Abstract
Peroxisome proliferator-activated receptor-γ (PPARγ) is a ligand-activated transcription factor of the nuclear hormone receptor superfamily. Increasing evidence suggests that PPARγ is involved in the regulation of vascular function and blood pressure in addition to its well recognized role in metabolism. Thiazolidinediones, PPARγ agonists, lower blood pressure and have protective vascular effects through largely unknown mechanisms. In contrast, loss-of-function dominant-negative mutations in human PPARγ cause insulin resistance and severe early onset hypertension. Recent studies using genetically manipulated mouse models have begun to specifically address the importance of PPARγ in the vasculature. In this minireview, evidence for a protective role of PPARγ in the endothelium and vascular smooth muscle, derived largely from studies of genetically manipulated mice, will be discussed.
Keywords: Endothelium, Gene Knockout, Smooth Muscle, Transcription Factors, Transgenic, Arterial Pressure, Hypertension, PPAR
Expression and Mechanism of Action
Two PPARγ2 isoforms termed PPARγ1 and PPARγ2, which differ by a 28-amino acid (30-amino acid in human) extension at the N terminus due to differential promoter usage and alternative splicing, have been identified (1). Despite nearly exclusive expression of PPARγ2 (the longer isoform) in adipose tissue, PPARγ1 is found in many tissues but at lower levels (1). Although a number of putative endogenous ligands have been identified, many exhibit low affinity, and thus, PPARγ may still be considered an orphan nuclear receptor. Proposed ligands include polyunsaturated fatty acids, oxidized fatty acids, and prostaglandin J2 (1). TZDs are high affinity synthetic PPARγ ligands that have been used to treat patients with insulin resistance and type 2 diabetes.
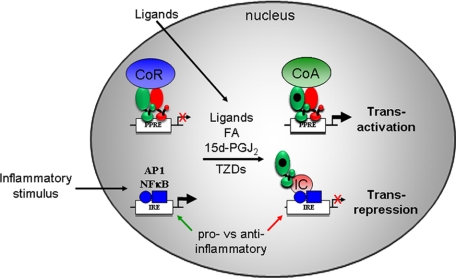
Mechanistically, in the absence of ligand, PPARγ/RXR heterodimers are bound with a complex of co-repressors to PPREs in the regulatory region of target genes, resulting in active transcriptional silencing. Ligand activation induces a conformational change that stimulates dissociation of co-repressors and replacement with a complex of co-activators to facilitate transactivation of PPARγ target genes (Fig. 1) (1). The co-activator complex includes proteins that modulate chromatin structure and bridge PPARγ with the transcriptional machinery. Genomic deletion of PPARγ results in higher basal target gene expression (2), consistent with a mechanism of active repression. PPARγ also controls expression of genes through an alternative mechanism termed “transrepression.” PPARγ-mediated transrepression occurs via interaction with other transcription factors (such as NF-κB and AP1), and although it is ligand-dependent, it apparently does not require binding of PPARγ to a PPRE. It has been suggested that the transrepression process plays important roles in inhibiting inflammatory gene expression (Fig. 1) (3). The transrepression pathway for PPARγ has been most intensively studied in macrophages as reported previously (3) and reviewed recently (4).
FIGURE 1.
Summary of PPARγ transcriptional mechanisms. In the transactivation pathway (top), unliganded PPARγ (green) and RXR (red) complex with co-repressors (CoR; blue) on the PPRE of a PPARγ target gene. This leads to active repression in the absence of PPARγ ligands. The level of repression and thus the level of basal transcription will depend on the cycling of co-repressors on and off the chromatin. Addition of endogenous or exogenous ligands induces a change in the complex that dismisses the co-repressors and recruits a complex of co-activators (CoA; green), leading to an increase in transcription of the target gene. This complex has many components, including enzymes that modify histones and mediators linking the PPARγ complex with the transcriptional machinery. In the transrepression pathway (bottom), an inflammatory stimulus activates the transcription of pro-inflammatory genes via NF-κB, AP1, and other pathways through an association of these factors with their cognate response element (inflammatory response element (IRE)). PPARγ ligands exert their anti-inflammatory action by association of liganded PPARγ with an “inhibitory complex” (IC; red), which decreases expression of the pro-inflammatory genes. Apparently, this does not require a PPRE or partnership with RXR. Components of the complex include post-translationally modified PPARγ, SUMO (small ubiquitin-like modifier)-protein ligases, ubiquitin-conjugating enzymes, and proteins that link these with co-repressors targeting AP1 and/or NF-κB. A comprehensive review of this pathway has been published recently (4). FA, fatty acid; 15d-PGJ2, 15-deoxy-Δ12,14-prostaglandin J2.
