Abstract
Heme oxygenases (HOs) -1 and -2 catalyze the breakdown of heme to release carbon monoxide, biliverdin, and ferrous iron, which may preserve cell function during oxidative stress. HO-1 levels decrease in endothelial cells exposed to hypoxia, whereas the effect of hypoxia on HO-2 expression is unknown. The current study was carried out to determine if hypoxia alters HO-2 protein levels in human endothelial cells and whether this enzyme plays a role in preserving their viability during hypoxic stress. Human umbilical vein endothelial cells (HUVECs), human aortic endothelial cells (HAECs), and human blood outgrowth endothelial cells were exposed to 21% or 1% O2 for 48 or 16 h in the presence or absence of tumor necrosis factor-α (10 ng/ml) or H2O2 (100 μm). In all three endothelial cell types HO-1 mRNA and protein levels were decreased following hypoxic incubation, whereas HO-2 protein levels were unaltered. In HUVECs HO-2 levels were maintained during hypoxia despite a 57% reduction in steady-state HO-2 mRNA level and a 43% reduction in total protein synthesis. Polysome profiling revealed increased HO-2 transcript association with polysomes during hypoxia consistent with enhanced translation of these transcripts. Importantly, inhibition of HO-2 expression by small interference RNA increased oxidative stress, exacerbated mitochondrial membrane depolarization, and enhanced caspase activation and apoptotic cell death in cells incubated under hypoxic but not normoxic conditions. These data indicate that HO-2 is important in maintaining endothelial viability and may preserve local regulation of vascular tone, thrombosis, and inflammatory responses during reductions in systemic oxygen delivery.
Keywords: Cell/Apoptosis, Enzymes/Heme, Enzymes/Oxidase, Oxygen/Hypoxia, Oxygen/Radicals, Protein/Stability, Protein/Synthesis
Introduction
Hypoxia is frequently observed in patients with shock and cardiopulmonary diseases and in normal subjects at high altitudes. The compensatory mechanisms that preserve blood flow to vital organs under these conditions are, in part, dependent on the release of endothelium-derived vasoregulatory factors (1–3). During prolonged exposure to hypoxia, endothelial function is impaired and the adaptive responses that they mediate are compromised (2–4). Investigation of mechanisms that preserve endothelial cell survival and function in this setting is needed so that therapeutic strategies to mitigate the vascular effects of hypoxia may be developed.
Heme oxygenases 1 and 2 (HO-1 and -2)4 are the rate-limiting enzymes in the heme catabolic pathway that cleaves heme to release carbon monoxide (CO), biliverdin, and ferrous iron (5). These products possess anti-apoptotic, anti-oxidant, and anti-inflammatory properties, and so, may ameliorate the deleterious effects of hypoxia on endothelial function. HO-1 and -2 are the heme oxygenase isoforms identified in the endothelium. HO-1 expression is suppressed during hypoxia in human endothelial cells, and this is mediated by increased expression of the transcription repressor Bach1 (6). Although a protective role for HO-2 has been suggested in other conditions associated with impaired endothelium-dependent vasoregulation (diabetes and oxidant injury), the effect of hypoxia on HO-2 expression in human endothelial cells is unknown (7–9). The current study was carried out to test the hypothesis that HO-2 expression is oxygen-regulated in human endothelial cells and to determine whether it plays a role in preserving endothelial cell viability during hypoxic stress.
MATERIALS AND METHODS
Endothelial Cell Culture
Pooled human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAECs), purchased from Lonza (Basel, Switzerland), were cultured in EGM-2 medium according to manufacturer's instructions. Human blood outgrowth endothelial cells (HBOECs) were derived from peripheral blood as described previously (10). Healthy volunteers underwent a mononuclear cell collection (100 ml, 3–8% hematocrit) using a Cobe Spectra apheresis system (Gambro, Denver, CO). Cells were cultured in EGM-2MV medium containing 20% human serum in tissue culture flasks pre-coated with fibronectin (10 μg/ml) between 7 and 14 days to obtain a pure population of HBOECs. HBOECs were characterized by the surface expression of CD34, VEGFR2, CD146, and CD31 and stained negatively for CD14 and CD45. Early passage HUVECs (passages 3 and 4) and HAECs (passages 5–7) derived from multiple donors were used in these studies.
Hypoxic Incubation
Cells exposed to hypoxia were grown to 70% confluence and transferred, after changing the medium, to a humidified Plexiglas chamber maintained at 37 °C and continuously flushed with gas composed of 1% O2/5% CO2/balance N2. Normoxic control cells were exposed to air/5% CO2/balance N2 under otherwise identical conditions.
HO-1 and -2 RNA Interference
HUVECs and HAECs (105 cells/cm2) were seeded in antibiotic-free EGM-2 media in 60-mm dishes for 16–24 h and transfected with human HO-1 small interfering RNA (siRNA), HO-2 siRNA, HO-1 and -2 siRNA, or nonspecific control siRNA using siRNA Transfection Reagent (Santa Cruz Biotechnology, Santa Cruz, CA) following the manufacturer's instructions. Transfected cells were replated after 24 h in either 60-mm dishes (for flow cytometry studies) or 12-well plates (for measuring reactive oxygen species and mitochondrial membrane depolarization studies).
Quantitative Real-time Reverse Transcription-PCR
Total RNA was extracted from HUVECs incubated at 1% or 21% O2 for 16 or 48 h using the Protein and RNA Isolation System from Applied Biosystems (Foster City, CA). The first-strand cDNAs were synthesized using a cDNA synthesis kit containing random primers (Bio-Rad Laboratories). All quantitative reverse transcription-PCR analyses were performed in triplicate using SYBR® green with the ABI PRISM 7900 HT sequence detection system (Applied Biosystems). Levels of HO-1 and -2 cDNA were detected using the following primers: HO-1 (sense, 5′-GTC CGC AAC CCG ACA G-3′; antisense, 5′-ACC AGC TTG AAG CCG TCT C-3′, exon 1/2); HO-2 (sense, 5′-CCC TGG ACC TGA ACA TGA A-3′; antisense, 5′-ACC CAT CCT CCA AGG TCT C-3′, exon 4/5). The exponential portion of the amplification curve for 1000 copies of target amplicon passed through the cycle threshold (CT1000) at 24.34 ± 0.63 cycles for HO-1 and 24.35 ± 0.30 cycles for HO-2. 28 S (sense, 5′-TTG AAA ATC CGG GGG AGA G-3′; antisense, 5′-ACA TTG TTC CAA CAT GCC AG-3′) ribosomal RNA levels were used as a controls for normalization. Results obtained under each experimental condition were compared with their corresponding normoxic control values.
