Abstract
Amiloride is a small molecule diuretic, which has been used to dissect sodium transport pathways in many different systems. This drug is known to interact with the epithelial sodium channel and acid-sensing ion channel proteins, as well as sodium/hydrogen antiporters and sodium/calcium exchangers. The exact structural basis for these interactions has not been elucidated as crystal structures of these proteins have been challenging to obtain, though some involved residues and domains have been mapped. This work examines the interaction of amiloride with acid-sensing ion channel-1, a protein whose structure is available using computational and experimental techniques. Using molecular docking software, amiloride and related molecules were docked to model structures of homomeric human ASIC-1 to generate potential interaction sites and predict which analogs would be more or less potent than amiloride. The predictions made were experimentally tested using whole-cell patch clamp. Drugs previously classified as NCX or NHE inhibitors are shown to also inhibit hASIC-1. Potential docking sites were re-examined against experimental data to remove spurious interaction sites. The voltage sensitivity of inhibitors was also examined. Using the aggregated data from these computational and experimental experiments, putative interaction sites for amiloride and hASIC-1 have been defined. Future work will experimentally verify these interaction sites, but at present this should allow for virtual screening of drug libraries at these putative interaction sites.
Keywords: Channels/Sodium, Methods/Computer Modelling, Methods/Computation, Acid-sensing Ion Channels (ASIC), High Throughput Screening (HTS), Amiloride, Small Molecule Docking
Introduction
Amiloride is a small molecule best known for its ability to inhibit channels formed by the epithelial sodium channel (ENaC)2/degenerin (Deg) family of proteins (1). Amiloride was discovered in 1965, a product of the concerted effort to find diuretics that conserved potassium while still leading to overall volume reduction and blood pressure reduction in hypertensive patients (2). Using a rodent model of hypertension, Bicking et al. (2) screened over 300 compounds to find that amiloride and some structurally related molecules were able to increase sodium excretion in deoxycorticosterone acetate-induced hypertensive rats, while maintaining potassium levels. Clinical trials found amiloride to be safe for the long-term treatment of hypertension, as it reduced the risk of hypokalemia, which was associated with other diuretics in use at the time, such as furosemide and ethacrynic acid (3).
Although the exact molecular targets of amiloride were not identified until the 1990s with the cloning of the ENaC proteins, it was apparent much earlier that this molecule could inhibit both sodium conducting and sodium exchange proteins, albeit with different affinities (1). However, even after identification of the ENaC/Deg proteins as high affinity binders for amiloride, showing an IC50 of ∼0.1 μm for the prototypical channel composed of αβγ-ENaCs (1), the mechanism of amiloride inhibition and the residues involved remained elusive. Using mutagenesis and chimeric proteins coupled to electrophysiological and radioligand binding data, model emerged where amiloride physically occluded the channel pore from the external aspect (1, 4), though there are data-implicating sites in the extracellular loops (5–9). As inhibitors of ENaC/Deg proteins could be useful in the treatment of pathologies ranging from cardiovascular disease and hypertension (10), cystic fibrosis (11), neurodegenerative diseases (12), and various cancers (13–15), in this study we have used computational techniques to examine the structural basis of the amiloride and ENaC/Deg relationship.
This is possible thanks to recent work by the Gouaux group, which has been able to define the crystal structure of channels made up of homomeric chicken acid-sensing ion channel 1 (ASIC-1) (16, 17). As galline ENaC/Deg channels are scantily described, a homology modeling approach has been used previously to examine the interactions of human ASIC models with the peptide inhibitor Psalmotoxin-1 (18, 19). This study focuses on the interactions of hASIC-1 and hASIC-3 with amiloride in order to define binding pockets for future inhibitor studies. Using a small molecule docking program, Autodock Vina (20), amiloride and 29 other related molecules were docked to channel models, created with MODELLER (21), based on the crystal structure of the nonfunctional cASIC-1, 2QTS, and that of the functional cASIC-1, 3HGC. Whole-cell patch clamp recordings were used to experimentally verify the predictions of the docking software with ASIC-1, whereas the findings of Kuduk et al. (22) were leveraged to validate the results of docking to ASIC-3. Comparisons of the docking sites against putative cation binding sites described in the crystal 3IJ4 (16) suggested sodium competition experiments with amiloride. By focusing the findings from these different studies on the ENaC/Deg interaction with amiloride (5–9, 22–26) and adding the computational and experimental results in this current study, putative interaction sites for amiloride with the ENaC/Deg proteins are described. This work also validates these sites for future virtual screening of larger drug libraries beyond the handful of amiloride derivatives used in this work.
