMRglc were exacerbated bilaterally in PND35 CCI KG-fed animals on days 1 and 7. Several mechanisms may contribute to this further decrease in CMRglc, including decreased plasma glucose, cell death, a reduction in the need for glucose metabolism, and/or increased ketone metabolism. Insulin-induced hypoglycemia has been shown to alter the lumped constant and decrease CMRglc (Nedergaard et al., 1988). While the plasma glucose decreased approximately 20% in the current study, it did not reach hypoglycemic levels, and therefore, this reduction is unlikely to explain the additional 14–37% decrease in CMRglc observed. While it is certainly possible that certain areas of the brain become dennervated or exhibit retrograde degeneration, which could cause a reduction in glucose metabolism, cell death is unlikely to explain the contralateral cortical decrease. In this case, it is more likely that the PND35 brain increased its metabolism of ketones bilaterally. It has been shown that accelerated rates of ketone metabolism can produce an acetyl-CoA pool that competes with pyruvate derived acetyl-CoA and leads to pyruvate oxidation inhibition (Randle et al., 1966; Ruderman et al., 1974). This may explain the mechanism by which cerebral ketone utilization results in further decreases in CMRglc after TBI and may require a certain minimum threshold of ketone metabolism to generate sufficient acetyl-CoA pool to cause inhibition (Fig. 5) and may explain the neuroprotective effect on cortical lesion volume.
FIG. 5.
Diagrammatic representation of the current hypothesis for changes in cerebral metabolic rates for glucose (CMRglc) after injury and ketosis among postnatal day 35 (PND35) and PND70 rats. The normal glucose metabolic pathway is shown for both sham PND35 and PND70 standard-fed rats (A). Both PND35 and PND70 rats show similar decreases in CMRglc after controlled cortical impact (CCI) on standard diet. Increased glucose processing through the pentose phosphate pathway has only been determined in the PND70 age group (B). Injured PND35 animals maintained on the ketogenic (KG) diet showed a further decrease in CMRglc, which may be directly related to greater ketone metabolism, which contributes more to the acetylCoA pool and consequently inhibits pyruvate oxidation (C). It remains unclear at this time whether there is a threshold of pyruvate oxidation inhibition that alters glucose uptake. PND70 rats on the KG diet showed normalization of the CMRglc rates (D). In this case, while ketones are likely to be metabolized, there are normal rates of glucose uptake, which may be subsequently shunted to other pathways. It is hypothesized that the increased activities in alternative metabolic pathways may contribute to this glucose uptake and may result in detrimental byproducts after traumatic brain injury.
While the PND35 sham animals on the KG diet did not show changes in cortical CMRglc, the contralateral cortex of PND35 CCI animals on the KG diet exhibited a decrease in CMRglc. This again emphasizes the fact that the contralateral hemisphere is affected by the injury and should not be used as a “control.” More importantly, the metabolic changes occurring post-injury on the contralateral side may reflect reorganizational changes that have been shown to take place during this time period. At 7 days post-cortical ischemia, stimulation of right vibrissae triggered increased 14C-2DG uptake in the contralateral barrel cortex, suggesting compensatory plasticity (Jablonka nd Kossut, 2006). fMRI studies have also shown that unilateral lesion of the sensorimotor cortex resulted in increased activation of the contralateral cortex and regions lateral to the injury upon hindlimb stimulation (Abo et al., 2001). Also, at 1–4 days after lateral TBI, cerebral blood flow increases in the contralateral cortex (Scremin et al., 2007). These types of contralateral compensatory processes have also been observed following human TBI and suggest altered synaptic connectivity in the contralateral cortex in response to injury-related dennervation. The decrease in CMRglc observed in the contralateral cortex among PND35 injured KG-fed rats may reflect an increase in ketone metabolism during reorganization sufficient to suppress glucose uptake.
Another explanation for the bilateral CMRglc decrease of the PND35 rats on the KG diet after TBI may be related to differential response to injury. At 3 days post-injury, PND35 rats show contralateral decreases in the temporal and posterior cortical regions, which were not observed among PND70 rats. This may indicate that the CCI injury has contralateral consequences of a different timecourse in the younger rat, and ketone-induced decreases in CMRglc may be observed in injured regions. The depression of CMRglc at 7 days among PND35 rats on the KG diet can be associated with the degree of ketosis that is maintained in this age group. PND70 rats on the KG diet do not maintain high levels of ketones, and plasma glucose is also no longer depressed at 7 days.