Western Blotting
Total protein was extracted from HUVECs, HAECs, and HBOECs incubated at 1% or 21% O2 for 16 or 48 h using the Protein and RNA Isolation System kit. After protein concentrations were determined by the Lowry method, total proteins (20 μg/lane) were resolved by 8–16% SDS-PAGE and transferred to nitrocellulose. To detect HO-1 and -2 protein, membranes were blocked in 5% milk-TTBS (50 mm Tris, 150 mm NaCl, pH 7.5) and incubated with rabbit polyclonal anti-HO-1 (1:2,000, Assay Designs, Ann Arbor, MI) or anti-HO-2 (1:2,000, Assay Designs) antibody overnight followed by peroxidase-conjugated anti-rabbit IgG (1:2,500). HO-1 and -2 protein levels were normalized to β-actin detected by reprobing the membranes with anti-β-actin monoclonal antibody (1:40,000, Sigma). The immunocomplexes were visualized with the ECL plus kit (Amersham Biosciences) and quantified by digital densitometry using the Quantity One software provided by Bio-Rad Laboratories. Results obtained under each experimental condition were compared with their corresponding normoxic control values.
[3H]Uridine and [3H]Leucine Incorporation
HUVECs plated in 12-well plates were incubated at 1% or 21% O2 for 16 or 48 h. As described (11), [3H]uridine or [3H]leucine (PerkinElmer Life Sciences) was added to media (10 μCi/well) for the last 15, 30, 45, or 60 min of normoxic or hypoxic exposure. Cells were washed with cold PBS, incubated in 10% trichloroacetic acid for 20 min, washed 3 times with 100% ethanol, and dried at 45 °C. The residues were dissolved in 0.3 n NaOH for 20 min and neutralized with 0.3 n HCl. The resultant mixture (400 μl) from each well was added to 5 ml of scintillation fluid, and radioactivity in each sample was counted in a liquid scintillation counter. The rate of [3H]leucine or [3H]uridine incorporation is represented by the slope of the radioactivity-incubation time relationship.
[35S]Methionine Incorporation
To determine the rate of HO-1 and -2 synthesis, HUVECs were incubated at 1% or 21% O2 for 14 h and then in methionine-free RPMI 1640 medium supplemented with [35S]methionine (10 μCi/ml, PerkinElmer Life Sciences) for an additional 2 h at the same O2 concentrations. To determine HO-1 and -2 protein stability, HUVECs were pulse-labeled with [35S]methionine (50 μCi/ml) in methionine-free RPMI 1640 medium at 21% O2 for 3 h and chased in EGM-2 media under 1% or 21% O2 for up to 24 h. Total proteins were extracted in radioimmune precipitation assay buffer (50 mm Tris, 150 mm NaCl, 50 mm NaF, 1 mm sodium orthovanadate, 5 mm benzamidine hydrochloride, 1 mm EDTA, 1% Igepal CA630, 0.5% sodium deoxycholate, and 0.1% SDS) and quantified by the Lowry method. Cell lysates (500 μg), precleared with protein G-Sepharose, were then immunoprecipitated using rabbit polyclonal anti-HO-1 (1:50) or anti-HO-2 (1:50) antibodies overnight. The resulting immunoprecipitates were separated by 10% SDS-PAGE, and bands were visualized by autoradiography.
Polysome Profiling
As described (12), HUVECs were incubated at 1% or 21% O2 for 6 h. At the end of the exposure periods, cells were washed with PBS containing 100 μg/ml cycloheximide and lysed using 200 μl of lysis buffer (100 mm KCl, 5 mm MgCl2, 10 mm HEPES, pH 7.4, 100 mg/ml cycloheximide, 0.5% Nonidet P-40, and 1000 units/ml RNAseOUT). After centrifugation to remove cell debris, supernatants were layered onto a 10-ml sucrose gradient (15–45%) and centrifuged for 2 h at 35,000 rpm. A programmable density gradient fraction collector with continuous spectrophotometric measurement at A254 nm was used to divide the gradient into 16 fractions. RNA extracted from each of the fractions was treated with DNase, reverse transcribed using random hexamers, and HO-2 transcripts in each fraction were quantified using quantitative real-time reverse transcription-PCR.
Measurement of Intracellular ROS
Intracellular reactive oxygen species (ROS) levels were measured in intact HUVECs using carboxy-5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA, Invitrogen). This method is based on the oxidation of non-fluorescent carboxy-H2DCFDA resulting in the formation of the fluorescent compound 2′,7′-dichlorofluorescein (DCF). The fluorescence generated by DCF is proportional to the rate of carboxy-H2DCFDA oxidation, which is in turn indicative of the cellular oxidizing activity and intracellular ROS levels. HUVECs grown to 70% confluence in 12-well plates were incubated with water, TNF-α (10 ng/ml), or H2O2 (100 μm) prior to incubation at 1% or 21% O2 for 16 or 48 h. Cells were washed twice in Hanks' balanced salt solution (HBSS) and then incubated in HBSS containing carboxy-H2DCFDA (15 μm) for 30 min in the dark at 37 °C. After rinsing with HBSS once to remove free probe, fluorescence (Ex484 nm/Em518 nm) from each well was measured using the Fluroskan Ascent & FL fluorescent plate reader (Thermo Fisher, Pittsburgh, PA). The cell number in each well was counted using the Z2 Coulter particle count and size analyzer (Beckman Coulter, Fullerton, CA) so that fluorescence could be normalized to cell number.
Mitochondrial Membrane Depolarization
Depolarization of the mitochondrial membrane was detected using the cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1, Invitrogen). JC-1 localizes to and aggregates within the mitochondria in proportion to mitochondrial membrane potential, emitting red fluorescence. Upon depolarization of the mitochondrial membrane JC-1 forms monomers that emit green fluorescence. Differences in the ratio of red to green fluorescence are representative of changes in mitochondrial membrane depolarization. HUVECs grown to 70% confluence in 12-well plates were incubated with water, TNF-α (10 ng/ml), or H2O2 (100 μm), prior to incubation at 1% or 21% O2 for 16 or 48 h. At the end of the exposure period, HUVECs were incubated with PBS containing JC-1 (2 μm) for 15 min in the dark. Cells were then washed once with PBS, and fluorescence (Ex485 nm/Em518 nm (green) and Ex544 nm /Em590 nm (red)) was measured using the Fluroskan Ascent & FL fluorescent plate reader (Thermo Fisher, Pittsburgh, PA).
Annexin V/Propidium Iodide labeling
The Annexin V-FLUOS staining kit (Roche Applied Science) was used to detect phosphatidylserine externalization (a marker of apoptosis) in HUVECs and HAECs incubated at 1% or 21% O2 for 16 or 48 h. HUVECs and HAECs treated with TNF-α or H2O2 were incubated at 1% or 21% O2 for 16 h. Cells in the media were included in the experiment. After trypsinization, cells were washed once with PBS before the addition of 100 μl of labeling solution containing 2 μl of Annexin V-Fluos labeling reagent and 2 μl of propidium iodide (PI) solution. Cells were analyzed using the cytomicsTM FC 500 flow cytometer (Beckman Coulter, Fullerton, CA).
