Abstract
Stathmin is an important microtubule (MT)-destabilizing protein, and its activity is differently attenuated by phosphorylation at one or more of its four phosphorylatable serine residues (Ser-16, Ser-25, Ser-38, and Ser-63). This phosphorylation of stathmin plays important roles in mitotic spindle formation. We observed increasing levels of phosphorylated stathmin in Epstein-Barr virus (EBV)-harboring lymphoblastoid cell lines (LCLs) and nasopharyngeal carcinoma (NPC) cell lines during the EBV lytic cycle. These suggest that EBV lytic products may be involved in the regulation of stathmin phosphorylation. BGLF4 is an EBV-encoded kinase and has similar kinase activity to cdc2, an important kinase that phosphorylates serine residues 25 and 38 of stathmin during mitosis. Using an siRNA approach, we demonstrated that BGLF4 contributes to the phosphorylation of stathmin in EBV-harboring NPC. Moreover, we confirmed that BGLF4 interacts with and phosphorylates stathmin using an in vitro kinase assay and an in vivo two-dimensional electrophoresis assay. Interestingly, unlike cdc2, BGLF4 was shown to phosphorylate non-proline directed serine residues of stathmin (Ser-16) and it mediated phosphorylation of stathmin predominantly at serines 16, 25, and 38, indicating that BGLF4 can down-regulate the activity of stathmin. Finally, we demonstrated that the pattern of MT organization was changed in BGLF4-expressing cells, possibly through phosphorylation of stathmin. In conclusion, we have shown that a viral Ser/Thr kinase can directly modulate the activity of stathmin and this contributes to alteration of cellular MT dynamics and then may modulate the associated cellular processes.
Keywords: Phosphorylation/Cytoskeletal Proteins, Phosphorylation/Kinases/Serine-Threonine, Microtubules, Tumor Viruses, Viral Protein, BGLF4, Epstein-Barr Virus, Microtubule Cytoskeleton, Stathmin
Introduction
The microtubule (MT)2 cytoskeleton is composed of tubulin heterodimers and is involved in a variety of cellular processes, such as maintaining cell polarity, supporting cell structures, segregation of chromosomes during mitosis, vesicular transportation, and cell motility. MTs undergo rapid transition between polymerized and depolymerized states and this is termed dynamic instability (1–3). Polymerization of MT is regulated by the MT-stabilizing proteins, a classic superfamily of microtubule-associated proteins (MAPs) (4, 5), and depolymerization of MT is regulated by two different families: the KinI family of kinesin-related-proteins (a family of MT motors) (6, 7) and the MT-destabilizing proteins (a family of oncoprotein 18/stathmin proteins) (4, 8). Stathmin is a ubiquitous cytosolic protein and is highly conserved in vertebrates (9, 10). It acts by promoting MT catastrophe (8) or by sequestering free tubulin heterodimers (11). Moreover, all above actions lead to depolymerization of MT. Phosphorylation of one to four of its N-terminal phosphorylatable residues has negative effects on stathmin (12–15) and contributes to correct assembly of the mitotic spindle and cell cycle progression during mitosis (13, 16–19).
It is known that some viruses rely on the host cell cytoskeleton for transportation to the site of replication or to the egress progeny virions to the extracellular environment, indicating that this trafficking is essential for their infection (20, 21). Accordingly, a variety of viruses have been found to use diverse approaches to regulate cellular MTs or actin cytoskeletons (21, 22). For example, adenovirus has been found to activate PKA and P38/MAPK pathways to boost MT-mediated targeting of the virus to the nucleus, and this can enhance virus infection (23). Additionally, vaccinia virus has been shown to induce the formation of actin tails and viral particles are propelled on the tips of the actin tails (24). Moreover, several viruses encode MAP-like proteins which possess MT-stabilizing activity (25, 26). Apparently, various viruses exploit different strategies to target and modulate the cellular MT network during infection. However, the approaches used by Epstein-Barr virus (EBV) to regulate the MT dynamics are obscure.
EBV, a human gammaherpesvirus, infects over 95% of the human population (27, 28). The infection is associated with many types of malignances (27, 28). Recently, increased levels of stathmin expression have been reported in EBV-infected primary B cells and in EBV transformed lymphoblastoid cell lines (LCLs) (29). Moreover, an EBV-encoded latent protein, LMP1, was shown to increase phosphorylation of stathmin in EBV-related nasopharyngeal carcinoma (NPC) (30, 31). However, the precise role of stathmin in EBV infection remains unclear. BGLF4 is the only kinase expressed by EBV during the lytic stage and it can phosphorylate a spectrum of viral and cellular factors (32–34). In this study, we investigated the expression and roles of stathmin in EBV-positive cell lines. We demonstrate that BGLF4, an EBV-encoded Ser/Thr kinase, can phosphorylate and then attenuate the activity of stathmin to alter MT dynamics.
EXPERIMENTAL PROCEDURES
Virus Harvest and Infection
EBV-positive B95.8 cells were cultured in complete RPMI 1640 medium and treated with 40 ng/ml tetradecanoyl phorbol acetate and 3 mm sodium butyrate for 72 h. Cell suspensions were centrifuged at 8,000 rpm for 30 min at 4 °C to remove the cell debris. The cell-free supernatant was ultracentrifuged at 15,000 rpm for 90 min at 4 °C. The pellet was resuspended in a volume of 1 ml of complete medium per 100 ml starting culture supernatant. The resuspended virus was then filtered through a 0.22-μm filter and stored at −80 °C until use.
Cell Culture and Transfection
Peripheral blood mononuclear cells (PBMC) were isolated from blood by Ficoll-Hypaque density gradient centrifugation (Amersham Biosciences). PBMC were then cultured at 2 × 106 cells per well in 6-well plates in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 1 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin and infected with EBV B95.8 virus. P1, P7, P9, P13, P14, and P15 cell lines were LCLs established by EBV infection of PBMCs.
The immortalized T lymphocyte cell line Jurkat was maintained in RPMI 1640 supplemented with 8% fetal calf serum. The HeLa cell line is derived from human cervical carcinoma, and cells are grown at 37 °C with 5% CO2 in Dulbecco's modified Eagle's medium (HyClone) supplemented with 8% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen).
Inducible human embryonic kidney 293 (HEK293) T-REx cells for BGLF4, BGLF4-KD, and vector expression were grown in Dulbecco's modified Eagle's medium with 8% tetracycline-free serum. These cells were derived by cloning the BGLF4 and BGLF4-KD open reading frames in pLenti4-CPO/V5/His (Invitrogen). The expression plasmids were transfected into HEK293 T-REx cells with Lipofectamine 2000 (Invitrogen) and selected in growth medium with 400 μg/ml zeocin and 5 μg/ml blasticidin, as reported previously (35). To induce protein expression, these cells were incubated with 10 ng/ml doxycycline (Invitrogen) for the times indicated in the figures. Expression lysates were collected in radioimmune precipitation assay buffer (RIPA, 50 mm Tris/HCl, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with a complete protease inhibitor mixture (Roche Applied Science) and then subjected to immunoblotting. NA cells are an EBV harboring NPC cell line infected with a recombinant Akata-EBV strain carrying a neomycin-resistance gene (36). NA cells were seeded at 1 × 105 cells per well in 12-well plates and then co-transfected with pSG5-Rta (37) and either si-BGLF4-1, si-BGLF4-2 or control siRNA fragments as described below. Cell lysates were harvested 36 h post-transfection. HeLa cells were seeded at a density of 3 × 105 cells per well in 6-well plates and transfected with 0.1, 0.3, 1, or 2.5 μg of pSG5-BGLF4 or PSG5-BGLF4-KD individually using Lipofectamine 2000. All cell lysates were harvested in RIPA buffer 24 h post-transfection.
