Abstract
Dendritic cells (DCs) are professional antigen presenting cells that can control immune responses against self and altered self, typically foreign, determinants. DCs can be divided into several subsets including CD8α+ and CD8α− DCs. These subsets possess specific functions. For example, mouse splenic CD8α+, but not CD8α− DCs, selectively take up dying cells and cross-present cell-associated antigens to naïve T cells. In this study, we identified genes that were more expressed in CD8α+ than CD8α− DCs by microarray analysis. Only one of these genes, when the extracellular domains were linked to human IgG Fc domain, could bind to late apoptotic or necrotic cells. This gene was a new member of the Triggering receptor expressed on myeloid cells (Trem) family, Trem-like 4 (Treml4). Treml4 mRNA and protein, the latter detected with a new monoclonal antibody, were predominantly expressed in spleen. Treml4, like other Trem family members, could associate with the adaptor molecule DAP12, but neither DAP10 nor FcRγ. Consistent with the microarray data, we confirmed that Treml4 protein was more expressed on CD8α+ than CD8 α− DCs, and we also found that Treml4 was expressed at high levels on splenic macrophages in spleen particularly red pulp and marginal metallophilic macrophages. In addition, Treml4 expression on DCs was not changed after maturation induced by Toll-like receptor ligands. Thus, Treml4 is a new Trem family molecule that is abundantly expressed on CD8α+ DCs and subsets of splenic resident macrophages, and can recognize dead cells by different types of phagocytes in spleen.
Keywords: Dendritic cells, Monocytes/Macrophages, Cell surface molecules
Introduction
Dendritic cells (DCs)3 consist of several subsets that are classified according to their expression of select cell surface molecules and distinct functions. In mouse spleen, for example, there are CD8α+ DEC-205+ CD11c+ and CD8α− DCIR2+ CD11c+ classical DCs, and B220+ CD11cdull plasmacytoid DCs. CD8α+ spleen DCs are the predominant producers of IL-12 upon stimulation with Toxoplasma gondii (1, 2), and selectively engulf different types of dying cells, including allogeneic cells killed by NK cells, irradiated tumor cells, and virus-infected cells (3, 4). Furthermore, CD8α+ DCs are superior cells for the cross-presentation of antigens on MHC class I molecules (5–7). Molecular mechanisms for these distinct functions are starting to be identified, e.g., CD8α+ DCs express higher levels of mRNA transcripts and proteins involved in the MHC class I processing pathway (7).
Apoptotic cells are generated continually in mammals and other species, and cell death is increased further during embryo development, tissue remodeling and inflammation. Potentially harmful cells that are eliminated by apoptosis include self reactive T and B cells, tumor cells, and cells infected with viruses and some bacteria. Engulfment of apoptotic cells in some instances suppresses production of inflammatory cytokines from activated macrophages (8). When dying cells are engulfed by DCs in the steady state in the absence of infection or immunological adjuvants, antigen-specific tolerance to antigens in the dying cells can be induced (3). On the other hand, if mice are given dying cells with adjuvants, antigen-specific T cell responses develop (3, 9). Analyses of genetically modified mice have shown that increased accumulation of apoptotic cells induces the development of autoimmune diseases (10–12). Thus, accumulating evidence indicates that the uptake of apoptotic cells is not only a clearance mechanism but is also capable of suppressing inflammation and modulating immune responses from antigen-specific tolerance to resistance (13).
In vitro studies indicate that many receptors can be involved in the uptake of dying cells by macrophages including scavenger receptors, the phosphatidyl serine receptor, integrins, CD14, C1q, C1qR, and CD36 (14–21). Recently, the T-cell immunoglobulin and mucin domain containing molecules, Tim-1 and Tim-4 were shown to recognize phosphatidyl serine and mediate apoptotic cell uptake (22–24). Recent in vivo studies have shown that the Mer tyrosine kinases (Mertk) and milk fat globule epidermal growth factor 8 (MFG-E8) contribute to the engulfment of apoptotic cells by macrophages (11, 12). Sen et al. also reported that Mertk mediates suppression of NF-κB activation in DCs that take up dying cells (25). Mertk also suppresses the maturation and cytokine production by DCs taking up apoptotic cells, and mediates induction of immunological tolerance (25, 26). CD36 and DEC-205 (CD205) are two receptors that are expressed more abundantly on CD8α+ than CD8α− DC subset in mouse spleen, but each of these molecules is dispensable for uptake of dying cells by CD8α+ DC in vivo (3, 27, 28).
To identify gene(s) involved in dying cell uptake and signaling by DCs, we compared gene expression by DNA microarrays from mouse splenic CD8α+ and CD8α− DCs, where the former are specialized to clear apoptotic cells. Several transmembrane genes were more expressed by CD8α+ DCs, but the soluble form of only one of them, the triggering receptor expressed on myeloid cells-like 4 (Treml4), exhibited binding to dead cells. We will describe these findings as well as the capacity of Treml4 to associate with an adaptor molecule DNAX activation protein of 12kDa (DAP12) and to serve as a marker for select groups of both DCs and macrophages. Surprisingly, macrophages in spleen but not several other organs expressed Treml4. Thus, we have identified a new type of molecule with the potential to recognize dead cells and signal different types of splenic phagocytes.
Materials and Methods
Mice
C57BL/6 and BALB/c mice were purchased from Charles River Laboratories, Taconic Firms, or Clea Japan. All mice were maintained under specific pathogen-free conditions and used at 6–12 wk of age under institutional guidelines of the Rockefeller University and Tokyo Medical and Dental University.
Cell lines
B16.Flt3L cells, which produce mouse Fms-like tyrosine kinase 3 ligand (Flt3L) were established via retroviral gene transfer to B16 melanoma cell line by Dranoff et al. (29) and kindly provided by Dr. Laura Santambrogio (Albert Einstein College of Medicine). A hybridoma clone, 2.4G2, was from American Type Culture Collection. Human embryonic kidney (HEK) 293A and 293T cells were cultured in DMEM supplemented with 10% fetal bovine serum and 10 mM HEPES.