PPARγ Mutations and Hypertension
Given that PPARγ is a critical regulator of adipogenesis, it is not surprising that mutations or polymorphisms of PPARγ in humans are often associated with impaired adiposity. However, rare mutations in PPARγ have been reported to cause HT. For instance, patients with heterozygous mutations (P467L or V290M) in the ligand-binding domain of PPARγ develop severe insulin resistance and HT at an early age (5). Mechanistic studies revealed that these mutants retain the ability to bind to DNA at consensus PPREs but exhibit impaired basal and ligand-induced transcriptional activity. Moreover, they can inhibit the transcriptional activation of coexpressed wild-type PPARγ. Consequently, it was concluded that they act in a DN manner. We reported recently that expression of DN PPARγ (mouse P465L, which is equivalent to human P467L) in aorta inhibits the expression of genes that are normally stimulated by TZDs (6). It remains unclear if these mutations affect the transrepression pathway. Another mutation in the ligand-binding domain (F360L) associated with HT has also been identified. However, the F360L mutant does not possess DN activity (7). In addition, other patients with rare heterozygous mutations (C114R, C131Y, or C162W) in the DNA-binding domain of PPARγ exhibit increased BP (8). These mutations also act in a DN manner but block transcriptional activation via a mechanism involving sequestration of RXR and co-activators. Although the prevalence of patients carrying these mutations is low, the evidence clearly implicates a significant role of PPARγ or PPARγ target genes in BP regulation.
Endothelial PPARγ
Endothelial dysfunction is a marker of cardiovascular disease and is closely associated with inflammation. PPARγ is expressed in ECs, and it exerts anti-inflammatory effects through various mechanisms (Fig. 2) (9). Expression of constitutively active PPARγ in cultured ECs reduces adhesion molecule expression and leukocyte recruitment via suppression of NF-κB and AP1 activation (10), presumably through a transrepression pathway similar to that occurring in macrophages (3). PPARγ also inhibits the inflammatory responses to other cytokines (interferon-γ and tumor necrosis factor-α) in human EC cultures (11). Inhibition of the protein kinase C pathway has also been reported as an anti-inflammatory target of PPARγ (12).
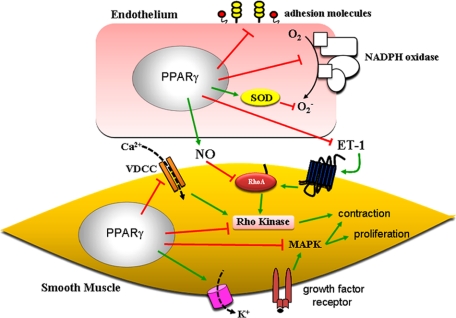
FIGURE 2.
Summary of PPARγ-mediated effects on pathways in the endothelium and vascular smooth muscle. PPARγ affects gene expression and consequently the vascular phenotype via PPRE-dependent transactivation and the transrepression mechanism as shown in Fig. 1. In ECs, activation of PPARγ reduces inflammation, reactive oxygen species, and ET-1 release while increasing NO bioavailability. In VSMCs, PPARγ ligand mediates inhibition of Ca2+ influx through the voltage-dependent Ca2+ channel (VDCC) and blunts Rho kinase activation. Moreover, TZDs prevent VSMC proliferation by inhibiting growth factor signaling. Therefore, PPARγ may control BP homeostasis by regulating the balance between vasodilation and vasoconstriction. SOD, superoxide dismutase; MAPK, mitogen-activated protein kinase.
ECs regulate vascular tone in part through the production of vasoactive agents. Activation of PPARγ enhances NO production (13), whereas disruption of PPARγ in ECs causes a reduction in NO release (14). Moreover, PPARγ ligand reduces reactive oxygen species production in ECs by decreasing expression of the NADPH oxidase subunits nox1, gp91phox, and nox4 and by increasing activity and expression of copper/zinc superoxide dismutase (15). These data suggest that activation of endothelial PPARγ can increase NO bioavailability. Besides modulation of NO, activation of PPARγ can affect vascular tone through suppression of ET-1 synthesis in ECs (16). Consequently, activation of PPARγ is beneficial for endothelial function by promoting an anti-inflammatory and antioxidant milieu and by maintaining the proper balance of vasodilators and vasoconstrictors, which would influence vascular tone.
Vascular Smooth Muscle PPARγ
Activation of PPARγ by TZDs can directly affect smooth muscle-mediated constriction. As reported in both VSMC culture and rat aortic rings, TZDs can inhibit the L-type Ca2+ current (17), thereby reducing vascular contraction (Fig. 2). Similar findings were also reported in a resistance vessel. Non-TZD PPARγ agonists blunt myogenic tone in pressurized mesenteric artery perhaps through inhibition of L-type Ca2+ channels (18). Interestingly, TZDs may promote vasorelaxation through stimulation of Ca2+-activated K+ channels, but the mechanism remains unclear (19). Caution must be taken before concluding that the vasodepressor action of these compounds occurs through PPARγ-dependent mechanisms because pharmacological concentrations of RZ (>1000 μm) can cause relaxation in isolated arteries, effects that are not blocked by a PPARγ antagonist (20).
