Abstract
Imaging can provide quantitative assessment of radiation-induced normal tissue effects. Identifying an early sign of normal tissue damage with imaging would have the potential to predict organ dysfunction, thereby allowing re-optimization of treatment strategies based upon individual patients’ risks and benefits. Early detection with non-invasive imaging may enable interventions to mitigate therapy-associated injury prior to its clinical manifestation. Further, successive imaging may provide an objective assessment of the impact of such mitigation therapies. However, many problems make application of imaging to normal tissue assessment challenging, and further work is required to establish imaging biomarkers as surrogate endpoints of clinical outcome. The performance of clinical trials where normal tissue injury is a clearly defined endpoint would greatly aid in realization of these goals.
Keywords: Normal tissue effects, imaging, biomarker
1. Introduction
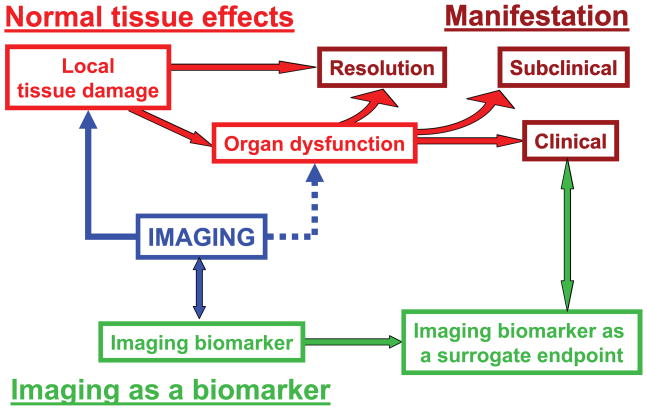
Radiation therapy (RT) may induce local tissue damage which in turn, depending on the severity and the volume affected, may lead to organ dysfunction (Figure 1). Organ dysfunction may be clinical (symptomatic) or sub-clinical (asymptomatic). When imaging to assess normal tissue effects is quantitative, it can represent a useful imaging biomarker (Figure 1). Imaging biomarkers are closely connected with anatomical, physiological and molecular changes that characterize the radiation induced tissue damage or organ dysfunction. However, only when imaging biomarkers are correlated to clinical endpoints, can they become surrogate endpoints of clinical injury (Figure 1). Identifying an early sign of normal tissue damage with imaging would have the potential to predict organ dysfunction, thereby allowing re-optimization of treatment strategies based upon individual patients’ risks and benefits. However, some imaging biomarkers may prove to be overly sensitive and too non-specific to be useful as surrogate endpoints.
Figure 1.
Relation between normal tissue effects and their clinical manifestation, and role of imaging to assess the effects.
Understanding of underlying pathophysiology of normal tissue damage, and the associated molecular and cellular processes that lead to long term effects such as cell death and apoptosis, can help identifying precursors of organ dysfunction, which can be explored as potential imaging biomarkers1. However, because knowledge of the normal tissue effects is incomplete, choice of imaging biomarkers is often pragmatic and markers may be identified without the researcher’s understanding the underlying molecular mechanisms. Even with incomplete understanding of underlying biology, imaging biomarkers may be successfully used as surrogate endpoints of clinical injury. In addition, they may help to elucidate the spatial and temporal development of underlying molecular processes that drive RT associated injury. Choosing imaging techniques that are sensitive to early biological processes of normal tissue damage is important.
Acute and late normal tissue injury occurs from a complex interaction between radiation-induced death of parenchymal cells, damage to the supporting vasculature, and associated inflammatory and fibrotic reactions. Long-term depletion of tissue-specific stem cells or progenitor cells can lead to fibrosis, organ dysfunction, and necrosis1. This interaction between basic cellular and molecular process and physiological expression divides imaging assessment into two distinguished biological levels: (a) Imaging of anatomical (structural) changes in affected organs, (b) Imaging of functional, molecular and cellular processes of RT-induced injury. Topics related to these assessments and the potential for further research are discussed here.