Antibodies and reagents
Purified anti-CD16/32 (clone 2.4G2) or Fluorescein isothiocyanate (FITC), Phycoerythrin (PE), Allophycocyanin (APC), or biotin conjugated anti-CD3ε (145-2C11), anti-B220 (RA3-6B2), anti-CD11c (HL3), anti-CD8α (53-6.7) were from BD Bioscience. Anti-CD11c beads (N418) and anti-PE beads were from MiltenyiBiotec. FITC conjugated anti-human IgG (H+L) was from Jackson ImmunoResearch. Purified anti-Treml4 mAb was biotinylated with EZ-link NHS-Biotin reagent (Pierce) according to manufacturer’s instructions. Lipopolysaccharide (LPS, O55:B5) was from Sigma-Aldrich. Polyinosine-polycytidylic acid (poly(I:C)) were from GE Healthcare or Invivogen. Phosphorothioate-stabilized CpG deoxyoligonucleotides (CpG DNA, 5’-TCCATGACGTTCCTGATGCT-3’) was purchased from Genelink or Operon.
Cell sorting
B16.Flt3L cells were cultured with DMEM containing 10% FBS and 5×106 cells were injected into the backs of mice. After 10–12 days, when the secreted Flt3L had greatly expanded the numbers of all major DC subsets (30), the spleens were collected and cut into small fragments. Single cell suspensions were prepared by treating with collagenase D (Roche Applied Science). After adding anti-CD16/32 mAb or 2.4G2 hybridoma supernatant to block non-specific binding of antibodies, the cells were stained with FITC-conjugated anti-CD3ε and anti-B220, and PE-conjugated anti-CD11c, and APC-conjugated anti-CD8α. After washing, CD11c+ DCs were enriched by positive selection using anti-PE magnetic beads and MACS columns (MiltenyiBiotec). CD8α+CD11c+ or CD8α−CD11c+ cells were then sorted from the electronically CD3ε- and B220-negative fractions by FACSvantage (BD Bioscience). The purity of each fraction was more than 98%.
Microarray analysis
Total RNA was isolated from the sorted cells by Trizol (Invitrogen) and subjected to microarray analysis using the mouse genome 430 2.0 array (Affymetrix). The data were analyzed by GeneSpring software (Agilent Technologies). Microarray data were deposited in NCBI’s Gene Expression Omunibus (GEO) with the accession number GSE13250.
RT-PCR and real-time PCR
Total RNA was isolated from sorted cells or tissues by Trizol and used to synthesize cDNA with Superscript III (Invitrogen). Semi-quantitative PCR were performed using primer pairs indicated in Supplemental Table I4. Real-time quantitative PCR was performed with the iCycler iQ real-time PCR detection system (Bio-Rad Laboratories).
Cloning of Treml4
Mouse Treml4 cDNA was amplified by PCR, cloned into pCR2.1 vector (Invitrogen), and sequenced. The sequence of cloned Treml4 ORF was identical to a clone registered on GenBank (accession number, BC117091). PCR primers were sense, 5’-GACTGGTATGGCCTGGAGGTACTC-3’ and anti-sense, 5’-GCCTGTTCTGCCTTAGTACCAGTT-3’.
Production of soluble proteins
To produce soluble forms of several transmembrane proteins, cDNA fragments encoding the extracellular region of each gene were amplified by PCR and cloned into an expression vector containing the exons for hinge, CH2, and CH3 region of human IgG1 (31). The fusion proteins were produced by transient transfection of HEK293T cells using calcium phosphate. Cells were cultured in serum free DMEM supplemented with 1% Nutridoma SP (Roche Applied Science) for 4–5 days. The fusion proteins were purified from culture supernatants using protein G sepharose beads (GE Healthcare Bioscience) after ammonium sulfate precipitation.
Preparation of dying or dead cells
Splenocytes or thymocytes were prepared from C57BL/6 mice, irradiated with γ-ray (1500 rads), and cultured for 5 hrs to induce apoptosis. To prepare necrotic cells, splenocytes or thymocytes were incubated at 56°C for 30 min or 100°C for 10 min. These dying or necrotic cells were incubated with Propidium Iodide (PI, BD Bioscience) or 7-Amino-actinomycin D (7-AAD, BD Bioscience) together with Annexin V (BD Bioscience) according to manufacturer’s instructions.
Flow cytometry
Dying or dead cells were incubated with anti-CD16/32 antibody to block non-specific Fc binding, and then incubated with soluble fusion proteins. After washing, binding to dying or necrotic cells were detected by incubating with FITC-conjugated anti-human IgG antibody (Jackson ImmunoResearch) and analyzed on a FACSCalibur (BD Bioscience).
To check for expression of Treml4 on splenic CD11c+ DCs, whole splenocytes, or lymph node cells, each population was first incubated with biotinylated anti-Treml4 and the indicated fluorescent antibodies, exposed APC- or PE-conjugated streptavidin (BD Bioscience), and analyzed on a FACSCalibur or Guava EazyCyte Mini (Guava Technologies). Data were analyzed by FlowJo software (Tree Star).
Co-immunoprecipitation assay and western blotting
To construct the expression vectors for FLAG-tagged Treml4 and myc-tagged DAP10, DAP12, and FcRγ, each cDNA was amplified by PCR, sequenced, and inserted into pcDNA3.1 vector. HEK293A cells were transiently transfected with the indicated combination of expression vectors using Lipofectamin2000 (Invitrogen). Whole cell lysates were prepared 36 hrs after transfection with lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1% Triton-X 100, and 10% Glycerol, and precipitated with anti-FLAG M2 antibody (Sigma-Aldrich) and protein G sepharose beads (GE Healthcare Bioscience). The immunoprecipitates were separated on a 5–20% polyacrylamide gradient gel (Bio-Rad Laboratories), and transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with HRP-conjugated anti-myc mAb (9E10, Roche). Peroxidase activity was detected with the ECL plus system (GE Healthcare Bioscience).