In contrast to the PND35 rats, PND70 injured standard-fed and KG-fed animals showed no difference from each other in their CMRglc changes, except at post-injury day 7, where CMRglc rates were greater among KG-fed animals. It appears at this time point that animals in this age group show “recovery” of their glucose metabolic rates when maintained on the KG diet for 7 days after injury. Given the tremendous body of literature reporting adult rodent brain capacity to metabolize ketones after prolonged ketosis, it is unlikely that the adult brain is not utilizing the substrate (Prins et al., 2004). There is also no evidence to contradict the inhibitory effects of ketone metabolism on pyruvate oxidation. There is, however, evidence that the adult brain bilaterally increases pentose pathway activity by 9–12% after CCI injury (Bartnik et al., 2005), and there is accumulation of propylene glycol (Hirt et al., 2007). Together these findings suggest that increased activity of alternative metabolic pathways may stimulate return of sham levels of glucose uptake (Fig. 5), which may have negative consequences on cortical contusion volume in the mature brain.
Changes in the lumped constant (LC) have been shown to cause over- or under-estimation of the cerebral metabolic rates of glucose. While the LC has been shown to decrease with age, between PND14 and PND90 there is little change (Takei et al., 1986), and it is not expected to contribute to the age groups in the current study. The LC does not change with mild hypoglycemia, but plasma glucose levels less than 5 mM cause and increase in the LC (Schuier et al., 1990). While animals maintained on the KG diet in the current study did show decreases in plasma glucose, they did not approach 5 mM. Finally, in regards to TBI, the global LC has been estimated to decrease from 0.65 to 0.43 in human patients (Wu et al., 2004) and may contribute to an underestimation of the CMRglc rates. The changes in the LC are not likely to explain the marked changes in CMRglc in the current study.
The results from the current study show that ketosis affects cerebral glucose metabolism in an age-dependent manner after TBI. While both age groups were similar in terms of glucose metabolism and contusion volume after TBI while on the standard diet, the presence of ketones further reduced CMRglc in PND35 and normalized CMRglc in PND70 rats after injury. The changes in CMRglc among PND35 TBI rats on the KG diet were associated with decreased cortical contusion volume, which was consistent with previous findings (Prins et al., 2005). These results suggest that conditions of reduced glucose utilization and increased alternative substrate metabolism may be preferable acutely after TBI in the juvenile brain. Clinically, conditions of low plasma glucose and elevated ketones were shown to improve nitrogen balance (Ritter et al., 1996). Future studies addressing optimal post-injury substrate conditions will help establish age-appropriate substrate management guidelines for TBI patients.
Acknowledgments
This work was supported by NS052406, NS27544, and NS37363.
Author Disclosure Statement
No competing financial interests exist.
References
- Abo M. Chen Z. Lai L.J. Reese T. Bjelke B. Functional recovery after brain lesion-contralateral neurmodulation: an fMRI study. Neuroreport. 2001;12:1543–1547. doi: 10.1097/00001756-200105250-00048. [DOI] [PubMed] [Google Scholar]
- Alessandri B. Reinert M. Young H.F. Bullock R. Low extracellular (ECF) glucose affects the neurochemical profile in severe head-injured patients. Acta Neurochir. 2000;Suppl. 76:425–430. doi: 10.1007/978-3-7091-6346-7_88. [DOI] [PubMed] [Google Scholar]
