Abstract
The main endogenous source of glutamine is de novo synthesis in striated muscle via the enzyme glutamine synthetase (GS). The mice in which GS is selectively but completely eliminated from striated muscle with the Cre-loxP strategy (GS-KO/M mice) are, nevertheless, healthy and fertile. Compared with controls, the circulating concentration and net production of glutamine across the hindquarter were not different in fed GS-KO/M mice. Only a ∼3-fold higher escape of ammonia revealed the absence of GS in muscle. However, after 20 h of fasting, GS-KO/M mice were not able to mount the ∼4-fold increase in glutamine production across the hindquarter that was observed in control mice. Instead, muscle ammonia production was ∼5-fold higher than in control mice. The fasting-induced metabolic changes were transient and had returned to fed levels at 36 h of fasting. Glucose consumption and lactate and ketone-body production were similar in GS-KO/M and control mice. Challenging GS-KO/M and control mice with intravenous ammonia in stepwise increments revealed that normal muscle can detoxify ∼2.5 μmol ammonia/g muscle·h in a muscle GS-dependent manner, with simultaneous accumulation of urea, whereas GS-KO/M mice responded with accumulation of glutamine and other amino acids but not urea. These findings demonstrate that GS in muscle is dispensable in fed mice but plays a key role in mounting the adaptive response to fasting by transiently facilitating the production of glutamine. Furthermore, muscle GS contributes to ammonia detoxification and urea synthesis. These functions are apparently not vital as long as other organs function normally.
Keywords: Amino Acid, Enzymes, Gene Knockout, Metabolism, Transgenic, Ammonia, Fasting, Glutamine, Glutamine Synthetase, Muscle-specific Knockout
Introduction
Glutamine is among the most abundant free amino acids in mammals. Almost 90% of the daily glutamine production originates from endogenous sources, because 30–35% of all nitrogen derived from protein catabolism is transported in the form of glutamine (1, 2). This glutamine can serve, after transport via the vasculature, as an oxidative fuel for enterocytes and immune cells, a precursor for purine and pyrimidine synthesis, a modulator of protein turnover, or an intermediate for gluconeogenesis and acid-base balance. The only enzyme capable of glutamine synthesis is glutamine synthetase (l-glutamate:ammonia ligase (ADP); EC 6.3.1.2). Because of the prominent role of glutamine in the interorgan transport of carbon and nitrogen, the plasma glutamine pool is turning over very rapidly (3). Glutamine tracer kinetic studies in humans have shown that ∼50% of plasma glutamine is oxidized and that of the remainder, 10–20% is used for gluconeogenesis, and most of the rest is used for protein synthesis and incorporation into macromolecules (2).
Thus far, the irreversible GS5 inhibitor methionine sulfoximine (MSO) was the tool of choice to interfere with cellular glutamine synthesis. Administration of MSO for 4–6 days results in a 40–50% decrease in plasma glutamine levels, a 55–65% decrease in intracellular muscle glutamine, and a 50% increase in muscle ammonia levels (4–6). An inherent drawback of the use of MSO is that GS activity is inhibited in all tissues. Furthermore, MSO has several side effects, including an increase in muscle perfusion (4), anorexia and weight loss (4, 6), neurotoxity (7, 8), and incomplete specificity (8, 9). To be able to study the tissue-specific functions of GS, we have generated a mouse strain in which the protein-coding region of the GS allele is flanked by loxP sequences (GSfl).
Skeletal muscle accounts for ∼70% of the endogenous production of glutamine in man (10, 11), ∼15% of which arises from proteolysis and the remainder from de novo synthesis (1, 10). Because of this quantitatively large contribution, muscular glutamine synthesis is thought to become crucial when circulating glutamine levels fall under catabolic conditions (12, 13), including fasting (11, 14), and when circulating ammonia levels rise because of liver failure (15, 16). To test the hypothesis that muscle becomes a major glutamine producer under these conditions, we crossed mice that had their GS allele floxed (GSfl) with mice that were transgenic for the Cre gene driven by the muscle creatine-kinase promoter/enhancer (MCK-Cre) (17). In such mice, GS expression is selectively and completely eliminated in striated muscles. Using these muscle-specific GS-deficient mice, we demonstrate that glutamine production by skeletal muscle is dispensable in both the fed and fasted states. Detoxification of circulating ammonia in these mice is, however, strongly compromised, underscoring the role of striated muscle in ammonia metabolism.
EXPERIMENTAL PROCEDURES
Generation and Characterization of Muscle-specific GS-deficient Mice
The LacZ knockin and floxed GS alleles (GSLacZ and GSfl, respectively) were constructed and introduced into the germ line as described in Refs. 18 and 19, respectively. The floxed GS allele is equally active as the wild-type allele (19). Accordingly, both GSfl/LacZ and GSfl/fl mice are phenotypically normal and fertile. MCK-Cre transgenic mice (MCK-Cretg/−), in which the Cre gene is expressed under the control of the muscle creatine kinase (MCK) promoter (17), were used to eliminate the floxed GS allele in skeletal muscle cells. To ensure complete removal of the floxed allele in cells that expressed MCK-Cre, we initially used animals in which one GS allele was already inactivated by replacing it in-frame by LacZ, whereas the other GS allele was floxed (Cretg/−/GSfl/LacZ mice; see Figs. 1 and 2). After we had established that GS deletion was equally efficient in Cretg/−/GSfl/fl as in Cretg/−/GSfl/LacZ mice, the experiments were continued with Cretg/−/GSfl/fl (GS-KO/M) mice (see Figs. 3–5). The genotype of mice was analyzed by PCR amplification of toe DNA, using the primer sets listed in supplemental Table S1.
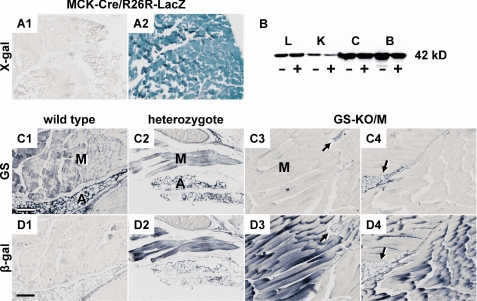
FIGURE 1.