2. Current approaches and status of normal tissue toxicity imaging
2.1 Anatomical imaging
Radiation therapy commonly causes changes that can be detected by planar X-ray or CT imaging. For example, in patients treated for lung or breast cancer, approximately 50–100% and 0–63%, respectively, have radiologic evidence of lung damage via chest X-ray (CXR) or CT2. These changes in imaging, however, do not necessarily correlate well with symptomatic injury. Similarly, in the liver, radiologic changes are often evident post-RT either prior to, or in the absence of, clinical symptoms3, 4. Post-RT CT and MRI can detect non-specific morphologic abnormalities in the brain that may reflect RT-induced tumor or normal tissue inflammation/necrosis, or surgery-induced changes (e.g. enhancement along the resection margin)5. A principal limitation of defining injury in this manner is that injury is often identified months to years following RT when any opportunity to intervene to ameliorate the effects has likely been lost.
2.2 Functional and molecular imaging
Functional imaging may provide an in vivo model of RT effects on both tumors and normal tissues. The potential advantage of functional and molecular imaging over anatomic imaging is that it may be more physiologically and clinically important, and may better reflect underlying pathophysiologic processes. Many functional and molecular imaging modalities have been used to monitor normal tissue responses; the most common being fluorodeoxyglucose (FDG) PET, though other isotopes have also been used (e.g. 15O for monitoring of blood flow changes). SPECT is often used to measure perfusion but can also be used to image radiolabeled receptors over-expressed in certain tumors. MRI has become useful to assess functional metrics such as regional perfusion (i.e. in the heart), ventilation (i.e. in the lung) and metabolic states (i.e. with MR Spectroscopy). Functional MRI (fMRI) has the ability of assessing regional brain activity in response to stimuli. Data for several organ systems such as lung6–8, heart9–13, liver14, 15, brain16–19 and parotid20, 21 have already revealed correlations of radiation dose with changes measurable by a variety of functional imaging modalities.
3. Opportunity and future for normal tissue toxicity imaging
3.1 Imaging as a precursor of injury manifestation
A large body of converging evidence, from histopathology, molecular biology, animal models and clinical observations, suggests that RT-induced normal tissue injury is a dynamic and progressive process1, 22. Given that it is extremely difficult to obtain human normal tissue after irradiation for histological and biological analysis and for longitudinal examination, it is important to establish in vivo functional and molecular imaging as a biomarker for early assessment and prediction of delayed or late organ dysfunction. Preclinical experiments, which can provide unique data on normal tissue injury dynamics, can be extremely helpful in this process 23, 24.
Identifying an early sign or precursor of normal tissue damage, e.g., during or shortly after the course of fractionated RT, could predict the delayed organ dysfunction. For example, cardiac functional imaging may allow for early detection of treatment-associated dysfunction. This is particularly important since these changes often do not manifest clinically for at least 10 years post treatment25. In patients treated for breast cancer, SPECT can detect myocardial perfusion defects in the irradiated left ventricle that are associated with wall motion abnormalities. However, there are no systematic changes in either ejection fraction or clinical cardiac events. Similarly, although SPECT lung perfusion imaging has been used quantitatively to relate changes in regional perfusion/ventilation (e.g. function) to the regional radiation dose6–8, there is limited correlation between the sum of these regional injuries (i.e. the integrated response) and changes in global lung function (e.g. pulmonary function tests). Changes in MRI-defined gadolinium enhancement kinetics may be associated with different phases of radiation pneumonitis26. Similarly, abnormalities in FDG PET studies may relate to symptomatic pneumonitis, and provide an objective measure of inter-patient variability of biological response27–29.
Re-optimization of treatment strategies based upon individual patients’ risks and benefits is another area that could benefit from normal tissue toxicity imaging. For example, the basic pathophysiology of RT-induced liver disease is venous occlusion. Symptoms generally occur 2 weeks to 2 months following completion of RT, and the clinical outcome ranges from mild, reversible damage to death. Therefore, early monitoring of venous perfusion would have the potential to select patients with pre-clinical signs of perfusion changes prior to the onset of symptomatic RT-induced injury. It has been shown that the reduction in regional portal venous perfusion during the course of radiation therapy and local dose distribution in the liver are two independent predictors for regional portal venous perfusion dysfunction one month post-RT14. Furthermore, it has been demonstrated that the regional liver venous perfusion dysfunction is associated with overall liver function30. Other alternative re-optimization strategy could be re-optimization of irradiation geometry based on local normal tissue damage31; however, it is questionable whether the changes would be detectable early enough to allow modification of already started treatment regimen.
