Abstract
The aim of this study was to investigate the occurrence of tissue hypoxia and apoptosis at different stages of tendinopathy and tears of the rotator cuff.
We studied tissue from 24 patients with eight graded stages of either impingement (mild, moderate and severe) or tears of the rotator cuff (partial, small, medium, large and massive) and three controls. Biopsies were analysed using three immunohistochemical techniques, namely antibodies against HIF-1α (a transcription factor produced in a hypoxic environment), BNip3 (a HIF-1α regulated pro-apoptotic protein) and TUNEL (detecting DNA fragmentation in apoptosis).
The HIF-1α expression was greatest in mild impingement and in partial, small, medium and large tears. BNip3 expression increased significantly in partial, small, medium and large tears but was reduced in massive tears. Apoptosis was increased in small, medium, large and massive tears but not in partial tears.
These findings reveal evidence of hypoxic damage throughout the spectrum of pathology of the rotator cuff which may contribute to loss of cells by apoptosis. This provides a novel insight into the causes of degeneration of the rotator cuff and highlights possible options for treatment.
Disease of the rotator cuff presents in a variety of ways. Despite a proposed continuum from chronic bursitis, through partial, complete to massive tears,1 very little research has been done to confirm this. However, if this continuum exists then the progression through the stages is not uniform.
The aetiology of disease of the rotator cuff is multifactorial. Both intrinsic failure of the tendon and extrinsic mechanical compression by the coracoacromial arch play an important role.2-4 However, their relative contribution and the initiating factors are not clear. Excessive apoptosis, programmed cell death, has been associated with tendinopathy5 and is present in degenerative tendons and has been observed in rotator cuffs with impingement6 and full thickness tears.7 Apoptosis plays a critical role in the homeostasis of normal tissue and it is important that organisms have a pathway to eliminate damaged or superfluous cells. Excessive apoptosis has been found in many diseases including osteoarthritis,8 rheumatoid arthritis,9,10 neoplasia11 and neurodegeneration.12 Apoptosis is a highly controlled form of cell suicide which is precipitated by intrinsic and extrinsic mechanisms. The former or mitochondrial pathway requires the BcL-2 family of pro- and anti-apoptotic proteins and the latter requires the binding of external death activators to specific receptors on the cell surface which transmit the apoptotic signal to the cytoplasm. BNip3 (Bcl-2 Nineteen kilodalton interacting protein) is a pro-apoptotic member of the Bcl-2 family in which it is unique as it is induced by hypoxic conditions as well as inflammation.13,14 Most cells are highly sensitive to oxygen levels and undergo apoptosis following periods of severe hypoxia, although tenocytes have not been studied well to date. BNip3 has been shown to play a role in hypoxia-induced death in many cell types, including synovial fibroblasts,15 myocytes16 and epithelial cells13 but not yet in human tenocytes.
Mechanical overload of the rotator cuff has been proposed as a primary cause of tendinopathy. Apoptosis has been induced following high strain mechanical loading of the tibialis anterior tendon of the rat17 and apoptotic genes have been upregulated by running overuse in the supraspinatus tendon of this animal.18 In cultured fibroblasts undergoing cyclical strain, overuse has also been shown to strongly induce Hypoxia Inducible Factor 1α (HIF-1α), a transcription factor which plays an important role in the intracellular hypoxic response.19
No author to our knowledge has examined how apoptosis and hypoxia may contribute to the cascade of pathological failure across the spectrum of disease of the rotator cuff.
We have examined the rotator cuff at different stages of failure, based on the continuum hypothesis and the appearance of the cuff. We investigated the incidence of hypoxia and apoptosis in the cuff with the hypothesis that the degree of hypoxia and consequent apoptosis worsens as the macroscopic appearance of the cuff deteriorates.