Total Caspase Activation
Total caspase activation was measured in HUVECs and HAECs incubated at 1% or 21% O2 for 16 or 48 h and in HUVECs and HAECs treated with TNF-α or H2O2 incubated at 1% or 21% O2 for 16 h. FITC-VAD-FMK is an FITC conjugate of the cell-permeable inhibitor of caspases (Promega, Madison, WI). This structure allows delivery of the inhibitor into the cell where binding to activated caspase serves as an in situ marker for apoptosis. After trypsinization, cells were suspended in PBS containing FITC-VAD-FMK (1 μm) at room temperature in the dark for 20 min. Cells were then washed, resuspended in PBS, and analyzed using the cytomicsTM FC 500 flow cytometer.
Data Analysis
Results are presented as mean ± S.E. for n number of independent experiments with p < 0.05 representing statistical significance. The significance of differences between individual means was determined by two-tailed Student's t test. Differences among multiple means were evaluated by analysis of variance corrected for multiple measures where appropriate, and, when overall differences were detected, differences between individual means were evaluated post-hoc using the Student-Newman-Keuls procedure.
RESULTS
Effect of Hypoxia on HO-1 and -2 mRNA and Protein Expression
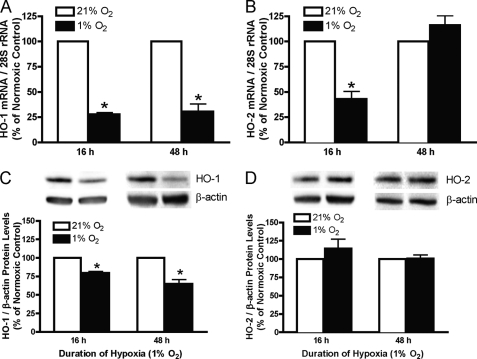
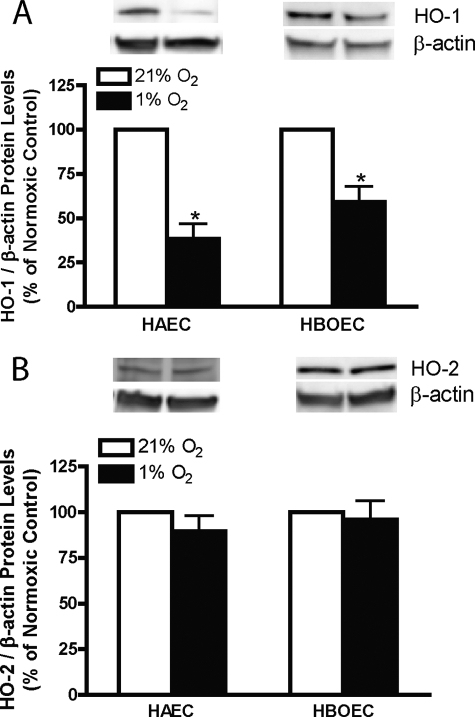
To determine the effects of hypoxia on the expression of HO-1 and -2 mRNA and protein, these levels were compared in HUVECs incubated under normoxic (21% O2) or hypoxic (1% O2) conditions for 16 and 48 h. After 16 and 48 h of hypoxia, steady-state HO-1 mRNA levels are reduced to 27.82 ± 1.80% and 30.71 ± 7.23% of the corresponding normoxic control values, respectively (Fig. 1A). Steady-state HO-2 mRNA expression was decreased (42.93 ± 7.52% of normoxic control, Fig. 1B) after 16 h of hypoxia and returned to the normoxic control level after 48 h. Steady-state HO-1 protein (Fig. 1C) is reduced after 16 and 48 h of hypoxia (79.57 ± 2.11% and 64.93 ± 5.75% of normoxic control values, respectively), whereas steady-state HO-2 protein levels were unaltered (Fig. 1D). To corroborate this finding in other systemic vascular endothelial cells, steady-state HO-1 and -2 proteins were also measured in HAECs and HBOECs incubated under normoxic or hypoxic conditions for 16 h. As shown in Fig. 2, hypoxia decreased steady-state HO-1 protein levels in both HAECs and HBOECs (38.50 ± 8.47% and 59.10 ± 8.85% of normoxic control values, respectively), but, as in HUVECs, steady-state HO-2 was unaltered.
FIGURE 1.
HO-1 and -2 mRNA (A and B) and protein (C and D) levels in HUVECs exposed to 21% or 1% oxygen for either 16 or 48 h. mRNA data are normalized to 28 S rRNA, and protein data are normalized to β-actin. Bars represent means ± S.E. (n = 6 independent experiments); *, p < 0.05 for differences from corresponding normoxic control value.
FIGURE 2.
HO-1 (A) and HO-2 (B) protein levels in HAECs and blood outgrowth endothelial cells exposed to 21% or 1% oxygen for 16 h. Bars represent means ± S.E. (n = 4 independent experiments); *, p < 0.05 for differences from corresponding normoxic control values.
Effect of Hypoxia on HO-1 and -2 Protein Synthesis and Degradation
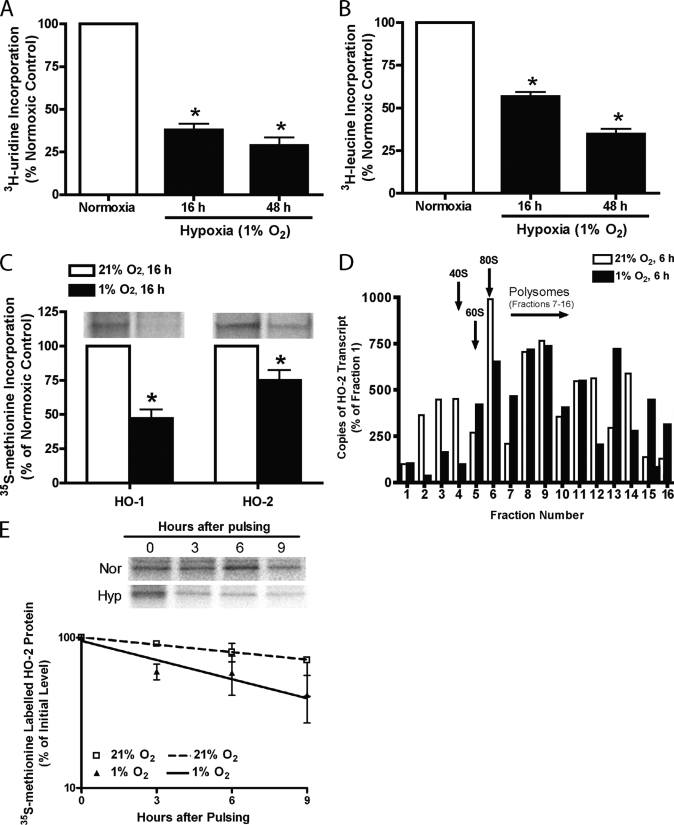
To compare the effects of hypoxia on HO-1 and -2 protein synthesis with its non-selective effects on total cellular mRNA and protein synthesis, [3H]uridine and [3H]leucine incorporation were assessed in HUVECs after 16 and 48 h of hypoxic incubation, and [35S]methionine incorporation into HO-1 and -2 protein was measured in HUVECs after exposure to hypoxia for 16 h (Fig. 3, A–C). Fig. 3A illustrates that total cellular RNA synthesis was decreased to 37.93 ± 3.71% and 28.78 ± 4.88% of normoxic control values, respectively, after 16 and 48 h of hypoxic exposure. Protein synthesis was reduced to 56.60 ± 2.77% at 16 h and 34.80 ± 2.97% of the normoxic control value (Fig. 3B) at 48 h. Results of [35S]methionine labeling and immunoprecipitation using HO-2 antibody (Fig. 3C) indicate that the synthesis of HO-2 protein was higher than HO-1 protein during hypoxia (74.80 ± 7.80% versus 47.01 ± 6.55% of normoxic control values, respectively). The relative preservation of HO-2 protein synthesis, despite the 57% reduction in HO-2 steady-state mRNA level and 43% reduction in total protein synthesis, suggests that HO-2 protein expression was preserved during hypoxia, possibly through enhanced translation. To directly determine the effect of hypoxia on HO-2 translation, ribosomal association of HO-2 mRNA was assessed by polysome profiling. As shown in Fig. 3D, HO-2 mRNA are located in higher polysome fractions after hypoxic incubation, which, together with the results of the metabolic labeling and immunoprecipitation studies, support enhanced translation of HO-2 transcripts during hypoxia. To assess the effect of hypoxia on HO-1 and -2 protein stability, HUVECs were pulsed with [35S]methionine for 3 h and chased with EGM-2 media for up to 24 h under normoxic or hypoxic conditions. As illustrated in Fig. 3E, hypoxia increased HO-2 protein degradation, reducing its half-life from 10.77 ± 2.73 h to 4.78 ± 0.74 h. In contrast, hypoxia had no dramatic effect on HO-1 protein stability. HO-1 protein half-life was 17.77 ± 8.68 h and 22.83 ± 6.22 h in HUVECs exposed to normoxia or hypoxia, respectively (supplemental Fig. S1).