Flag-tagged expression plasmids of different UL13 homologues, such as pTAG-UL13 of herpes simplex virus type-1 (HSV-1), pTAG-UL97 of human cytomegalovirus (HCMV), and pTAG-36 of murine herpesvirus 68 (MHV68) were generated independently by cloning BamHI-KpnI viral kinase gene fragments into pTAG-attR-C1 (Invitrogen) and were kindly provided by Ren Sun (UCLA) (38). Flag-tagged ORF36 of Kaposi's sarcoma-associated herpesvirus (KSHV) was generated by cloning the Flag-KSHV ORF36 gene fragment into the RsrII site of pLenti4-V5 (Invitrogen) (39). HeLa cells were seeded at a density of 3 × 105 cells per well in 6-well plates and transfected with 4 μg of either Flag-tagged plasmids of different UL13 homologues using Lipofectamine 2000 according to the manufacturer's instructions. All cell lysates were harvested in RIPA buffer 24 h post-transfection.
Plasmids and Interfering RNAs
pSG5-BGLF4 and pSG5-BGLF4-KD (K102I) are plasmids expressing wild-type BGLF4 and a BGLF4 kinase dead mutant, respectively (40). The full-length stathmin cDNA, stathmin-4A, and stathmin-4E mutants, which were kindly provided by Dr. Martin Gullberg (19), were cloned in the pSG5 vector, generating pSG5-stathmin-Flag, pSG5-stathmin-4E-Flag, and pSG5-stathmin-4A-Flag. The plasmids expressing pSG5-stathmin-SAAA-Flag, pSG5-stathmin-ASAA-Flag, pSG5-stathmin-AASA-Flag, and pSG5-stathmin-AAAS-Flag were generated using a single primer-based in vitro mutagenesis strategy (41) with primers primer-SASS gcttttgagctgattctcgcccctcggtcaaaagaa, primer-SSAS tccagaattcccccttgcccctccaaa gaagaa, primer-SAAA ctggagaagcgtgcctcaggccaggcttttg and primer AAAS gaagaaagacgcaagtcccatgaagctgagg. The plasmids generated are pSG5-stathmin-SAAA-Flag (Ser-25, -38, and -63 were replaced by alanine), pSG5-stathmin-ASAA-Flag (Ser-16, -38, and -63 were replaced by alanine), pSG5-stathmin-AASA-Flag (Ser-16, -25, and -63 were replaced by alanine) and pSG5-stathmin-AAAS-Flag (Ser-16, -25, and -38 were replaced by alanine). To purify GST-tagged stathmin and its mutants, the cDNAs from pSG5-stathmin-Flag and pSG5-stathmin mutants were further subcloned to the Escherichia coli expression plasmid, pGEX-4T-1, with an N-terminal GST tag. The plasmids generated are pGEX-4T1-stathmin-Flag, pGEX-4T1-stathmin-SAAA-Flag, pGEX-4T1-stathmin-ASAA-Flag, pGEX-4T1-stathmin-AASA-Flag, and pGEX-4T1-stathmin-AAAS-Flag.
The RNA fragments targeting the expression of BGLF4 were purchased from Invitrogen, including si-BGLF4-1 (5′-CCCUCUAUGUAAAGCUGCCGGAGAA), si-BGLF4-2 (5′-UGGGUAGGCUGGUCCUGACUGAUUA) and a control siRNA fragment (5′-CCCGUAUAAAUGUCGGGCCACUGAA) (42).
Co-immunoprecipitation
HeLa cells were seeded at a density of 90% in 10-cm Petri dishes and co-transfected with 7 μg of pSG5-BGLF4 and 7 μg of pSG5-stathmin-Flag with Lipofectamine 2000. At 18 h post-transfection, cell lysates were collected in Nonidet P-40 lysis buffer 50 mm Tris, pH 8.0, 150 mm NaCl, 2 mm EDTA, and 1 mm Na3VO4). Cell lysates were centrifuged at 16,000 × g for 20 min at 4 °C, and then the supernatant was precleared with 200 μl of 20% protein G-Sepharose beads (Amersham Biosciences) with rotation for 1 h at 4 °C. After centrifugation, the precleared supernatant was incubated with 3 μg of anti-BGLF4 (40), anti-Flag M2 mAb (Sigma), or irrelevant control antibodies (mIgG) at 4 °C for 1 h and then 250 μl of protein G-Sepharose beads were added to precipitate the immunocomplexes with rotation for 1 h at 4 °C. The recovered immunocomplexes were washed extensively in cold phosphate-buffered saline and resolved with sodium dodecyl sulfate (SDS) sample buffer, and subjected to immunoblotting analysis. HeLa cells were also transfected with 10 μg of pSG5-BGLF4, and lysates were subjected to co-immunoprecipitation as described above. The anti-stathmin polyclonal Ab (Santa Cruz Biotechnology) was used to precipitate the endogenous stathmin.
Immunoblotting and Quantification of Band Densities
Cell lysates were resolved by SDS-polyacrylamide gel electrophoresis and further transferred to Hybond-C Extra membranes (Amersham Biosciences). The membranes were incubated with 5% nonfat dry milk and the primary antibodies (Abs) used were anti-BGLF4 (2224 or 2216 clones) mAbs (40), anti-EBNA2 mAb (43), anti-GAPDH mAb (Biodesign), anti-stathmin polyclonal Ab (Calbiochem), anti-phosphorylated Ser-16 stathmin polyclonal Ab (Santa Cruz Biotechnology), anti-Flag M2 mAb (Sigma), anti-Zta mAb (44), and anti-Rta mAb (45). After hybridization with secondary antibodies, the membranes were developed using an enhanced chemiluminescence kit (Amersham Biosciences).
ImageQuant software was used to quantify the expression levels of the proteins detected by immunoblotting. Briefly, the value of the band density was quantified and then normalized to its corresponding internal control. Then, the results were shown relative to vector transfectant or cell line of interest.
Two-dimensional Gel Electrophoresis
BGLF4, BGLF4-KD and vector control inducible HEK 293 T-REx cells were grown in Dulbecco's modified Eagle's medium with 8% tetracycline-free serum and protein expression was induced by incubation with 10 ng/ml doxycycline for 24 h. Total lysates from BGLF4, BGLF4-KD or vector control cells were collected in two-dimensional lysis buffer (40 mm Tris, 7 m urea, 2 m thiourea, 4% CHAOS, pH 9) and subjected to two-dimensional PAGE according to the manufacturer's instructions. Briefly, isoelectric focusing was carried out using pH 3–10 carrier ampholytes and separated in 12.5% polyacrylamide gels. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes (Millipore) and probed with anti-stathmin polyclonal Ab or anti-phosphorylated Ser-16 stathmin polyclonal Ab. After hybridization with secondary antibodies, the membranes were developed using an enhanced chemiluminescence kit (Amersham Biosciences).