- Al-Mudallal A.S. Levin B.E. Lust W.D. Harik S.I. Effects of unbalanced diets on cerebral glucose metabolism in the adult rat. Neurology. 1995;45:2261–2265. doi: 10.1212/wnl.45.12.2261. [DOI] [PubMed] [Google Scholar]
- Andersen B.J. Marmarou A. Isolated stimulation of glycolysis following traumatic brain injury. In: Hoff J.T., editor; Betz A.L., editor. Intracranial Pressure VII. Springer-Verlag; Berlin: 1989. pp. 575–580. [Google Scholar]
- Andersen B.J. Marmarou A. Post-traumatic selective stimulation of glycoslysis. Brain Res. 1992;585:184–189. doi: 10.1016/0006-8993(92)91205-s. [DOI] [PubMed] [Google Scholar]
- Bartnik B.L. Sutton R.L. Fukushima M. Harris N.G. Hovda D.A. Lee S.M. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J. Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- Bartnik B.L. Lee S.M. Hovda D.A. Sutton R.L. The fate of glucose during the period of decreased metabolism after fluid percussion injury: a 13C NMR study. J. Neurotrauma. 2007;24:1079–1092. doi: 10.1089/neu.2006.0210. [DOI] [PubMed] [Google Scholar]
- Bergsneider M. Hovda D.A. Shalmon E. Kelly D.F. Vespa P.M. Martin N.A. Phelps M.E. McArthur D.L. Caron M.J. Kraus J.F. Becker D.P. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Chen S.F. Richards H.K. Smielewski P. Johnstrom P. Salvador R. Pickard J.D. Harris N.G. Relationship between flow-metabolism uncoupling and evolving axonal injury after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2004;24:1025–1036. doi: 10.1097/01.WCB.0000129415.34520.47. [DOI] [PubMed] [Google Scholar]
- Chen T. Qian Y.Z. Di X. Rice A. Zhu J.P. Bullock R. Latate/glucose dynamics after rat fluid percussion brain injury. J. Neurotrauma. 2000a;17:135–142. doi: 10.1089/neu.2000.17.135. [DOI] [PubMed] [Google Scholar]
- Chen T. Qian Y.Z. Di X. Rice A. Zhu J.P. Bullock R. Brain lactate uptake increases at the site of impact after traumatic brain injury. Brain Res. 2000b;861:281–287. doi: 10.1016/s0006-8993(00)01992-2. [DOI] [PubMed] [Google Scholar]
- Cherel Y. Burnol A.F. Leturque A. LeMaho Y. In vivo glucose utilization in rat tissues during the three phases of starvation. Metabolism. 1988;37:1033–1039. doi: 10.1016/0026-0495(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Cherian L. Hlatky R. Robertson C.S. Comparison of tetrahydrobiopterin and l-arginine on cerebral blood flow after controlled cortical impact injury in rats. J. Neurotrauma. 2004;21:1196–1203. doi: 10.1089/neu.2004.21.1196. [DOI] [PubMed] [Google Scholar]
- Cherian L. Robertson C.S. Goodman J.C. Secondary insults increase injury after controlled cortical impact in rats. J. Neurotrauma. 1996;13:371–383. doi: 10.1089/neu.1996.13.371. [DOI] [PubMed] [Google Scholar]
- Corddry D.H. Rapoport S.I. London E.D. No effect of hyperketonemia on local cerebral glucose utilization in conscious rats. J. Neurochem. 1982;38:1637–1641. doi: 10.1111/j.1471-4159.1982.tb06644.x. [DOI] [PubMed] [Google Scholar]
- Cochran A. Schaife E.R. Hansen K.W. Downey E.C. Hyperglycemia and outcomes from pediatric traumatic brain injury. J. Trauma. 2003;55:1035–1038. doi: 10.1097/01.TA.0000031175.96507.48. [DOI] [PubMed] [Google Scholar]
- Dietrich W. Alonso O. Busto R. Ginsberg M.D. Widespread metabolic depression and reduced somatosensory circuit activation following traumatic brain injury in rats. J. Neurotrauma. 1994;11:629–640. doi: 10.1089/neu.1994.11.629. [DOI] [PubMed] [Google Scholar]
- Feise G. Kogure K. Busto R. Scheinberg P. Reinmuth O.M. Effect of insulin hypoglycemia upon cerebral energy metabolism and EEG activity in the rat. Brain Res. 1976;126:263–280. doi: 10.1016/0006-8993(77)90725-9. [DOI] [PubMed] [Google Scholar]