EXPERIMENTAL PROCEDURES
Template Structures
The work of Gouaux's group described the structure of Gallus gallus ASIC-1 arranged to form a homomeric channel, available as PDB ID 2QTS and PDB ID 3HGC (16, 17). There are differences between the structures, both in resolution and constructs used, and thus both are used in this study as templates. Hetero-atoms/residues other than the chloride ion were removed from both. For 2QTS, chains A, B, and C were used, as these chains were of higher resolution than D, E, and F, while the biological unit was used for 3HGC to create a similar trimeric channel structure.
Target Sequences
The amino acid sequences for full-length chicken ASIC-1, human ASIC-1b, hASIC-2b, hASIC-3a, hASIC-4a, α-hENaC, β-hENaC, and γ-hENaC were obtained from the NCBI Protein Data Base. Alignments were performed using ClustalX 2.0.9 (27). Care was taken to realign regions as initial alignments did not conserve cysteines across the family, leading to better conservation of disulfide bonding order between the template and models. Results of the alignments, as well as accession numbers, are shown in Table 1, whereas the alignment itself is presented as supplemental Fig. S1. Identity and similarity statistics were calculated and formatted using the Sequence Manipulation Suite (28). Splice variants were chosen to minimize gaps in the alignments.
TABLE 1.
Protein alignments of the human ASIC/ENaC proteins against chicken ASIC-1
Multiple protein sequence alignment was performed using ClustalX2, with care taken to realign cysteine residues to better conserve disulfide bonding. Supplemental Fig. S1 provides the exact alignment.
| Protein name | NCBI accession no. | Similar | Identical |
|---|---|---|---|
| % | % | ||
| cASIC-1 | NP_001035557.1 | 100.0 | 100.0 |
| hASIC-1b | NP_001086.2 | 92.8 | 89.0 |
| hASIC-2b | NP_001085.2 | 73.2 | 64.7 |
| hASIC-3a | NP_004760.1 | 58.9 | 47.0 |
| hASIC-4a | NP_061144.2 | 46.3 | 37.2 |
| α-hENaC | NP_001029.1 | 27.6 | 16.0 |
| β-hENaC | NP_000327.2 | 28.4 | 16.9 |
| γ-hENaC | NP_001030.2 | 27.5 | 17.0 |
Homology Modeling
MODELLER 9v7 was used to perform automatic homology modeling of the target sequences using templates from cASIC-1 (21). N and C termini were removed from target sequences as no data are available for them within either of the crystal structures of cASIC-1 (16, 17). Support for the chloride ions was enabled in MODELLER, and they were considered during the modeling of the channels. The scripts used for modeling, in addition to adding support for the chloride ions, increased the thoroughness of the default optimization protocol. The variable target function method optimization was set to slow with the maximum iterations set at 100. The molecular dynamics with simulated annealing optimization was also set to slow, and the entire process was repeated three times to generate 128 models. The model with the best average rank from both the discrete optimized protein energy score (29) and molecular probability density function value (30) were selected for energy minimization in Gromacs 4, using the GROMOS96 43a1 force field and solvated with simple point charge water model (31, 32). The system was energy minimized using the steepest descents algorithm with no position restraints until the system converged to machine precision on the Cheaha computer cluster at the University of Alabama at Birmingham. Models were created using either the 2QTS or 3HGC template leading to a total of four new models, two of hASIC-1b and two of hASIC-3a. Attempts to create models based on both templates were problematic as there is divergence in the transmembrane domains of the two templates (16, 17). Models were validated against their templates using the NIH Structural Analysis and VErification Server.
Small Molecule Docking
Structures for amiloride and related molecules were retrieved from PubChem as three-dimensional SDF files. The drugs chosen for this study were either readily available or were hits in a search for the string “amiloride” in the NCBI PubChem webserver. OpenBabel 2.2.3 was used to create MOL2 files. For comparison with the work of Kuduk et al. (22), small molecule PDBQT files were generated using the Dundee PRODRG web server (33). The receptor and ligands were prepared for docking with Vina (20). The search space was defined as the size of the molecule plus 15 Å in each dimension, centered at the geometric center ± 5 Å for two randomly chosen dimensions to reduce center bias. Unlike older versions of Autodock which used grid spacing and various other settings to define the thoroughness of docking and the search algorithm parameters, Vina has been redesigned to use an iterated local search algorithm (20), taking as input primarily the search space and an exhaustiveness setting to determine the time spent per docking, with the default set at 8. For this work, as the search space was relatively large, between 1.2 and 1.5 million Å3, the exhaustiveness setting was increased to 256. Up to 1000 binding poses within 10 kcal/mol of the lowest binding energy were outputted and analyzed. For each pose, a center of mass (COM) was computed, and the COMs were clustered using a quality threshold clustering algorithm (34) defining a 2 Å distance for clusters, with those clusters containing one or two poses considered as outliers and removed from further analyses.