Glutamine synthetase expression in MCK-Cretg/−/GSfl/LacZ mice. A, MCK-Cre expression in muscle. Cryostat sections of gastrocnemius muscle of a 5-day-old neonatal and a 1-month-old MCK-Cre/R26R-lacZ transgenic mouse were stained for the presence of β-galactosidase activity. MCK-Cre-mediated recombination of the R26R locus, which causes LacZ expression, is hardly detectable at 5 days of age (panel A1) but is complete at 1 month of age (panel A2). B, GS protein expression in liver (L), kidney (K), cerebral cortex (C), and brown adipose tissue (B) is unaffected in GSfl/LacZ mice with (−) or without (+) muscle-specific GS elimination. C and D, expression of GS and β-galactosidase in the muscle tissue. Serial sections of gastrocnemius muscle from Cretg/−/GS+/+ (panels C1 and D1), Cretg/−/GS+/LacZ (panels C2 and D2), and Cretg/−/GSfl/LacZ (panels C3, C4, D3, and D4) were stained immunohistochemically for GS (C) and β-galactosidase (D). In Cretg/−/GS+/LacZ mice, GS protein (panel C2) and β-galactosidase (panel D2) co-localize in muscle and adipose tissue. The expression pattern of β-galactosidase in Cretg/−/GS+/LacZ and Cretg/−/GSfl/LacZ mice is similar (panels D2–D4). In contrast, GS protein was absent in muscle tissue from Cretg/−/GSfl/LacZ mice, whereas the neighboring adipose tissue maintained normal GS expression (panels C3 and C4 versus panels D3 and D4). M, muscle tissue; A (arrow), adipose tissue. Scale bars, 200 μm.
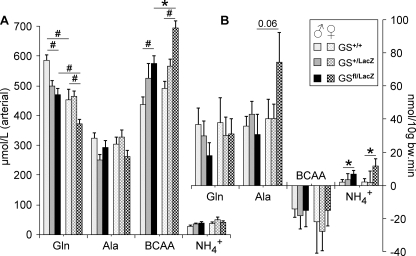
FIGURE 2.
Effect of genotype and gender on circulating concentrations and metabolism of glutamine, alanine, branched chain amino acids, and ammonia in the hindquarter of 4-hours fasted mice. A, arterial plasma concentration. B, net production (positive values) or consumption (negative values) across the hindquarter. All mice were transgenic for MCK-Cre. White bars, GS+/+; gray bars, GS+/LacZ; black bars, GSfl/LacZ mice; bars without hatching, male mice; bars with hatching, female mice. *, p < 0.05; #, p < 0.01.
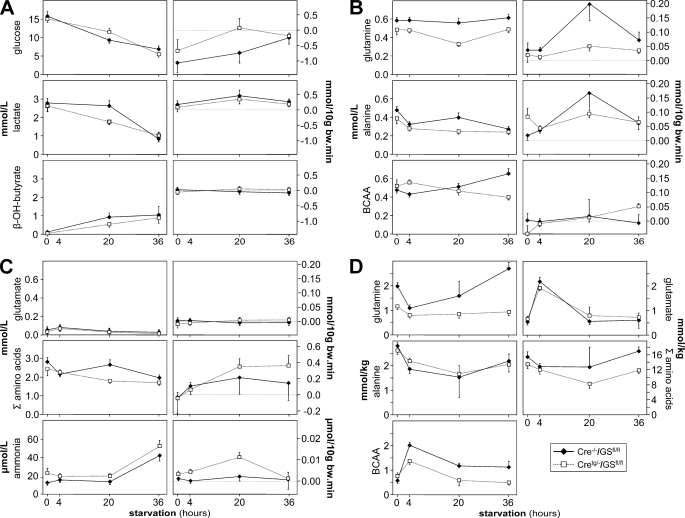
FIGURE 3.
The effect of fasting on circulating arterial concentrations and net production or consumption across the hindquarter of male control and GS-KO/M mice. A, glucose, lactate, and β-hydroxybutyrate (β-OH-butyrate). B, glutamine, alanine, and branched chain amino acids. C, glutamate, summed amino acids, and ammonia. The arterial levels of the respective compounds are shown in the left column, whereas production (positive values) or consumption (negative values) are shown in the right column. D, tissue concentration of glutamine, alanine, branched chain amino acids, glutamate, and summed amino acids in calf muscle. Filled diamonds represent control mice; open squares represent GS-KO/M mice. Significant effects of genotype or duration of fasting are mentioned in the text.
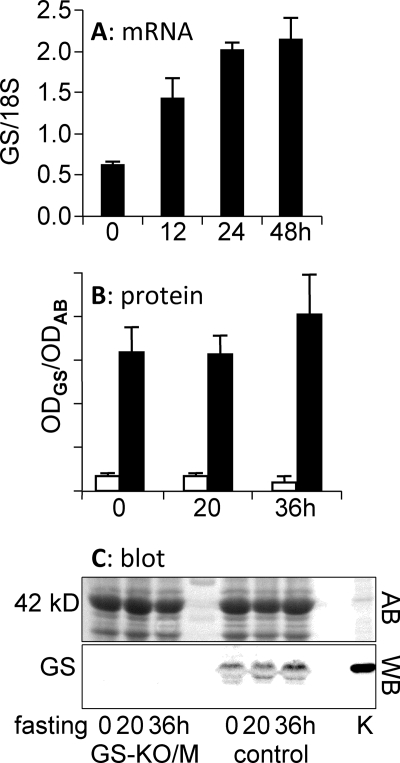
FIGURE 4.
Changes in GS mRNA and protein in calf muscle of fasting control and GS-KO/M mice. A, GS mRNA. B, GS protein. C, representative example of a Western blot used to quantify muscle GS protein. Black columns, control muscle; white columns, GS-KO/M muscle. AB, Western blot stained with Amido Black; WB, the same blot stained for the presence of GS. Note the difference in appearance of the GS band (42 kDa) in muscle and kidney (K) caused by the abundant presence of α1-actin (also 42 kDa) in muscle (see AB).
FIGURE 5.
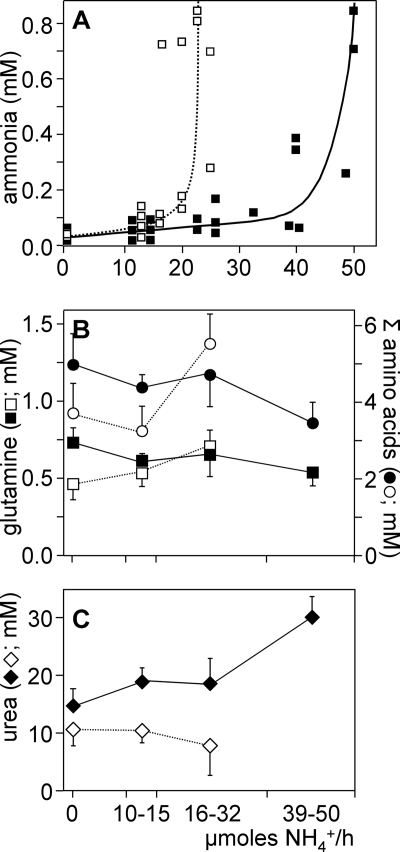
The effect of infusion of incremental amounts of NH4HCO3 into the external jugular vein on circulating ammonia levels in control and GS-KO/M mice. Control (filled symbols) and GS-KO/M male mice (open symbols) were subjected to stepwise increments (every 80 min) in the rate of ammonia infusion. A, plasma concentration of ammonia. B, plasma glutamine and total amino acid concentration. C, plasma urea concentration. Plasma concentrations as a function of the infusion rate of ammonia were measured at the end of each 80-min infusion period. A shows individual observations of ammonia concentration at different infusion rates, whereas the concentrations of glutamine and total amino acids (B) and urea (C) are binned into groups with the indicated infusion rate. Black symbols, control mice; white symbols, GS-KO/M mice.