Activation of PPARγ can also affect Ca2+ sensitization. In primary rat VSMCs, PIO promotes the activation of myosin light chain phosphatase, thus reducing phosphorylation of myosin light chain (21). Similarly, Wakino et al. (22) demonstrated that PIO and troglitazone treatment suppresses Ang II-stimulated Rho kinase activity in vitro. PIO treatment lowers BP in SHRs, which is associated with inhibition of Rho kinase activity in the vasculature. As discussed in detail below, we reported recently that aortas from mice with vascular smooth muscle-targeted expression of the DN PPARγ mutation P467L exhibit a marked contraction response to ET-1, which is significantly blunted by Rho kinase inhibition (23). Taken together, these data indicate that PPARγ may have a profound effect on vasoconstrictor pathways.
Evidence from Human Studies
TZDs moderately decrease BP in individuals with insulin resistance, presumably, but not exclusively, by improving insulin sensitivity. However, when comparing the effects of TZDs with other insulin-sensitizing drugs or insulin secretagogues on BP in insulin-resistant individuals (24), only TZDs were associated with a reduction in BP. These observations suggest that TZDs may exert vasodepressor effects independently of their insulin-sensitizing properties. As observed in the PROactive Trial (Prospective Pioglitazone Clinical Trial in Macrovascular Events), a PIO-mediated reduction in mean systolic BP of 3 mm Hg is associated with a significant reduction in cardiovascular mortality in patients with type 2 diabetes (25). Therefore, although TZDs cause a small reduction in BP, it appears that this is sufficient to lower cardiovascular risk (24).
Interestingly, the beneficial cardiovascular effects of TZDs might extend beyond the lowering of BP. In the DREAM Trial (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication), RZ substantially reduced the risk of diabetes or death (26, 27). Ramipril, an inhibitor of the renin-angiotensin system (an angiotensin-converting enzyme inhibitor), did not reduce risk despite superior BP reduction. The substudy of the DREAM Trial (STARR, Study of Atherosclerosis with Ramipril and Rosiglitazone) that evaluated carotid-intima media thickness, a measure of vascular disease progression, demonstrated that RZ modestly reduces carotid-intima media thickness progression (28). Consequently, PPARγ may play a role in vascular structure and growth (discussed below).
It is important to recognize that although TZDs appear to have some cardiovascular protective actions, the beneficial effect of TZDs may be counterbalanced by an increase in the incidence of heart failure and systemic edema. For example, RZ is associated with a higher risk of heart failure as reported in the DREAM Trial and the interim report from the RECORD Trial (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes) (29). The caution regarding the effects of RZ on the risk of myocardial infarction and cardiovascular death remains under debate.
Evidence from Animal Studies
TZDs have been reported to reduce BP in both insulin-resistant and non-insulin-resistant models of HT. For example, RZ reduces the development of HT in insulin-resistant models, fructose-fed rats (30) and obese Zucker rats (31). The BP-lowering effect of TZDs in these models may be due in part to metabolic improvements. Although results from non-insulin-resistant HT animal models are somewhat controversial, most studies have demonstrated beneficial effects of TZDs on HT and vascular function.
In Ang II-induced HT, TZDs attenuate the development of HT without affecting the lipid profile. Activation of PPARγ by TZDs reduces vascular inflammation, prevents up-regulation of the Ang II type 1 receptor, and improves endothelium-dependent dilation in mesenteric artery (32). Interestingly, RZ lowers the BP and improves the vascular function of a lifelong HT mouse model expressing both human renin and human angiotensinogen transgenes. However, RZ has no effect on expression of endothelial nitric-oxide synthase, Ang II type 1 receptor, or prepro-ET-1 (20). In addition, neurologic HT induced by injection of Ang II into the rostral ventral lateral medulla of the brain is prevented by RZ (33).
Four-week treatment with PIO lowers BP in SHRs (34). It is notable that expression of PPARγ in mesenteric arteries is increased in the SHR (35), which may facilitate a greater vasodepressor response to TZDs. Similarly, RZ reduces BP and improves vascular relaxation responses to NO in SHRs by a mechanism associated with a reduction in oxidative stress (36). In DOCA-salt-induced HT rats, treatment with RZ partially prevents the increase in BP and preserves endothelial function. Prepro-ET-1 mRNA is significantly increased in mesenteric arteries and aortas of DOCA-salt-treated rats, and this is abrogated by RZ (37).