Patients and Methods
With approval of the local ethical committee and informed written consent, 27 patients had samples taken from the rotator cuff (Table I). They were placed according to their macroscopic appearance into nine groups, each of three patients. The groups were mild, moderate and severe impingement, partial articular tear, small, medium, large and massive full thickness tear and control. The impingement groups were classified according to the appearance of the bursa and tendon beneath the anterior acromion as mild (injected and oedematous), moderate (fibrillated with minor scuffing) or severe (major scuffing and fibrillation). The size of the tear was based on the classification of Post, Silver and Singh,20 measuring its longest diameter. Small tears are < 1 cm, medium < 2 cm, large < 5 cm and massive > 5 cm. All specimens were taken from fresh supraspinatus tendon except the control group which was from fresh tendon of subscapularis. The samples for the impingement and partial tear groups were taken using a punch biopsy during subacromial decompression from the bursal surface of the rotator cuff tendon in the impingement group and in the partial tears from the proximal edge of the tear. In the full thickness tear groups, the sample was taken during either arthroscopic subacromial decompression or open repair of the rotator cuff. Tissue was harvested from up to 1.5 cm from the proximal edge of the tear. The control sample was a full thickness biopsy of subscapularis obtained during open operations for stabilisation. The tissue was placed immediately into 10% buffered formalin and set in paraffin. Sections were cut at 5 μm using a Leica-LM microtome (Leica Microsystems, Wetzler, Germany), then placed onto Snowcoat X-tra glass slides (Surgipath, Peterborough, United Kingdom). These were deparaffinised in Xylene, rehydrated through graded alcohol and subjected to microwave-based antigen-retrieval as detailed below.
Table I.
This shows the distribution of demographic and pre-operative clinical features of the three patients in each macroscopic group
| Impingement |
Tear size |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Partial articular tear | Small | Medium | Large | Massive | Control | |
| Mean age in yrs (range) | 42.0 (39 to 45) |
48.0 (45 to 52) |
48.0 41 to 53) |
52.7 (40 to 67) | 61.0 (52 to 69) |
54.3 (47 to 61) |
56.7 (49 to 65) |
61.0 (52 to 75) |
19.7 (17 to 23) |
| Gender | |||||||||
| M:F | 1:2 | 2:1 | 2:1 | 3 M | 2:1 | 2:1 | 2:1 | 2:1 | 3 M |
| Mean length of symptoms in mths (range) |
22 (6 to 36) | 30 (12 to 48) | 14 (6 to 22) | 12 (9 to 17) | 22 (15 to 36) | 69 (11 to 174) | 12 (6 to 24) | 41 (2 to 84) | |
| Mean no. of steroid injections (range) |
1.7 (1 to 3) | 3 (2 to 4) | 1.6 (1 to 3) | 1.3 (1 to 2) | 2.7 (1 to 5) | 2.3 (1 to 3) | 2 (1 to 4) | 2.6 (2 to 4) | |
| Last steroid injection (mths) | 5 (2 to 6) | 6 (4 to 9) | 4 (2 to 7) | 4 (2 to 5) | 7 (5 to 8) | 14 (6 to 24) | 12 (5 to 18) | 9 (2 to 24) | |
BNip3
Immunohistochemical staining was carried out using the Vectastain Universal Elite ABC system (Vector Laboratories, Burlingame, California), which labels the primary antibody with a biotinylated secondary antibody and then with a preformed Avidin and Biotinylated horseradish peroxidase macromolecule. This can be visualised by using 3, 3′-diaminobenzidine (DAB) as a peroxidase substrate.