Histology
The general histology was assessed in hematoxylin- and azophloxin-stained sections. MCK-Cre mice were crossed with R26R-lacZ reporter mice to demonstrate the tissue specificity of GS elimination (20). Calf muscle from 5-day- and 1-month-old MCK-Cretg/−/R26R-lacZ mice was embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA), flash-frozen in N2-cooled isopentane, and stored at −80 °C. Cryostat sections (10 μm) were fixed in 0.5% buffered glutaraldehyde for 10 min at room temperature. β-Galactosidase activity was visualized by applying 5 mm K3[Fe(CN)]6, 5 mm K4[Fe(CN)6], 10 mm EDTA (pH 8.0), 5 mm MgCl2, 20 mm NaCl, 0.1% (w/v) 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside, as described (18). For immunohistochemistry, the cryosections were briefly washed in phosphate-buffered saline to remove the optimal cutting temperature compound, postfixed in 4% buffered formaldehyde for 10 min at room temperature, washed again in phosphate-buffered saline, and boiled for 5 min in 10 mm sodium citrate (pH 6.0) to retrieve epitopes (21) and to inactivate endogenous alkaline phosphatase. The sections were blocked in Teng-T (10 mm Tris-HCl, 5 mm EDTA, 150 mm NaCl, 0.25% (w/v) gelatin, and 0.05% (v/v) Tween 20, pH 8.0) for 30 min and incubated overnight with the first antibody diluted in Teng-T (monoclonal anti-GS, 1:1,500 (Transduction Laboratories, Lexington, KY) and monoclonal anti-β-galactosidase, 1: 500 (Promega Benelux, Leiden, The Netherlands)). After washing, the sections were incubated with a 1:100 dilution in Teng-T of goat anti-mouse IgG covalently coupled to alkaline phosphatase (Sigma), washed again, and incubated with alkaline-phosphatase substrate (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate; Roche Applied Science) for 30 min (18). Incubations from which the first antibody was omitted served as negative controls.
Animal Experiments
The mice were maintained on a 12-h light/12-h dark cycle at 20 °C with free access to water and food. The studies were carried out in accordance with the Dutch guidelines for the care and use of laboratory animals and approved by the Ethical Committee for Animal Research of the University of Amsterdam and Maastricht University.
Effects of Genotype and Gender on Hindquarter Metabolism
Cretg/−/GS+/+, Cretg/−/GS+/LacZ, and Cretg/−/GSfl/LacZ male and female mice (20–30 g; n = 8–9/group) were fasted for 4 h prior to surgical instrumentation between 12:00 and 4:00 p.m. Anesthesia, fluid, and temperature maintenance and catheterization of the jugular vein, carotid artery, abdominal aorta, and inferior caval vein below the level of the renal veins were performed as described (22). Plasma flow across the hindquarter was measured using the indicator-dilution technique with [14C]para-aminohippuric acid (PerkinElmer Life Sciences) infusion into the abdominal aorta, as described (22). Blood was collected from the carotid artery (arterial blood) and inferior caval vein, just above the bifurcation to collect venous blood coming from the hindquarter, and centrifuged. For determination of amino acid concentrations, 80 μl of plasma was added to 6.4 mg of lyophilized sulfosalicylic acid, vortexed, frozen in liquid nitrogen, and stored at −80 °C. The production or consumption of amino acids, ammonia, glucose, and lactate across the hindquarter was calculated by multiplying the inferior caval venous − arterial concentration difference with the hindquarter plasma flow (22, 23), with a positive number referring to net production and a negative number to net consumption.
At the end of the experiment, calf muscle samples were freeze-clamped in liquid N2 and stored at −80 °C. Approximately 50 mg of tissue was added to 250 μl of a 2.5% sulfosalicylic acid solution containing 100 mg of glass beads (1-mm diameter), homogenized for 30 s in a Mini-Bead Beater (Biospec Products, Bartlesville, OK) at maximum speed, and centrifuged. The supernatant was stored at −80 °C. Plasma and muscle tissue amino acid concentrations were measured using a fully automated high pressure liquid chromatography system (24).
Effects of Fasting
Male Cretg/−/GS+/+ (control) and Cretg/−/GSfl/fl (GS-KO/M) mice (∼30 g; n = 8–16/group and time point) were fasted for 0, 4, 20, or 36 h with free access to drinking water. Surgical instrumentation and blood sampling were performed as described above. Blood ammonia concentrations were determined with the ammonia checker II (Type AA-4120; Kyoto Daiichi Kagaka, Japan). Plasma amino acid concentrations were determined as described above. Plasma lactate and glucose concentrations were measured enzymatically on HClO4-treated, Mes/KOH-neutralized samples. The average hindquarter plasma flow was used in animals, in which the flow measurement had failed. Hindquarter plasma flow did not change with fasting, as was also reported for rats (25).
Ammonia Challenge
4-h fasted male control and GS-KO/M mice (25–30 g; n = 6/group) were anesthetized and instrumented for NH4HCO3 infusion into the external jugular vein and blood sampling from the carotid artery, as described above. A solution containing 0.25 m mannitol and 125 or 250 mm NH4HCO3 was infused at a flow rate of 50–200 μl/h. Preliminary experiments showed that blood ammonia concentration reached a plateau within 60 min after initiation of such an ammonia challenge. In the experiments described, blood ammonia concentration was measured every 80 min following the initiation of a challenge. Thereafter, the infusion rate of NH4HCO3 was increased, followed 80 min later by another ammonia measurement and an increase in the infusion rate or the NH4HCO3 concentration.
GS mRNA Assay
Total RNA was extracted from frozen calf muscle with TRIzol (Sigma). First strand cDNA was transcribed as described (26). PCR amplification was carried out using a LightCyclerTM (Roche Applied Science). The data were analyzed using LinRegPCR (27). The primers are listed in supplemental Table S1. If reverse transcriptase was omitted, no product formed. GS mRNA levels were expressed relative to 18 S rRNA content.
Western Blot Analysis
Calf muscle homogenates (50 μg) were heated for 3 min at 100 °C in the presence of 2% SDS and 40 mmol/liter dithiothreitol, size fractionated on a discontinuous 10% SDS-PAGE system, and blotted onto polyvinylidene difluoride transfer membranes (Immobilon-P; Millipore, Bedford, MA). Equal loading of the membranes was verified with Amido Black staining. Nonspecific binding of proteins to the membrane was blocked with 5% nonfat milk in 50 mmol/liter Tris-HCl (pH 7.5), 0.15 mol/liter NaCl, and 0.1% Tween 20. The membranes were sequentially incubated with monoclonal anti-GS and goat anti-mouse IgG conjugated to alkaline phosphatase (Sigma). The resulting immune complexes were detected with CDP-star (Roche Applied Science). Chemoluminescence was quantified with the Lumi-Imager (Roche Applied Science).