Unique Insights from Genetic Mouse Models
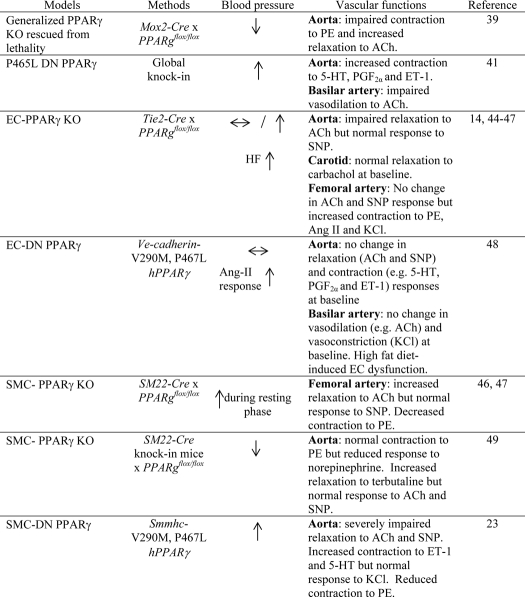
Taken together, accumulating evidence from humans and animal models supports the hypothesis that activation of PPARγ may promote antihypertensive effects and prevent vascular dysfunction. However, most previous studies investigating PPARγ have relied heavily, if not exclusively, on systemic treatment with TZDs. Because of its role as an insulin sensitizer, caution must be taken to conclude that the cardioprotective effects of TZDs are attributable to the direct actions of PPARγ in the vasculature. Moreover, the majority of studies using TZDs have not demonstrated whether the vasodepressor effect is mediated through PPARγ. To resolve these issues, investigators have developed genetic models causing either ablation or interference with PPARγ. In initial studies, this was performed systemically, but in later studies, it was targeted specifically to the endothelium or vascular smooth muscle. These studies have now provided unique insights into the specific vascular role of PPARγ (summarized in Table 1).
TABLE 1.
Summary of BP and vascular phenotypes of different genetic models used to study the role of PPARγ in the vasculature
5-HT, 5-hydroxytryptamine (serotonin); PGF2α, prostaglandin F2α; SNP, sodium nitroprusside; hPPARγ, human PPARγ; SMMHC, smooth muscle myosin heavy chain.
Systemic Models
Ablation of PPARγ
Classic PPARγ KO mice die in utero due to embryonic defects in placental vascularization and myocardial thinning (38), suggesting a critical role for PPARγ first in early fetal and then in later cardiovascular development. Subsequently, viable PPARγ KO mice were generated by preserving PPARγ expression in trophoblasts (39). Despite severe lipodystrophy and insulin resistance in this mouse model, total loss of PPARγ surprisingly leads to hypotension. These findings are counterintuitive because insulin resistance is normally associated with increased BP, and PPARγ activation lowers BP (39). High salt treatment increases BP in both wild-type and PPARγ KO mice similarly, and the BP in PPARγ KO mice remains lower than that in wild-type mice. These data suggest that other mechanisms rather than salt handling could account for lower BP. It is notable that aortas from these mice exhibit a blunted contraction response to PE and an enhanced relaxation response to ACh, which may explain the hypotensive phenotype (39). What remains unresolved is how to reconcile the contradiction that PPARγ activators lower BP and improve vascular function, whereas PPARγ deficiency does the same.
DN Mutation of PPARγ
Patients heterozygous for DN mutations in PPARγ were noted to have severe insulin resistance, partial lipodystrophy, and early onset HT (5). Homozygous knock-in mice carrying the equivalent to the P467L mutation in humans (P465L in mice) die in utero, consistent with this allele lacking transactivation capacity (40). Similar to P467L patients, heterozygous P465L/+ (one normal and one mutant allele) mice have abnormal fat distribution and have HT. However, the knock-in mice do not exhibit insulin resistance as observed in human patients. Further studies have indicated that renal salt and water handling is normal (40). These observations provide evidence that the effects of PPARγ on BP regulation may be uncoupled from its effects on insulin sensitivity. We therefore tested the hypothesis that P465L knock-in mice would exhibit vascular dysfunction. We reported that cerebral arteries and arterioles exhibit severe endothelial dysfunction, which is restored by a scavenger of superoxide (41). This suggests that PPARγ protects vascular function by preventing oxidative stress. We also observed vascular remodeling and hypertrophy in cerebral arterioles from these mice (41). Both of these structural changes and impaired vasodilation could reduce vasodilator capacity and cerebral blood flow and could potentially contribute to increased peripheral vascular resistance and thus BP. These studies also suggest that cerebral vessels are particularly sensitive to effects of PPARγ interference (41).
Tissue-specific Models
To differentiate the specific role of PPARγ in the vasculature, cell-specific promoters have been used to modulate PPARγ expression in either the endothelium or vascular smooth muscle.
Genetic Models of Endothelial PPARγ
Ablation of Endothelial PPARγ
Most studies investigating the role of PPARγ in ECs are based on the use of Tie2 promoter-driven Cre recombinase. This promoter is widely utilized as “an EC-specific promoter.” However, the data obtained from the models must be interpreted cautiously because expression of Tie2-Cre is also found in hematopoiesis-derived cell types. Indeed, ablation of PPARγ by Tie2-Cre results in osteopetrosis (42) and production of toxic milk in pregnancy (43), effects unlikely to be directly attributable to EC PPARγ. Therefore, loss of PPARγ in non-EC types in which Tie2 is active could lead to confounding results.