The prepared slides were initially deparaffinised in xylene and rehydrated in graded alcohol (100%, 90%, 80% and then 70%). The sections were then placed in a solution of 3% H2O2 and 100% alcohol for 20 minutes in order to quench endogenous peroxidase activity. Antigen retrieval was performed using a microwave at 800 W for 10 minutes with 400 ml of Dako retrieval solution. The slides were then placed in a rack in a plastic container and allowed to cool in water for 10 minutes. The following steps required 100 μL of solution to be used per slide which was washed in phosphate buffered saline (PBS) between stages. Horse serum (1:100 in PBS) was applied for 20 minutes to block the non-specific staining of antibodies. Primary monoclonal anti-BNip3 (Sigma, Poole, United Kingdom; 1:100 in PBS) was applied overnight at 4°C. A secondary biotinylated antibody, Streptavidin-horse-peroxidase was allowed to stand for 30 minutes, then applied for 20 minutes at room temperature. The slide was then coloured with DAB (metal enhanced DAB, Roche, Penzberg, Germany) for eight minutes (1:10 with peroxidase buffer). The cells were counterstained in the dark at room temperature with DAPI (4 6-Diamidino-2-phenylindole dihydrochloride) for 20 minutes. They were then washed in distilled water and a coverslip applied with a Flurosave reagent. They were stored in a closed box to prevent exposure to light. In order to control for specificity, the BNip3 antibody was used to detect the protein by Western blot in cultured tenocytes subjected to a timed course of total hypoxia (0.1% O2). Specific 26 kD bands were detected after eight and 16 hours. Tendon sections were also stained using secondary antibody only and with the appropriate isotype control. Positive cells were counted in 10 high powered fields in which the total cell number was counted using the DAPI stain under ultraviolet light. The BNip3 scoring was done by RTB.
HIF-1 α
Immunohistochemical staining was carried out using the DAKO Envision system to visualise the antigen-antibody complex. Slides were prepared according to the BNip3 protocol and antigen retrieval carried out using EDTA (pH 8) in a microwave. The slides were allowed to cool. The following steps required 100 μL of solution to be used per slide which was washed in PBS between stages. Horse serum (1:100 in PBS) was applied for 20 minutes to block the non-specific staining of antibodies. Sections were stained at room temperature using a 1:100 dilution in PBS of a mouse monoclonal antibody directed against HIF-1α (Clone 54, BD Biosciences, Oxford, United Kingdom). Staining was visualised using the Envision Peroxidase/DAB/Mouse detection kit. Positive cells were counted in 10 high powered fields. HIF-1α staining was scored according to the following scale: 0 = no HIF-1α positive tenocytes; 1 = 1% to 10%; 2 = 11% to 25%; 3 = 26% to 50%; 4 = > 50%. The scoring was done by HJK and confirmed by PAH.
Apoptosis was assessed by the terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick end labelling method (TUNEL) using the in situ all-death detection kit (Roche). This technique detects DNA cleavage within the nuclei which results from the apoptotic cascade. These cleavages are identified by the enzyme terminal deoxynucleotidyl transferase (TdT) and it catalyses the addition of deoxyuridine triphosphate (dUTP). The dUTPs are then labelled secondarily with horse radish peroxidase.
The sections were deparaffinised in xylene and rehydrated by being passed through graded alcohol (100% to 70%). The tissue was incubated for 10 minutes in 3% H2O2 to inactivate endogenous peroxidase activity. The tissue was permeabilised with Triton X 0.5% for six minutes. Fragmented DNA was then end-labelled with dUTP using TdT at 37°C for 60 minutes. The signal was then detected using biotylated anti-FITC antibody and incubating the sample in Streptoavidin-HRP at 37°C for 30 minutes. The slide was placed in DAB at room temperature for 4.5 minutes to develop the colour. Cells were counterstained with DAPI, then washed in distilled water and a coverslide applied with Flurosave reagent. The TUNEL positive control was a section of colon provided by the supplier and negative control was exclusion of TdT enzyme. Positive cells were counted in10 high powered fields. In the same fields, the total cell number was counted using the DAPI stain under ultraviolet light. Apoptotic scoring was done by RTB.
Statistical analysis
This was carried out on all result variables using Graph Pad Prism software (Graph Pad Software, LaJolla, California). The association between the differing appearances of the rotator cuff and the controls was analysed using an unpaired t-test for BNip-3 and apoptosis. The association between age and length of symptoms was tested using the Pearson correlation coefficient. A pvalue ≤ 0.05 was considered significant.