Statistical Analysis
Gender and genotype effects on biochemical data were analyzed by two-way analysis of variance; a significant difference was followed up by analysis per factor. Gender effects on biochemical data (ammonia, amino acids) were tested with Student's t test. Genotype effects on biochemical data were tested with one-way analysis of variance. For the fasting data, genotype effects were tested with a t test between both genotypes per fasting condition. The effects of fasting were tested with a t test between fed and 20 h of fasting/genotype. Linear regression analysis with comparison of slopes was used to test whether the effects of ammonia infusion on glutamine and urea plasma levels (see Fig. 5) could be fitted better to a common slope or separate slopes per group. To remove scaling differences, standardized values were used in this analysis. The data are expressed as the means ± S.E.
RESULTS
Characteristics of MCK-Cretg/−/GSfl/LacZ Mice
General Features
Breeding MCK-Cretg/−/GS+/LacZ mice with GSfl/fl mice yielded Cretg/−/GSfl/LacZ mice with the expected Mendelian distribution. MCK-Cretg/−/GSfl/LacZ mice were viable and fertile with no obvious phenotype. The tissue-specific deletion of the GSfl allele was verified by PCR genotyping of various tissues of 2-month-old mice and could be demonstrated in skeletal muscle and heart only. Other tissues, including liver, kidney, brain, stomach, and small intestine, revealed no detectable Cre-mediated recombination events (not shown). In agreement, GS protein expression was not affected in liver, kidney, brain, and brown adipose tissue (Fig. 1B). Quantitative PCR analysis showed low (∼10%) residual GS mRNA expression in the hindquarter of GS-KO/M mice compared with controls. This residual expression can be attributed to GS-positive adipose tissue (Fig. 1, panels C3 and C4).
Histological Features
MCK-Cre/R26R-LacZ mice were produced to check the onset and efficiency of Cre-mediated excision. Fig. 1A shows no Cre-mediated LacZ expression in the muscle of 5-day-old neonatal mice (panel A1) but virtually complete LacZ expression throughout the muscle tissue of 1-month-old mice (panel A2).
In MCK-Cretg/−/GS+/LacZ mice, β-galactosidase activity expressed from the GSLacZ allele co-localized with GS protein expressed from the GS+ allele in muscle and adipose tissue (Fig. 1, panels C2 and D2). Therefore, the β-galactosidase reporter was used as a marker of cells in which GS was inactivated (Fig. 1, panels C3, C4, D3, and D4). In sections of MCK-Cretg/−/GSfl/LacZ mice, GS protein had completely disappeared from muscle tissue, whereas the adjacent adipose tissue maintained normal GS expression (Fig. 1, C3 and C4). These results show that GS expression was completely eliminated from Cretg/−/GSfl/LacZ muscle, without an effect on muscle architecture.
Metabolic Differences in the Hindquarters of Wild-type and GS-deficient Mice
Comparison of Cretg/−/GS+/+, Cretg/−/GS+/LacZ and Cretg/−/GSfl/LacZ Mice
Food was withdrawn 4 h prior to the experiment. Significant effects of genotype on the amino acid concentration in the arteries and inferior caval vein were observed for glutamine (∼20% lower in GS-deficient mice) and the branched chain amino acids (∼35% higher in GS-deficient mice; arterial valine, isoleucine, and leucine concentrations all increased from wild-type mice via heterozygous mice to GS-deficient mice; Fig. 2A). No differences in alanine were found. The concentration of glutamine was ∼20% higher in male than in female mice, irrespective of the presence or absence of GS. The ammonia levels in the arteries and inferior caval vein did not differ between the genotypes and genders.
Plasma flow was not different between genotypes and genders, the average flow being 0.71 ± 0.04 ml/10 g of body weight/min. Because the venous concentration of glutamine and alanine was ∼10 and ∼20%, respectively, higher than the arterial concentration, glutamine and alanine were produced across the hindquarter (Fig. 2B), without differences between genotypes and genders. In contrast, the venous concentration of the branched chain amino acids was ∼6% lower than the arterial concentration, indicating a small consumption of these amino acids. The production of ammonia across the hindquarter was significantly lower in control than in GS-deficient mice (Fig. 2B).
Muscle glutamine levels were 25–30% lower in male and female GS-deficient mice compared with control mice (Table 1). MCK-Cretg/−/GS+/LacZ mice had an intermediate decline in muscle glutamine content (not shown). Muscle alanine concentration was 30–40% higher in males than in females but was not different between GS-deficient and control mice.
TABLE 1.
Concentration of glutamine, alanine, branched chain amino acids, and glutamate in calf muscle
The mice were fasted for 4 h. The values are expressed in μmol/kg of tissue.
| Genotype | Gin |
Ala |
BCAA |
Glu |
||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Cretg/−/GS+/+ | 1090 ± 50 | 1070 ± 75 | 1755 ± 175a | 1205 ± 165 | 2035 + 295 | 1830 ± 160 | 2159 ± 200 | 2340 + 190 |
| Cretg/−/GSfl/LacZ | 845 ± 75b | 680 ± 55b | 2250 ± 14011 | 1535 ± 145 | 1605 ± 150 | 1965 ± 170 | 2470 ± 135 | 2020 ± 115 |
a p = 0.04, male relative to female.
b p = 0.08 (male) and <0.01 (female) relative to control.
Role of Muscular GS during Fasting
To study the response of MCK-Cretg/−/GSfl/fl (GS-KO/M) mice to fasting, we compared fed, postabsorptive (4-h fasted) and fasted (20 and 36 h fasted) male mice. GS-KO/M and control mice had a similar body weight in the fed state, but GS-KO/M mice lost slightly more weight than control mice during the first 20 h of fasting (12 ± 1 and 10 ± 0.5%, respectively; p = 0.04). After 36 h of fasting, the difference in weight loss (19 ± 1 and 17 ± 1%, respectively) was no longer significant (p = 0.15). The relative liver weight was similar in all groups (∼4% of body weight).
Arterial glucose, lactate, and β-hydroxyburyrate levels did not differ between control and GS-KO/M mice, except for lactate at 20 h of fasting (p = 0.02). Glucose and lactate levels declined to ∼40% of fed values after 36 h of fasting (p < 0.001; Fig. 3A), whereas β-hydroxybutyrate levels increased from hardly detectable to ∼1 mmol/liter (p < 0.01). These findings show that fasting had the anticipated effects on carbohydrate metabolism in both control and GS-KO/M mice. In control mice, glucose consumption across the hindquarter tended to decline (p = 0.08 at 36 h), whereas lactate and β-hydroxybutyrate production did not change. In GS-KO/M mice, neither glucose consumption nor lactate production changed with fasting (at 20 h of fasting, the p value of the difference in glucose consumption between GS-KO/M and control mice amounted to 0.10).