BGLF4 in Vitro Kinase Assay
Wild-type stathmin (pGEX-4T1-stathmin-Flag) and stathmin mutated at specific serine residues (pGEX-4T1-stathmin-SAAA-Flag, pGEX-4T1-stathmin-ASAA-Flag, pGEX-4T1-stathmin-AASA-Flag, and pGEX-4T1-stathmin-AAAS-Flag) were transformed to E. coli and then treated with isopropylthio-β-d-galactoside (IPTG) to induce recombinant protein expression. The recombinant proteins were purified with glutathione-Sepharose according to the manufacturer's instructions (Amersham Biosciences). BGLF4 kinase assay was performed according to a previous report (38). For in vitro kinase assays, immunoprecipitates of BGLF4 or BGLF4-KD were incubated in 30 μl of kinase buffer containing 25 μm ATP, 2.5 μCi of [γ-32P]ATP, and 2 μg of GST-stathmin-Flag. All mixtures were incubated at 30 °C for 30 min and then the reactions stopped by adding 10 μl of sample buffer (50 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5 mm EDTA, and 0.02% bromphenol blue) and heating at 95 °C for 5 min. Thereafter, all samples were subjected to immunoblotting and autoradiography. The other stathmin mutants, as well as the positive control protein, Histone H1, and negative control protein, GST protein, were used as substrates in similar experiments to those described above.
Indirect Immunofluorescence Assay (IFA)
Slide-cultured HeLa cells were transfected with 5 μg or 10 μg of pSG5 vector, pSG5-BGLF4, pSG5-BGLF4-KD, pSG5-stathmin-4E-Flag, pSG5-stathmin-4A-Flag, or pSG5-stathmin-Flag using Lipofectamine 2000. Twenty-four hours after transfection, cells were fixed in 4% paraformaldehyde at room temperature for 20 min. Fixed slides were washed in phosphate-buffered saline (145 mm NaCl, 1.56 mm Na2HPO4, 1 mm KH2PO4, pH 7.2) and then permeabilized by 0.1% Triton X-100 at room temperature for 5 min. Fixed cells were then stained with rabbit anti-BGLF4 polyclonal Ab (1:100) (40), mouse anti-Flag M2 Ab (1:250) or rabbit polyclonal to beta tubulin Ab (1:200) (Abcam) at 37 °C for 1.5 h. After washing with phosphate-buffered saline three times, slides were incubated with rhodamine-conjugated or fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (Cappel) or anti-mouse IgG antibodies (Cappel) at 37 °C for 1 h. DNA was stained by Hoechst 33258 at room temperature for 90 s, and slides were observed by fluorescence microscopy (Zeiss).
Statistical Analysis
Statistical analyses employed the correlation coefficient, Student's t test, and 2 × 2 Contingency Table of chi-squared test using Microsoft EXCEL. Briefly, the expression levels of BGLF4, phosphorylated and nonphosphorylated stathmin in twenty LCLs were quantified as described above. The correlation coefficients between levels of BGLF4 and stathmin (either phosphorylated or nonphosphorylated) were calculated separately. Student's t test was used to compare the treated samples with the corresponding controls.
RESULTS
Elevated Stathmin Phosphorylation Is Associated with BGLF4 Expression in the EBV Lytic Cycle
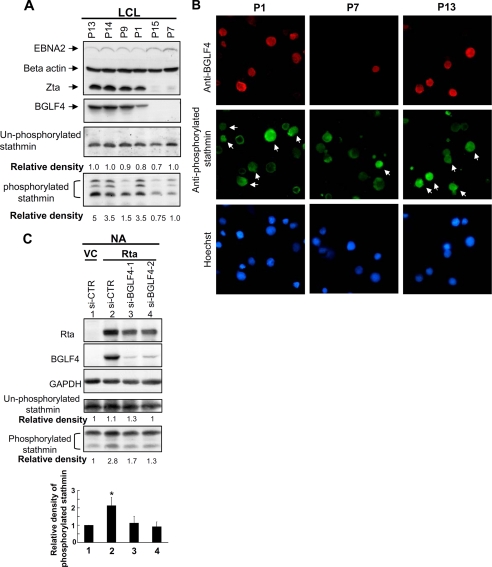
To test whether any EBV products affect the activity of stathmin, we compared the results from LCL cells with and without spontaneous lytic cycle progression. Six representative LCLs are shown in Fig. 1A. All LCL lines expressed the EBV latent protein, EBNA2. In addition, expression of the EBV lytic kinase BGLF4 was observed clearly in Zta-expressing LCLs, including P1, P9, P13, and P14, but not in P15 or P7. Based on the relative density of total phosphorylated stathmin, levels of phosphorylated stathmin were apparently correlated with viral lytic protein expression, compared with lower or non-lytic protein-expressing cell lines (P15 and P7 cells). This suggests that stathmin phosphorylation might be regulated by EBV-lytic products. BGLF4 is the only EBV-encoded serine/threonine kinase and has a similar biological function to the cellular cdc2 kinase (33, 34), which is a protein kinase for stathmin (46, 47). Thus, the correlation coefficient was calculated between levels of BGLF4 and phosphorylated and nonphosphorylated stathmin from twenty LCLs in total (data not shown). Levels of BGLF4 and phosphorylated stathmin are positively correlated (the correlation coefficient is 0.59); whereas levels of BGLF4 and nonphosphorylated stathmin are not correlated (the correlation coefficient is 0.12).
FIGURE 1.
Increasing phosphorylation of stathmin in LCL with spontaneous lytic cycle progression. A, cell lysates were harvested from EBV-transformed LCL cell lines (P1, P7, P9, P13, P14, and P15) and subjected to immunoblotting analysis. Cell lysates were probed by specific antibodies against EBNA2, BGLF4, Zta, phosphorylated stathmin at serine-16, nonphosphorylated stathmin, and β-actin. The band densities of nonphosphorylated and total phosphorylated stathmin of each LCL (P13, P14, P1, and P15) relative to the P7 were quantified and normalized to the intensity of the corresponding internal control using ImageQuant software. These densities are indicated below the corresponding bands. B, P1, P7, and P13 LCL were stained with anti-BGLF4 (red color) and anti-phosphorylated stathmin (green color) antibodies. Nuclear DNA was stained with Hoechst 33258. BGLF4-expressing cells are indicated by arrows. C, an EBV-positive nasopharyngeal cell line, NA, was co-transfected with pSG5-Rta and si-BGLF4-1, si-BGLF4-2 or control siRNA fragments. Cell lysates were harvested 36 h post-transfection and subjected to immunoblotting. The band densities of nonphosphorylated and total phosphorylated stathmin of each transfection relative to the vector and si-CTR transfection (lane 1) were quantified and normalized to the intensity of the corresponding internal control using ImageQuant software. These densities are indicated below the corresponding panels, and relative band densities of phosphorylated stathmin from three independent experiments are statistically analyzed and plotted. *, significant difference from vector control (p < 0.05).
To determine whether stathmin is hyper-phosphorylated and can act as a substrate of BGLF4, an IFA was performed in P1, P7, and P13 cells. In Fig. 1B, increasing fluorescent intensity of phosphorylated stathmin was observed in BGLF4-positive cells, compared with BGLF4-negative cells. To verify the importance of BGLF4 presence for stathmin phosphorylation, especially in reactivated EBV-positive cells, BGLF4 expression was knocked down in Rta-induced EBV-harboring NA cells. In Fig. 1C, the phosphorylation of stathmin was increased in NA cells with EB viral lytic cycle progression (lanes 1 and 2). However, the phosphorylation was abolished when BGLF4 expression was knocked down by siRNA (Fig. 1C, lanes 3 and 4). These data indicate that BGLF4 could be the protein responsible for stathmin phosphorylation during viral lytic cycle.