Results
The distribution of demographic and pre-operative clinical features in each group is shown in Table I.
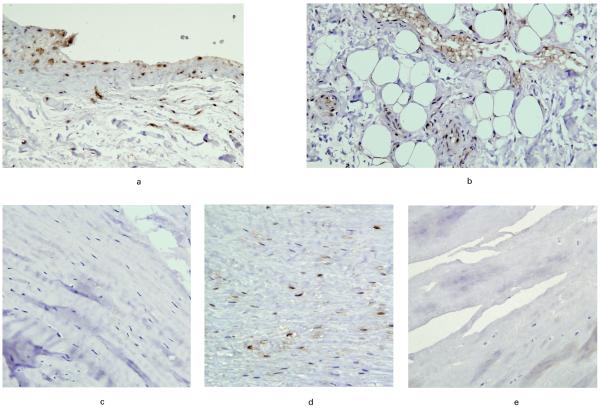
HIF-1α was present in all macroscopic groups, not only within the substance of the tendon but also in blood vessels and bursa where these were present in the sections. HIF-1α was absent in the controls. The average apoptotic scores were as follows: mild impingement, 2.7; moderate impingement, 0.7; severe impingement, 0.3; partial tear, 3.0; small tear, 1.7; medium tear, 2.5; large tear, 2.0; massive tear, 1.3 and control 0. These results demonstrate the highest expression for HIF-1α being in the mild impingement group and the partial, medium and large tear groups. The highest levels of HIF-1α staining were seen in the blood vessels and bursae. Examples of HIF-1α staining are shown in Figure 1.
Fig. 1.
Photomicrograph giving examples of HIF-1α staining in a) bursa, b) blood vessels, c) moderate impingement, d) medium tear and e) massive tear.
The expression of BNip3 in the different macroscopic groups is seen in Figure 2. The control group had the lowest proportion of BNip3 positive cells. Mild, moderate and severe impingement had similar proportions of BNip3 positive cells. There was a marked increase in such cells between severe impingement and partial tear. Partial, small, medium and large tears had a significantly higher proportion of BNip3 positive cells than controls and all the impingement groups (p < 0.001). Between large and massive tears there was a fall in the proportion of positive cells. Massive tears still had a significantly higher proportion of BNip3 positive cells than controls (p < 0.05).
Fig. 2.
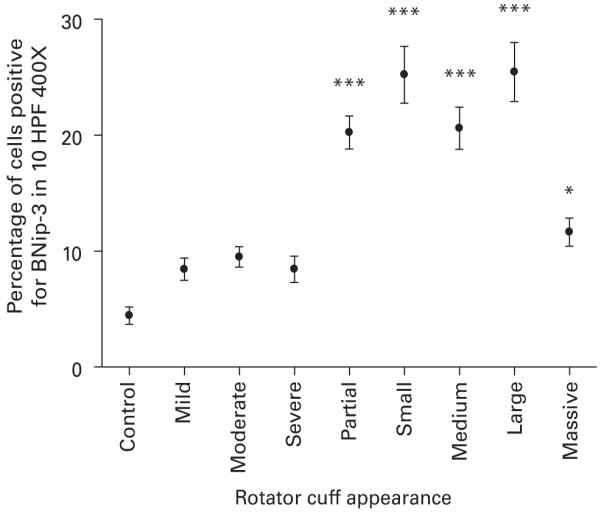
A graph showing the percentage of cells positive for BNip3 in each of the different macroscopic groups of the rotator cuff in 10 HPF 400X.*** significant difference to control of p < 0.001, * significant difference to control of p < 0.05.
There was a correlation between the proportion of BNip3 positive cells and the age of the patient (r = 0.63, p < 0.001), but not with their length of symptoms.
Apoptosis was present in all the specimens. The apoptotic index averaged between 6.8 and 21 throughout the spectrum of rotator cuff failure and the distribution of apoptotic indices is shown in Figure 3. Apoptosis was lowest in the control and mild impingement groups. Moderate and severe impingement and partial tears had similar indices. There was a marked increase in the apoptotic index between partial and small full thickness tears. Massive tears had the greatest index. Small, medium, large and massive tears had a significantly higher apoptotic index than controls (p < 0.001).