FIGURE 3.
Rate of [3H]uridine (A) and [3H]leucine (B) incorporation into RNA and protein in HUVECs exposed to 21% or 1% oxygen for 16 or 48 h. C, rate of [35S]methionine incorporation into HO-1 and -2 protein in HUVECs exposed to 21% or 1% oxygen for 16 h. Bars represent means ± S.E. (n = 4 independent experiments); *, p < 0.05 for differences from the corresponding normoxic control values. D, quantification of the abundance of HO-2 mRNA in various polysome fractions from HUVECs exposed to 21% (white bars) or 1% oxygen (black bars) for 6 h. E, level of radiolabeled HO-2 protein after immunoprecipitation with anti-HO-2 antibodies in HUVECs pulsed with [35S]methionine for 3 h and chased in EGM-2 medium under 21% or 1% oxygen for the times indicated. The result represents the average of four independent experiments. The protein level at 0 h was defined as 100%.
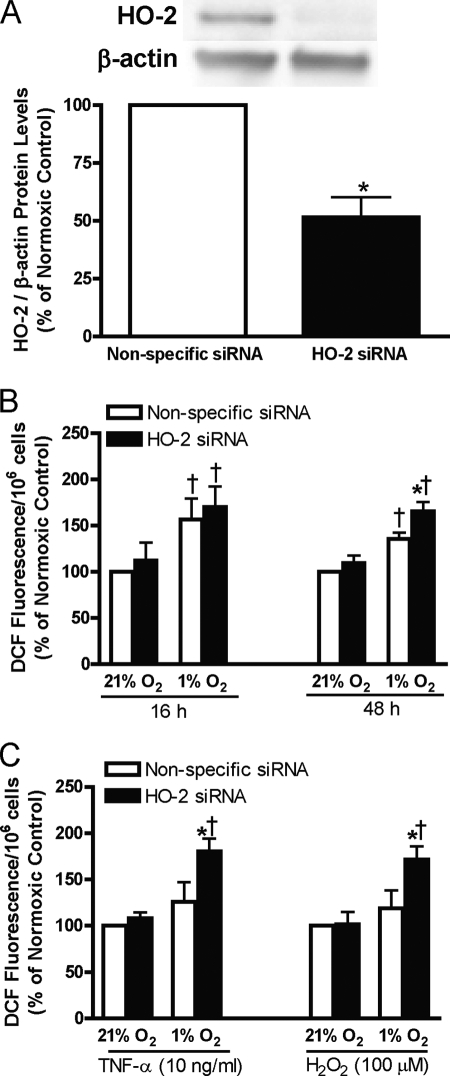
Effect of Decreased HO-2 Protein Level on ROS Production in Human Endothelial Cells during Hypoxia with or without Treatment with TNF-α or H2O2
HO-2 protects cells from oxidative stress by reducing intracellular concentrations of heme (a pro-oxidant) and by increasing levels of bilirubin and ferritin, which are potent anti-oxidants. To determine the importance of HO-2 in protecting cells from oxidative stress during hypoxia, we compared the level of ROS in HUVECs transfected with nonspecific or HO-2 siRNA exposed to normoxia or hypoxia for 16 or 48 h, and also in HUVECs treated with TNF-α or H2O2 exposed to normoxia or hypoxia for 16 h. Fig. 4A is a representative blot of the extent of HO-2 protein inhibition using HO-2 siRNA. Inhibition of HO-2 protein expression had no effect on HO-1 protein level under any of the conditions tested (data not shown). As illustrated in Fig. 4B, exposure to hypoxia for 16 or 48 h increases ROS levels in HUVECs. Compared with the nonspecific siRNA control, inhibition of HO-2 expression increased ROS levels in HUVECs exposed to hypoxia for 48 h, but had no effect at the 16-h time point or in cells incubated under normoxic conditions. In HUVECs exposed to hypoxia for 16 h that were also treated with TNF-α or H2O2, inhibition of HO-2 expression increased ROS levels (Fig. 4C).
FIGURE 4.
A, representative blots and quantification of HO-2 protein in HUVECs transfected with nonspecific or HO-2 siRNA. B, cellular oxidant levels (fluorescence of 2′,7′-dichlorofluorescein, DCF) in HUVECs transfected with nonspecific or HO-2 siRNA exposed to 21% or 1% oxygen for 16 or 48 h. C, oxidant levels in HUVECs transfected with nonspecific or HO-2 siRNA exposed to 21% or 1% oxygen for 16 h treated with TNF-α or H2O2. Bars represent means ± S.E. (n = 6 independent experiments); *, p < 0.05 for differences from nonspecific siRNA control. †, p < 0.05 for differences from corresponding normoxic control values.