For western blotting of tissue lysates, spleen, liver, lung, bone marrow, peritoneal lavage and peripheral lymph nodes (popliteal, cervical, inguinal, axillary and brachial) were collected. Organs were meshed with two frozen slides. The cells were collected and solublized in RIPA buffer (25 mM Tris pH7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 5% protease inhibitor cocktail, Sigma-Aldrich) for 15 min at 4°C. Cell lysates were sonicated to homogenize and then quantified by DC protein assay (BioRad). Equal amounts of protein were separated by 4–12% poly-acrylamide NuPage Bis-Tris gels (Invitrogen), and transferred to PDVF membranes (Invitrogen). Membranes were incubated with the primary antibody, washed, and incubated with peroxidase-conjugated species-specific anti-body (Supplemental Table IIa). After washing, membranes were developed with ECL Plus System. To control for protein loading, membranes were incubated in Restore Western Blot Stripping Buffer (Thermo Scientific), washed, and immunoblotted using an anti-Actin mAb (Sigma-Aldrich). Developed films were analyzed by densitometry using computerized image analysis software (MCID-M5, Imaging Research), and the data was normalized as follows: protein band (density × area) / actin (density × area). The Student t test was applied to reveal significant differences in protein expression between BALB/c and C57BL/6 mice.
Production of anti-Treml4 monoclonal antibody
A chimeric protein consisting of Treml4 extracellular portion and human IgG1 Fc portion was used to immunize Wistar Rats 4 times. One month after fourth immunization, purified FLAG-tagged extracellular domain of Treml4 was injected for a final boost. 3 days later, splenocytes were fused with myeloma cell line P3×63.Ag14. Production of monoclonal antibody against Treml4 was screened by ELISA assay using Treml4-FLAG protein. Two hybridoma clones, named 16E5 and 32D11, were established but they produced the identical antibody (IgG1, κ light chain).
Immunofluorescence staining
Spleens were harvested and frozen in Tissue-Tek OCT compounds (Sakura Finetek). Frozen tissue was sectioned at 10 µm in thickness on a microtome (Microm Laborgeräte), fixed 15 min with cold acetone, rehydrated in PBS and blocked with Avidin/Biotin Blocking reagents (Zymed) according to the manufacturer’s instructions. Sections were first blocked with 5% mouse serum in FACS buffer (2% FCS in PBS) for 60 min at room temperature, and then stained in humidified chamber overnight at 4°C with primary antibodies (Supplementary Table IIb). After washing in FACS buffer, sections were stained with anti-FITC Alexa 488 and Streptavidin Alexa 555 (Molecular Probes, Invitrogen) diluted in FACS buffer/2% mouse serum. SIGNR1 staining was performed using anti-Hamster FITC (Jackson ImmunoResearch) following by anti-FITC Alexa 488. Sections were mounted in Aqua-Poly Mount (Polysciences) and were stored at 4°C until microscopic examination. The images were acquired with a Zeiss LSM 510 system (Carl Zeiss MicroImaging) at the Rockefeller University Bio-Imaging Resource Center.
Results
Microarray analysis of mouse splenic DC subsets
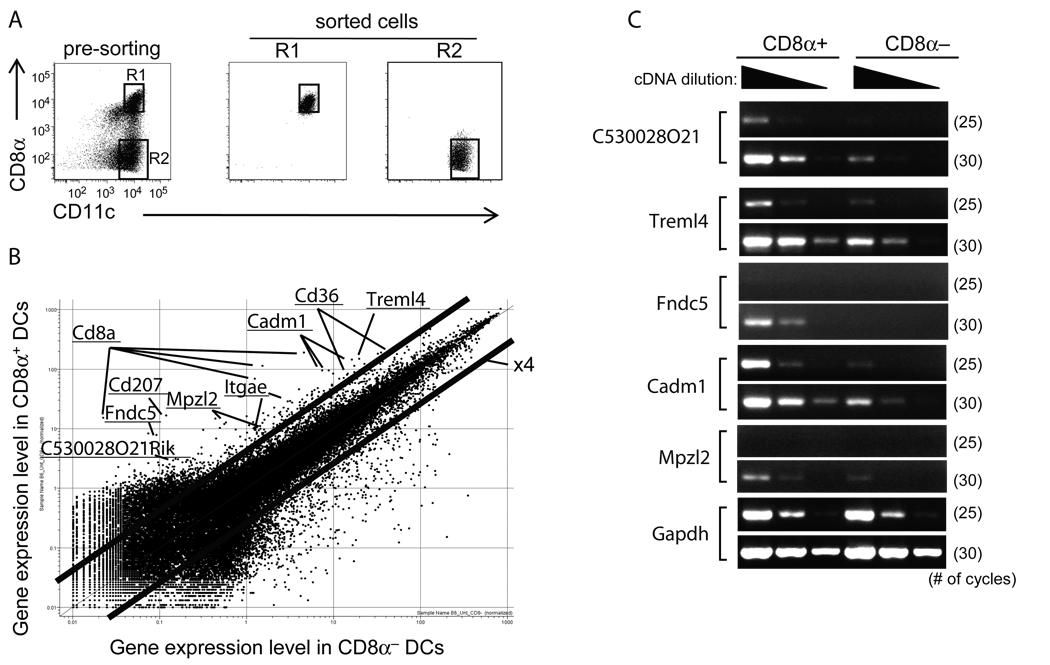
To identify gene(s) involved in the uptake and signaling of dying cells by DCs, we conducted a DNA microarray analysis using sorted mouse splenic CD8α+ and CD8α− DCs. Because the number of splenic DCs is quite low, we initiated s.c. tumors with B16.Flt3L cells that had been retrovirally transduced to express mouse Flt3L (29) to expand DCs. These in vivo expanded DCs showed similar phenotypes to that observed in non-tumor carrying animals, and functioned similarly in terms of cytokine production in response to bacterial products, stimulation of T cells in the mixed leukocyte reaction, and uptake of dying cells by CD8α+ DCs (data not shown). 10–12 days after injection of Flt3L tumor cells, splenic CD11c+ DCs were enriched by MACS and further purified by FACS cell sorter into CD8α+ and CD8α− subsets. Their purities were more than 90% (Fig. 1A). On microarray, several genes for predicted transmembrane proteins were differentially expressed by the CD8α+ subset that takes up dying cells (Fig. 1B). We selected genes showing greater than 4 fold mRNA abundance in CD8α+ cells for further investigation, e.g., CD36 and CD207, which were previously reported to be more abundantly expressed in CD8α+ DCs (3, 27, 28, 32). We confirmed the gene expression results either by FACS analysis (CD36, CD103, and CD207; data not shown) or semi-quantitative RT-PCR (C530028O21Rik, Fndc5, Cadm1, Mpzl2, and Treml4; Fig. 1C). Thus several transmembrane proteins were available as potential receptors on CD8α+ DCs for dying cells.