Fig. 3.
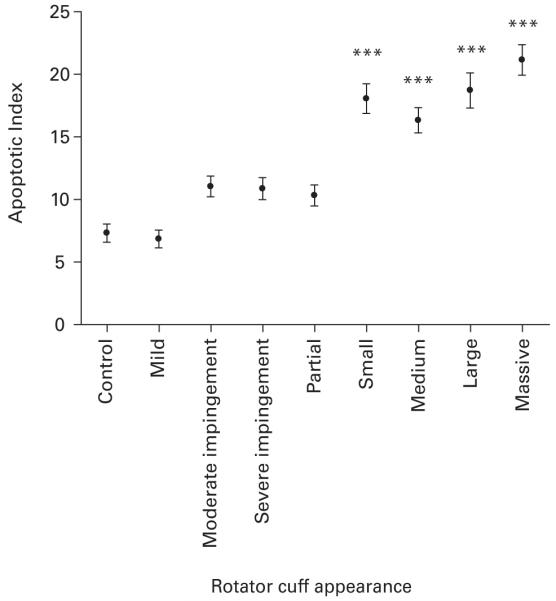
Graph showing the average apoptotic index in each of the different macroscopic groups of the rotator cuff. *** Significant difference to control of p < 0.001.
There was a correlation between the apoptotic index and the age of patients (r = 0.65; p < 0.001) but not with the duration of symptoms.
Discussion
Although apoptosis is a physiological process in normal healthy tissue, excessive apoptosis has been associated with tendinopathy.5,6,21 In our study, excessive apoptosis was found within full thickness tears of the rotator cuff, with almost a threefold increase compared with the control or mild groups. Yuan et al7 reported similar findings. In our study the control patients were younger (mean age 19.7 years (17 to 23)) than those with impingement and no full thickness tear (mean age 47.7 years (39 to 67)) or those with full thickness tear (mean age 58.3 years (47 to 75)). We found a correlation between the proportion of apoptotic cells and the age of the patients, whereas in Yuan et al’s7study, there was no correlation between the proportion of apoptotic cells and age, duration of symptoms or size of tear.
Excessive apoptosis has been postulated as a primary cause of tendinopathy and tearing within the supraspinatus tendon, rather than a secondary effect of degeneration.7 The reduced cell proliferation and increasing chondroid metaplasia as the rotator cuff tear deteriorates22 would support the view that the cellular characteristics of the tendon change with worsening pathology. For example, abnormal tenocytes are more prevalent than collagen disruption in early tendinosis in the patellar tendons of athletes.23
The apoptotic cells in the rotator cuff have been identified as fibroblast or fibroblast-like7 which have an important role in the maintenance of the tendon extracellular matrix.24 Reduction in their number and function would result in an impaired homeostasis of the collagen matrix and reduced healing response to microtrauma. Hypoxic environments in vitro have significantly reduced collagen synthesis.25
The regulation of apoptosis in tendinopathy is poorly understood. Several factors, including mechanical overuse, hypoxia and oxidative stress are thought to contribute to its pathogenesis. In a rat model overuse has been shown to lead to supraspinatus tendinopathy.26 Pro-apoptotic genes are induced in overuse models and have been found to be upregulated in the torn human supraspinatus compared with controls.18 Stress-activated protein kinase, an upstream regulator of apoptosis, has been found in human and canine patellar tendons undergoing cyclical strain.27,28 HIF-1α can be induced during cyclical strain of tenocytes and this can stimulate vascular endothelial growth factor, a potent angiogenic cytokine which has also been implicated in tendon degeneration.29
Degeneration of the tendon occurs mainly in areas of poor blood supply. In the rotator cuff, a critical zone of hypovascularity has been identified histologically30-32 and coincides with the most common site for tears of the rotator cuff. Conversely, Doppler flow examinations33 and histological biopsies34 at the site of tears of the rotator cuff support areas of hyper vascularisation. This difference in findings is likely to reflect neovascularisation within the tendons.29 Levy et al,35 using laser Doppler flowmetry, found areas of hypoperfusion within the rotator cuff tendon in patients with impingement. Hypoxia is a strong regulator of apoptosis and, although not exclusive to the hypoxic environment, expression of HIF-1α and BNip3 suggest it has a role in degeneration of the rotator cuff.