Nicol et al. (44) first reported that disruption of PPARγ in ECs (E-PPARγ null mice) does not alter base-line BP. However, E-PPARγ null mice exhibit HT after being fed a HF diet. RZ decreases BP in wild-type mice during a HF diet but fails to reduce BP in E-PPARγ null mice (44), suggesting that endothelial PPARγ is required for the BP-lowering effects of TZDs in response to HF. Further studies from the same mouse model have addressed the role of endothelial PPARγ in systemic metabolism. The investigators in this study recognized the potential confounding influence of Tie2-Cre expression in hematopoietic cells and performed bone marrow transplantation to ensure that the effects were due to endothelial PPARγ. The results were surprising, as loss of PPARγ specifically in ECs protected against HF diet-induced adiposity and insulin resistance (45). Endothelium-dependent relaxation remained impaired in carotid arteries from these mice after a HF diet, suggesting a complicated mechanism by which endothelial PPARγ regulates both systemic metabolism and vascular function. These data suggest the provocative hypothesis that EC PPARγ may be beneficial in the vessel but have some other detrimental effects in other cell types.
The mechanisms responsible for the BP actions of endothelial PPARγ are not completely understood. The following studies proposed potential mechanisms. PPARγ deletion in ECs causes hypercontraction responses to PE, Ang II, and KCl in the femoral artery without altering ACh-mediated relaxation. This is accompanied by a loss of rhythmicity of the clock gene Bmal1 in the blood vessel (46). It remains unclear how changes in the rhythmicity of gene expression in the blood vessel could play a role in the reduction of BP during the dark phase. In a separate study by the same group, the HT caused by DOCA-salt was similar between E-PPARγ null mice and control littermates. RZ significantly lowers BP only in DOCA-treated control mice but not in E-PPARγ null mice (47), consistent with the earlier report by Nicol et al. (44) that endothelial PPARγ is required for the BP-lowering effect of TZDs.
Using the same E-PPARγ null mouse model, Kleinhenz et al. (14) reported quite different findings. Here, E-PPARγ null mice exhibited significantly increased base-line BP, although the BP response to Ang II infusion was similar in E-PPARγ null and control mice. Vascular reactivity of the aorta in response to ACh was markedly impaired, whereas the response to NO donor was normal, indicating endothelial dysfunction. As expected, lack of PPARγ in ECs diminished NO production and caused oxidative stress. Elevated NF-κB binding activity was also found in aortas from these mice, suggesting a loss of the anti-inflammatory actions of PPARγ. This study provides additional compelling evidence that endothelial PPARγ exerts vascular protection by reducing oxidative stress and inflammation.
DN Mutation of Endothelial PPARγ
The roles of endothelial PPARγ in regulating vascular function have been explored by targeting expression of DN mutant PPARγ (P467L or V290M) specifically in ECs under the control of the vascular endothelial cadherin promoter (48). These are the same mutations that cause HT in human patients (5). Neither the aortas nor basilar arteries from transgenic mice fed a normal diet exhibited vascular dysfunction. However, after 12 weeks of a HF diet, basilar arteries from transgenic mice exhibited markedly impaired endothelium-dependent dilation, which was reversed by a superoxide scavenger. Endothelial dysfunction became evident in aortas from transgenic mice after prolonged HF diet treatment (25 weeks) (48). These data suggest that interference of PPARγ in ECs is clearly deleterious. An array of genes related to oxidative stress such as NADPH oxidase subunits was found to be increased in ECs derived from transgenic mice. Although there was no difference in BP between transgenic and control mice, the pressor response to Ang II was augmented in transgenic mice carrying endothelium-specific DN PPARγ (48). All of these data support the notion that PPARγ in ECs plays a critical role in protecting the blood vessel against dysfunction in response to a HF diet. Moreover, cerebral vessels appear particularly susceptible to vascular dysfunction after the combination of interference of EC PPARγ and a HF diet.
Genetic Models of Smooth Muscle PPARγ
Ablation of Smooth Muscle PPARγ
Recent studies by Chang et al. (49) demonstrated that VSMC-selective PPARγ deficiency leads to hypotension. Again, this is particularly interesting because ligand-mediated activation of PPARγ typically reduces BP, suggesting that genes related to BP regulation could be suppressed by PPARγ. β2-Adrenergic receptor expression is increased by PPARγ deficiency, which may account for the increased dilation in response to β-adrenergic receptor agonists and the hypotensive phenotype observed in these mice (49). In contrast, Wang et al. (46) demonstrated that loss of smooth muscle PPARγ leads to abnormalities in circadian rhythm and HT during the resting phase. The discrepancy between these two studies may be attributable to differences in engineering of SMC-targeted Cre recombinase mice, with the former model being a knock-in allele with very accurate SMC targeting. Moreover, Wang et al. (47) reported that DOCA-salt treatment increases BP similarly in wild-type and VSMC-targeted PPARγ null mice and that RZ successfully reduces BP in both. On the basis of this study, the authors concluded that smooth muscle PPARγ is not required for the antihypertensive actions of TZDs, unlike the results from the E-PPARγ null mice. Unexpectedly, the relaxation response to ACh in femoral arteries from VSMC-targeted PPARγ null mice was improved, whereas the contraction response to PE was slightly reduced (47).