Figure 1. Microarray analysis of two Flt3L-expanded DC subsets from mouse spleen.
A, In vivo expanded mouse splenic DCs (methods) were sorted from CD3ε− B220− CD11c+ fractions (left panel). The purities of CD8α+ and CD8α− DCs were 94 and 91%, respectively (middle and right panel). B, DNA microarray analysis was performed using RNAs from CD8α+ and CD8α− DCs. Names of representative genes are indicated. Cadm1, cell adhesion molecule1; Itgae, integrin αE; Mpzl2, myelin protein zero-like 2; Fndc5, fibronectin type III domain containing 5; Treml4, triggering receptor expressed on myeloid cells-like 4. C, A semi-quantitative RT-RCR was carried out in two DC subsets. The numbers in parenthesis indicate the number of PCR cycles.
Binding of Fc fusion proteins to apoptotic or necrotic cells
To determine if the identified CD8α+ DC proteins could recognize apoptotic or necrotic cells, we generated soluble chimeric fusion forms of C530028O21Rik, Fndc5, Cadm1, Mpzl2, and Treml4 proteins. These fusion proteins consisted of their extracellular domain fused to the human IgG1 Fc domain and were produced mainly as dimers (data not shown). Apoptosis of splenocytes was induced by γ-ray irradiation, and then these dying cells were incubated with the individual chimeric proteins. As shown in Fig. 2A, we could not detect any binding of fusion proteins to living (PI− Annexin V−) or early apoptotic (PI− Annexin V+) cells. Interestingly, Treml4 bound to late apoptotic (PI+ Annexin V+) splenocytes. Also Treml4 bound to various other sources of dying cells, including thymocytes and the 293, EL4, and CHO tumor cell lines (Fig. 2B and data not shown). Because late apoptotic cells, also called secondary necrotic cells, show similar membrane integrity to necrotic cells, we next investigated whether these fusion proteins bound to necrotic cells. Thymocytes were incubated at 56°C for 30 min or 100°C for 10 min, and then stained with these fusion proteins. Only Treml4, but not the other fusion proteins, bound to necrotic cells that were positive for PI staining (Fig. 2C and data not shown). Thus, a chimeric Treml4 fusion protein binds to PI positive, late apoptotic and necrotic cells, but not living or early apoptotic cells.
Figure 2. Binding of chimeric fusion proteins to apoptotic or necrotic cells.
Splenocytes (A) or thymocytes (B) were exposed to γ-irradiation to induce apoptosis, and then stained with the indicated chimeric fusion proteins. The binding of fusion proteins were evaluated by FACS. Apoptosis were monitored by staining with propidium iodine (PI) and annexin V. C, Thymocytes were incubated at 100°C for 10 min to induce necrosis, stained with fusion proteins, and then analyzed by FACS. Representative data from at least two independent experiments were shown.
Treml4 associates with DAP12
Mouse Treml4 showed similar structural features to other members of the Trem family (reviewed in 33), with a single V-type immunoglobulin like motif in the extracellular portion and a short cytoplasmic tail (Fig. 3A). Treml4 mRNA was predominantly expressed in spleen (Fig. 3B). Trem1, 2, 3, and PDC-Trem are known to associate with and transduce signals through an adaptor molecule DAP12 (34–37). This association is mediated by a cationic lysine residue in the transmembrane domain, which was also found in the transmembrane region of Treml4. To determine if Treml4 associated with DAP12 or other adaptor molecules such as DAP10 and FcRγ, HEK293A cells were transiently transfected with an expression vector for FLAG-tagged Treml4 together with expression vectors for myc-tagged DAP12, DAP10 or FcRγ, and then their association was evaluated by co-immunoprecipitation assay. As shown in Fig. 3C, we found that Treml4 associated with DAP12, but not DAP10 nor FcRγ.
Figure 3. Association of Treml4 with DAP12.
A, Amino acid sequence alignment of human and mouse Treml4. Human (GenBank accession number, NM198153) and mouse Treml4 share an overall amino-acid identity of 39.9%. The predicted signal peptide (mouse, residues 1–30; human, 1–23) and transmembrane segments (TM; mouse, 200–333) are indicated. B, Real-time RCR analysis of Treml4 mRNA. Values are relative to expression of the gene encoding GAPDH. BMCs, bone marrow cells; LNs, lymph nodes. C, HEK293 cells were transiently transfected with the indicated combination of Treml4-FLAG, myc-FcRγ, myc-DAP10, and myc-DAP12 (above lane). After 36 hours, cell lysates were immunopreciptated (IP) with anti-FLAG antibody, followed by blotting (IB) with anti-myc antibody. WCL, whole cell lysate; n.s., non-specific bands. D, Thymocytes were exposed to γ-irradiation to induce apoptosis and then stained with the indicated chimeric fusion proteins. E, Thymocytes were incubated at 56°C for 30 min and then stained with fusion proteins and analyzed by FACS. Representative data from at least two independent experiments were shown.
Although it is well known that Trem members can modulate cellular responses to bacterial products (reviewed in 33), the identity of natural ligands for them are largely unknown. It has been reported that Trem2 recognizes anionic ligands expressed on bacteria (38), and more recently Takahashi et al. reported that Trem2 is involved in the clearance of apoptotic neurons by microglia (39). Therefore, we purified soluble forms (Fc fusion proteins) of Trem1, 2 and 3, to investigate whether they could bind to apoptotic or necrotic cells. We found that Trem2, like Treml4, bound to late apoptotic and necrotic cells, whereas Trem1 and Trem3 did not exhibit detectable binding. Thus, Trem2- and Treml4-Fc fusion proteins can bind to dead cells (Fig. 3D and E).