BGLF4 Induces Stathmin Phosphorylation in Vivo
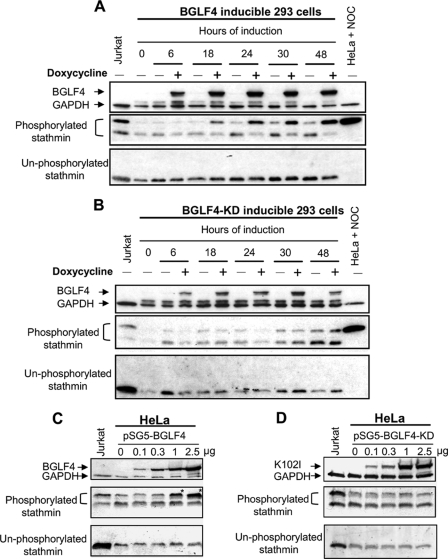
As mentioned above, BGLF4 contributes to stathmin phosphorylation during EBV lytic cycle. Next, we used doxycycline-induced BGLF4-expressing 293 cells to confirm the effects of BGLF4 on phosphorylation of stathmin. In Fig. 2A, increasing phosphorylation of stathmin was clearly observed by following BGLF4 expression at 18, 24, 30, and 48 h, compared with the non-induction controls. In contrast, expression of BGLF4-kinase dead (KD) protein, which has a point mutation in the kinase domain of BGLF4, did not increase the phosphorylation of stathmin (Fig. 2B). Thus, this experiment demonstrated that BGLF4 expression is associated with stathmin phosphorylation. Moreover, comparing non-doxycycline-treated cells across all the time points for the level of phosphorylated stathmin (Fig. 2, A and B), we found a cell-cycle dependent increase in stathmin phosphorylation. This finding is reasonable because stathmin is known to be cell cycle-regulated (16, 46, 48). Further, as shown in Fig. 2C, phosphorylation of stathmin increased with BGLF4 expression in a dose-dependent manner. Again, BGLF4-KD expression did not affect the phosphorylation of stathmin (Fig. 2D). Taken together, the EBV-encoded BGLF4 kinase can mediate stathmin phosphorylation in vivo.
FIGURE 2.
Increasing phosphorylation of stathmin in BGLF4-expressing 293 cells. A and B, doxycycline-driven BGLF4 or BGLF4 kinase dead mutant (BGLF4-KD) expressing 293 cells were induced by 10 ng/ml doxycycline for indicated times (induction: +; non-induction: −). C and D, HeLa cells were transfected with various amounts of BGLF4- or BGLF4-KD-expressing plasmids and then lysates were harvested at 24 h post-transfection. Cell lysates were analyzed by immunoblotting and proteins of interest were detected by specific antibodies against BGLF4, nonphosphorylated stathmin, phosphoserine 16 stathmin, and GAPDH. Positive controls of hyperphosphorylated stathmin were harvested from Jurkat or HeLa cells treated with nocodazole (NOC). The same blot was stripped and then re-probed with anti-C-terminal stathmin antibody. Hyperphosphorylated forms of stathmin are indicated by the asterisks in C.
BGLF4 Directly Phosphorylates Stathmin in Vitro
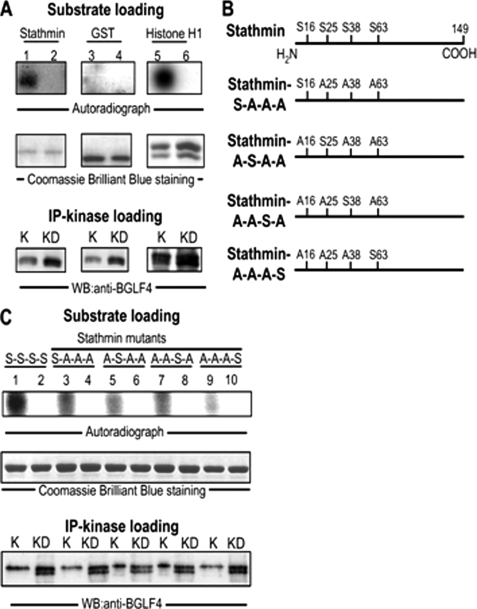
Based on the kinase dead data above and the fact that several cellular proteins are the substrates of BGLF4, we assumed that the kinase activity of BGLF4 is required for phosphorylation of stathmin (33, 34). An in vitro kinase assay was performed to determine whether BGLF4 can phosphorylate stathmin directly. GST-tagged stathmin and GST control protein were purified from E. coli as substrates for a BGLF4 kinase activity assay. In Fig. 3A, we clearly demonstrated that BGLF4 can phosphorylate GST-stathmin, but not a GST control protein, directly. It is also shown that BGLF4 can phosphorylate a common substrate, the histone H1 protein, served as a positive control (Fig. 3A). It is well-documented that there are four serine residues that can be phosphorylated on stathmin, residues 16, 25, 38, and 63 (Fig. 3B) (12–15). Therefore, in Fig. 3C, to elucidate further the serine residues on stathmin that might be targeted by BGLF4, purified GST-stathmin serine mutants were used in assays similar to that described above. BGLF4 was shown to phosphorylate stathmin on serine residues 16, 25, and 38, but to phosphorylate serine residue 63 weakly. Thus, BGLF4 can indeed phosphorylate stathmin directly in vitro.
FIGURE 3.
Stathmin is phosphorylated by BGLF4 in vitro. A, recombinant GST-stathmin, GST control protein, and a positive control protein, Histone H1, were used as substrates. HeLa cells were transfected with BGLF4 or BGLF4-kinase dead expression plasmids and BGLF4 (K) and its kinase dead mutant (KD) were obtained by immunoprecipitation with anti-BGLF4 antibody from total cell lysates 24 h post-transfection. IP kinase assays were carried out for 30 min as described under “Experimental Procedures.” The amounts of substrates and kinase loaded are shown. The experiment was performed twice, and a representative example is shown. BGLF4 was observed to phosphorylate GST-stathmin and Histone H1 protein but not GST control protein. B, positions of serine phosphorylation sites in wild-type stathmin and purified stathmin mutants are shown. Phosphorylatable residues of GST-tagged stathmin mutants, which can only be phosphorylated at one of four target residues in the N terminus of stathmin, such as SAAA (only phosphorylatable on residue 16), ASAA (only phosphorylatable on residue 25), AASA (only phosphorylatable on residue 38) and AAAS (only phosphorylatable on residue 63) are indicated. C, substrates of GST-tagged stathmin mutants in B were used in the same reaction as described in A. BGLF4 can be seen to phosphorylate GST-stathmin mutants mainly at residues 16, 25, and 38. The experiment was performed twice, and a representative example is shown.
BGLF4 Mediates Stathmin Phosphorylation at Serines 16, 25, and 38 in Vivo
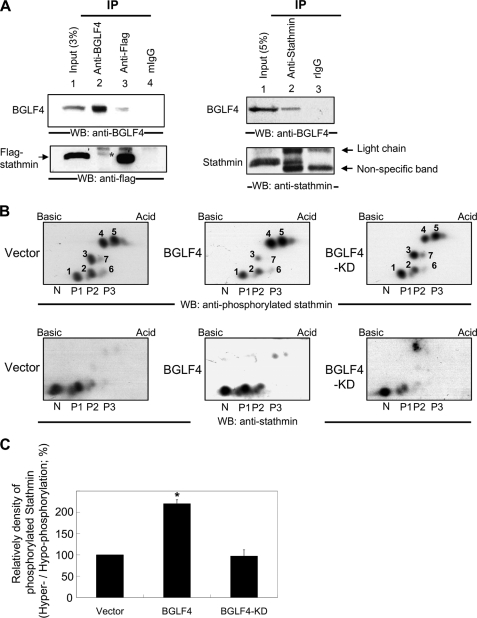
To determine whether BGLF4 can phosphorylate stathmin in vivo, we first asked whether BGLF4 can interact physically with stathmin in cells (Fig. 4A). Co-immunoprecipitation assays showed that BGLF4 can be detected in immunoprecipitates of Flag-tagged stathmin (lane 3, upper panel). In the bottom panel of lane 2, stathmin was also detected in immunocomplexes precipitated by BGLF4 antibody. These results from reciprocal co-IP Western blotting demonstrated that BGLF4 interacts with stathmin in cells. Moreover, a co-immunoprecipitation assay was also performed to determine the relationship between BGLF4 and endogenous stathmin (Fig. 4A, right panel). BGLF4 can be detected in immunocomplexes precipitated by stathmin antibody. Thus, BGLF4 indeed can interact with endogenous stathmin.