The arterial concentration of glutamine was similar in fed GS-KO/M and control mice (p = 0.13) but was ∼20% lower in GS-KO/M mice at 4 (p < 0.001) and 36 h of fasting (p < 0.001) and even ∼40% at 20 h of fasting (p < 0.001; Fig. 3B). During the first 4 h of fasting, arterial alanine concentration declined to ∼70% of the fed condition in both GS-KO/M and control animals (p < 0.05). Upon continued fasting, the arterial alanine concentration did not change in GS-KO/M mice but rose transiently in control mice to ∼160% of that in experimental mice at 20 h of fasting (p < 0.05; Fig. 3B). In GSKO/M mice, arterial BCAA levels were significantly higher (∼125%) than in controls at 4 h (p < 0.005) and significantly lower (∼60%) than in controls at 36 h of fasting (p < 0.001; Fig. 3B). Arterial plasma glutamate levels did not differ between GS-KO/M and control mice (Fig. 3C). The sum of all of the amino acids followed a similar pattern as alanine and was ∼35% lower in GS-KO/M than in control mice at 20 h of fasting (p < 0.005; Fig. 3B). The arterial concentrations of amino acids, which are not mentioned in this paragraph, are provided in supplemental Table S2. Arterial ammonia levels were higher in GS-KO/M than in control mice at 0 and 20 h of fasting (p < 0.04 at both time points). Furthermore, ammonia concentrations doubled between 20 and 36 h of fasting in both control and GS-KO/M mice (p < 0.001; Fig. 3C).
Fig. 3B shows that, compared with the fed condition, the production of glutamine and alanine across the hindquarter increased >4-fold during the first 20 h of fasting in control mice (both p < 0.04), but the production of these amino acids returned to the fed values during the next 16 h of fasting. The production of glutamine and alanine in GS-KO/M mice did, in contrast, not change with fasting and was significantly lower than that in control mice at 20 h of fasting for glutamine (p < 0.02; p = 0.21 for alanine). The production or consumption of BCAA (Fig. 3B) and glutamate (Fig. 3C) was small in experimental and control mice, with only the change from BCAA consumption in the fed condition to production in the 36 h-fasted condition being significant (p = 0.014). The production of all amino acids summed increased in GS-KO/M mice (p = 0.14 at 20 h and p < 0.05 at 36 h of fasting), but that in control mice did not. The difference in hindquarter amino acid production between control and GS-KO/M mice after 36 h of fasting was, nevertheless, not significant (p = 0.25; Fig. 3C). These findings show that the hindquarter only contributed to whole body glutamine and alanine production in fasting control mice and also that this contribution was transient with a peak at 20 h of fasting. In the absence of GS expression in muscle, amino acid production was no longer channeled into glutamine and alanine. Ammonia production did not change appreciably in fasting control mice. In GS-KO/M mice, however, the production of ammonia was ∼5-fold higher than that of control mice during the first 20 h of fasting (p < 0.005 and p < 0.012 at 4 and 20 h, respectively) but returned to control values after 36 h of fasting (p = 0.94).
In postprandial control mice, the tissue concentration of glutamine in muscle had declined to ∼50% of the fed value (p < 0.001), whereas that in GS-KO/M mice did not change significantly (Fig. 3D). Muscle alanine concentration declined 30–35% in both genotypes (p < 0.01), whereas the sum of amino acids showed the same trend (Fig. 3C). At the same time, tissue BCAA and glutamate concentrations increased 3–4-fold in control mice (both p < 0.001) and somewhat less in GS-KO/M mice (p < 0.01 and 0.001, respectively). All of these changes were transient and returned to fed values at 20 or 36 h of fasting. The tissue concentrations of amino acids, which are not mentioned in this paragraph, are provided in supplemental Table S2.
To investigate whether the decline of glutamine production after 20 h of fasting resulted from a decline in GS expression, GS mRNA and protein content were determined in calf muscle (Fig. 4). GS mRNA expression in control muscle increased ∼3-fold during the first day of fasting and plateaued thereafter (Fig. 4A). GS protein content, on the other hand, did not increase in control muscle during 36 h of fasting (Fig. 4B). Similarly, GS protein content in GS-KO/M muscle, which amounted to only 10% of that in control muscle, did not change. As stated earlier, this residual GS content originates from adipose tissue that is present inside calf muscle (Fig. 1).
Role of Muscular GS in Ammonia Detoxification
To study the response of GS-KO/M mice to an intravenous challenge with ammonia, NH4HCO3 was infused in stepwise increments into the external jugular vein. This protocol caused arterial ammonia levels to gradually rise in both control and GS-KO/M mice. Rising ammonia levels will increase the flux through the ammonia-detoxifying enzymes until their maximal capacity is reached (Fig. 5A). When the infusion rate exceeded 40 μmol of NH4HCO3/25g of body weight/h in control mice, circulating ammonia levels began to rise rapidly, indicating that ammonia detoxification failed. In GS-KO/M mice, circulating ammonia levels already began to increase when 15 μmol of NH4HCO3/25 g of body weight/h was infused. In both control and GS-KO/M mice, the direct relation between the amount of ammonia infused and plasma ammonia levels broke down, when plasma ammonia levels exceeded ∼150 μmol/liter. Because the absence of GS from striated muscle is the only difference between control and GS KO/M mice, these data show that the body can detoxify ∼25 μmol of ammonia/25 g of body weight/h in a muscle GS-dependent manner.
The infusion of ammonia tended to decrease the circulating concentration of glutamine (p = 0.07) and the sum of amino acids (p = 0.08) in control mice (Fig. 5B), whereas it increased circulating urea levels (Fig. 5C; p = 0.02). The ammonia load-dependent trends in plasma glutamine and urea concentration were best described by two separate lines (p = 0.04) with different slopes (p < 0.001). In contrast, the infusion of ammonia tended to increase the circulating concentration of glutamine and the sum of amino acids in GS-KO/M mice (p = 0.1), whereas circulating levels of urea even tended to decline (p = 0.08). Both ammonia load-dependent changes in concentration did not differ in trend in GS-KO/M mice. The reciprocal changes in plasma glutamine and urea concentrations in wild-type and GS-KO/M mice show that ammonia is converted to urea in wild-type mice and into amino acids, including glutamine, in GS-KO/M mice. This observation demonstrates that muscle glutamine synthesis plays a key role in (liver) urea synthesis and that glutamine synthetase outside muscle tissue remains functional.