Treml4 is expressed on a subset of DCs: FACS analyses
To further pursue the expression and biology of Treml4, we generated an anti-Treml4 monoclonal antibody (Fig. 4A). We first investigated the types of leucocytes that express Treml4 by FACS analysis. CD3ε+ T cells or B220+ B cells in spleen or lymph nodes did not express Treml4 (Fig. 4B). For splenic DCs, CD11c+ cells were enriched with anti-CD11c beads. Treml4 was more abundantly expressed on CD8α+ DCs than CD8α− DCs, which was consistent with results from the microarray analyses and RT-PCR of splenic DCs (Fig. 4B). Another DC subset, plasmacytoid DCs which were B220+ CD11c+ cells, showed at best low expression of Treml4 (Fig. 4B). A similar expression pattern was observed with DCs from lymph nodes (Fig. 4B). Thus, Treml4 is expressed on subsets of DCs, especially on CD8α+ DCs, but not on lymphocytes.
Figure 4. Distribution of Treml4 by FACS.
A, Parental CHO cells or Treml4 stable transfectants were stained with anti-Treml4 monoclonal antibody. B, Splenocytes or lymph node cells were prepared with collagenase digestion, and the cells were stained with control antibody (open histogram) and anti-Treml4 antibody (gray histogram). The expression of Treml4 was monitored by FACS on B220+ B and CD3ε+ T cells, CD8α+ and CD8α− DCs (CD8α+ CD11c+ and CD8α−CD11c+, respectively) and B220+ plasmacytoid DCs (B220+ CD11c+).
Treml4 is abundant on two groups of macrophages in tissue sections of spleen
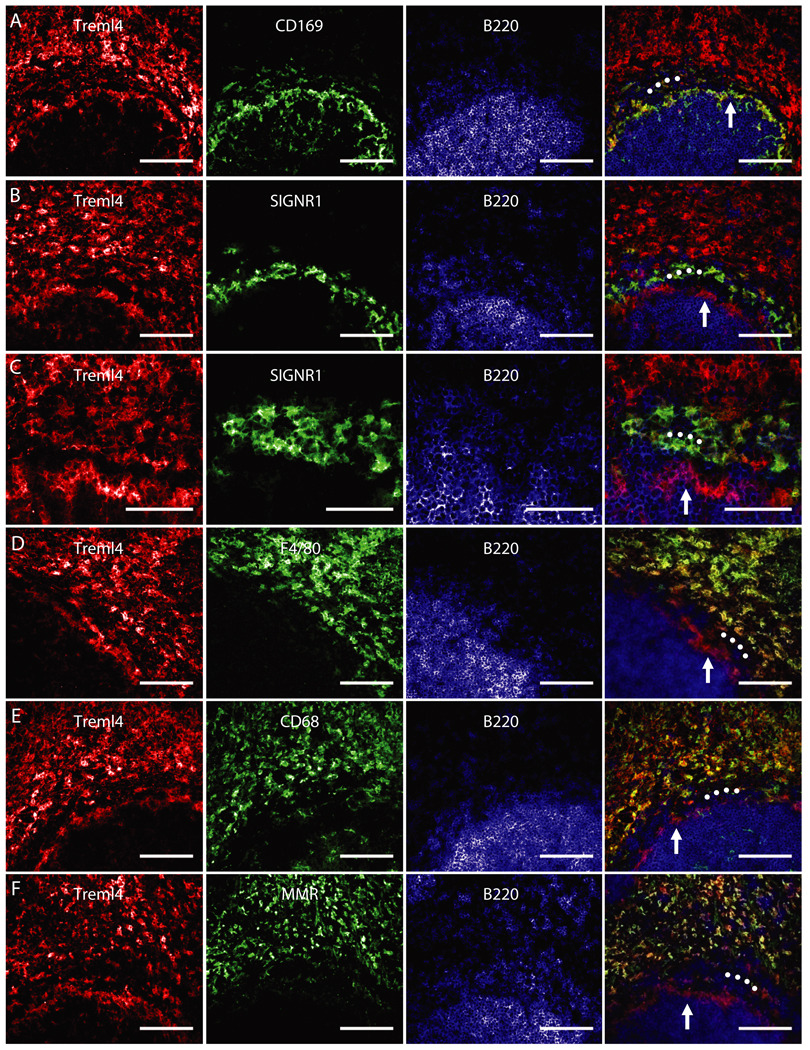
To investigate in more detail the distribution of Treml4, we stained sections from spleen and lymph node with mAbs to Treml4 and various macrophage markers. It is known that there are several distinct types and locations of macrophages in spleen. Red pulp macrophages express F4/80, CD68, and the macrophage mannose receptor/CD206, marginal zone macrophages are positive for SIGN-R1/CD209b+, while marginal metallophilic macrophages express sialoadhesin/CD169 (reviewed in 40). Double staining for Treml4 mAb and antibodies to either F4/80, SIGN-R1, or CD169 showed that Treml4 was expressed on F4/80+ red pulp and CD169+ marginal metallophilic macrophages, but only very weakly on SIGN-R1+ marginal zone macrophages (Fig 5). In lymph node, however, we observed little if any staining of macrophages for Treml4, indicating again that Treml4 is restricted to selected populations of macrophages (data not shown). These data indicate that Treml4 is primarily expressed in spleen, consistent with the mRNA analyses (Fig. 3B), and in spleen, Treml4 is predominantly expressed on macrophages but also some DCs.
Figure 5.
Views of the border of red pulp (top) and white pulp (bottom) of BALB/c mouse spleen. The B cell follicle of the white pulp was marked in blue by the anti-B220 mAb, while the macrophages of the red pulp and marginal zones were stained in green for the indicated molecules. The signal for Treml4 was stained in red. Between the B cell follicle and red pulp are concentric layers of two types of macrophages: the marginal metallophilic macrophages (white vertical arrows), stained in green for CD169 (A), and the SIGNR1+ marginal zone macrophages (B and C for higher magnification; white circles), which have weak staining for Treml4. D, F4/80 antigen (green) is found on red pulp macrophages but not marginal metallophilic (white vertical arrow) and marginal zone macrophages (white circles). E, CD68 is abundant on red pulp macrophages (green) but not on Treml4+ (red) marginal metallophilic macrophages (white vertical arrows) or marginal zone macrophages (white circles). F, As in E, but MMR (green) is abundant on red pulp macrophages but not in marginal metallophilic (white arrows) or marginal zone macrophages (white circles). Scale = 100 µm; but C, where scale = 50 µm. MMR, Macrophage mannose receptor.