Structural Visualization
Models were visualized using Visual Molecular Dynamics from University of Illinois. Figures are rendered using Tachyon (35).
Cell Culture and Transfection
CHO-K1 cells were maintained in 1:1 Dulbecco's modified Eagle's medium/F12 (Hyclone) supplemented with 10% fetal bovine serum (FBS, Hyclone) and 1% penicillin/streptomycin (Invitrogen). CHO-AP1 cells were a generous gift of Sergio Grinstein (Hospital for Sick Children, Toronto, ON) and were maintained in αMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 26 mm NaHCO3. Cells were transfected with plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol at a ratio of 1:2.5 μg: μl of the bicistronic pBi-eGFP/hASIC-1b plasmid DNA to lipid. Transfected cells were replated onto sterile glass coverslips and patched.
Patch Clamp
Micropipettes were prepared using a Narashigi PP-83 two-stage micropipette puller with an electrical resistance of 3–5 MΩ when filled with 120 mm KCl, 5 mm NaCl, 10 mm HEPES, 0.4 mm CaCl2, 2 mm MgCl2, 1 mm EGTA, and 2 mm MgATP (pH 7.4). The whole-cell configuration was achieved by abutting the pipette tip with a cell, applying suction, forming a >1 GΩ seal, and rupturing the membrane to achieve cytoplasmic access using either suction or the Zap function of the Axopatch 200B patch-clamp amplifier. The barrels of a VC-77 MCS perfusion system (Warner Instruments) were moved adjacent to the cell for rapid, local perfusion. Signals were recorded with pCLAMP 9 using a DigiData 1320 digitizer (Molecular Devices). The signal was sampled at 5 kHz and low pass filtered at 5 kHz with the 200B four-pole Bessel filter. Cells were perfused with a modified Krebs buffer containing130 mm NaCl, 2 mm CaCl2, 10 mm d-glucose, 10 mm HEPES, and 10 mm MES, pH 7.4 with HCl. For sodium competition measurements, NaCl was replaced with N-methyl-d-glucamine, and calcium was omitted to create a high Na+ solution with 10 mm N-methyl-d-glucamine/120 mm NaCl and a low Na+ solution with 120 mm N-methyl-d-glucamine/10 mm NaCl. Cells were held at −60 mV and pH 6.0 pulses of 3-s duration were applied every 30 s, with 200 ms (−100 to +100 mV) applied once before the acid pulse and once during the latter part of the acid pulse. Little to no desensitization was seen in the absence of access resistance changes. Thus, data were normalized to the pulse immediately prior to initiation of the experimental pulse.
Statistics
Data were analyzed using Clampfit (Molecular Devices), Excel 2007 (Microsoft), and R 2.9.2 (36). Data are presented as averages ± S.D. as calculated by Excel 2007. One-way ANOVAs were performed in R, with α = 0.05, to assay for differences between groups. Tukey's HSD post-hoc test was used to define different groups.
RESULTS
Homology Modeling
Models were created for hASIC-1b and hASIC-3a against the template structures of 2QTS (17) and 3HGC (16). The best model for human ASIC-1b and human ASIC-3a were created and analyzed using the NIH SAVES web server. The models scored well as compared with the initial templates, as would be expected by the level of identity shown in Table 1, similar to what was found for prior ASIC models(18). NIH SAVES results are shown in supplemental Table S1 for the individual models and the template molecules.
Small Molecule Docking to hASIC-1 Models
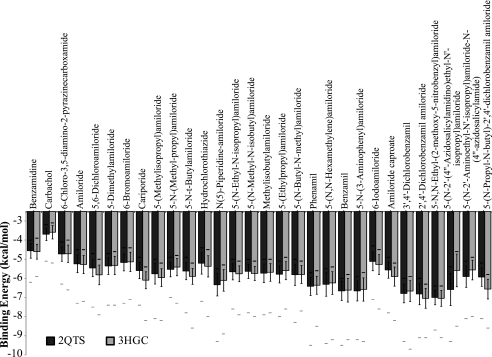
Small molecules were blind docked, screening the flexible ligands against the entirety of the rigid protein models. The top 1000 best docked poses were computed for each drug/model combination. For each docking position, a COM for the molecule was computed. Using a quality threshold clustering approach, poses whose COMs were less than 2.0 Å from at least one other pose were removed to reduce outliers. A visual example of this data reduction protocol is shown in Fig. 1. The average binding energy for the remaining poses for each drug was computed and is shown in Fig. 2. Using a one-way ANOVA followed by a Tukey-HSD post-hoc test, many statistically significant differences were observed. First, examining differences between ligands binding to either 2QTS-based hASIC-1b or 3HGC-based hASIC-1b shows that at least 16 of the ligands score statistically differently between the two templates. However, the statistically significant differences in binding energies for the same ligand between different templates were minimal, averaging 0.29 kcal/mol. The largest error was for 5-(N-2′-(4″-azidosalicylamidino)ethyl-N′-isopropyl)amiloride, which showed a difference of 0.99 kcal/mol between the two models, and was also the largest molecule tested with 33 non-hydrogen atoms, suggesting that this may be an issue of sampling the conformational space of the molecule rather than true discordance between the models. Further, supporting this hypothesis is correlation of the molecular mass of the ligands with the average difference between the two templates, with a Pearson correlation coefficient of 0.56, which is statistically significant at a p < 0.01 for n = 30. This suggests that there is no significant difference between models based on the two structures, at least from an inhibitor docking standpoint.