FIGURE 4.
BGLF4 interacts with stathmin and induces phosphorylation of stathmin in vivo. A, HeLa cells were transiently transfected with either pSG5-BGLF4 or pSG5-BGLF4 combined with pSG5-stathmin-Flag-expressing plasmids. Cell lysates were harvested 18 h post-transfection and immunoprecipitated by anti-BGLF4, anti-Flag antibodies, or mouse IgG antibodies. Immunoprecipitates were subjected to immunoblotting and then probed with anti-BGLF4, anti-Flag, or anti-stathmin antibodies. The Flag-tagged stathmin is indicated by the asterisk. B, inducible BGLF4, BGLF4-KD (BGLF4 kinase dead form), or vector control 293 T-REx cells were induced with 10 ng/ml doxycycline for 24 h. Lysates were subjected to two-dimensional PAGE and probed with anti-stathmin or anti-phosphorylated Ser-16 stathmin antibodies. N, nonphosphorylated form of stathmin. P1, stathmin isoforms which are phosphorylated at one phosphorylatable residue (dot 1); P2, stathmin isoforms which are phosphorylated at two phosphorylatable residues (dots 2 and 3); P3, stathmin isoforms which are phosphorylated at three phosphorylatable residues (dots 4 and 5). C, ratio for relatively density of hyperphosphorylated stathmin (dots 4 and 5) and hypophosphorylated stathmin isoforms (dots 1, 2, 3, 6, and 7) from the two experiments are plotted and statistically analyzed. *, significant difference to vector control (p < 0.05).
Previous studies have shown that various kinases can phosphorylate stathmin at different serine residues (49), leading to different degrees of activity. For example, cdc2 phosphorylates serine residues of 25 and 38 (46, 47) and calcium-calmodulin dependent protein kinase only phosphorylates serine 16 (50). Thus, it is important to know which serine residue(s) of stathmin are phosphorylated by BGLF4 in vivo. In this study, the phosphorylated forms of stathmin were detected using an antibody against stathmin phosphorylated at Ser-16 (Figs. 1 and 2). This indicates that BGLF4 can at least target Ser-16 of stathmin in vivo. Moreover, the phosphorylated patterns in our Fig. 2 are similar to those of stathmins resolved by one-dimensional PAGE and known to be phosphorylated at serines 16, 25, 38 ± 63 (51). Therefore, our data suggest that expression of BGLF4 leads to phosphorylation of stathmin at serines 16, 25, 38 ± 63 in vivo. To confirm this, a two-dimensional PAGE analysis was used to resolve the various molecular forms of phosphorylated or nonphosphorylated stathmin in cells expressing BGLF4 (Fig. 4B). The phosphorylation status of different isoforms is indicated under each panel, as described previously (51). The phosphorylation patterns of phosphorylated isoforms were observed to be similar in vector- and BGLF4-KD-expressing cells (Fig. 4B, upper panels; dots 1–7). However, in cells expressing BGLF4, relatively low levels of low-phosphorylated isoforms of stathmin (upper panel; dots 1–3) and relatively high levels of hyperphosphorylated forms of stathmin (upper panel; dots 4 and 5) were observed. Furthermore, the ratio for relative density of hyperphosphorylated stathmin (dots 4 and 5) and hypophosphorylated stathmin (dots 1, 2, 3, 6, and 7) isoforms are also calculated and shown in Fig. 4C. This indicates that BGLF4 expression indeed led to an increase in at least two phosphorylated isoforms of stathmin, the isoform that is phosphorylated on serine residues 16, 25 and 38 (Fig. 4B, P3; dot 4) and the isoform that is phosphorylated possibly at all phosphorylatable serine residues (dot 5), recognized by two-dimensional PAGE as described previously (51). Therefore, BGLF4 expression can indeed mediate phosphorylation at residues 16, 25, 38 ± 63 of stathmin in vivo, which is consistent with our in vitro kinase assay (Fig. 3). On the other hand, comparing the upper and lower panels, the anti-stathmin antibody is only sensitive to the nonphosphorylated or low-phosphorylated forms of stathmin, as also shown in Figs. 1 and 2.
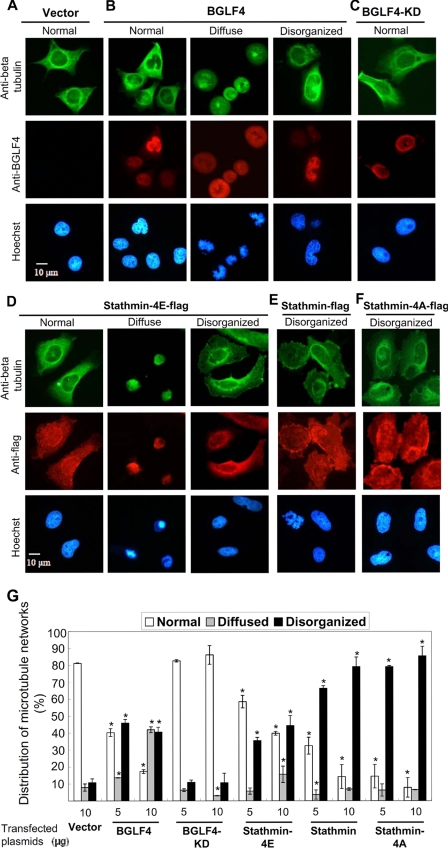
Microtubule Networks Are Reorganized in Cells Expressing BGLF4
It is shown above that BGLF4 expression increases the number of phosphorylated isoforms of stathmin in cells, suggesting that BGLF4 expression can negate the depolymerization activity of stathmin. Thus, we hypothesized that the balance between MT-destabilizer and stabilizer in cells would be interfered with by BGLF4 expression. To test this, HeLa cells were transfected with various amounts plasmid of control vector, BGLF4, BGLF4-KD, stathmin-4E (phosphor-mimic stathmin), stathmin-4A (non-phosphorylatable stathmin) or stathmin, and then the MT networks in the transfected cells were examined by immunofluorescence assays. As shown in Fig. 5G, the percentages of alteration of MT networks in the various transfected cells were calculated. As expected, MT arrays were observed as normal-organized in most of the vector (Fig. 5A) and BGLF4-KD-expressing cells (Fig. 5C). Of note, increasingly disorganized and diffuse types of MT network were present in BGLF4-expressing (Fig. 5B) and phospho-mimic stathmin 4E cells (Fig. 5D). Furthermore, 70–90% of disorganized pattern was seen in stathmin (Fig. 5E) and in nonphosphorylatable stathmin 4A-expressing cells (Fig. 5F). These data indicate that BGLF4 expression can alter the organization of MT networks severely through phosphorylation of stathmin. However, we cannot exclude the possibility that BGLF4 may also target other factors that contribute to modulating the MT networks.
FIGURE 5.