DISCUSSION
The main findings of the present study is that the elimination of expression of GS in skeletal muscle of MCK-Cretg/−/GSfl/LacZ or MCK-Cretg/−/GSfl/fl (GS-KO/M) mice was complete and specific for striated muscle (Fig. 1). Even though the hindquarter consists, in addition to striated muscle, of adipose and connective tissue, bone, and skin, we were able to deduce the specificity of our experimental findings from comparing wild-type and GS-KO/M mice. The absence of GS expression in muscle did not affect the health or reproductive activity of the animal. In agreement, no differences in the metabolism of glutamine, any other amino acid, or simple carbohydrates across the hindquarter were found in fed mice. Upon fasting, however, the production of glutamine across the hind limb did not increase in GS-KO/M mice, whereas it did in control mice (Fig. 3). Because the elimination of GS from muscle was complete, the remaining glutamine production across the hindquarter must originate from other GS-expressing tissues, such as fat (Fig. 1) (28, 29) or skin (30). Furthermore, the capacity to detoxify intravenously infused ammonia was ∼2-fold lower in GS-KO/M than in control mice (Fig. 5). The use of genetic tools to eliminate GS selectively and completely from muscle has therefore allowed us to demonstrate that GS function in muscle is minimal in the unchallenged, fed condition and becomes manifest only in the stressed condition, that is, upon food withdrawal or during an ammonia challenge.
The developmental appearance of GS in striated muscle reveals a peak in activity in the first postnatal week, which exceeds the adult level by ∼3-fold and which is followed by a decline toward weaning (31). This neonatal peak in GS activity coincides with the period of terminal differentiation and nerve-induced growth of the myotubes (32). Because glutamine transport in skeletal muscle is electrogenic and dependent on innervation (33), the decline in GS activity may well reflect an innervation-dependent increase in the intracellular concentration of glutamine (34), which is known to decrease the half-life of GS protein (4–6, 35). Cre expression in transgenic MCK-Cre striated muscle begins just prior to birth and reaches its maximal level only at ∼10 days after birth (17). In agreement, we did not detect any Cre-mediated recombination in 5-day-old neonatal MCK-Cre/ROSA26 mice. Our conclusion that GS activity in muscle is dispensable in the fed condition therefore only applies to postweaning mice with fully developed striated muscles.
A substantial effect of gender on amino acid concentration, including glutamine, was found in the circulation and in muscle tissue. Glutamine concentrations were higher in male than in female mice, irrespective of whether GS was present or absent in muscle. GS protein content and activity in muscle were similar in male and female mice (29). Similarly, no gender-dependent difference in the production of glutamine or any other amino acid across the hindquarter was found. Apparently, muscle glutamine levels do not directly reflect the flux through GS in mice. In this respect, it is of interest to note that muscle glutamine levels in mice (∼1 mmol/kg) are 3–7-fold lower than those in rats (25, 36) and even 9–12-fold lower than those in humans (1, 10, 37, 38). Because plasma glutamine concentrations are similar in these species, the differences must arise from differences in glutamine transport across the sarcolemma and/or metabolism.
In the fed condition, muscle glutamine production was low and did not differ between GS-deficient and control muscle. This finding demonstrates that the flux through muscle GS is very low in the fed condition. Only a slightly increased BCAA consumption and an increased alanine production distinguished the hindquarter of GS-KO/M from wild-type mice, suggesting that the decreased muscle concentration of glutamine increased BCAA import and released the amino groups of these BCAAs as alanine. If present, this conversion of BCAA into alanine is confined to the fed condition.
When muscle protein breakdown accelerates as a result of fasting, skeletal muscle increases the release of amino acids, in particular glutamine and alanine, into the circulation (14, 39). Our observation that GS-deficient muscle was unable to increase its production of glutamine (and alanine) upon fasting therefore demonstrates that the flux through muscle GS is very low in the fed condition and becomes noteworthy in the postprandial state only. The increase in hindquarter glutamine production in wild-type mice between 4 and 20 h of fasting was preceded by a ∼2-fold decline in muscle glutamine content, whereas the decline in glutamine production between 20 and 36 h of fasting was accompanied by a continued increase in muscle glutamine content. In GS-KO/M mice, neither muscle glutamine content nor hindquarter glutamine production changed with fasting. Together, these findings support the hypothesis that changes in muscular glutamine concentration regulate proteolysis (40, 41).
The production of glutamine and alanine in muscle is dependent on the supply of glutamate via transamination of amino acids that are released upon proteolysis (14, 39, 42) and possibly, but not in our study, from glutamate that is taken up from the circulation (43). The consumption of glucose in 20-h fasted muscle was marginally higher in GS-KO/M than in control mice, but the absence of a simultaneously increased production of alanine demonstrates that the “glucose-alanine” cycle (14, 39, 44) did not compensate for the deleted production of glutamine. The reciprocal changes in muscle glutamine and glutamate concentration in control mice are consistent with an increased production of glutamate as an early, postabsorptive response to fasting and with the production of glutamine and, to a lesser extent, alanine from glutamate increasing toward 20 h of fasting. Between 20 and 36 h of fasting, muscle glutamine levels continue to rise. In conjunction with the declining production of glutamine, this finding implies an inhibition of glutamine transport across the sarcolemma. In contrast to the decrease in glutamine production that is seen in mouse muscles upon continued fasting, fasting rat muscles progressively increase glutamine production up to at least 112 h of fasting (25). A decreased glutamine production was also found in the muscle of hypercatabolic burn patients (37), but in these subjects, a compensatory increase in alanine synthesis occurred that did not develop in long term fasting mouse muscle. Based on the declining glutamine production without the compensatory increase in alanine production, the simultaneously declining ammonia production, and the increasing BCAA production in GS-KO/M hindquarter during fasting, we hypothesize that glutamate production in muscle declines upon prolonged fasting in the mouse.
GS mRNA levels in wild-type mouse muscle are increased ∼3-fold at 24 and 48 h of fasting (45). Our finding that this adaptive increase in mRNA expression corresponded with a transient rather than a sustained increase in glutamine production has, to the best of our knowledge, not been reported before. In agreement with the transient increase in amino acid production, we observed in a series of independent experiments that control muscle lost 13 ± 4% weight during the first 24 h of fasting and only 3 ± 2% between 24 and 48 h of fasting. We therefore repeated the mRNA and protein measurements and confirmed that GS mRNA levels in control muscle increased during the first day of fasting to a ∼3-fold higher plateau (Fig. 4). GS protein levels, however, did not follow this increase in muscle mRNA content, pointing to a stringent (post)translational level of control. In agreement with an earlier report (6), this control may be exerted by the increasing muscle concentration of glutamine, a known determinant of GS protein stability (4–6, 35). Because GS protein and activity levels correspond closely in all tissues that we investigated (29), we hypothesize that glutamine synthesis in wild-type mice becomes limited by precursor availability upon prolonged fasting, that is, by a decline in muscle proteolysis. The reciprocal changes in muscle tissue concentrations of free BCAA and glutamate on the one hand and of glutamine and alanine on the other hand are compatible with this hypothesis. The observed rise in ketone bodies may underlie the decline in muscle amino acid production during prolonged fasting, because studies in vitro have demonstrated that ketone bodies decrease muscle proteolysis by inhibiting glycolysis and decreasing pyruvate availability (46, 47).