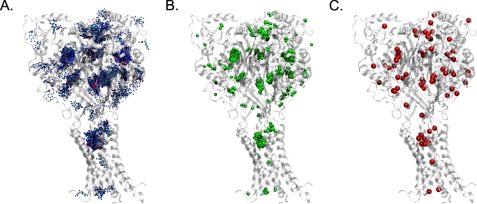
FIGURE 1.
This visualization shows the data processing performed to remove outliers using amiloride interacting with the model of hASIC-1b based on 2QTS as an example. In panel A are 1000 poses computed for a flexible amiloride molecule docking to the rigid model, with each non-hydrogen atom of amiloride shown as a dot (carbon and chloride in cyan, oxygen in red, nitrogen in blue). In panel B, shown by green beads are the computed COMs for each pose. Panel C shows how by using a simple quality threshold clustering algorithm, these 1000 COMs can be replaced by a smaller number of red beads helping to remove outlier poses and also to visualize areas where the poses cluster.
FIGURE 2.
The average binding energies ± S.D. are shown for the 30 ligands docked to hASIC-1b models based on either the 2QTS or 3HGC. Solid white bars are the maximal or worst energies computed for the dockings, whereas the dark black or gray bars represent the minimum or best binding energy for the docking results. Though there are significant differences for docking between the two models (one-way ANOVA, α = 0.05, Tukey-HSD), the discordance between models is at most 1 kcal/mol, averaging 0.29 kcal/mol.
5 of the 30 drugs were chosen for further examination: Amiloride, the serine protease inhibitor benzamidine, the amiloride analog benzamil, the NHE inhibitor 5-(N,N-hexamethylene) amiloride, and the NCX inhibitor 3′,4′-dichlorobenzamil. Amiloride was chosen as it is a well described inhibitor of ENaC/Deg channels (1, 37). Benzamil is known to be a more potent inhibitor of ENaC channels (37), and there is evidence for it being a more potent ASIC inhibitor (38). Though the other 3 drugs have known biological functions, their functional effects on ASICs are undefined and allow for functional testing of the screening system.
Experimental Testing of Docking
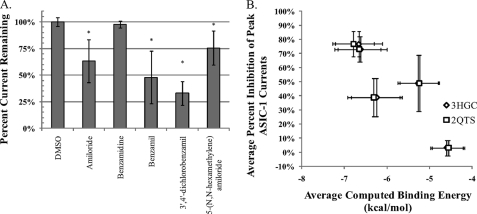
Using the whole-cell patch clamp technique in CHO-K1 cells transiently transfected with hASIC-1b, the effects of 25 μm of the selected drugs were tested on the pH 6.0 induced current. As the chosen drugs have a variety of known functions, a quick testing protocol was chosen where the drug was present only during the acid pulse. The reported Ki for amiloride with ASIC-1 is in the 1–10 μm range (1); thus 25 μm with this rapid protocol was chosen as it was expected not to be maximally inhibitory. As suggested by the computational docking, the protease inhibitor benzamidine had no statistically significant effect on the acid-induced currents, as compared with the vehicle alone (Fig. 3). With this protocol, amiloride reduced currents by about 40%, and benzamil was slightly more effective, inhibiting ASIC-1 currents by about 50%. More interestingly, 5-(N,N-hexamethylene) amiloride, and the 3′,4′-dichlorobenzamil also both were able to inhibit the ASIC-1 currents, as predicted by the computational screening. 5-(N,N-Hexamethylene) amiloride reduced currents by ∼25%, whereas 3′,4′-dichlorobenzamil proved to be most potent, inhibiting the ASIC current by about 70%. These results are generally consistent with the computational data, although it was expected that 5-(N,N-hexamethylene) amiloride would be much more effective than amiloride. This discrepancy may in part be due to the lack of flexibility of the azepane moiety; the 7-member heterocycle ring was held in a rigid minimal energy conformation, which likely led to the overestimation of the binding energy. Future docking trials may attempt to increase the flexibility of the azepane moiety, but this would require software more robust than Autodock.