3.2 Imaging as a biomarker
Imaging biomarkers are characteristics that can be objectively imaged as indicators of normal biological processes, pathogenic processes, or pharmacologic responses to therapeutic interventions. Imaging biomarkers as surrogate endpoints are imaging biomarkers that are intended to substitute for clinical endpoints. Surrogate endpoints are expected to predict clinical benefit (or harm or lack of benefit or harm) based on epidemiologic, therapeutic, pathophysiologic, or other scientific evidence.
Imaging biomarkers should be discussed in the context of molecular biomarkers, which are described in detail elsewhere (Bentzen et al, same issue). Molecular biomarkers have biophysical properties, which allow their measurements in biological samples, like in plasma, serum, cerebrospinal fluid or biopsy samples. Molecular biomarkers can detect molecular and cellular changes with high sensitivity, but might not be very specific. For example, the hematocrit or other blood counts can be a sensitive measure of marrow function. However, they can also be affected by dysfunction caused by other conditions (e.g. bowel disease or malnutrition affecting hematocrit). Further, molecular markers lack spatial information. Thus, they may be useful in the realm of whole organ irradiation (e.g. blood counts for total body irradiation or liver function tests for whole liver irradiation), but may not be sensitive for regional organ effects. Similar limitations hold for molecular biomarkers obtained from biopsy samples, which sample only few points in the organ, thus not being able to assess the regional organ resoponse heterogeneity. On the contrary, imaging has the unique potential for detecting the spatial distribution of the tissue damage that can lead to organ dysfunction. Common to imaging and molecular biomarkers32 is that they may be used to identify patients at increased or decreased risk for radiation treatment-related injury. In some settings, imaging and molecular biomarkers may be synergistic, and their combined use may overcome inherent limitations of each single approach, thereby increasing overall sensitivity and specificity of the assessment.
Multiple obstacles lie in the path toward establishment of imaging biomarkers as surrogate endpoints for assessment and prediction of clinical injury. Challenges for quantitative imaging are discussed in the next section. Other obstacles include unknown temporal dynamics of RT-induced injury, understanding of normal biology, and the relationship between early normal tissue damage and late symptomatic organ dysfunction. In addition, RT is often combined with chemotherapy and molecular targeted therapies, where normal tissue toxicity can result from either modality separately or through a synergistic effect of combined therapies. New knowledge about the mechanisms of normal tissue toxicity, and potential inhibition of the effects, for example with anti-inflammatory compounds (e.g., inhibitors of prostaglandin and leukotriene formation, NFkB and IL-1 signaling) might significantly affect RT-induced toxicity management. Monitoring induction, resolution and mitigation of radiation-induced toxicity will be essential in the development of clinically successful normal tissue preserving strategies. Molecular imaging might be particularly useful to study and monitor these changes. However, much work still needs to be done before relevant molecular probes are developed to fully explore the potential that molecular imaging offers for questions relevant to human clinical imaging..
3.3 Need for quantitative imaging of normal tissue toxicity
Imaging modalities and techniques, primarily developed with clinical diagnostic application in mind, are often not quantitative enough to raise imaging to the level of a biomarker.. Imaging, even though having a quantitative physical basis, is very often burdened with significant uncertainties, preventing characterization of small changes that are characteristic of moderate normal tissue injury. It is important that quantification is ensured through the whole procedure – from image acquisition and image reconstruction to image analysis. The assessment of imaging as either a biomarker or surrogate endpoint also requires quantitative clinical endpoints. However, while imaging endpoints are usually continuous, most clinical endpoints are dichotic. Using a continuous variable for measurement of an organ function, or stage of injury, could improve statistical power for correlative analysis relating imaging to clinical events, thereby reducing the number of patients required for studies. While qualitative diagnostic imaging does not carry the same value as quantitative imaging, it can still be useful in the diagnosis of normal tissue effects (e.g. esophageal stricture seen on barium swallow, cerebral edema on CT/MRI).