Because the absence of GS from striated muscle is the only difference between control and GS-KO/M mice, the present study also revealed that striated mouse muscle can detoxify ∼1 μmol/g of body weight/h of ammonia in a GS-dependent fashion. Assuming that muscle accounts for ∼40% of body weight (48), this capacity corresponds with a rate of ∼2.5 μmol/g of muscle/h. This calculation assumes that GS is exclusively eliminated from muscle (which was confirmed by the analysis of MCK-Cre/R26R-LacZ crosses) and that there was no compensatory change of GS expression in other tissues (than those shown in Fig. 1B). In rats with a porto-caval shunt and acute liver failure caused by hepatic artery ligation, a similar capacity for ammonia detoxification in muscle was deduced from arterio-venous balance studies across the hindquarter (49). In our study, ammonia detoxification by muscle began to fail when plasma ammonia levels exceeded ∼150 μm, that is, when concentrations begin to exceed the Km of GS for ammonia (50). At physiological ammonia levels, muscles still detoxify ∼200–300 nmol/g of body weight/h. Our mice consumed ∼3 g/day of feed containing 18% protein. Because proteins cannot be stored, the mice had to detoxify ∼5 mmol of ammonia/day or ∼8 μmol/g of body weight/h. Murine muscle, therefore, contributes ∼3% to the daily whole body ammonia detoxification. If circulating ammonia levels rise to a supraphysiological concentration of >100 μm, the contribution can increase to ∼10%. In control mice, detoxification of ammonia by muscle glutamine synthesis resulted in a decline of plasma glutamine and a rise in plasma urea. These reciprocal changes demonstrate that ammonia detoxification by muscle actually leads to elimination of ammonia as urea and are compatible with activation of liver glutaminase. However, when considering the potentially supportive function of muscles in ammonia detoxification, it should be kept in mind that muscle mass has often declined because of concurrent catabolic conditions.
An inherent drawback of genetic modification is that the modification may have induced a functional adaptation in another tissue. If such an adaptation is present in GS-KO/M mice, its capacity is low, because an overnight fast is apparently sufficient to unmask the deficiency. Compared with the MSO model of irreversible systemic GS enzyme inactivation (4–9), our model is mild and far more amenable to the inclusion of control conditions. Unfortunately, the heterogeneous tissue composition of the hindquarter model with, in addition to muscle, extensive expression of GS in fat (Fig. 1) (28, 29) and skin (30) makes it less suitable for more analytical biochemical studies with stable isotopes. Furthermore, although tissue glutamine levels in murine muscle appear to be ∼10-fold lower than in human muscle (10, 37, 38), it will be necessary to infuse labeled glutamine for a prolonged period via a permanent jugular vein catheter to reach steady-state levels (38). Irrespective of these considerations, the use of a muscle-specific GS-deficient mouse has made it possible to quantify the contribution of muscle to glutamine production and ammonia detoxification in a fairly physiological condition.
Supplementary Material
Acknowledgments
We thank Nicole Worms for the contribution as a student to this study. The MCK-Cre mouse line was kindly provided by Dr. Ronald Kahn (Joslin Diabetes Center, Harvard Medical School, Boston, MA).
This work was supported by Netherlands Organization for Scientific Research (NWO) Grant SGC210009 (to W. H. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
- GS
- glutamine synthetase
- MCK
- muscle-specific creatine kinase
- Cre
- cyclization-recombination enzyme of bacteriophage P1
- BCAA
- branched chain amino acid
- MSO
- methionine sulfoximine
- Mes
- 2-(N-morpholino)ethanesulfonic acid.
REFERENCES
- 1.Kuhn K. S., Schuhmann K., Stehle P., Darmaun D., Fürst P. (1999) Am. J. Clin. Nutr. 70, 484–489 [DOI] [PubMed] [Google Scholar]
- 2.Biolo G., Zorat F., Antonione R., Ciocchi B. (2005) Int. J. Biochem. Cell Biol. 37, 2169–2179 [DOI] [PubMed] [Google Scholar]
- 3.Curthoys N. P., Watford M. (1995) Annu. Rev. Nutr. 15, 133–159 [DOI] [PubMed] [Google Scholar]
- 4.Heeneman S., Deutz N. E. (1993) Clin. Nutr. 12, 182–190 [DOI] [PubMed] [Google Scholar]
- 5.Olde Damink S. W., de Blaauw I., Deutz N. E., Soeters P. B. (1999) Clin. Sci. 96, 639–646 [DOI] [PubMed] [Google Scholar]
- 6.Labow B. I., Souba W. W., Abcouwer S. F. (1999) Am. J. Physiol. Endocrinol. Metab. 276, E1136–E1145 [DOI] [PubMed] [Google Scholar]
- 7.Apostolakis M., Anogianakis G., Kallaras C., Zaraboukas T., Liangouris J., Nowack-Apostolaki E., Economou L. (1989) Brain Res. Bull. 23, 257–262 [DOI] [PubMed] [Google Scholar]
- 8.Rao V. L., Murthy C. R. (1991) Neurosci. Lett. 126, 13–17 [DOI] [PubMed] [Google Scholar]
- 9.Sellinger O. Z. (1982) J. Neurochem. 38, 1676–1685 [DOI] [PubMed] [Google Scholar]
- 10.Biolo G., Fleming R. Y., Maggi S. P., Wolfe R. R. (1995) Am. J. Physiol. Endocrinol. Metab. 268, E75–E84 [DOI] [PubMed] [Google Scholar]
- 11.Nurjhan N., Bucci A., Perriello G., Stumvoll M., Dailey G., Bier D. M., Toft I., Jenssen T. G., Gerich J. E. (1995) J. Clin. Invest. 95, 272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melis G. C., ter Wengel N., Boelens P. G., van Leeuwen P. A. (2004) Curr. Opin. Clin. Nutr. Metab. Care 7, 59–70 [DOI] [PubMed] [Google Scholar]