Microtubule networks are altered in cells expressing BGLF4, stathmin-4E (pseudo-phosphorylated stathmin), wild-type stathmin, and stathmin-4A (nonphosphorylatable stathmin). Slide-cultured HeLa cells were transfected with vector, BGLF4, BGLF4-KD, stathmin-4E, stathmin, or stathmin-4A expression plasmids. Twenty-four hours post-transfection, cells were fixed in 4% paraformaldehyde and stained with anti-BGLF4 Ab, anti-β tubulin Ab, anti-Flag Ab, and Hoechst 33258. Microtubule networks can be categorized by normal, diffuse, or disorganized types in vector- (A), BGLF4- (B), BGLF4-KD- (C), stathmin-4E- (D), stathmin- (E), and stathmin-4A-expressing cells (F). G, variations of microtubule networks observed in different transfections are calculated from two independent experiments and more than 200 cells were counted in each transfection. The counts of each categorical variable (disorganized, diffuse, or normal type of MT) in one treatment (BGLF4-, BGLF4-KD-, stathmin-4E, stathmin-Flag, or stathmin-4A-expresion cells) were compared with that in vector control cells by using the 2 × 2 Contingency Table of chi-squared test. *, significant difference to vector control (p < 0.05).
The above data demonstrate that BGLF4 expression can reorganize the MT networks through phosphorylation of stathmin. To confirm that BGLF4 plays a role in MT turnover, another approach was applied to measure the levels of assembled tubulin in BGLF4-expression cells. We found that a slightly higher content of polymerized MT was observed in BGLF4-expressing cells in comparison with that in vector control cells (data not shown). Thus, this also supports that BGLF4 can play a role in the regulation of MT dynamics.
Increasingly Phosphorylated Forms of Stathmin Are Observed in Cells Transfected with UL13 Homologues
Conserved herpesviral protein kinases (CHPKs) is a group of serine/threonine kinases that are conserved in all Herpesviridae. Among the CHPKs, UL13 is encoded by HSV, and similar CHPKs encoded by other herpesvirus are called UL13 homologues (52, 53). Members of this group likely play similar roles in infection by targeting common host substrates. Thus, whether these homologues may interact with stathmin was also tested here. In Fig. 6, expression of BGLF4 (positive control), UL13 (HSV-1), UL97 (HCMV), and ORF36 (KSHV), but not mORF36 (MHV68), led to increasing phosphorylation of stathmin in cells. Therefore, these data suggest that UL13, UL97, and ORF36 could phosphorylate the same host substrate, stathmin, and that mORF36 of a murine herpesvirus may not target human cellular stathmin.
FIGURE 6.
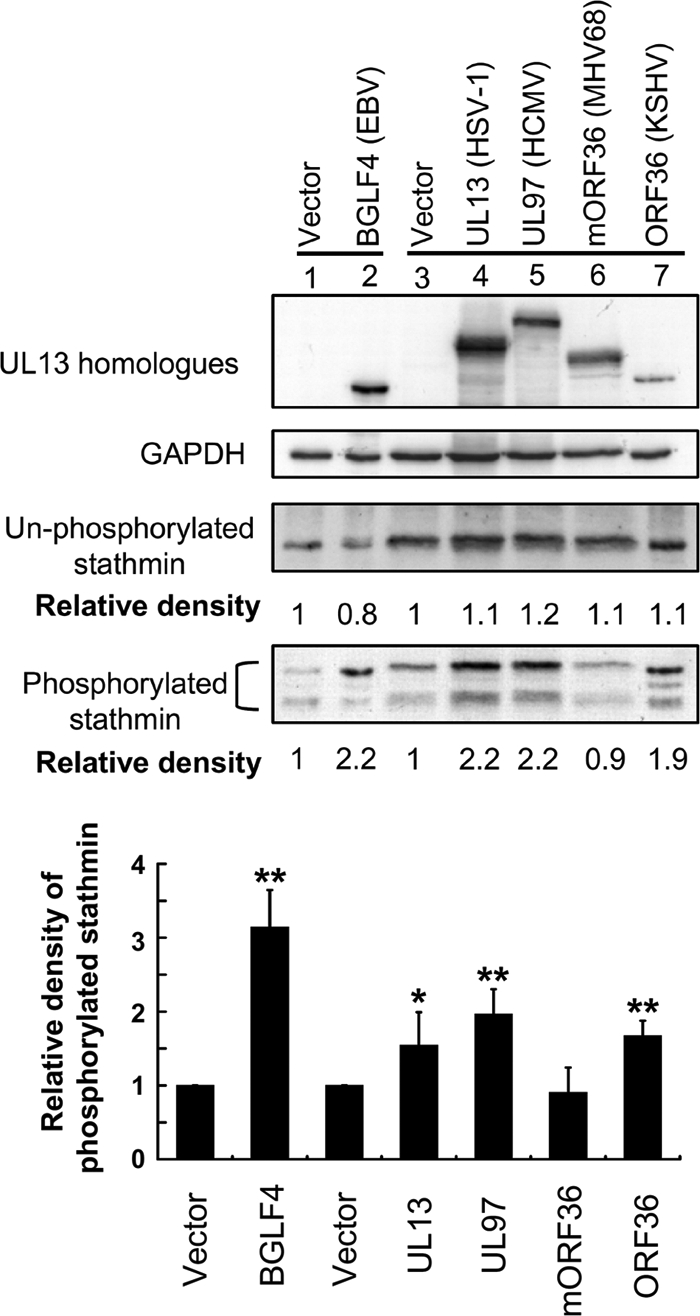
UL13 homologues induce phosphorylation of stathmin in vivo. HeLa cells were transiently transfected with vector and Flag-tagged UL13 (HSV-1), UL97 (HCMV), mORF36 (MHV68), or ORF36 (KSHV)-expressing plasmids. A BGLF4 expression plasmid was transfected as the positive control. Cell lysates were harvested 24 h post-transfection and subjected to immunoblotting analysis. The membrane was probed with specific antibodies against BGLF4, Flag, phosphoserine 16 stathmin, and GAPDH. The blot was then stripped and re-probed with anti-C-terminal stathmin Ab. The band densities of nonphosphorylated or total phosphorylated stathmin of each transfection relative to the vector control were quantified and normalized to the intensity of its corresponding internal control by using ImageQuant software. Relative densities are indicated below the corresponding panels, and relative densities of phosphorylated stathmin from three independent experiments are statistically analyzed and plotted. *, significant difference to vector control (p < 0.05); **, significant difference to vector control (p < 0.01).
DISCUSSION
Stathmin is an important microtubule regulator in cells and phosphorylation of stathmin is required for correct cell cycle progression during mitosis (13, 16–19). BGLF4 is the only EBV-encoded viral kinase and is expressed as an abundant early lytic protein during EBV reactivation (40, 54). It is known that this viral kinase can target many cellular factors (32, 33). In this study, we tried to determine the roles of viral kinase to regulate the cellular MT dynamics. Herein, we demonstrate that EBV BGLF4 kinase is responsible for increased phosphorylation of stathmin in reactivated EBV-positive cells (Fig. 1). Furthermore, the results from an in vitro kinase assay and in vivo two-dimensional electrophoresis revealed that BGLF4 phosphorylates stathmin mainly on serine residues 16, 25, and 38 (Figs. 3 and 4). The MT destabilizing activity of stathmin is reduced by certain amounts by phosphorylation of one to four of its target residues and phosphorylation of serines at 16, 25, and 38 of stathmin is sufficient for inactivation of its depolymerization activity (12, 50, 55). Thus, our findings indicate that BGLF4 expression can inactivate the stathmin, and this also hinted that the balance between MT-stabilizer and MT-destabilizer within cells may be disturbed in cells expressing BGLF4. Indeed, MT networks, as well as the content of polymerized MT, were found to be altered in BGLF4-expressing cells (Figs. 5 and 6). Thus, it is assumed here that BGLF4 expression may phosphorylate stathmin in a localized fashion in vivo, leading to reduction or inhibition of the activity of stathmin in a localized environment (56–58). Importantly, many viruses have been reported to use diverse approaches to regulate the MT networks, however, to our knowledge, this is the first report that a viral product that can directly regulate the activity of stathmin.