FIGURE 3.
Using whole-cell electrophysiology in CHO-K1 cells transiently transfected with hASIC-1b, the effect of 5 of the 30 drugs were tested for interaction with the channel. A, peak acid-induced currents in the presence of 25 μm drug were normalized to the peak prior. All drugs were dissolved in DMSO, and compared with the pH 6.0 + vehicle, amiloride, 5-(N,N-hexamethylene) amiloride, benzamil, and 3′,4′-dichlorobenzamil were able to significantly inhibit the peak acid-induced current as predicted by the computational docking. Benzamidine was not statistically different from vehicle. (n > = 4, ± S.D.,*, significant versus DMSO, ANOVA w/Tukey-HSD, α = 0.05). B, average percent inhibition (±S.D.) correlated well with the average computed binding energy (±S.D.) to models based on either the 3HGC or 2QTS structures (panel B), showing a Pearson correlation coefficient of ∼0.87, which is statistically significant at a p < 0.10 for a Student's two-tailed test.
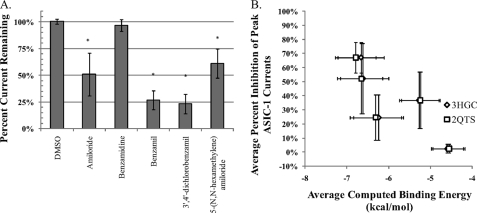
As it was noted that some of these drugs have described biological functions, it is possible that their off-target effects are leading to apparent inhibition of the channel. This should be relatively unlikely as the drugs are present only during the acid pulse, but it is a possibility. For example, CHO-K1 cells are described as having endogenous NHE activity (39, 40), the inhibition of which by 5-(N,N-hexamethylene) amiloride would perturb cytosolic pH. This could lead to alterations in ASIC-1 function, as intracellular pH has been shown to modulate ASIC currents in murine primary cortical neurons (41). To test for this, a CHO cell line devoid of endogenous NHE activity, CHO-AP1 cells (42), were transiently transfected with hASIC-1b, and the drugs were rescreened in these cells (Fig. 4). Similar to the CHO-K1 cells, amiloride, 5-(N,N-hexamethylene) amiloride, benzamil, and 3′,4′-dichlorobenzamil drugs were able to inhibit the acid-induced currents whereas benzamidine showed no statistically significant effect. These data further rule out a secondary mechanism of action, suggesting that the ligands are acting directly on hASIC-1b.
FIGURE 4.
Using whole-cell electrophysiology in CHO-AP1 cells transiently transfected with hASIC-1b, the effect of the drugs were tested for interaction with the channel in the absence of a possible interaction with endogenous NHE in CHO-K1 cells. A, peak acid-induced currents in the presence of 25 μm drug were normalized to the peak prior. As compared with the DMSO vehicle control, amiloride, 5-(N,N-hexamethylene) amiloride, benzamil, and 3′,4′-dichlorobenzamil were able to significantly inhibit the peak acid-induced current as predicted by the computational docking. Benzamidine was again not statistically different from vehicle. (n > = 4, ± S.D., *, significant versus DMSO, ANOVA w/Tukey-HSD, α = 0.05). B, average percent inhibition (±S.D.) correlated well with the average computed binding energy (±S.D.) to models based on either the 3HGC (panel A) or 2QTS structures (panel B), showing a Pearson correlation coefficient of ∼0.82 which is statistically significant at a p < 0.10 for a two-tailed test.
Defining a Docking Site for Amiloride
With functional data verifying the computational data of this blind docking, work was done to define a docking site for these drugs. Initial examinations of the docking clusters for these drugs show that there are a large number of putative interaction sites, with 69–104 clusters found per docking of the five chosen ligands to the hASIC-1b models. This is to be expected with the unbiased blind docking approach used, the large number of docking runs for each set, and the flexibility of the ligand allowing for many possible energetic minimums to be found.