- 13.Tjader I., Berg A., Wernerman J. (2007) Crit. Care Med. 35, (suppl.) S553–S556 [DOI] [PubMed] [Google Scholar]
- 14.Chang T. W., Goldberg A. L. (1978) J. Biol. Chem. 253, 3685–3693 [PubMed] [Google Scholar]
- 15.Clemmesen J. O., Kondrup J., Ott P. (2000) Gastroenterology 118, 1131–1139 [DOI] [PubMed] [Google Scholar]
- 16.Olde Damink S. W., Deutz N. E., Dejong C. H., Soeters P. B., Jalan R. (2002) Neurochem. Int. 41, 177–188 [DOI] [PubMed] [Google Scholar]
- 17.Brüning J. C., Michael M. D., Winnay J. N., Hayashi T., Hörsch D., Accili D., Goodyear L. J., Kahn C. R. (1998) Mol. Cell 2, 559–569 [DOI] [PubMed] [Google Scholar]
- 18.He Y., Hakvoort T. B., Vermeulen J. L., Lamers W. H., van Roon M. A. (2007) Dev. Dyn. 236, 1836–1875 [DOI] [PubMed] [Google Scholar]
- 19.He Y., Hakvoort T. B., Vermeulen J. L., Labruyère W. T., de Waart D. R., van der Hel W. S., Ruijter J. M., Uylings H. B., Lamers W. H. (2010) Glia 58, in press [DOI] [PubMed] [Google Scholar]
- 20.Soriano P. (1999) Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- 21.Shan-Rong S., Jiang G., Taylor C. R. (2000) Antigen Retrieval Techniques: Imunohistochemistry and Molecular Morphology, Eaton Publishing, Natick, MA [Google Scholar]
- 22.Hallemeesch M. M., Ten Have G. A., Deutz N. E. (2001) Lab. Anim. 35, 101–110 [DOI] [PubMed] [Google Scholar]
- 23.Dejong C. H., Deutz N. E., Soeters P. B. (1993) J. Clin. Invest. 92, 2834–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Eijk H. M., Rooyakkers D. R., Deutz N. E. (1993) J. Chromotogr. 620, 143–148 [DOI] [PubMed] [Google Scholar]
- 25.de Blaauw I., Deutz N. E., von Meyenfeldt M. F. (1996) Clin. Sci. 90, 457–466 [DOI] [PubMed] [Google Scholar]
- 26.Lekanne Deprez R. H., Fijnvandraat A. C., Ruijter J. M., Moorman A. F. (2002) Anal. Biochem. 307, 63–69 [DOI] [PubMed] [Google Scholar]
- 27.Ruijter J. M., Ramakers C., Hoogaars W. M., Karlen Y., Bakker O., van den Hoff M. J., Moorman A. F. (2009) Nucleic Acids Res. 37, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandari B., Burns D. M., Hoffman R. C., Miller R. E. (1986) Mol. Cell. Endocrinol. 47, 49–57 [DOI] [PubMed] [Google Scholar]
- 29.van Straaten H. W., He Y., van Duist M. M., Labruyère W. T., Vermeulen J. L., van Dijk P. J., Ruijter J. M., Lamers W. H., Hakvoort T. B. (2006) Biochem. Cell Biol. 84, 215–231 [DOI] [PubMed] [Google Scholar]
- 30.Danielyan L., Zellmer S., Sickinger S., Tolstonog G. V., Salvetter J., Lourhmati A., Reissig D. D., Gleiter C. H., Gebhardt R., Buniatian G. H. (2009) PLoS ONE 4, e4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arola L., Palou A., Remesar X., Alemany M. (1981) Arch. Int. Physiol. Biochim. 89, 189–194 [DOI] [PubMed] [Google Scholar]
- 32.Witzemann V. (2006) Cell Tissue Res. 326, 236–271 [DOI] [PubMed] [Google Scholar]
- 33.McGivan J. D., Bungard C. I. (2007) Front. Biosci. 12, 874–882 [DOI] [PubMed] [Google Scholar]
- 34.Tang H., Cheung W. M., Ip F. C., Ip N. Y. (2000) Mol. Cell. Neurosci. 16, 127–140 [DOI] [PubMed] [Google Scholar]
- 35.Lie-Venema H., Hakvoort T. B., van Hemert F. J., Moorman A. F., Lamers W. H. (1998) Prog. Nucleic Acids Res. 61, 243–308 [DOI] [PubMed] [Google Scholar]
- 36.Jepson M. M., Bates P. C., Broadbent P., Pell J. M., Millward D. J. (1988) Am. J. Physiol. Endocrinol. Metab. 255, E166–E172 [DOI] [PubMed] [Google Scholar]
- 37.Biolo G., Fleming R. Y., Maggi S. P., Nguyen T. T., Herndon D. N., Wolfe R. R. (2000) Clin. Sci. 99, 189–194 [PubMed] [Google Scholar]
- 38.Van Acker B. A., Hulsewé K. W., Wagenmakers A. J., Deutz N. E., Van Kreel B. K., Halliday D., Matthews D. E., Soeters P. B., Von Meyenfeldt M. F. (1998) Clin. Sci. 95, 339–346 [PubMed] [Google Scholar]
- 39.Chang T. W., Goldberg A. L. (1978) J. Biol. Chem. 253, 3677–3684 [PubMed] [Google Scholar]
- 40.MacLennan P. A., Smith K., Weryk B., Watt P. W., Rennie M. J. (1988) FEBS Lett. 237, 133–136 [DOI] [PubMed] [Google Scholar]
- 41.Hickson R. C., Czerwinski S. M., Wegrzyn L. E. (1995) Am. J. Physiol. Endocrinol. Metab. 268, E730–E734 [DOI] [PubMed] [Google Scholar]
- 42.Darmaun D., Matthews D. E., Bier D. M. (1988) Am. J. Physiol. Endocrinol. Metab. 255, E366–E373 [DOI] [PubMed] [Google Scholar]
- 43.Rutten E. P., Engelen M. P., Schols A. M., Deutz N. E. (2005) Curr. Opin. Clin. Nutr. Metab. Care 8, 41–51 [DOI] [PubMed] [Google Scholar]
- 44.Felig P. (1973) Metabolism 22, 179–207 [DOI] [PubMed] [Google Scholar]
- 45.Jagoe R. T., Lecker S. H., Gomes M., Goldberg A. L. (2002) FASEB J. 16, 1697–1712 [DOI] [PubMed] [Google Scholar]
- 46.Finn P. F., Dice J. F. (2006) Nutrition 22, 830–844 [DOI] [PubMed] [Google Scholar]
- 47.Thompson J. R., Wu G. (1991) Comp. Biochem. Physiol. B Mol. Biol. 100, 209–216 [DOI] [PubMed] [Google Scholar]
- 48.Li B., Higgins J. E., Jefferson L. S. (1979) Am. J. Physiol. Endocrinol. Metab. 5, E222–E228 [Google Scholar]
- 49.Chatauret N., Desjardins P., Zwingmann C., Rose C., Rao K. V., Butterworth R. F. (2006) J. Hepatol. 44, 1083–1088 [DOI] [PubMed] [Google Scholar]
- 50.Listrom C. D., Morizono H., Rajagopal B. S., McCann M. T., Tuchman M., Allewell N. M. (1997) Biochem. J. 328, 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.