Treml4 is expressed primarily in spleen: western blotting
We further investigated the tissue distribution of Treml4 by western blotting. We developed a protocol (see Methods) to homogenize various tissues for quantitative blotting studies. Endogenous Treml4 protein was readily detected in a spleen lysate (Fig. 6A). However, the expression of Treml4 in lymph nodes was less than that in spleen, because larger samples and longer exposure times for development were required to obtain even a weak western blotting signal, whereas other macrophage antigens like CD68 and F4/80 were readily detected in lymph node (Fig. 6B). Among several tissues, Treml4 protein was clearly detected only in spleen, even though other macrophage markers were observed in all tissues investigated, suggesting that Treml4 protein, as consistent with RT-PCR analysis, is predominantly expressed in spleen (Fig. 6C). Moreover, although CD68 expression was not different between two strains of mice, BALB/c and C57BL/6, Treml4 was more abundantly expressed in the spleen from BALB/c than C57BL/6 mice (Fig. 6D). Thus Treml4 is a distinctive marker in that it is peculiarly abundant in spleen rather than other organs.
Figure 6. Tissue distribution of Treml4 by western blotting.
A, Representative data of the immunoblot signals for Treml4 and CD68 in BALB/c spleen lysates from two mice (#1 and #2). B, Expression of Treml4, F4/80 and CD68 was assayed in lymph nodes of BALB/c (B) or C57BL/6 (C) mice using different amounts of protein (10 or 20 µg) and different film exposure times (1, 3 or 10 min) to illustrate that signals were acquired under limiting conditions for quantification. C, The expression of the indicated molecules was monitored by western blotting. The indicated organs from BALB/c (B) or C57BL/6 (C) mice were lysed and 10 µg protein (except for CD68 and F4/80 markers in peritoneal lavage, 2 or 1 µg protein were loaded, respectively) were separated by SDS-PAGE. LNs, lymph nodes; BM, bone marrow; P. lavage, peritoneal lavage. D, Treml4 and CD68 were assayed by Western Blot (upper panel) and densitometry (lower panel) from three BALB/c (B; #1, 2 and 3) and three C57BL/6 (C; #1, 2 and 3) mice. Asterisk represents statistically difference between both mice strains: **, P<0.01.
Treml4 expression is not altered after DC maturation
Bacterial products are reported to upregulate expression of TREM1 on human monocytes and neutrophils (34, 41), while maturation stimuli downregulate expression of human TREM2 on immature DCs or mouse Trem2 on bone marrow-derived macrophages (42). Recently, a new Trem family member, termed PDC-Trem, was found to be expressed on activated plasmacytoid DCs, but not immature plasmacytoid DCs (37). Thus, the expression of Trem family members can be differentially regulated. We therefore investigated Treml4 expression after DC activation. MACS enriched splenic CD11c+ DCs were stimulated with Toll-like receptor (TLR) ligands such as poly (I:C) (TLR3), LPS (TLR4), or CpG DNA (TLR9) to activate DCs, and the expression of Treml4 on DCs was monitored 24 hrs after stimulation. The expression of Treml4 was clearly detected on freshly isolated DCs. Although the relative frequency of CD8α+ DCs was slightly reduced when the cells were cultured without any stimulus (medium in Fig. 7), the expression of Treml4 on CD8α+ DCs was retained. Treml4 on CD8α+ DCs was neither down- nor up-regulated, even though DCs were activated by some TLR ligands as indicated by upregulation of CD40 (Fig. 7). Thus, Treml4 expression was regulated distinctly from other Trem family members.
Figure 7. Regulation of Treml4 expression on DCs.
MACS enriched CD11c+ splenic DCs were left unstimulated (med) or stimulated with poly(I:C) (10 µg/ml), LPS (1 µg/ml) or CpG DNA (1 µM). 24hrs later, the expression of Treml4 and CD40 was monitored by FACS. CD40 was used as a marker for DC activation by the TLR ligands.
Discussion
We have identified a new member of the Trem family in mice and found that it has a distinctive tissue distribution being primarily expressed on subsets of splenic macrophages (red pulp and marginal metallophilic macrophages) and DCs (CD8α+ DCs). We also found that the soluble form of Treml4 has binding affinity to necrotic cells.
DNA microarray analysis revealed that many genes were differentially expressed between splenic CD8α+ and CD8α− DCs, where the former DCs selectively take up dying cells in vivo (3). Consistent with previous reports, our microarray data indicated that CD36, CD205 (DEC-205), CD207 (Langerin), and CD103 (αE integrin) mRNA were each more abundantly expressed in CD8α+ DCs. However, it had been reported that CD36- or DEC205-deficient DCs could take up dying cells in vivo (3, 27, 28). Since CD207 is weakly expressed on the surface of splenic DCs from C57BL/6 mice (43), CD207 was not likely to be required for dying cell uptake. Thus, none of these molecules expressed by CD8α+ DCs seemed essential for their uptake of dying cells.
We further considered the C530028O21Rik, Fndc5, Cadm1, Mpzl2, and Treml4 genes that were more abundantly expressed in CD8α+ than CD8α− DCs. Functional analyses of Cadm1 and Mpzl2 knockout mice had not assessed uptake of dying cells (44–47). When we prepared Fc fusion proteins of each of these 5 genes, to see if they could bind to dying cells, only Treml4 showed binding to necrotic PI+ Annexin V+ cells, but not early apoptotic cells in our study.
The predicted amino acid sequence of Treml4 showed characteristic features of the Trem family, including the presence of a V-type immunoglobulin-like motif in the extracellular portion and a short cytoplasmic tail (reviewed in 33). Many Trem members including Trem1, 2, 3, and PDC-Trem have a cationic lysine residue in their transmembrane domain, which is essential for association with ITAM-bearing adaptor molecule, DAP12. This lysine residue was also observed in the transmembrane domain of Treml4, and consistent with this, we observed a Treml4/DAP12 association by immunoprecipitation assay.