- Fineman I. Hovda D.A. Smith M. Yoshino A. Becker D.P. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45Ca autoradiographic study. Brain Res. 1993;624:94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- Goodman J.C. Valadka A.B. Gopinath S.P. Uzura M. Robertson C.S. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit. Care Med. 1999;27:2063–2064. doi: 10.1097/00003246-199909000-00041. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Andrus P.K. Yonkers P.A. Brain hdroxyl radical generation in acute experimental head injury. J. Neurochem. 1993;60:588–594. doi: 10.1111/j.1471-4159.1993.tb03189.x. [DOI] [PubMed] [Google Scholar]
- Hirt D. Kadri M. Martin N.A. Que Hee S. Eliseo M. Berg J. Vespa P.M. Glenn T.C. Propylene glycol after human traumatic brain injury: novel biomarker of altered glucose metabolism. Brain. 2007:B051. [Google Scholar]
- Ip E.Y. Zanier E.R. Moore A.H. Lee S.M. Hovda D.A. Metabolic, neurochemical, and histological responses to vibrissa motor cortex stimulation after traumatic brain injury. J. Cereb. Blood Flow Metab. 2003;23:900–910. doi: 10.1097/01.WCB.0000076702.71231.F2. [DOI] [PubMed] [Google Scholar]
- Jablonka J. Kossut M. Focal stroke in the barrel cortex of rats enhances ipsilateral response to vibrissal input. Acta Neurobiol. Exp. 2006;66:261–266. doi: 10.55782/ane-2006-1614. [DOI] [PubMed] [Google Scholar]
- Katayama Y. Becker D.P. Tamura T. Hovda D.A. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Kawamata T. Katayama Y. Hovda D.A. Yohino A. Becker D.P. Administration of excitatory amino acid antagonists via microdialysis attenuates the increasein glucose utilization seen following concussive brain injury. J. Cereb. Blood Flow Metab. 1992;12:12–24. doi: 10.1038/jcbfm.1992.3. [DOI] [PubMed] [Google Scholar]
- Miller A.L. Regional glucose and beta-hydroxybutyrate use by developing rat brain. Metab. Brain Dis. 1986;1:53–61. doi: 10.1007/BF00998477. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Jakobsen J. Diemer N.H. Autoradiographic determination of cerebral glucose content, blood flow, and glucose utilization in focal ischemia of the rat brain: influence of the plasma glucose concentration. J. Cereb. Blood Flow Metab. 1988;8:100–108. doi: 10.1038/jcbfm.1988.13. [DOI] [PubMed] [Google Scholar]
- Nehlig A. Pereira de Vasconcelos A. Boyet S. Quantitative autoradiographic measurement of local cerebral glucose utilization in freely moving rats during postnatal development. J. Neurosci. 1988;8:2321–2333. doi: 10.1523/JNEUROSCI.08-07-02321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M.T. Seal A. Nortje J. Al-Rawi P.G. Coles J.P. Fryer T.D. Menon D.K. Pickard J.D. Hutchinson P.J. Glucose metabolism in traumatic brain injury: a combined microdialysis and [18F]-2-fluoro-2-deoxy-d-glucose-positron emission tomography (FDG-PET) study. Acta Neurochir. 2005;Suppl. 95:165–168. doi: 10.1007/3-211-32318-x_35. [DOI] [PubMed] [Google Scholar]
- Osteen C.L. Moore A.H. Prins M.L. Hovda D.A. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J. Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- Passineau M.J. Zhao W. Buto R. Dietrich W. Alonso O. Loor J.Y. Bramlett H.M. Ginsberg M.D. Chronic metabolic sequelae of traumatic brain injury: prolonged suppression of somatosensory activation. Am. J. Physiol. Heart Circ Physiol. 2000;279:H924–H931. doi: 10.1152/ajpheart.2000.279.3.H924. [DOI] [PubMed] [Google Scholar]
- Prins M.L. Lee S.M. Fujima L. Hovda D.A. Increased cerebral uptake and oxidation of exogenous betaHB improves ATP following traumatic brain injury in adult rats. J. Neurochem. 2004;90:666–672. doi: 10.1111/j.1471-4159.2004.02542.x. [DOI] [PubMed] [Google Scholar]
- Prins M.L. Fujima L.S. Hovda D.A. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J. Neurosci. Res. 2005;82:413–420. doi: 10.1002/jnr.20633. [DOI] [PubMed] [Google Scholar]
- Prins M.L. Giza C.C. Induction of monocarboxylate transporter-2 expression and ketone transport following traumatic brain injury in juvenile and adult rats. Dev. Neurosci. 2006;28:447–456. doi: 10.1159/000094170. [DOI] [PubMed] [Google Scholar]