DN Mutation of Smooth Muscle PPARγ
We have generated transgenic mice harboring a DN PPARγ mutation under the control of the smooth muscle myosin heavy chain promoter. These mice exhibit HT and severe aortic dysfunction (23). Relaxation in response to ACh and sodium nitroprusside is significantly impaired in the thoracic aorta in vitro, and reduced relaxation in response to a cGMP analogue provides evidence that the PPARγ-mediated defect is downstream of cGMP. Strikingly, the contractile responses of aortas from transgenic mice to agonists such as ET-1 and serotonin are markedly elevated despite a normal receptor-independent contraction response to KCl (23). These transgenic mice display an elevation of BP, consistent with mice and humans carrying these mutations. Interestingly, cerebral arterioles in S-P467L mice show hypertrophy and inward remodeling (23). Our data highlight a critical role of smooth muscle PPARγ in the regulation of vascular structure and function.
Concluding Remarks
Most of the evidence clearly demonstrates that vascular PPARγ plays an important role as a regulator of vascular function and BP. However, there is substantial conflict in the literature regarding the importance and role of PPARγ as gleaned from cell-specific knock-out studies. The reason for these conflicts may include differences in the specificity of Cre recombinase models employed, the genetic background, the specific vessels studied, and the measurements of BP. Each of these factors could affect interpretation of the data. Indeed, our studies of endothelium-specific interference with PPARγ illustrate that there is differential susceptibility to dysfunction among vascular beds.
One aspect of these studies that requires additional analysis is whether genetic ablation (Cre-loxP) is functionally identical to genetic interference (though DN mutations). It is quite likely that these models of PPARγ ablation are not identical. For example, PPARγ deficiency may cause a loss of active repression of certain target genes that occurs in the absence of ligand. In contrast, DN PPARγ causes increased repression due to increased occupancy of PPARγ/RXR·co-repressor complexes at PPREs (50). Therefore, the mode of ablation needs to be considered in the further development of models designed to interrogate PPARγ. Our choice of the DN mutant approach was motivated by the observation that these are bona fide missense mutations causing early onset HT in human patients.
Supplementary Material
This is the second of five articles in the “Biochemistry in Medicine: Hypertension Minireview Series.” This minireview will be reprinted in the 2010 Minireview Compendium, which will be available in January, 2011.
- PPARγ
- peroxisome proliferator-activated receptor-γ
- TZD
- thiazolidinedione
- RXR
- retinoid X receptor
- PPRE
- peroxisome proliferator response element
- HT
- hypertension/hypertensive
- DN
- dominant-negative
- BP
- blood pressure
- EC
- endothelial cell
- ET-1
- endothelin-1
- VSMC
- vascular smooth muscle cell
- RZ
- rosiglitazone
- PIO
- pioglitazone
- Ang II
- angiotensin II
- SHR
- spontaneous hypertensive rat
- DOCA
- deoxycorticosterone acetate
- KO
- knock-out
- PE
- phenylephrine
- ACh
- acetylcholine
- HF
- high fat.