In our study there appeared to be an incremental increase in apoptosis with a worsening macroscopic appearance. There appeared to be two major steps between mild and moderate appearances in the cuff, and between partial and small full thickness tears. After complete rupture of the tendon, the increase in apoptosis could not be a result of excessive tensile loading and other mechanisms must play a role. For example, in vitro studies of stress deprivation in tendon have demonstrated elevated levels of apoptosis which were even greater than when the tendon was cylically loaded.36 Our group have previously reported a genetic component to tears of the rotator cuff which may be expressed through the levels of apoptosis within it.37
There was a large increase in the expression of BNip3 and HIF-1α once the supraspinatus tendon was torn. The rise in BNip3 expression between severe impingement and partial tear appears to precede the rise in apoptosis between partial and small tears which would be expected if BNip3 affects apoptosis. Its fall in massive tears may be a consequence of the loss of BNip3-positive cells due to apoptosis and the adaptation of surviving tenocytes into chondrocyte-like cells which cope better with reduced blood perfusion. There is no evidence to show that oxygen supply to the tissues improves in massive tears. Indeed, there is evidence that these have the poorest blood supply.22
In the mild impingement group, although HIF-1α was highly expressed, BNip3 expression was low. This may represent an early stage of HIF expression prior to an upregulation to BNip3, or an effect of other inhibitory factors in the local micro-environment. Both moderate and severe impingement groups showed less evidence of HIF-1α, which may indicate that the vascular remodelling induced earlier has restored oxygenation. This may arise when the tendon reacts to a hypoxic insult by producing HIF-1α, which in turn is a potent stimulator of angiogenesis. The vascular response would then improve blood flow to the tendon.38 There may also be other differences such as the severity of the bursitis and/or tissue swelling between these stages, which may affect perfusion and should be studied further.
The pattern of worsening apoptosis and hypoxia-induced apoptosis supports a continuum of failure of the rotator cuff. However, the mechanisms by which they are acting may be very different before and afterwards. By examining the rotator cuff at different stages of failure, we can try to identify features which may contribute to the aetiology of its disease, within which we are yet to understand the significance of each factor and the size of its effect on failure of the cuff. Our paper supports the presence of hypoxia and apoptosis in disease of the cuff but does not establish a relationship between cause and effect.
There are limitations to the study which must be highlighted. The controls were neither age nor tendon matched, which is a common problem with histological research in tendons. However, subscapularis has been used as a control for supraspinatus in other histological studies.22 Secondly, despite both being in the critical zone, the site of the biopsy was different between the intact and the torn supraspinatus. Thirdly, in the study there was a correlation between the age of the patient and the apoptotic index. Disease of the rotator cuff worsens with increasing age and the number of apoptotic cells will naturally increase. As our controls were not age matched it is impossible to say whether disease progression or age is the predominant cause for the rise in apoptosis.
The impact of excessive apoptosis on tendon function has not been studied. Although, we have shown that its presence increases with deteriorating macroscopic appearance of the cuff, its effect on the integrity and function of the tissues can only be assumed. Reduction in cell numbers would result in an impaired healing response following repair of the tendon. This may account for the high rates of re-rupture following repairs of the rotator cuff.
Apoptosis is a potentially reversible process. Pro- and anti-apoptotic proteins compete within the cell to determine their fate. If we could manipulate the production of these proteins we should theoretically be able to stimulate a greater healing response at the time of repair. This could also be achieved using stem cell therapy to replace lost tenocytes.