Clearly, these problems call for a wide cooperative effort among various governmental, professional and industrial entities. Some of these efforts have already begun. Initial efforts started within individual professional societies: Radiological Society of North America (RSNA), American College of Radiology (ACR), American Association of Physicists in Medicine (AAPM), Society of Nuclear Medicine (SNM), International Society for Magnetic Resonance in Medicine (ISMRM); however, it was soon realized that the problems are too significant to be solved by a single professional society. The first organized effort was initiated by National Cancer Institute (NCI) and Association of American Cancer Institutes (AACI) in 2003. It lead to the formation of Image Response Assessment Teams (IRAT) with the purpose to facilitate development of multi-disciplinary teams in NCI-designated comprehensive cancer centers to advance the role of imaging in assessment of response to therapy. The IRAT project’s primary objective was to increase collaboration between imaging scientists and oncology investigators to enhance the use of quantitative anatomic, functional, and molecular imaging endpoints in clinical therapeutic trials. The IRAT initiative is currently being augmented with the efforts within Clinical and Translational Science Award (CTSA), particularly the Imaging Working Group. The second large effort was a workshop “Imaging as a Biomarker: Standards for Change Measurements in Therapy” organized by National Institute of Standards and Technology (NIST) in 2006. It included US Food and Drug Administration (FDA), Pharmaceutical Research and Manufacturers of America (PhRMA), National Institutes of Health (NIH), academia and societies. Key summary points from this workshop were: 1) variability is too high in the multi-center trials that use imaging, 2) standards for imaging clinical trials are lacking, and 3) sharing of imaging data is inadequate partially due to insufficient infrastructure and under-developed processes. In 2008 the Quantitative Imaging Biomarkers Alliance (QIBA) between drug and equipment industries and imaging societies has been formed to develop and advance standards for the use of volumetric CT, FDG-PET, and DCE-MRI in clinical trials. More organized efforts following from these initiatives are underway warranting a significant shift from qualitative to quantitative imaging in the future.
While these efforts are important in making imaging quantitative and more useful, they do not specifically address or consider RT-induced normal tissue toxicity imaging as an endpoint. In order to make normal tissue imaging more successful, a more coherent effort should be initiated between all interested parties: clinicians, physicists, radiobiologists, radiologists, their representative societies, such as American Society for Therapeutic Radiology and Oncology (ASTRO), European Society for Therapeutic Radiology and Oncology (ESTRO), American Association of Physicists in Medicine (AAPM), Radiation Research Society (RRS), and cooperative clinical trial groups such as Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Therapy of Cancer (EORTC). For example, a cross-society task group could be formed to systematically approach normal tissue imaging, prepare normal tissue imaging guidance documents, and share the expertise between the interested parties. More collaborative clinical trials focusing on normal tissue imaging, as main or secondary endpoints, should be initiated, optimally within one of the cooperative groups.
4. Conclusions
Imaging has been successfully used to assess radiation-induced injury within several organs. The extent and severity of normal tissue damage has also been successfully related to clinically observed changes in global organ dysfunction. Since current assessment of normal tissue damage and organ dysfunction mostly relies on established anatomical and functional imaging techniques, the full potential of discovering and applying imaging biomarkers has not been explored. Use of molecular imaging, even though potentially much more powerful in identifying radiation-induced injury, has yet to be thoroughly investigated. New knowledge and understanding of the onset, dynamics and resolution of RT-induced injury mechanisms will likely lead to development of more specific molecular imaging techniques. There are many questions that make application of imaging to normal tissue assessment challenging – we do not know when to image and what to image and how imaging changes correlate to the clinically observed effects. In addition, establishing imaging as a biomarker, particularly rising it to the level of surrogate endpoints of clinically relevant outcome, is still relatively weak.