REFERENCES
- 1.Bensinger S. J., Tontonoz P. (2008) Nature 454, 470–477 [DOI] [PubMed] [Google Scholar]
- 2.Guan H. P., Ishizuka T., Chui P. C., Lehrke M., Lazar M. A. (2005) Genes Dev. 19, 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascual G., Fong A. L., Ogawa S., Gamliel A., Li A. C., Perissi V., Rose D. W., Willson T. M., Rosenfeld M. G., Glass C. K. (2005) Nature 437, 759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perissi V., Jepsen K., Glass C. K., Rosenfeld M. G. (2010) Nat. Rev. Genet. 11, 109–123 [DOI] [PubMed] [Google Scholar]
- 5.Barroso I., Gurnell M., Crowley V. E., Agostini M., Schwabe J. W., Soos M. A., Maslen G. L., Williams T. D., Lewis H., Schafer A. J., Chatterjee V. K., O'Rahilly S. (1999) Nature 402, 880–883 [DOI] [PubMed] [Google Scholar]
- 6.Keen H. L., Halabi C. M., Beyer A. M., de Lange W. J., Liu X., Maeda N., Faraci F. M., Casavant T. L., Sigmund C. D. (2010) Arterioscler. Thromb. Vasc. Biol. 30, 518–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegele R. A., Cao H., Frankowski C., Mathews S. T., Leff T. (2002) Diabetes 51, 3586–3590 [DOI] [PubMed] [Google Scholar]
- 8.Agostini M., Schoenmakers E., Mitchell C., Szatmari I., Savage D., Smith A., Rajanayagam O., Semple R., Luan J., Bath L., Zalin A., Labib M., Kumar S., Simpson H., Blom D., Marais D., Schwabe J., Barroso I., Trembath R., Wareham N., Nagy L., Gurnell M., O'Rahilly S., Chatterjee K. (2006) Cell Metab. 4, 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan S. Z., Usher M. G., Mortensen R. M. (2008) Circ. Res. 102, 283–294 [DOI] [PubMed] [Google Scholar]
- 10.Wang N., Verna L., Chen N. G., Chen J., Li H., Forman B. M., Stemerman M. B. (2002) J. Biol. Chem. 277, 34176–34181 [DOI] [PubMed] [Google Scholar]
- 11.Lombardi A., Cantini G., Piscitelli E., Gelmini S., Francalanci M., Mello T., Ceni E., Varano G., Forti G., Rotondi M., Galli A., Serio M., Luconi M. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 718–724 [DOI] [PubMed] [Google Scholar]
- 12.Verrier E., Wang L., Wadham C., Albanese N., Hahn C., Gamble J. R., Chatterjee V. K., Vadas M. A., Xia P. (2004) Circ. Res. 94, 1515–1522 [DOI] [PubMed] [Google Scholar]
- 13.Polikandriotis J. A., Mazzella L. J., Rupnow H. L., Hart C. M. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1810–1816 [DOI] [PubMed] [Google Scholar]
- 14.Kleinhenz J. M., Kleinhenz D. J., You S., Ritzenthaler J. D., Hansen J. M., Archer D. R., Sutliff R. L., Hart C. M. (2009) Am. J. Physiol. Heart Circ. Physiol. 297, H1647–H1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang J., Kleinhenz D. J., Lassègue B., Griendling K. K., Dikalov S., Hart C. M. (2005) Am. J. Physiol. Cell Physiol. 288, C899–C905 [DOI] [PubMed] [Google Scholar]
- 16.Delerive P., Martin-Nizard F., Chinetti G., Trottein F., Fruchart J. C., Najib J., Duriez P., Staels B. (1999) Circ. Res. 85, 394–402 [DOI] [PubMed] [Google Scholar]
- 17.Buchanan T. A., Meehan W. P., Jeng Y. Y., Yang D., Chan T. M., Nadler J. L., Scott S., Rude R. K., Hsueh W. A. (1995) J. Clin. Invest. 96, 354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heppner T. J., Bonev A. D., Eckman D. M., Gomez M. F., Petkov G. V., Nelson M. T. (2005) Pharmacology 73, 15–22 [DOI] [PubMed] [Google Scholar]
- 19.Eto K., Ohya Y., Nakamura Y., Abe I., Fujishima M. (2001) Eur. J. Pharmacol. 423, 1–7 [DOI] [PubMed] [Google Scholar]
- 20.Ryan M. J., Didion S. P., Mathur S., Faraci F. M., Sigmund C. D. (2004) Hypertension 43, 661–666 [DOI] [PubMed] [Google Scholar]
- 21.Atkins K. B., Irey B., Xiang N., Brosius F. C., 3rd (2009) Am. J. Physiol. Cell Physiol. 296, C1151–C1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakino S., Hayashi K., Kanda T., Tatematsu S., Homma K., Yoshioka K., Takamatsu I., Saruta T. (2004) Circ. Res. 95, e45–e55 [DOI] [PubMed] [Google Scholar]
- 23.Halabi C. M., Beyer A. M., de Lange W. J., Keen H. L., Baumbach G. L., Faraci F. M., Sigmund C. D. (2008) Cell Metab. 7, 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giles T. D., Sander G. E. (2007) Curr. Hypertens. Rep. 9, 332–337 [DOI] [PubMed] [Google Scholar]
- 25.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. (2002) Lancet 360, 1903–1913 [DOI] [PubMed] [Google Scholar]