In this study, the activity of stathmin has been shown to be modulated by EBV during its lytic cycle. In addition, expression of stathmin has been reported previously to be up-regulated in EBV-infected primary B cells and in EBV-transformed LCLs (29). Moreover, an EBV-encoded latent protein, LMP1, has been shown to mediate phosphorylation of stathmin in EBV-related NPC by enhancing cdc2 kinase activity (30). These previous reports indicate that stathmin is regulated during EBV infection but the purpose of this regulation is unclear. Herein, we further demonstrate that the cellular MT dynamics are modulated by phosphorylation of stathmin through BGLF4 mediation, and especially the MT organization is drastically disorganized in most BGLF4-expressing cells. Thus, EBV infection may indirectly affect some MT-associated cellular processes.
Cellular cdc2 is known as a proline-directed kinase. Otherwise, BGLF4 has been documented as a cdc2-mimicking kinase because BGLF4 and cdc2 can target the same phosphorylatable site at EF-1δ (59). Consistently, it is shown here that BGLF4 can phosphorylate sites 25 and 38 of stathmin (Fig. 3 and 4), which are the target sites for cdc2 in an in vitro kinase assay (48). Indeed, the phosphorylation sequence for sites 25 and 38 of stathmin is [serine-proline] and the serine is phosphorylatable by BGLF4 and cdc2. Thus, our data confirm that BGLF4 is a cdc2-mimicking kinase and can target proline-directed serine residues. Of note, we found that BGLF4 can also phosphorylate on site 16 of stathmin in vitro and in vivo (Figs. 3 and 4), which is not a typical proline-directed sequence in that the phosphorylatable residue is serine in the sequence [arginine-alanine-serine-glycine]. In fact, a recent study also indicates that BGLF4 can target a non-proline directed sequence on the EBV transactivator BZLF1 (60). Thus, this suggests that BGLF4 can recognize not only proline-directed but can also target to non proline-directed residues on its substrates. Also, another report demonstrated that BGLF4 can phosphorylate more residues than cdc2 on the same lamin A protein (39). Consistently, a study using a protein array to identify systematically potential BGLF4 substrates has shown that about 21 of 60 viral proteins are phosphorylated by BGLF4, but approximately half of these proteins can be phosphorylated by cdc2 (32). Taken together, these data clearly indicate that BGLF4 can target more residues or more proteins than the cellular cdc2 kinase. Thus, it is suggested that the ability of BGLF4 to recognize a broader range of residues than cdc2 can enable EBV to modulate its cellular or viral targets more easily.
CHPKs is a group of kinases that are conserved in all Herpesviridae and these kinases are believed to play a conserved role in viral infection by targeting common cellular and viral substrates (33). Therefore, whether these homologues also modulate the activity of stathmin was also investigated in this study. Among them, our data demonstrated that expression of UL13, UL97, and ORF36, but not mORF36, led to elevation of the levels of phosphorylated stathmin in cells (Fig. 6). This finding suggests that most of the CHPKs tested may target to and then modulate the activity of stathmin in cells, and this also implies that these CHPKs may play conserved roles in the regulation of MT networks during herpesvirus infection. These findings are consistent with a previous report that expression of UL13, UL97, ORF36, and BGLF4, but not mORF36, can cause disassembly of nuclear lamina (39).
In conclusion, it has been shown in this study that stathmin is phosphorylated in EBV-positive cells during the lytic cycle, implying reduced activity of stathmin when EBV-positive cells are reactivated. We further provide evidence that an EBV kinase, BGLF4, is responsible for phosphorylating stathmin directly. Furthermore, BGLF4 expression is shown to alter the MT dynamics and also drastically alter the MT organization. Thus, this study shows for the first time that a viral infection can exploit and modulate stathmin to alter MT organization.
Acknowledgments
We thank Dr. Tim J. Harrison of University College London (UCL) Medical School, London, UK and Dr. Shao-yin Chen of Taiwan Stem Cell Bank, The Food Industry Research and Development Institute, Hsinchu, Taiwan, for reviewing the manuscript critically. We thank the services from Proteomics & Protein Function Core Laboratory in the Center of Genomic Medicine, National Taiwan University, and also thank Dr. Martin Gullberg of Dept. of Molecular Biology, Umeå University, Sweden for providing several stathmin-related expression plasmids.
This work was supported by the National Health Research Institute (NHRI-EX98-9726BI and NHRI-EX99-9726BI), National Science Council (Grants NSC97-2320-B-002-003, NSC98-3112-B-002-033, and NSC99-3112-B-002-022), Frontier and Innovative Research of National Taiwan University (97R0331), and a research training fellowship from the National Taiwan University.
- MT
- microtubule
- EBV
- Epstein-Barr virus
- MAP
- microtubule-associated protein
- LCL
- lymphoblastoid cell lines
- NPC
- nasopharyngeal carcinoma
- KSHV
- Kaposi's sarcoma-associated herpesvirus
- CHPK
- conserved herpesviral protein kinases
- GST
- glutathione S-transferase
- KD
- kinase dead.