The answers to these questions will only be obtained by performing clinical trials that focus on normal tissue injury, and include imaging as an investigative modality as well as one of the endpoints. The importance of well-designed clinical trials where normal tissue injury is a clearly defined endpoint is paramount. In addition, preclinical studies of RT-induced normal tissue injury can greatly help understanding complicated patophysiology. As we better understand the mechanisms of RT injury elucidated by such studies, we will be able to more rationally plan radiotherapeutic management to minimize treatment related complications and intervene in injury processes to maximally improve outcomes and QOL for our patients. The establishment of imaging as a biomarker holds great promise for realization of these goals.
Footnotes
Conflict of interest: No conflicts of interest noted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Rodemann HP, Blaese MA. Responses of normal cells to ionizing radiation. Semin Radiat Oncol. 2007;17:81–88. doi: 10.1016/j.semradonc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Marks LB. Oncology. Vol. 8. Williston Park, N.Y.: 1994. The pulmonary effects of thoracic irradiation; pp. 89–106. discussion 100, 103. [PubMed] [Google Scholar]
- 3.Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15:279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 5.Vos MJ, Hoekstra OS, Barkhof F, et al. Thallium-201 single-photon emission computed tomography as an early predictor of outcome in recurrent glioma. Journal of Clinical Oncology. 2003;21:3559–3565. doi: 10.1200/JCO.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Fan M, Marks LB, Hollis D, et al. Can we predict radiation-induced changes in pulmonary function based on the sum of predicted regional dysfunction? Journal of Clinical Oncology. 2001;19:543–550. doi: 10.1200/JCO.2001.19.2.543. [DOI] [PubMed] [Google Scholar]
- 7.Fan M, Marks LB, Lind P, et al. Relating radiation-induced regional lung injury to changes in pulmonary function tests. International Journal of Radiation Oncology Biology Physics. 2001;51:311–317. doi: 10.1016/s0360-3016(01)01619-4. [DOI] [PubMed] [Google Scholar]
- 8.Seppenwoolde Y, De Jaeger K, Boersma LJ, et al. Regional differences in lung radiosensitivity after radiotherapy for non-small-cell lung cancer. International Journal of Radiation Oncology Biology Physics. 2004;60:748–758. doi: 10.1016/j.ijrobp.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Seddon B, Cook A, Gothard L, et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiotherapy & Oncology. 2002;64:53–63. doi: 10.1016/s0167-8140(02)00133-0. [DOI] [PubMed] [Google Scholar]
- 11.Marks LB, Zhou S, Yu X. The impact of irradiated left ventricular volume on the incidence of radiation-induced cardiac perfusion changes. Int J Radiat Oncol Biol Phys. 2003:57. [Google Scholar]
- 12.Marks LB, Prosnitz RG, Hardenbergh PH. Functional consequences of radiation (RT)-induced perfusion changes in patients with left-sided breast cancer. Int J Radiat Oncol Biol Phys. 2002;54:3–4. [Google Scholar]
- 13.Prosnitz RG, Hubbs JL, Evans ES, et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer. 2007;110:1840–1850. doi: 10.1002/cncr.22965. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y, Platt JF, Francis IR, et al. The prediction of radiation-induced liver dysfunction using a local dose and regional venous perfusion model. Med Phys. 2007;34:604–612. doi: 10.1118/1.2431081. [DOI] [PubMed] [Google Scholar]
- 15.Munden RF, Erasmus JJ, Smythe WR, et al. Radiation injury to the liver after intensity-modulated radiation therapy in patients with mesothelioma: an unusual CT appearance. AJR Am J Roentgenol. 2005;184:1091–1095. doi: 10.2214/ajr.184.4.01841091. [DOI] [PubMed] [Google Scholar]