- Randle P.J. Garland P.B. Hales C.N. Newsholme E.A. Denton R.M. Pogson C.I. Interactions of metabolism and the physiological role of insulin. Recent Prog. Horm. Res. 1966;22:1–48. doi: 10.1016/b978-1-4831-9825-5.50004-x. [DOI] [PubMed] [Google Scholar]
- Richards H.K. Simac S. Piechnik S. Pickard J.D. Uncoupling of cerebral blood flow and metabolism after cerebral contusion in the rat. J. Cereb. Blood Flow Metab. 2001;21:779–781. doi: 10.1097/00004647-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Ritter A.M. Robertson C.S. Goodman J.C. Contant C.F. Grossman R.G. Evaluation of carbohydrate free diet for patients with severe head injury. J. Neurotrauma. 1996;13:473–485. doi: 10.1089/neu.1996.13.473. [DOI] [PubMed] [Google Scholar]
- Rovlias A. Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46:335–342. doi: 10.1097/00006123-200002000-00015. [DOI] [PubMed] [Google Scholar]
- Ruderman N.B. Ross P.S. Berger M. Goodman M.N. Regulation of glucose and ketone-body metabolism in brain of anesthetized rats. Biochem. J. 1974;138:1–10. doi: 10.1042/bj1380001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuier F. Orzi F. Suda S. Lucignani G. Kennedy C. Sokoloff L. Influcence of plasma glucose concentration on lumped constant of the deoxyglucose method: effects of hyperglycemia in the rat. J. Cereb. Blood Flow Metab. 1990;10:765–773. doi: 10.1038/jcbfm.1990.134. [DOI] [PubMed] [Google Scholar]
- Scremin O.U. Li M.G. Scremin A.M.E. Cortical contusion induces trans-hemispheric reorganization of blood flow maps. Brain Res. 2007;1141:235–241. doi: 10.1016/j.brainres.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Reivich M. Kennedy C. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sutton R.L. Hovda D.A. Adelson P.D. Benzel E.C. Becker D.P. Metabolic changes following cortical contusion: relationship to edema and morphological changes. Acta Neurochir. 1994;Suppl. 60:446–448. doi: 10.1007/978-3-7091-9334-1_122. [DOI] [PubMed] [Google Scholar]
- Takei H. Fredericks W.R. Rapoport S.I. The lumped constant in the deoxyglucose procedure declines with age in Fisher-344 rats. J. Neurochem. 1986;46:931–938. doi: 10.1111/j.1471-4159.1986.tb13059.x. [DOI] [PubMed] [Google Scholar]
- Thomas S. Prins M.L. Samii M. Hovda D.A. The cerebral metabolic response to traumatic brain injury sustained early in development: a 2-deoxy-d-glucose autoradiography study. J. Neurotrauma. 2000;17:649–665. doi: 10.1089/089771500415409. [DOI] [PubMed] [Google Scholar]
- Vespa P. Boonyaputthikul R. McArthur D.L. Miller C. Etchepare M. Bergsneider M. Glenn T. Martin N. Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit. Care Med. 2006;34:400–411. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- Wu H.M. Huang S.C. Hattori N. Glenn T.C. Vespa P.M. Yu C.L. Hovda D.A. Phelps M.E. Bergsneider M. Selective metabolic reduction in gray matter acutely following human traumatic brain injury. J. Neurotrauma. 2004;21:149–161. doi: 10.1089/089771504322778613. [DOI] [PubMed] [Google Scholar]
- Yoshino A. Hovda D.A. Kawamata T. Katayama Y. Becker D.P. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- Yoshino A. Hovda D.A. Katayama Y. Kawamata T. Becker D.P. Hippocampal CA3 lesion prevents postconcussive metabolic dysfunction in CA1. J. Cereb. Blood Flow Metab. 1992;12:996–1006. doi: 10.1038/jcbfm.1992.137. [DOI] [PubMed] [Google Scholar]
- Zauner A. Doppenberg E. Woodward J.J. Allen C. Jebraili S. Young H.F. Bullock R. Multiparametric continuous monitoring of brain metabolism and substrate delivery in neurosurgical patients. Neurol. Res. 1997;19:265–273. doi: 10.1080/01616412.1997.11740812. [DOI] [PubMed] [Google Scholar]