REFERENCES
- 1.Desai A., Mitchison T. J. (1997) Annu. Rev. Cell Dev. Biol. 13, 83–117 [DOI] [PubMed] [Google Scholar]
- 2.Mitchison T., Kirschner M. (1984) Nature 312, 237–242 [DOI] [PubMed] [Google Scholar]
- 3.Schulze E., Kirschner M. (1988) Nature 334, 356–359 [DOI] [PubMed] [Google Scholar]
- 4.Andersen S. S. (2000) Trends Cell Biol. 10, 261–267 [DOI] [PubMed] [Google Scholar]
- 5.Cassimeris L. (1999) Curr. Opin. Cell Biol. 11, 134–141 [DOI] [PubMed] [Google Scholar]
- 6.Desai A., Verma S., Mitchison T. J., Walczak C. E. (1999) Cell 96, 69–78 [DOI] [PubMed] [Google Scholar]
- 7.Joshi H. C. (1998) Curr. Opin. Cell Biol. 10, 35–44 [DOI] [PubMed] [Google Scholar]
- 8.Belmont L. D., Mitchison T. J. (1996) Cell 84, 623–631 [DOI] [PubMed] [Google Scholar]
- 9.Koppel J., Boutterin M. C., Doye V., Peyro-Saint-Paul H., Sobel A. (1990) J. Biol. Chem. 265, 3703–3707 [PubMed] [Google Scholar]
- 10.Maucuer A., Moreau J., Méchali M., Sobel A. (1993) J. Biol. Chem. 268, 16420–16429 [PubMed] [Google Scholar]
- 11.Howell B., Larsson N., Gullberg M., Cassimeris L. (1999) Mol. Biol. Cell 10, 105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawler S. (1998) Curr. Biol. 8, R212–R214 [DOI] [PubMed] [Google Scholar]
- 13.Marklund U., Larsson N., Gradin H. M., Brattsand G., Gullberg M. (1996) EMBO J. 15, 5290–5298 [PMC free article] [PubMed] [Google Scholar]
- 14.Di Paolo G., Antonsson B., Kassel D., Riederer B. M., Grenningloh G. (1997) FEBS Lett. 416, 149–152 [DOI] [PubMed] [Google Scholar]
- 15.Holmfeldt P., Larsson N., Segerman B., Howell B., Morabito J., Cassimeris L., Gullberg M. (2001) Mol. Biol. Cell 12, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson N., Melander H., Marklund U., Osterman O., Gullberg M. (1995) J. Biol. Chem. 270, 14175–14183 [DOI] [PubMed] [Google Scholar]
- 17.Larsson N., Marklund U., Gradin H. M., Brattsand G., Gullberg M. (1997) Mol. Cell. Biol. 17, 5530–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin C. I., Atweh G. F. (2004) J. Cell. Biochem. 93, 242–250 [DOI] [PubMed] [Google Scholar]
- 19.Holmfeldt P., Brännström K., Stenmark S., Gullberg M. (2006) Mol. Biol. Cell 17, 2921–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sodeik B. (2000) Trends Microbiol. 8, 465–472 [DOI] [PubMed] [Google Scholar]
- 21.Ploubidou A., Way M. (2001) Curr. Opin. Cell Biol. 13, 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cudmore S., Reckmann I., Way M. (1997) Trends Microbiol. 5, 142–148 [DOI] [PubMed] [Google Scholar]
- 23.Suomalainen M., Nakano M. Y., Boucke K., Keller S., Greber U. F. (2001) EMBO J. 20, 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cudmore S., Cossart P., Griffiths G., Way M. (1995) Nature 378, 636–638 [DOI] [PubMed] [Google Scholar]
- 25.Ploubidou A., Moreau V., Ashman K., Reckmann I., González C., Way M. (2000) EMBO J. 19, 3932–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott G., O'Hare P. (1998) J. Virol. 72, 6448–6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J. I. (2000) N. Engl. J. Med. 343, 481–492 [DOI] [PubMed] [Google Scholar]
- 28.Young L. S., Rickinson A. B. (2004) Nat. Rev. Cancer 4, 757–768 [DOI] [PubMed] [Google Scholar]
- 29.Baik S. Y., Yun H. S., Lee H. J., Lee M. H., Jung S. E., Kim J. W., Jeon J. P., Shin Y. K., Rhee H. S., Kimm K. C., Han B. G. (2007) Cell Prolif. 40, 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin X., Liu S., Luo X., Ma X., Guo L., Li L., Li Z., Tao Y., Cao Y. (2009) Int. J. Cancer 124, 1020–1027 [DOI] [PubMed] [Google Scholar]
- 31.Yan G., Li L., Tao Y., Liu S., Liu Y., Luo W., Wu Y., Tang M., Dong Z., Cao Y. (2006) Proteomics 6, 1810–1821 [DOI] [PubMed] [Google Scholar]
- 32.Zhu J., Liao G., Shan L., Zhang J., Chen M. R., Hayward G. S., Hayward S. D., Desai P., Zhu H. (2009) J. Virol. 83, 5219–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gershburg E., Pagano J. S. (2008) Biochim. Biophys. Acta 1784, 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi Y., Kato K. (2003) Rev. Med. Virol. 13, 331–340 [DOI] [PubMed] [Google Scholar]
- 35.Sankar S., Chan H., Romanow W. J., Li J., Bates R. J. (2006) Cell Signal. 18, 982–993 [DOI] [PubMed] [Google Scholar]
- 36.Chang Y., Tung C. H., Huang Y. T., Lu J., Chen J. Y., Tsai C. H. (1999) J. Virol. 73, 8857–8866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chua H. H., Lee H. H., Chang S. S., Lu C. C., Yeh T. H., Hsu T. Y., Cheng T. H., Cheng J. T., Chen M. R., Tsai C. H. (2007) J. Virol. 81, 2459–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee C. P., Chen J. Y., Wang J. T., Kimura K., Takemoto A., Lu C. C., Chen M. R. (2007) J. Virol. 81, 5166–5180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee C. P., Huang Y. H., Lin S. F., Chang Y., Chang Y. H., Takada K., Chen M. R. (2008) J. Virol. 82, 11913–11926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J. T., Yang P. W., Lee C. P., Han C. H., Tsai C. H., Chen M. R. (2005) J. Virol. 86, 3215–3225 [DOI] [PubMed] [Google Scholar]
- 41.Makarova O., Kamberov E., Margolis B. (2000) BioTechniques 29, 970–972 [DOI] [PubMed] [Google Scholar]
- 42.Wang J. T., Doong S. L., Teng S. C., Lee C. P., Tsai C. H., Chen M. R. (2009) J. Virol. 83, 1856–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young L., Alfieri C., Hennessy K., Evans H., O'Hara C., Anderson K. C., Ritz J., Shapiro R. S., Rickinson A., Kieff E. (1989) N. Engl. J. Med. 321, 1080–1085 [DOI] [PubMed] [Google Scholar]
- 44.Tsai C. H., Liu M. T., Chen M. R., Lu J., Yang H. L., Chen J. Y., Yang C. S. (1997) J. Biomed. Sci. 4, 69–77 [DOI] [PubMed] [Google Scholar]
- 45.Hsu T. Y., Chang Y., Wang P. W., Liu M. Y., Chen M. R., Chen J. Y., Tsai C. H. (2005) J. Virol. 86, 317–322 [DOI] [PubMed] [Google Scholar]
- 46.Luo X. N., Mookerjee B., Ferrari A., Mistry S., Atweh G. F. (1994) J. Biol. Chem. 269, 10312–10318 [PubMed] [Google Scholar]
- 47.Marklund U., Osterman O., Melander H., Bergh A., Gullberg M. (1994) J. Biol. Chem. 269, 30626–30635 [PubMed] [Google Scholar]
- 48.Brattsand G., Marklund U., Nylander K., Roos G., Gullberg M. (1994) Eur. J. Biochem./FEBS 220, 359–368 [DOI] [PubMed] [Google Scholar]
- 49.Cassimeris L. (2002) Curr. Opin. Cell Biol. 14, 18–24 [DOI] [PubMed] [Google Scholar]
- 50.Melander Gradin H., Marklund U., Larsson N., Chatila T. A., Gullberg M. (1997) Mol. Cell. Biol. 17, 3459–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gavet O., Ozon S., Manceau V., Lawler S., Curmi P., Sobel A. (1998) J. Cell Science 111, 3333–3346 [DOI] [PubMed] [Google Scholar]
- 52.Chee M. S., Lawrence G. L., Barrell B. G. (1989) J. Gen. Virol. 70, 1151–1160 [DOI] [PubMed] [Google Scholar]
- 53.Smith R. F., Smith T. F. (1989) J. Virol. 63, 450–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gershburg E., Marschall M., Hong K., Pagano J. S. (2004) J. Virol. 78, 12140–12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manna T., Thrower D. A., Honnappa S., Steinmetz M. O., Wilson L. (2009) J. Biol. Chem. 284, 15640–15649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersen S. S., Ashford A. J., Tournebize R., Gavet O., Sobel A., Hyman A. A., Karsenti E. (1997) Nature 389, 640–643 [DOI] [PubMed] [Google Scholar]
- 57.Budde P. P., Kumagai A., Dunphy W. G., Heald R. (2001) J. Cell Biol. 153, 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Küntziger T., Gavet O., Manceau V., Sobel A., Bornens M. (2001) Mol. Biol. Cell 12, 437–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawaguchi Y., Kato K., Tanaka M., Kanamori M., Nishiyama Y., Yamanashi Y. (2003) J. Virol. 77, 2359–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asai R., Kato A., Kawaguchi Y. (2009) J. Gen. Virol. 90, 1575–1581 [DOI] [PubMed] [Google Scholar]