HO-2 Preserves Human Endothelial Cell Viability during Hypoxia in the Presence and Absence of TNF-α or H2O2
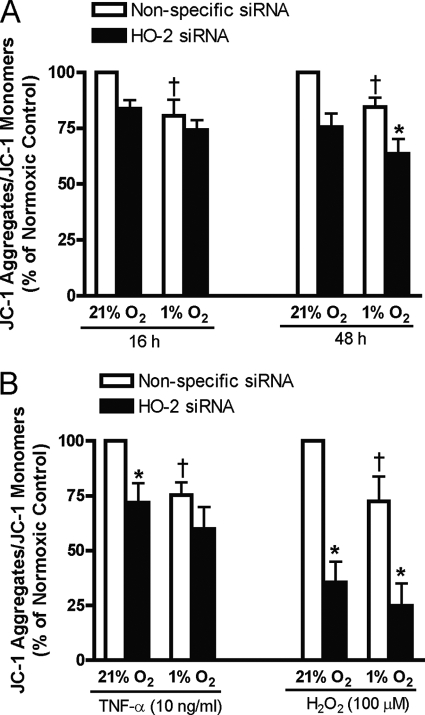
Both hypoxia and oxidative stress may trigger programmed cell death (apoptosis) or necrosis depending on their severity/magnitude. To determine the functional significance of preservation of HO-2 levels during hypoxia, the effect of inhibiting HO-2 expression using HO-2 siRNA on HUVEC viability during hypoxia in the presence or absence of TNF-α or H2O2 was assessed. Mitochondrial membrane depolarization is an early event in the intrinsic apoptotic pathway activated by hypoxia. As shown in Fig. 5A the ratio of JC-1 aggregates/JC-1 monomer was decreased during hypoxia, indicating mitochondrial membrane depolarization (13). Inhibition of HO-2 expression was associated with mitochondrial membrane depolarization in cells incubated under normoxic conditions and exacerbated it in cells exposed to hypoxia for 48 h (Fig. 5A) indicating that HO-2 protein or its activity increased the capacity to maintain mitochondrial membrane potential. In HUVECs treated with TNF-α, inhibition of HO-2 enhanced mitochondrial membrane depolarization in normoxic but not in hypoxic cells. Inhibition of HO-2 enhanced mitochondrial membrane depolarization in both normoxic and hypoxic cells treated with H2O2 (Fig. 5B).
FIGURE 5.
A, mitochondrial membrane depolarization (JC-1 disaggregation) in HUVECs transfected with nonspecific or HO-2 siRNA exposed to 21% or 1% oxygen for 16 or 48 h. B, mitochondrial membrane depolarization in HUVECs transfected with nonspecific or HO-2 siRNA exposed to 21% or 1% oxygen and treated with TNF-α or H2O2. Bars represent means ± S.E. (n = 6 independent experiments); *, p < 0.05 for differences from nonspecific siRNA controls; †, p < 0.05 for differences from corresponding normoxic control values.
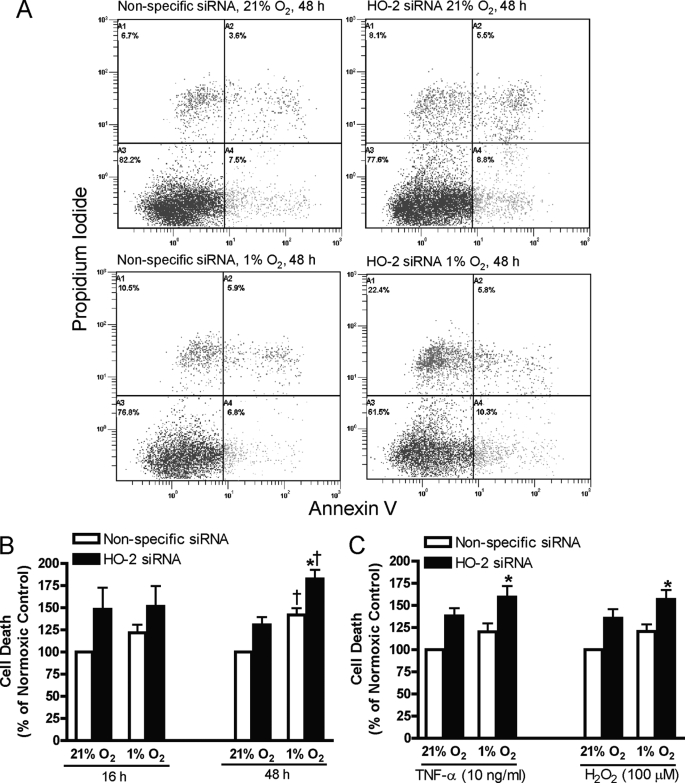
Annexin V/PI staining was used to detect externalization of phosphatidylserine, an early event in apoptosis, and cell membrane permeabilization, an indicator of cell death. Representative plots of annexin V/PI staining are shown in Fig. 6A. Non-viable cells are cells that are stained by annexin V and/or PI (Fig. 6A). Fig. 6B demonstrates that exposure of HUVECs to hypoxia for 48 h increased cell death and that cell death was further increased by inhibition of HO-2 expression. In HUVECs treated with TNF-α or H2O2, inhibition of HO-2 expression had no effect on normoxic cells and increased cell death after hypoxic exposure for 16 h (Fig. 6C). To confirm that the effect of decreased HO-2 activity on cell viability was mediated by increased apoptosis, caspase activation was assessed in HUVECs exposed to normoxia or hypoxia for 16 or 48 h and in HUVECs treated with TNF-α or H2O2 exposed to normoxia or hypoxia for 16 h. In HUVECs in which HO-2 protein expression was inhibited, exposure to hypoxia for 48 h increased activated caspase levels by 1.5-fold compared with nonspecific siRNA control (supplemental Fig. S2A). Total activated caspase was also increased in HUVECs treated with TNF-α or H2O2 and exposed to hypoxia for 16 h (supplemental Fig. S2B).
FIGURE 6.
A, representative annexin V/PI staining plots of HUVECs transfected with nonspecific or HO-2 siRNA exposed to 21% or 1% oxygen for 48 h. Cell death (% cells staining positive for Annexin V and/or PI) in HUVECs transfected with nonspecific or HO-2 siRNA exposed to 21% or 1% oxygen for 16 or 48 h (B) or exposed to 21% or 1% oxygen for 16 h in the presence of TNF-α or H2O2 (C). Bars represent means ± S.E. (n = 6 independent experiments); *, p < 0.05 for differences from nonspecific siRNA controls; †, p < 0.05 for differences from corresponding normoxic control values.
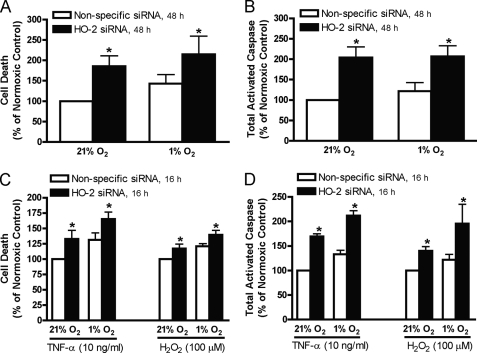
To confirm the cytoprotective effect of HO-2 protein during hypoxia is not specific to HUVECs, annexin V/PI staining and total activated caspase levels were assessed in HAECs exposed to normoxia or hypoxia for 48 h and in HAECs treated with TNF-α or H2O2 exposed to normoxia or hypoxia for 16 h. Inhibition of HO-2 increased cell death in normoxic and hypoxic HAECs (Fig. 7A) and in HAECs treated with TNF-α or H2O2 (Fig. 7C). When HO-2 expression was suppressed, the total activated caspase level increased in HAECs exposed to normoxia or hypoxia for 48 h and in normoxic and hypoxic HAECs treated with TNF-α or H2O2 (Fig. 7, B and D).