References
- 1.Neer CS., 2nd Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg [Am] 1972;54-A:41–50. [PubMed] [Google Scholar]
- 2.Budoff JE, Rodin D, Ochiai D, Nirschl RP. Arthroscopic rotator cuff debridement without decompression for the treatment of tendinosis. Arthroscopy. 2005;21:1081–9. doi: 10.1016/j.arthro.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Bunker T. Rotator cuff disease. Curr Orthop. 2002;16:223–33. [Google Scholar]
- 4.Rees JL. The pathogenesis and surgical treatment of tears of the rotator cuff. J Bone Joint Surg [Br] 2008;90-B:827–32. doi: 10.1302/0301-620X.90B7.19874. [DOI] [PubMed] [Google Scholar]
- 5.Lian Ø , Scott A, Engebretsen L, et al. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35:605–11. doi: 10.1177/0363546506295702. [DOI] [PubMed] [Google Scholar]
- 6.Tuoheti Y, Itoi E, Pradhan RL, et al. Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg. 2005;14:535–41. doi: 10.1016/j.jse.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Yuan J, Murrell GA, Wei AQ, Wang MX. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002;20:1372–9. doi: 10.1016/S0736-0266(02)00075-X. [DOI] [PubMed] [Google Scholar]
- 8.Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27:455–62. [PubMed] [Google Scholar]
- 9.Dubikov AI, Belogolovykh LA, Medved EE. Apoptosis as a mechanism of autoimmune inflammation in human knee joint. Bull Exp Biol Med. 2004;138:568–70. doi: 10.1007/s10517-005-0129-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim HA, Song YW. Apoptotic chondrocyte death in rheumatoid arthritis. Arthritis Rheum. 1999;42:1528–37. doi: 10.1002/1529-0131(199907)42:7<1528::AID-ANR28>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Schulze-Bergkamen H, Krammer PH. Apoptosis in cancer: implications for therapy. Semin Oncol. 2004;31:90–119. doi: 10.1053/j.seminoncol.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson MD. Anti-apoptosis therapy: a way of treating neural degeneration? Curr Biol. 1998;8:418–21. doi: 10.1016/s0960-9822(98)70267-2. [DOI] [PubMed] [Google Scholar]
- 13.Kothari S, Cizeau J, McMillan-Ward E, et al. BNIP3 plays a role in hypoxic cell death in human epithelial cells that is inhibited by growth factors EGF and IGF. Oncogene. 2003;22:4734–44. doi: 10.1038/sj.onc.1206666. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Ray R, Dubik D, et al. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J Exp Med. 1997;186:1975–83. doi: 10.1084/jem.186.12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kammouni W, Wong K, Ma G, et al. Regulation of apoptosis in fibroblast-like synoviocytes by the hypoxia-induced Bcl-2 family member Bcl-2/adenovirus E1B 19-kd protein-interacting protein 3. Arthritis Rheum. 2007;56:2854–63. doi: 10.1002/art.22853. [DOI] [PubMed] [Google Scholar]
- 16.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–31. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 17.Scott A, Khan KM, Heer J, et al. High strain mechanical loading rapidly induces tendon apoptosis: an ex vivo rat tibialis anterior model. Br J Sports Med. 2005;39:25. doi: 10.1136/bjsm.2004.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg [Br] 2009;91-B:417–24. doi: 10.1302/0301-620X.91B3.21652. [DOI] [PubMed] [Google Scholar]
- 19.Petersen W, Varoga D, Zantop T, et al. Cyclic strain influences the expression of the vascular endothelial growth factor (VEGF) and the hypoxia inducible factor 1 alpha (HIF-1 alpha) in tendon fibroblasts. J Orthop Res. 2004;22:847–53. doi: 10.1016/j.orthres.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Post M, Silver R, Singh M. Rotator cuff tear: diagnosis and treatment. Clin Orthop. 1983;173:78–91. [PubMed] [Google Scholar]