Examination of amiloride binding to the ENaC/Deg proteins has shown that there may be multiple binding regions of differing affinities. This would be consistent with the multiple clusters retained from the computational data. Specifically, there are data in support of binding to a pore region (5, 24, 25) and in support of amiloride binding domains further from the pore (5–9). The electrophysiological examination of amiloride inhibition of ENaC (43) and ASIC (5) currents shows a voltage dependence to the block, increasing at more negative potentials, likely due to the active form of amiloride being positively charged at physiological pH (44). This voltage sensitivity was observed for the tested inhibitors, except for benzamidine, in the whole-cell patch clamp experiments performed (data not shown). This functional data would suggest that the inhibition of ENaC by amiloride occurs either in a manner so as to expose the drug to the effects of transmembrane voltage, either by a voltage-induced conformational change or by mimicking the effects of membrane voltage on cations. The concept of voltage-induced conformational changes has been examined in ENaCs by use of the electroneutral amiloride analog 6-chloro-3,5-diamino-pyrazine-2-carboxamide, also known as CDPC, which showed no voltage dependence to inhibition suggesting the charge of amiloride is directly sensing the voltage rather than a charge on the channel protein (45). Taken together, these observations suggest that an additional constraint of being within the conductive pathway be applied to the possible docking sites.
Unfortunately, the conductive pathway of the ENaC/Deg channels is poorly defined. However, using the cesium binding sites in the crystal structure (16), a conduction route can be deduced from the multiple vestibules found in the models. The 3IJ4 structure gives a starting point, one of three symmetrically distributed Cs+ ions binding in the extracellular vestibule, and an ending point with 2 Cs+ ions binding in the channel pore (Fig. 5, panel A). There is also a large central vestibule in the channel where no cations have been found, but as it is directly in the path between the Cs+ ions, it could be expected to be involved in transit from the extracellular sites to the pore region. An additional Cs+ ion binding site is located on the external aspect of the channel but does not appear to be involved in a conductive pathway.
FIGURE 5.
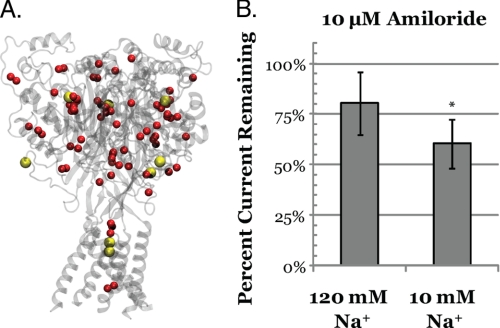
Following data reduction and clustering, many putative docking pockets remained. To narrow down the scope, examination of the putative conductive pathway was conducted. In panel A, the hASIC-1b model based on 3HGC is shown as a transparent gray ribbon diagram. The red sphere represents the clustering results for amiloride. The yellow van Der Waal surfaces represent cesium cations cocrystallized with cASIC-1 aligned to the model structure. Visually, overlap between some of the putative binding sites and the cesium cations can be observed in both the extracellular and pore region. This suggests sodium cations may directly compete with amiloride when interacting with hASIC-1b. In panel B, this prediction is verified by testing the effects of sodium concentration on the effect of 10 μm amiloride on hASIC-1b expressed in CHO-K1 cells. There is ∼200% more amiloride inhibition seen in 10 mm Na+ (39.5%) as compared with 120 mm Na+ (19.7%). (n = 9, ± S.D., 2-tailed unpaired Student's t test, *, p < 0.01).
Using these locations as additional criteria, it can be observed that there is overlap between the computed docking clusters and Cs+ ions (Fig. 5, panel A). This would suggest that cations and amiloride may compete for binding to hASIC-1b, an observation that has been noted (3, 43, 45) and disputed (46) for amiloride-sensitive ENaC channels in epithelia or heterologous expression systems. To test this predicted competition between sodium for amiloride inhibition of hASIC-1b, the relative inhibition of pH 6.0 induced currents was computed for CHO-K1 cells transiently transfected with hASIC-1b in 10 and 130 mm Na+ solutions. The impermeant cation N-methyl-d-glucamine was used to maintain osmotic balance and thus Na+ and H+ were the only conductive cations. At high sodium concentrations, 10 μm amiloride was able to inhibit only ∼20% of the high sodium acid-induced current, whereas at low sodium, 10 μm amiloride was able to inhibit ∼40% of the low sodium acid-induced currents. The observation that sodium and amiloride appear to compete, confirm that the clusters docking near the Cs+ cations are likely valid.