FIGURE 7.
Cell death (A) and total activated caspase level (B) in HAECs transfected with nonspecific or HO-2 siRNA exposed to 21% or 1% oxygen for 48 h. Cell death (C) and total activated caspase level (D) in HAECs transfected with nonspecific or HO-2 siRNA exposed to 21% or 1% oxygen for 16 h and treated with TNF-α or H2O2. Bars represent means ± S.E. (n = 5 independent experiments); *, p < 0.05 for differences from nonspecific siRNA controls.
DISCUSSION
The results of this study show that in human endothelial cells incubated under hypoxic conditions: 1) steady-state HO-1 mRNA and protein levels were decreased; 2) steady-state HO-2 protein level was unaltered despite a 57% reduction in steady-state HO-2 mRNA level, a 43% reduction in total protein synthesis, and increased HO-2 protein degradation; 3) HO-2 protein level was maintained through enhanced translation of HO-2 transcripts; and 4) inhibition of HO-2 expression increased oxidative stress, mitochondrial membrane depolarization, and apoptotic cell death.
Previous studies indicate cell type- and species-dependent differences in the regulation of HO expression by hypoxia. Aortic HO-2 protein is increased in rats exposed to hypoxia (7), remains unchanged in cultured rat aortic smooth muscle cells and human cytotrophoblasts (14, 15), and is decreased in Jurkat, K562, and YN-1 cells after hypoxic incubation (16). HO-1 mRNA and protein levels decrease in HUVECs, human astrocytes, and human coronary artery endothelial cells (6, 17) but increase in bovine aortic and rat pulmonary artery endothelial cells and in human fibroblasts and smooth muscle cells after hypoxic incubation (18–21). In HUVECs, decreased expression of HO-1 after hypoxia is mediated by induction of the transcription repressor Bach1 (6). Global suppression of mRNA expression and protein synthesis by hypoxia may also contribute. The current study, the first to directly compare the effects of hypoxia on HO-1 and -2 expression in human endothelial cells, demonstrates that, although both HO-1 and -2 mRNA levels were decreased, HO-2, but not HO-1, steady-state protein level remained unchanged. HO-1 and -2 protein levels were, therefore, differentially regulated by oxygen tension.
HO-1 is primarily regulated transcriptionally, and its proximal promoter regions contain cis-acting response elements that bind transcription factors, including HIF-1α, AP1, SP1, as well as the heme response element GC-NNNGTCA (5). In contrast, the 5′-flanking region of the HO-2 gene contains no regulatory elements corresponding to transcription factors known to participate in the response to hypoxia (22–25). Not surprisingly, therefore, hypoxic incubation did not result in increased HO-2 mRNA levels in the current study, or in any of the cell culture systems in which it has previously been evaluated (14, 26). Nevertheless, HO-2 expression is not entirely constitutive. Development stage-specific changes in HO-2 protein levels have been reported, and HO-2 protein is increased in the aortic endothelium of rats exposed to hypoxia without a corresponding increase in HO-2 mRNA (7, 27–29). Similarly, spatial and temporal dissociation between HO-2 protein and mRNA expression have been noted in the rodent brain and testis (23, 24, 28). Our current results, therefore, reconcile these observations by demonstrating that HO-2 expression is regulated at the post-transcriptional level.
Hypoxia results in decreased cap-dependent translation due to increased formation of the eIF4E/4E-BP1 inhibitory complex and increased phosphorylation of eIF2F-α (30). When hypoxia is severe, or prolonged, transcription is also inhibited and mRNA levels decrease, as observed in the present study. Accordingly, maintenance of protein levels under these conditions requires enhanced translation of existing mRNA transcripts and/or reduced degradation of protein. Our current observation that HO-2 transcripts are localized to larger polysome fractions after hypoxic incubation (Fig. 3D) supports enhanced translation as an important mechanism by which HO-2 protein levels are preserved. Consistent with this conclusion, we observed that the reduction in HO-2 protein synthesis after exposure to hypoxia for 16 h is small given that hypoxia decreased HO-2 mRNA levels and increased HO-2 protein degradation. In other transcripts for which this has been described, structural features that enhance cap-dependent (neuronal nitric-oxide synthase) and cap-independent (Tie-2) ribosomal association have been identified (1, 31). Using the BD Marathon-Ready human testis cDNA library, Zhang et al. have demonstrated that HO-2 transcription is initiated from multiple sites (16). Thus, translation of HO-2 could be enhanced through selective transcriptional activation of a promoter that produces more efficiently translated mRNA species during hypoxia, a mechanism we have previously shown to mediate hypoxic enhancement of neuronal nitric-oxide synthase expression in vascular smooth muscle (1). In support of this concept, Sun et al. have demonstrated the presence of two HO-2 homologous transcripts (1.3 and 1.9 kb) in the rat brain and note that the 1.3-kb mRNA is translated more efficiently than the 1.9-kb mRNA (28). In view of the current findings, therefore, further examination of HO-2 mRNA structure and its functional relevance in the regulation of HO-2 protein expression during hypoxia are now warranted.
Oxidative injury occurs when there is an imbalance between the formation of ROS and the antioxidant capacity of the cell and is implicated in the pathogenesis of organ dysfunction in diseases associated with reduced oxygen delivery (32, 33). Previous studies demonstrate that HO-2 is a component of the endogenous cell defense against oxidative stress; HO-2 gene deletion increases hemin-induced injury in astrocytes and sensitizes cerebral vascular endothelial cells to glutamate- and TNF-α-induced apoptosis (9, 34, 35). The results of the current study confirm the essential role that HO-2 plays in oxidative stress defense in human endothelial cells exposed to hypoxia, because inhibition of its expression increases intracellular ROS levels after 48 h of hypoxic incubation (Fig. 4B). Compensation of HO activity by increased HO-1 expression was not observed. To corroborate the conclusion that HO-2 is important in modulating oxidative stress, the effect of inhibition of HO-2 expression on the response to other oxidative stimuli (TNF-α or H2O2) was also evaluated. In cells deficient in HO-2, significant increases in ROS levels were observed only after hypoxic incubation. Its role, relative to other defense mechanisms, therefore, is specifically enhanced during hypoxia. HO activity is required for catabolism of the pro-oxidant heme and for production of bilirubin, a scavenger of superoxide and peroxyl radicals (5). HO-2 also plays a specific role in regulating intracellular free iron, which increases the generation of reactive hydroxyl radicals through the Fenton reaction (36, 37). Because HO-1 expression is inhibited by hypoxia, HO-2 becomes the predominant isoform under these conditions and a significant mechanism of defense against oxidative stress and hypoxic injury.