- 26.Gerstein H. C., Yusuf S., Bosch J., Pogue J., Sheridan P., Dinccag N., Hanefeld M., Hoogwerf B., Laakso M., Mohan V., Shaw J., Zinman B., Holman R. R. (2006) Lancet 368, 1096–1105 [DOI] [PubMed] [Google Scholar]
- 27.Bosch J., Yusuf S., Gerstein H. C., Pogue J., Sheridan P., Dagenais G., Diaz R., Avezum A., Lanas F., Probstfield J., Fodor G., Holman R. R. (2006) N. Engl. J. Med. 355, 1551–1562 [DOI] [PubMed] [Google Scholar]
- 28.Lonn E. M., Gerstein H. C., Sheridan P., Smith S., Diaz R., Mohan V., Bosch J., Yusuf S., Dagenais G. R. (2009) J. Am. Coll. Cardiol. 53, 2028–2035 [DOI] [PubMed] [Google Scholar]
- 29.Home P. D., Pocock S. J., Beck-Nielsen H., Gomis R., Hanefeld M., Jones N. P., Komajda M., McMurray J. J. (2007) N. Engl. J. Med. 357, 28–38 [DOI] [PubMed] [Google Scholar]
- 30.Sharabi Y., Oron-Herman M., Kamari Y., Avni I., Peleg E., Shabtay Z., Grossman E., Shamiss A. (2007) Am. J. Hypertens. 20, 206–210 [DOI] [PubMed] [Google Scholar]
- 31.Walker A. B., Chattington P. D., Buckingham R. E., Williams G. (1999) Diabetes 48, 1448–1453 [DOI] [PubMed] [Google Scholar]
- 32.Diep Q. N., El Mabrouk M., Cohn J. S., Endemann D., Amiri F., Virdis A., Neves M. F., Schiffrin E. L. (2002) Circulation 105, 2296–2302 [DOI] [PubMed] [Google Scholar]
- 33.Chan S. H., Wu C. A., Wu K. L., Ho Y. H., Chang A. Y., Chan J. Y. (2009) Circ. Res. 105, 886–896 [DOI] [PubMed] [Google Scholar]
- 34.Wakino S., Hayashi K., Tatematsu S., Hasegawa K., Takamatsu I., Kanda T., Homma K., Yoshioka K., Sugano N., Saruta T. (2005) Hypertens. Res. 28, 255–262 [DOI] [PubMed] [Google Scholar]
- 35.Diep Q. N., Schiffrin E. L. (2001) Hypertension 38, 249–254 [DOI] [PubMed] [Google Scholar]
- 36.Potenza M. A., Gagliardi S., De Benedictis L., Zigrino A., Tiravanti E., Colantuono G., Federici A., Lorusso L., Benagiano V., Quon M. J., Montagnani M. (2009) Am. J. Physiol. Endocrinol. Metab 297, E685–E694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iglarz M., Touyz R. M., Amiri F., Lavoie M. F., Diep Q. N., Schiffrin E. L. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 45–51 [DOI] [PubMed] [Google Scholar]
- 38.Barak Y., Nelson M. C., Ong E. S., Jones Y. Z., Ruiz-Lozano P., Chien K. R., Koder A., Evans R. M. (1999) Mol. Cell 4, 585–595 [DOI] [PubMed] [Google Scholar]
- 39.Duan S. Z., Ivashchenko C. Y., Whitesall S. E., D'Alecy L. G., Duquaine D. C., Brosius F. C., 3rd, Gonzalez F. J., Vinson C., Pierre M. A., Milstone D. S., Mortensen R. M. (2007) J. Clin. Invest. 117, 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai Y. S., Kim H. J., Takahashi N., Kim H. S., Hagaman J. R., Kim J. K., Maeda N. (2004) J. Clin. Invest. 114, 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beyer A. M., Baumbach G. L., Halabi C. M., Modrick M. L., Lynch C. M., Gerhold T. D., Ghoneim S. M., de Lange W. J., Keen H. L., Tsai Y. S., Maeda N., Sigmund C. D., Faraci F. M. (2008) Hypertension 51, 867–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan Y., Chong L. W., Evans R. M. (2007) Nat. Med. 13, 1496–1503 [DOI] [PubMed] [Google Scholar]
- 43.Wan Y., Saghatelian A., Chong L. W., Zhang C. L., Cravatt B. F., Evans R. M. (2007) Genes Dev. 21, 1895–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicol C. J., Adachi M., Akiyama T. E., Gonzalez F. J. (2005) Am. J. Hypertens. 18, 549–556 [DOI] [PubMed] [Google Scholar]
- 45.Kanda T., Brown J. D., Orasanu G., Vogel S., Gonzalez F. J., Sartoretto J., Michel T., Plutzky J. (2009) J. Clin. Invest. 119, 110–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang N., Yang G., Jia Z., Zhang H., Aoyagi T., Soodvilai S., Symons J. D., Schnermann J. B., Gonzalez F. J., Litwin S. E., Yang T. (2008) Cell Metab. 8, 482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang N., Symons J. D., Zhang H., Jia Z., Gonzalez F. J., Yang T. (2009) Toxicol. Pathol. 37, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beyer A. M., de Lange W. J., Halabi C. M., Modrick M. L., Keen H. L., Faraci F. M., Sigmund C. D. (2008) Circ. Res. 103, 654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang L., Villacorta L., Zhang J., Garcia-Barrio M. T., Yang K., Hamblin M., Whitesall S. E., D'Alecy L. G., Chen Y. E. (2009) Circulation 119, 2161–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigmund C. D. (2010) Hypertension 55, 437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.