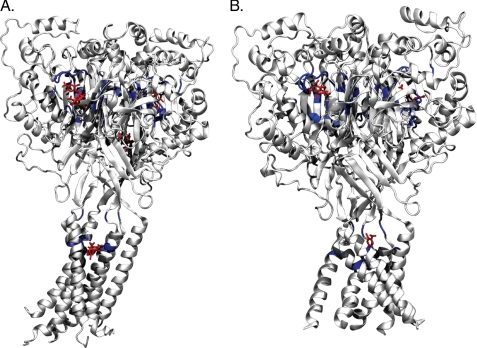
Isolating the COMs that are within 10 Å of Cs+ ions, based on 3HGC model returns ∼532 of the 947 non-outlier docked poses while the 2QTS-based model returned 470 of 982 non-outlier poses. Isolating the poses with the most negative calculated energies in these clusters and defining residues with atoms within 5 Å of the docking sites returns 42/33 residues for the 2QTS/3HGC-based models, shown visually in Fig. 6 and described in supplemental Table S3. For 2QTS, multiple minimal poses returned the same binding energy for some Cs+ pockets, with one binding pocket located at a very different location. Of these, 24 residues are shared between the two models. These residues are not identical to residues implicated by functional mutagenesis data for ENaC or ASIC proteins (5–9, 24, 25); however, they are within similar regions. For example, in murine αENaC S583, and the equivalent glycine residues in β- and γ-ENaC, have been implicated in interacting with amiloride (1). Though this residue was not implicated by our docking, a 5 amino acid tract (GDIGG) located two residues before this residue and the L residue just after this site were implicated (supplemental Fig. S2 or supplemental Table S3). Using this information, it is now possible to define these regions within the channel as sites for larger scale virtual screening experiments and better guided experimental verification.
FIGURE 6.
The lowest docked poses within 10 Å of the Cs+ binding pockets were extracted and are shown as red licorice models. Residues with atoms within 5 Å of the amiloride molecules are shown as solid gray-colored ribbon diagrams, with the solid blue ribbon diagram representing the residues shared between the 3HGC (panel A) and 2QTS (panel B) models. The transparent gray outlines show the overall ribbon structure of the molecules. Individual residues are stated in supplemental Table S3 as well as diagrammed in the ENaC/ASIC protein alignment in supplemental Fig. S1.
Small Molecule Docking to hASIC-3 Models
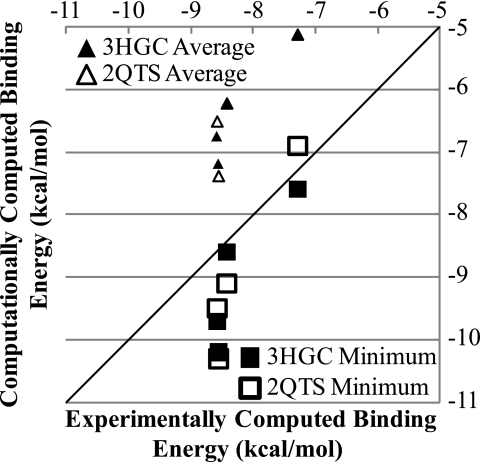
To test the validity of small molecule screening for novel drugs based on amiloride, the experimental results of Kuduk et al. (22) were compared with the computational results found for hASIC-3 models based on 2QTS or 3HGC. Although 60 drugs were synthesized and screened using an automated patch clamp technique with HEK293 cells stably expressing ASIC-3a, only 4 were chosen for in-depth dose response calculations (22). The correlation between the experimental and computed binding energies for these four drugs were examined in Fig. 7, finding that the Pearson correlation for the lowest binding energy correlated best with the experimental data for the 2QTS-based models (0.96), whereas the average binding energy correlated best with the experimental data for the 3HGC-based data (0.93). This recapitulation of the physical data based on the computational work further validates the strength of this methodology for finding novel small molecule inhibitors of ASICs.
FIGURE 7.
Using data generated by Kuduk et al. (22), the capabilities of the docking algorithm to recapitulate experimental amiloride-based ligand binding to ASIC-3 were examined. Computing the experimental binding energy as RTln(10 − pKi), the average and best minimal computationally calculated energies were correlated to experimental data for the 4 thoroughly examined drugs. In this small set, the Pearson correlation coefficient for the average energies was 0.88 for the 2QTS-based model and 0.93 for the 3HGC-based model. For the best or minimum binding energy, the correlation was 0.96 and 0.87 for the 2QTS- and 3HGC-based models. The critical value for the significance of the Pearson correlation coefficient at p < 0.05 for n = 4 is 0.95, and thus only the correlation of the minimum binding energy of the 2QTS-based models is statistically significant. However, the other values also trend to significance, and this further validates the virtual screening methodology applied, suggesting it can be used to predict small molecule binding to ASIC models.
DISCUSSION
The interaction of amiloride with the ENaC/Deg proteins has been studied extensively because they were elucidated as the target of this sodium channel inhibitor (1). However, until the description of the chicken ASIC1 structure (16, 17), there was little known about the structure of the target proteins beyond the topology and inferences made from mutagenesis and functional experiments (1). Using computational methods, we have docked amiloride against the channel models. Although this is computationally expensive, the methodology used allows for unbiased determination of docking regions and appears robust enough to help define novel small molecule inhibitors of the channels. However, there are important considerations that can affect the interpretation of these results.