Apoptosis may be triggered in response to stimuli extrinsic or intrinsic to the affected cell. Hypoxia-induced apoptosis occurs mainly through the intrinsic pathway (38, 39). The lack of oxygen limits ATP synthesis required for maintenance of the mitochondrial membrane potential. Depolarization of the mitochondrial membrane elevates cytoplasmic ROS levels and further inhibits oxidative ATP synthesis, because the electromotive force for electron transport is reduced. This, in turn, activates Bax and leads to cytochrome c release into the cytosol, caspase activation, and chromatin fragmentation (40). In the current study, we demonstrate mitochondrial membrane depolarization after exposure to hypoxia for 16 h, whereas increased cell death (annexin V/PI staining) and total activated caspase activity were detected only after 48 h. These results support involvement of the intrinsic pathway, because mitochondrial membrane depolarization precedes caspase activation. HO-2 protects against apoptotic cell death induced by TNF-α and glutamate in cerebrovascular endothelial cells and by hydrogen peroxide in HEK cells (9, 34, 41, 42). In the present study, inhibition of HO-2 expression exacerbated mitochondrial membrane depolarization and increased cell death and activated caspase levels in hypoxic, but not normoxic human endothelial cells. When cells where concomitantly treated with TNF-α or H2O2, the anti-apoptotic effect of HO-2 was detected after a shorter hypoxic exposure. These results indicate that HO-2 also protects against hypoxia-induced apoptosis in human endothelial cells and plays an even greater role in preserving cell viability during concomitant oxidative stress induced by TNF-α or H2O2. This may be because generalized inhibition of protein synthesis by hypoxia suppresses the expression of components of conventional cytoprotective pathways.
Consistent with previous studies, our present results support a central role for ROS in triggering apoptotic endothelial death induced by hypoxia, TNF-α, and H2O2. Elevated ROS levels induce apoptosis through activation of the c-Jun N-terminal kinase (JNK)-c-Jun pathway and/or by damaging the mitochondrial membrane resulting in reduced mitochondrial membrane potential (43). These effects may be abrogated by the anti-oxidative effects of HO-2, although several other mechanisms may have been invoked. For example, CO inhibits TNF-α-induced apoptosis by activation of p38 MAPK (44). In addition HO-2 protects cell viability through mechanism(s) separate from its role in heme degradation, because transfection of HEK cells with a catalytically inactive HO-2 mutant protects against oxidative injury (41).
Although HO-2 and HO-1 catalyze the same reaction, the differences between these enzymes could provide insight into the advantages of specifically maintaining HO-2 expression. In HEK cells transfected with plasmids containing either HO-1 or HO-2 and treated with hydrogen peroxide, HO-2 was found to colocalize with its redox partner NAPH-cytochrome P450 reductase in the microsomal fraction, whereas HO-1 was more widely dispersed (42). Accordingly, HO-2 may provide a more efficient pathway for heme degradation, hence greater cytoprotective capacity, due to its subcellular localization. Additionally, HO-2 contains Cys-Pro repeats, termed heme regulatory domains, not present in HO-1 that provide heme binding sites distinct from the heme catalytic domain (5). During hypoxia or ischemia injury, large amounts of pro-oxidant heme are released by cells undergoing necrosis or apoptosis. HO-2, but not HO-1, could sequester this excess free heme. Finally, differential regulation of the expression of these isoforms enables fine control of the antioxidant capacity of the endothelium; by maintaining HO-2 and down-regulating HO-1 during hypoxia, endothelial cells reserve the capacity to increase HO activity in response to additional stress.
We have previously identified a role for HO-2 in preserving endothelium-dependent modulation of vasoconstrictor responses to endothelin-1 and phenylephrine in rats exposed to prolonged hypoxia (7). HO-2 knock-out mice exhibit hypoxemia and myocardial hypertrophy while breathing room air, indicating that HO-2 contributes to pulmonary ventilation-perfusion matching (45). Our current results highlight the importance of HO-2 in modulating endothelial cell apoptosis, which is a prominent feature in a variety of diseases, including atherosclerosis, ischemia/reperfusion injury, and transplantation. Accumulating evidence, therefore, supports a central role for HO-2 in the cardiopulmonary adaptation to hypoxia and in the pathophysiology of disorders in which endothelial injury contributes to vascular dysfunction. Accordingly it represents a potential novel target for therapeutic intervention.
Supplementary Material
Acknowledgment
We thank Effat Rezaei for her expert technical assistance.
This work was supported in part by the Canadian Institutes of Health Research (CIHR) and the Heart and Stroke Foundation (Grant T-5834).
This report is dedicated in honor of Dr. Michael Ward, deceased (November 2009).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- HO
- heme oxygenase
- DCF
- 2′,7′-dichlorofluorescein
- HAEC
- human aortic endothelial cell
- HBOEC
- human blood outgrowth endothelial cell
- HUVEC
- human umbilical vein endothelial cell
- JC-1
- 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide
- PI
- propidium iodide
- ROS
- reactive oxygen species
- siRNA
- small interfering RNA
- TNF
- tumor necrosis factor
- PBS
- phosphate-buffered saline
- HBSS
- Hanks' balanced salt solution
- MAPK
- mitogen-activated protein kinase
- carboxy-H2DCFDA
- carboxy-5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate.
REFERENCES
- 1.Ward M. E., Toporsian M., Scott J. A., Teoh H., Govindaraju V., Quan A., Wener A. D., Wang G., Bevan S. C., Newton D. C., Marsden P. A. (2005) J. Clin. Invest. 115, 3128–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward M. E. (1999) J. Appl. Physiol. 86, 1644–1650 [DOI] [PubMed] [Google Scholar]
- 3.Auer G., Ward M. E. (1998) J. Appl. Physiol. 85, 411–417 [DOI] [PubMed] [Google Scholar]