To begin to study the biological features of Treml4, we found a unique expression pattern. Real-time PCR and western blotting analysis indicated that Treml4 was predominantly expressed in the spleen, and its expression was more abundant in BALB/c than C57BL/6 spleen. Immunofluorescent staining of tissue sections and FACS analysis of cell suspensions from spleen revealed that Treml4 was expressed on DCs as well as spleen resident macrophages, especially in red pulp and marginal metallophilic macrophages. Our quantitative western blotting data indicated that the expression of Treml4 was primarily confined to macrophages in spleen, which is an unusual feature relative to many other known macrophage products that we studied such as F4/80, CD68, CD169, and CD206.
Based on genomic sequencing data, the human TREML4 sequence seems to be truncated around the predicted transmembrane domain of its mouse counterpart (Fig. 3A, and reviewed in 33), suggesting that human TREML4 may be produced only as soluble form and/or is not functional. Mouse Trem2 is reported to be involved in the clearance of apoptotic neurons by microglia (39). Human TREM2 is expressed on macrophages and monocyte-derived DCs, but mouse Trem2 is not expressed on splenic DCs (data not shown). In our experiments, Trem2-Fc fusion protein could bind to AnnexinV+ PI+ late apoptotic or necrotic cells just like Treml4-Fc. At present, the ligand for Treml4 is not yet identified and we do not know whether Trem2- and Treml4-Fc fusion proteins bind to the same molecules. A report suggests that ligands for Trem2 are found on the surface of microbes and they are anionic molecules (38). However Treml4-Fc fusion protein did not bind to Escherichia coli or Staphylococcus aureus (supplemental Fig.). However, Trem2- and Treml4-Fc fusion protein bound to necrotic cells which were incubated at 100°C, indicating that the ligand for Trem2 and/or Treml4 in necrotic cells are thermostable substances. Therefore, we cannot rule out a possibility that Trem2 and Treml4 may compensate functions for each other in mice.
It has been reported that signals from Trem members affect the activation status of DCs and macrophages (33). Although both Trem1 and Trem2 associate with DAP12, they can have contrasting effects on immune cells. For example, while activation of Trem1 showed a synergistic effect with TLR ligands (41), Trem2-deficient macrophages show enhanced cytokine production in response to TLR ligands (42). Furthermore, DAP12-deficient cells showed enhanced or reduced cytokine production in response to TLR ligands depending on the cell type or experimental conditions (48, 49). It remains unclear whether activation of Treml4 can affect cellular activation of DCs and reciprocally, whether TLR ligands intersect with Treml4 function on these cells.
Further studies are required to clarify whether Treml4 is involved in the uptake of dying or necrotic cells and mediates immune recognition in vivo. It has been reported that some C-type lectins including Lox-1, Mannose-binding lectin, and Mincle can recognize dead cells or molecules leaked from them (50–52). Trem2, which showed affinity to dead cells in our experiments (Fig. 3E), is expressed on DCs and macrophages such as GM-CSF-induced bone marrow derived DCs and bone marrow derived or thioglycollate-elicited peritoneal macrophages in mice (42, 53). Therefore, it is difficult to discriminate a role of Treml4 engagement from the activation of other C-type lectins by dead cells in wild-type DCs and macrophages. Treml4-deficient mice and cells will help to clarify these points.
Supplementary Material
Acknowledgements
The authors thank to Dr. Takashi Suda (Kanazawa University, Kanazawa, Japan) for kindly providing the expression vector for human IgG1 Fc fusion protein, and Dr. Laura Santambrogio (Albert Einstein College of Medicine) for B16.Flt3L cells, Klara Velinzon for expert sorting, Alison North and the Bio-Imaging Resource Center of Rockefeller University, Margarita Oks for technical help, Judy Adams for help with figures. We also thank Drs. Kayo Inaba (Kyoto University, Kyoto, Japan), Yoshiko Iwai (Tokyo Medical and Dental University, Tokyo, Japan), Olga Mizenina, Sayuri Yamazaki, Anna Charalambous, and Godwin Nchinda for valuable discussions. We gratefully acknowledge Jay Overholser and Dr. Frances Weis-Garcia (Sloan-Kettering Cancer Center) for their help with hybridoma production, and Dr. Bruz Marzolf (Institute for Systems Biology, Seattle, WA) for DNA microarray analysis.
Footnotes
Disclosure
The authors have no financial conflict of interest.
This work was supported by NIH grant AI13013 to R.M.S. and in part by the Program for Improvement of Research Environment for Young Researchers from Special Coordination Funds for Promoting Science and Technology (SCF) commissioned by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and Grant-in-Aid for Young Scientists (19890063 and 20790299, to H.H.) from MEXT of Japan. H.H. was a JSPS Postdoctoral Fellow for Research Abroad.
Abbreviation used in this paper
DCs, dendritic cells; Trem, Triggering Receptor Expressed on Myeloid cells; Treml4, Trem-like 4; DAP12, DNAX activation protein 12kDa; Flt3L, fms-like tyrosine kinase 3 ligand, BM, bone marrow, LNs, lymph nodes.
The online version of this article contains supplemental material.