- 21.Yuan J, Wang MX, Murrell GA. Cell death and tendinopathy. Clin Sports Med. 2003;22:693–701. doi: 10.1016/s0278-5919(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 22.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon: reduction in potential for repair as tear size increases. J Bone Joint Surg [Br] 2006;88-B:489–95. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 23.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–8. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Blevins FT, Djurasovic M, Flatow EL, Vogel KG. Biology of the rotator cuff tendon. Orthop Clin North Am. 1997;28:1–16. doi: 10.1016/s0030-5898(05)70260-1. [DOI] [PubMed] [Google Scholar]
- 25.Rempel D, Abrahamsson SO. The effects of reduced oxygen tension on cell proliferation and matrix synthesis in synovium and tendon explants from the rabbit carpal tunnel: an experimental study in vitro. J Orthop Res. 2001;19:143–8. doi: 10.1016/S0736-0266(00)00005-X. [DOI] [PubMed] [Google Scholar]
- 26.Soslowsky LJ, Thomopoulos S, Tun S, et al. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 27.Arnoczky SP, Tian T, Lavagnino M, et al. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: the effects of strain frequency, strain magnitude, and cytosolic calcium. J Orthop Res. 2002;20:947–52. doi: 10.1016/S0736-0266(02)00038-4. [DOI] [PubMed] [Google Scholar]
- 28.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching of human patellar tendon fibroblasts: activation of JNK and modulation of apoptosis. Knee Surg Sports Traumatol Arthrosc. 2003;11:122–9. doi: 10.1007/s00167-002-0322-y. [DOI] [PubMed] [Google Scholar]
- 29.Pufe T, Petersen WJ, Mentlein R, Tillmann BN. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005;15:211–22. doi: 10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 30.Rothman R, Parke W. The vascular anatomy of the rotator cuff. Clin Orthop. 1965;41:176–86. [PubMed] [Google Scholar]
- 31.Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop. 1990;254:35–8. [PubMed] [Google Scholar]
- 32.Rathbun JB, Macnab I. The microvascular pattern of the rotator cuff. J Bone Joint Surg [Br] 1970;52-B:540–53. [PubMed] [Google Scholar]
- 33.Swiontkowski MF, Iannotti JP, Boulas HJ, Esrerhai JL. Intraoperative assessment of rotator cuff vascularity using laser Doppler flowmetry. In: Post M, Morrey BF, Hawkins RJ, editors. Surgery of the shoulder. Mosby-Year Book; St. Louis: 1990. pp. 208–12. [Google Scholar]
- 34.Goodmurphy CW, Osborn J, Akesson EJ, et al. An immunocytochimical analysis of torn rotator cuff tendon taken at the time of repair. J Shoulder Elbow Surg. 2003;12:368–74. doi: 10.1016/s1058-2746(03)00034-x. [DOI] [PubMed] [Google Scholar]
- 35.Levy O, Relwani J, Zaman T, et al. Measurement of blood flow in the rotator cuff using laser Doppler flowmetry. J Bone Joint Surg [Br] 2008;90-B:893–8. doi: 10.1302/0301-620X.90B7.19918. [DOI] [PubMed] [Google Scholar]
- 36.Egerbacher M, Arnoczky SP, Caballero O, Lavagnino M, Gardner KL. Loss of homeostatic tension induces apoptosis in tendon cells: an in vitro study. Clin Orthop. 2008;466:1562–8. doi: 10.1007/s11999-008-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvie P, Ostlere SJ, Teh J, et al. Genetic influences in the aetiology of tears of the rotator cuff: sibling risk of a full-thickness tear. J Bone Joint Surg [Br] 2004;86-B:696–700. doi: 10.1302/0301-620x.86b5.14747. [DOI] [PubMed] [Google Scholar]
- 38.Benson RT, Rees JL, Hulley PA, et al. Evidence for vascular remodelling and inflammatory cytokine response in rotator cuff failure; Procs British Shoulder and Elbow Society Meeting; 2008. [Google Scholar]