- 4.Zacour M. E., Toporsian M., Auer G., Cernacek P., Ward M. E. (1998) Pulm. Pharmacol. Ther. 11, 197–199 [DOI] [PubMed] [Google Scholar]
- 5.Ryter S. W., Alam J., Choi A. M. (2006) Physiol. Rev. 86, 583–650 [DOI] [PubMed] [Google Scholar]
- 6.Kitamuro T., Takahashi K., Ogawa K., Udono-Fujimori R., Takeda K., Furuyama K., Nakayama M., Sun J., Fujita H., Hida W., Hattori T., Shirato K., Igarashi K., Shibahara S. (2003) J. Biol. Chem. 278, 9125–9133 [DOI] [PubMed] [Google Scholar]
- 7.Govindaraju V., Teoh H., Hamid Q., Cernacek P., Ward M. E. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H962–H970 [DOI] [PubMed] [Google Scholar]
- 8.Goodman A. I., Chander P. N., Rezzani R., Schwartzman M. L., Regan R. F., Rodella L., Turkseven S., Lianos E. A., Dennery P. A., Abraham N. G. (2006) J. Am. Soc. Nephrol. 17, 1073–1081 [DOI] [PubMed] [Google Scholar]
- 9.Basuroy S., Bhattacharya S., Tcheranova D., Qu Y., Regan R. F., Leffler C. W., Parfenova H. (2006) Am. J. Physiol. Cell Physiol. 291, C897–C908 [DOI] [PubMed] [Google Scholar]
- 10.Foltz W. D., Ormiston M. L., Stewart D. J., Courtman D. W., Dick A. J. (2008) Biomaterials 29, 1844–1852 [DOI] [PubMed] [Google Scholar]
- 11.Bitzan M. M., Wang Y., Lin J., Marsden P. A. (1998) J. Clin. Invest. 101, 372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fish J. E., Matouk C. C., Yeboah E., Bevan S. C., Khan M., Patil K., Ohh M., Marsden P. A. (2007) J. Biol. Chem. 282, 15652–15666 [DOI] [PubMed] [Google Scholar]
- 13.Cossarizza A., Baccarani-Contri M., Kalashnikova G., Franceschi C. (1993) Biochem. Biophys. Res. Commun. 197, 40–45 [DOI] [PubMed] [Google Scholar]
- 14.Morita T., Perrella M. A., Lee M. E., Kourembanas S. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 1475–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newby D., Cousins F., Myatt L., Lyall F. (2005) Placenta 26, 201–209 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Furuyama K., Kaneko K., Ding Y., Ogawa K., Yoshizawa M., Kawamura M., Takeda K., Yoshida T., Shibahara S. (2006) FEBS J. 273, 3136–3147 [DOI] [PubMed] [Google Scholar]
- 17.Nakayama M., Takahashi K., Kitamuro T., Yasumoto K., Katayose D., Shirato K., Fujii-Kuriyama Y., Shibahara S. (2000) Biochem. Biophys. Res. Commun. 271, 665–671 [DOI] [PubMed] [Google Scholar]
- 18.Lee P. J., Jiang B. H., Chin B. Y., Iyer N. V., Alam J., Semenza G. L., Choi A. M. (1997) J. Biol. Chem. 272, 5375–5381 [PubMed] [Google Scholar]
- 19.Hartsfield C. L., Alam J., Choi A. M. (1999) Am. J. Physiol. 277, L1133–L1141 [DOI] [PubMed] [Google Scholar]
- 20.Motterlini R., Foresti R., Bassi R., Calabrese V., Clark J. E., Green C. J. (2000) J. Biol. Chem. 275, 13613–13620 [DOI] [PubMed] [Google Scholar]
- 21.Panchenko M. V., Farber H. W., Korn J. H. (2000) Am. J. Physiol. Cell Physiol. 278, C92–C101 [DOI] [PubMed] [Google Scholar]
- 22.McCoubrey W. K., Jr., Eke B., Maines M. D. (1995) Biol. Reprod. 53, 1330–1338 [DOI] [PubMed] [Google Scholar]
- 23.McCoubrey W. K., Jr., Ewing J. F., Maines M. D. (1992) Arch. Biochem. Biophys. 295, 13–20 [DOI] [PubMed] [Google Scholar]
- 24.McCoubrey W. K., Jr., Maines M. D. (1994) Gene 139, 155–161 [DOI] [PubMed] [Google Scholar]
- 25.Raju V. S., McCoubrey W. K., Jr., Maines M. D. (1997) Biochim. Biophys. Acta 1351, 89–104 [DOI] [PubMed] [Google Scholar]
- 26.Yang Z. Z., Zou A. P. (2001) Am. J. Physiol. Renal Physiol. 281, F900–F908 [DOI] [PubMed] [Google Scholar]
- 27.Ewing J. F., Maines M. D. (1995) Endocrinology 136, 2294–2302 [DOI] [PubMed] [Google Scholar]
- 28.Sun Y., Rotenberg M. O., Maines M. D. (1990) J. Biol. Chem. 265, 8212–8217 [PubMed] [Google Scholar]
- 29.Kim Y. M., Choi B. M., Kim Y. S., Kwon Y. G., Kibbe M. R., Billiar T. R., Tzeng E. (2008) BMB Rep. 41, 164–169 [DOI] [PubMed] [Google Scholar]
- 30.Koritzinsky M., Magagnin M. G., van den Beucken T., Seigneuric R., Savelkouls K., Dostie J., Pyronnet S., Kaufman R. J., Weppler S. A., Voncken J. W., Lambin P., Koumenis C., Sonenberg N., Wouters B. G. (2006) EMBO J. 25, 1114–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park E. H., Lee J. M., Blais J. D., Bell J. C., Pelletier J. (2005) J. Biol. Chem. 280, 20945–20953 [DOI] [PubMed] [Google Scholar]
- 32.Zapelini P. H., Rezin G. T., Cardoso M. R., Ritter C., Klamt F., Moreira J. C., Streck E. L., Dal-Pizzol F. (2008) Mitochondrion 8, 211–218 [DOI] [PubMed] [Google Scholar]
- 33.Di Filippo C., Cuzzocrea S., Rossi F., Marfella R., D'Amico M. (2006) Cardiovasc. Drug Rev. 24, 77–87 [DOI] [PubMed] [Google Scholar]
- 34.Parfenova H., Basuroy S., Bhattacharya S., Tcheranova D., Qu Y., Regan R. F., Leffler C. W. (2006) Am. J. Physiol. Cell Physiol. 290, C1399–C1410 [DOI] [PubMed] [Google Scholar]
- 35.Chen J., Regan R. F. (2004) Biochem. Biophys. Res. Commun. 318, 88–94 [DOI] [PubMed] [Google Scholar]
- 36.Dennery P. A., Spitz D. R., Yang G., Tatarov A., Lee C. S., Shegog M. L., Poss K. D. (1998) J. Clin. Invest. 101, 1001–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dennery P. A., Visner G., Weng Y. H., Nguyen X., Lu F., Zander D., Yang G. (2003) Free Radic. Biol. Med. 34, 124–133 [DOI] [PubMed] [Google Scholar]
- 38.Stempien-Otero A., Karsan A., Cornejo C. J., Xiang H., Eunson T., Morrison R. S., Kay M., Winn R., Harlan J. (1999) J. Biol. Chem. 274, 8039–8045 [DOI] [PubMed] [Google Scholar]
- 39.Kim J. Y., Park J. H. (2003) FEBS Lett. 549, 94–98 [DOI] [PubMed] [Google Scholar]
- 40.Ly J. D., Grubb D. R., Lawen A. (2003) Apoptosis 8, 115–128 [DOI] [PubMed] [Google Scholar]
- 41.Kim Y. S., Doré S. (2005) Free Radic. Biol. Med. 39, 558–564 [DOI] [PubMed] [Google Scholar]
- 42.Kim Y. S., Zhuang H., Koehler R. C., Doré S. (2005) Free Radic. Biol. Med. 38, 85–92 [DOI] [PubMed] [Google Scholar]
- 43.Cai H. (2005) Cardiovasc. Res. 68, 26–36 [DOI] [PubMed] [Google Scholar]
- 44.Brouard S., Otterbein L. E., Anrather J., Tobiasch E., Bach F. H., Choi A. M., Soares M. P. (2000) J. Exp. Med. 192, 1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adachi T., Ishikawa K., Hida W., Matsumoto H., Masuda T., Date F., Ogawa K., Takeda K., Furuyama K., Zhang Y., Kitamuro T., Ogawa H., Maruyama Y., Shibahara S. (2004) Biochem. Biophys. Res. Commun. 320, 514–522 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.