Reference
- 1.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 2.Maldonado-López R, Maliszewski C, Urbain J, Moser M. Cytokines regulate the capacity of CD8α+ and CD8α− dendritic cells to prime Th1/Th2 cells in vivo. J Immunol. 2001;167:4345–4350. doi: 10.4049/jimmunol.167.8.4345. [DOI] [PubMed] [Google Scholar]
- 3.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman R, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz O, Diebold S, Chen M, Näslund T, Nolte M, Alexopoulou L, Azuma Y, Flavell R, Liljeström P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 5.den Haan J, Lehar S, Bevan M. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan R, Smith C, Belz G, van Lint A, Wakim L, Heath W, Carbone F. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 7.Dudziak D, Kamphorst A, Heidkamp G, Buchholz V, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee H, Park C, Steinman R, Nussenzweig M. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 8.Huynh M, Fadok V, Henson P. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii S, Liu K, Smith C, Bonito A, Steinman R. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botto M, Dell'Agnola C, Bygrave A, Thompson E, Cook H, Petry F, Loos M, Pandolfi P, Walport M. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 11.Scott R, McMahon E, Pop S, Reap E, Caricchio R, Cohen P, Earp H, Matsushima G. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 12.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 13.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 14.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci U S A. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadok V, Bratton D, Rose D, Pearson A, Ezekewitz R, Henson P. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 16.Mevorach D, Mascarenhas J, Gershov D, Elkon K. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 18.Devitt A, Moffatt O, Raykundalia C, Capra J, Simmons D, Gregory C. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 19.Ogden C, deCathelineau A, Hoffmann P, Bratton D, Ghebrehiwet B, Fadok V, Henson P. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor P, Carugati A, Fadok V, Cook H, Andrews M, Carroll M, Savill J, Henson P, Botto M, Walport M. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadok V, Warner M, Bratton D, Henson P. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (αv β3) J Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- 22.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi N, Karisola P, Peña-Cruz V, Dorfman D, Jinushi M, Umetsu S, Butte M, Nagumo H, Chernova I, Zhu B, Sharpe A, Ito S, Dranoff G, Kaplan G, Casasnovas J, Umetsu D, Dekruyff R, Freeman G. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santiago C, Ballesteros A, Martínez-Muñoz L, Mellado M, Kaplan G, Freeman G, Casasnovas J. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen P, Wallet M, Yi Z, Huang Y, Henderson M, Mathews C, Earp H, Matsushima G, Baldwin AJ, Tisch R. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-κB activation in dendritic cells. Blood. 2007;109:653–660. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallet M, Sen P, Flores R, Wang Y, Yi Z, Huang Y, Mathews C, Earp H, Matsushima G, Wang B, Tisch R. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205:219–232. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belz G, Vremec D, Febbraio M, Corcoran L, Shortman K, Carbone F, Heath W. CD36 is differentially expressed by CD8+ splenic dendritic cells but is not required for cross-presentation in vivo. J Immunol. 2002;168:6066–6070. doi: 10.4049/jimmunol.168.12.6066. [DOI] [PubMed] [Google Scholar]
- 28.Schulz O, Pennington D, Hodivala-Dilke K, Febbraio M, Reis e Sousa C. CD36 or αvβ3 and αvβ5 integrins are not essential for MHC class I cross-presentation of cell-associated antigen by CD8 α+ murine dendritic cells. J Immunol. 2002;168:6057–6065. doi: 10.4049/jimmunol.168.12.6057. [DOI] [PubMed] [Google Scholar]
- 29.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- 31.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahara K, Omatsu Y, Yashima Y, Maeda Y, Tanaka S, Iyoda T, Clausen B, Matsubara K, Letterio J, Steinman R, Matsuda Y, Inaba K, Clusen B. Identification and expression of mouse Langerin (CD207) in dendritic cells. Int Immunol. 2002;14:433–444. doi: 10.1093/intimm/14.5.433. [DOI] [PubMed] [Google Scholar]
- 33.Klesney-Tait J, Turnbull I, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 34.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 35.Bouchon A, Hernández-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung D, Seaman W, Daws M. Characterization of TREM-3, an activating receptor on mouse macrophages: definition of a family of single Ig domain receptors on mouse chromosome 17. Eur J Immunol. 2002;32:59–66. doi: 10.1002/1521-4141(200201)32:1<59::AID-IMMU59>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 37.Watarai H, Sekine E, Inoue S, Nakagawa R, Kaisho T, Taniguchi M. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Natl Acad Sci U S A. 2008;105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daws M, Sullam P, Niemi E, Chen T, Tchao N, Seaman W. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171:594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K, Rochford C, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 41.Bouchon A, Facchetti F, Weigand M, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 42.Turnbull I, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 43.Cheong C, Idoyaga J, Do Y, Pack M, Park S, Lee H, Kang Y, Choi J, Kim J, Bonito A, Inaba K, Yamazaki S, Steinman R, Park C. Production of monoclonal antibodies that recognize the extracellular domain of mouse langerin/CD207. J Immunol Methods. 2007;324:48–62. doi: 10.1016/j.jim.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeMonte L, Porcellini S, Tafi E, Sheridan J, Gordon J, Depreter M, Blair N, Panigada M, Sanvito F, Merati B, Albientz A, Barthlott T, Ozmen L, Blackburn CC, Guttinger M. EVA regulates thymic stromal organisation and early thymocyte development. Biochem Biophys Res Commun. 2007;356:334–340. doi: 10.1016/j.bbrc.2007.02.131. [DOI] [PubMed] [Google Scholar]
- 45.van der Weyden L, Arends MJ, Chausiaux OE, Ellis PJ, Lange UC, Surani MA, Affara N, Murakami Y, Adams DJ, Bradley A. Loss of TSLC1 causes male infertility due to a defect at the spermatid stage of spermatogenesis. Mol Cell Biol. 2006;26:3595–3609. doi: 10.1128/MCB.26.9.3595-3609.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita E, Kouroku Y, Ozeki S, Tanabe Y, Toyama Y, Maekawa M, Kojima N, Senoo H, Toshimori K, Momoi T. Oligo-astheno-teratozoospermia in mice lacking RA175/TSLC1/SynCAM/IGSF4A, a cell adhesion molecule in the immunoglobulin superfamily. Mol Cell Biol. 2006;26:718–726. doi: 10.1128/MCB.26.2.718-726.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada D, Yoshida M, Williams YN, Fukami T, Kikuchi S, Masuda M, Maruyama T, Ohta T, Nakae D, Maekawa A, Kitamura T, Murakami Y. Disruption of spermatogenic cell adhesion and male infertility in mice lacking TSLC1/IGSF4, an immunoglobulin superfamily cell adhesion molecule. Mol Cell Biol. 2006;26:3610–3624. doi: 10.1128/MCB.26.9.3610-3624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turnbull IR, McDunn JE, Takai T, Townsend RR, Cobb JP, Colonna M. DAP12 (KARAP) amplifies inflammation and increases mortality from endotoxemia and septic peritonitis. J Exp Med. 2005;202:363–369. doi: 10.1084/jem.20050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 51.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 52.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 53.Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.