- 16.Hahn CA, Zhou SM, Dunn RH, et al. Dose-Dependent Effects of Radiation Therapy on Cerebral Blood Flow, Metabolism and Neurocognitive Dysfunction. int J Radiat Biol Phys. 2005;62(2):S1–S67. doi: 10.1016/j.ijrobp.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 17.Rutkowski T, Tarnawski R, Sokol M, et al. 1H-MR spectroscopy of normal brain tissue before and after postoperative radiotherapy because of primary brain tumors. Int J Radiat Oncol Biol Phys. 2003;56:1381–1389. doi: 10.1016/s0360-3016(03)00327-4. [DOI] [PubMed] [Google Scholar]
- 18.Usenius T, Usenius JP, Tenhunen M, et al. Radiation-induced changes in human brain metabolites as studied by 1H nuclear magnetic resonance spectroscopy in vivo. Int J Radiat Oncol Biol Phys. 1995;33:719–724. doi: 10.1016/0360-3016(95)02011-Y. [DOI] [PubMed] [Google Scholar]
- 19.Waldrop SM, Davis PC, Padgett CA, et al. Treatment of brain tumors in children is associated with abnormal MR spectroscopic ratios in brain tissue remote from the tumor site. AJNR Am J Neuroradiol. 1998;19:963–970. [PMC free article] [PubMed] [Google Scholar]
- 20.Buus S, Grau C, Munk OL, et al. 11C-methionine PET, a novel method for measuring regional salivary gland function after radiotherapy of head and neck cancer. Radiother Oncol. 2004;73:289–296. doi: 10.1016/j.radonc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Murata Y, Ishida R, et al. Functional evaluation with intravoxel incoherent motion echo-planar MRI in irradiated salivary glands: a correlative study with salivary gland scintigraphy. J Magn Reson Imaging. 2001;14:223–229. doi: 10.1002/jmri.1177. [DOI] [PubMed] [Google Scholar]
- 22.Brush J, Lipnick SL, Phillips T, et al. Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol. 2007;17:121–130. doi: 10.1016/j.semradonc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Atwood T, Payne VS, Zhao W, et al. Quantitative magnetic resonance spectroscopy reveals a potential relationship between radiation-induced changes in rat brain metabolites and cognitive impairment. Radiat Res. 2007;168:574–581. doi: 10.1667/RR0735.1. [DOI] [PubMed] [Google Scholar]
- 24.Atwood T, Robbins ME, Zhu JM. Quantitative in vivo proton MR spectroscopic evaluation of the irradiated rat brain. J Magn Reson Imaging. 2007;26:1590–1595. doi: 10.1002/jmri.21095. [DOI] [PubMed] [Google Scholar]
- 25.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 26.Muryama S, Akamine T, Sakai S, et al. Risk Factor of Radiation Pneumonitis: Assessment with Velocity-Encoded Cine Magnetic Resonance Imaging of Pulmonary Artery. Journal of Computer Assisted Tomography. 2004;28:204–208. doi: 10.1097/00004728-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Guerrero T, Johnson V, Hart J, et al. Radiation pneumonitis: local dose versus [18F]-fluorodeoxyglucose uptake response in irradiated lung. Int J Radiat Oncol Biol Phys. 2007;68:1030–1035. doi: 10.1016/j.ijrobp.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Hart JP, McCurdy MR, Ezhil M, et al. Radiation pneumonitis: correlation of toxicity with pulmonary metabolic radiation response. Int J Radiat Oncol Biol Phys. 2008;71:967–971. doi: 10.1016/j.ijrobp.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassaballa HA, Cohen ES, Khan AJ, et al. Positron emission tomography demonstrates radiation-induced changes to nonirradiated lungs in lung cancer patients treated with radiation and chemotherapy. Chest. 2005;128:1448–1452. doi: 10.1378/chest.128.3.1448. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Pan C, Balter JM, et al. Liver function after irradiation based on computed tomographic portal vein perfusion imaging. Int J Radiat Oncol Biol Phys. 2008;70:154–160. doi: 10.1016/j.ijrobp.2007.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G, Dilling TJ, Stevens CW, et al. Functional lung imaging in thoracic cancer radiotherapy. Cancer Control. 2008;15:112–119. doi: 10.1177/107327480801500203. [DOI] [PubMed] [Google Scholar]
- 32.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, et al. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol. 2007;17:89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]