The Validity of a Homology Modeling and Docking Approach
At the core of homology modeling is an assumption that there is structural similarity between proteins related at the primary sequence level, but the technique is capable of recapitulating valid structures based on as little as 25% identity between target and template (47). However, this requires that other criteria are met such as a shared function between the target and template. As this work attempts to leverage functional data from both ENaC and ASIC proteins, it should be appreciated that there are significant functional differences between the prototypical ENaC and the prototypical ASIC-containing channels (1). For example, ENaCs are generally considered to be constitutively active or more recently, modified by proteases to become active, whereas ASICs are gated by a drop in extracellular pH (1). They are still, however, cation channels and share in inhibition by amiloride (1), so they are likely structurally similar enough to allow for some functional data from ENaCs to be applied to ASICs and for some homology modeling attempts (48).
The crystal structure templates were considered separately during the modeling and docking process. This is due to the structural differences between the two and the resolution difference between the two template models (16, 17). Although the 2QTS structure was of higher quality, the 3HGC structure was of a construct that showed the ability to conduct cations unlike the 2QTS structure. Models based on the individual subunits could have been created, but this would have lost data regarding the subunit-subunit interactions that may affect docking of amiloride analogs.
There are valid issues raised by the approaches used in this work. For example, the selectivity filter in ENaCs/ASICs has been described as being a conserved G/SXS motif in the transmembrane helices (1). The structural data suggest this corresponds to an area within the cytoplasmic vestibule of the channel (16), which is inconsistent with some prestructural expectations (1). This could be due to the desensitized state of the ASIC structures (16–18), a functional state which is not well described for ENaC-containing channels, and it highlights the importance of not expecting a one-to-one translation of functional data to structural data.
Another important point is the assumption of amiloride as interacting with the channel only in an open state. There is generally an absence of amiloride interaction data for nonfunctioning or closed channels, though it should be possible to perform these experiments with radioligand binding assays for ENaCs resistant to protease cleavage (49), but it has been shown for ASIC-2 that amiloride is capable of interacting with the channel when it is not conducting at the whole-cell level (5). This suggests that although the channel structures are closed and desensitized, valid binding data or sites may still be deduced.
Amiloride Docking to ASIC-1
Examining amiloride binding to the model, at least two distinct regions were found where amiloride can bind to hASIC-1 channels, corresponding to the cation binding sites in the crystal structures deduced by Gonzales et al. (16). This agrees with experimental ENaC and ASIC data suggesting amiloride directly competes with sodium cations and is in the conducting pathway (5, 43). Another putative site was detected in the acidic central midline vestibule created by the β-sheets of the palm domain (16); however, with the absence of a defined cation within this chamber, we refrain from defining this as another possible amiloride binding pocket due to the lack of experimental validation.
In terms of known locations affecting amiloride binding, the two best described motifs are the WYRFHY tract found in ENaCs and residues in the pore and pre-M2 region (6–9, 23, 25, 26, 45, 46). Unfortunately, the WYRFHY domain itself is not directly conserved in ASICs (supplemental Fig. S1), although there is a QYYFHY tract near the pore region of which Tyr-67 in the hASIC-1b model based on 3HGC was found to be near the best docked pose (supplemental Fig. S1 or Table S3). As for residues within the pore region and pre-transmembrane region 2, inferences from ENaC experiments implicate that multiple residues are involved. Functional data are clouded by the role of these residues in conductance and permeability of the cations. As noted earlier, although the docking results do not directly recapitulate residues implicated by functional data, this could be due to the desensitized state of the crystal structure. There are some expectations that were recapitulated and tested functionally, such as the competition of amiloride with cations in the channel (Fig. 6) (1). Residues implicated in binding cations by the Cs+ cocrystallized structure 3IJ4 (16), such as Gly-432 and Asp-433 at the pore and Thr-237—Thr-240 in the extracellular domain of cASIC-1, are also implicated by the docking results.
Although we have defined residues that may mediate the interaction, we are better able to define pockets where amiloride binds and interacts with the channel than specific residues for mutagenesis. Because of the flexibility introduced to the ligand, the rigidity of the receptor, the absence of explicit water molecules, and the lack of a true open conformation of the channel, this work is somewhat limited. However, the goal of defining binding pockets makes the ability to virtually screen or rationally design small molecule inhibitors more computationally feasible.
Virtual Screening of Amiloride Analogs
To show the feasibility of computational determination of analog affinity to ASIC models, a group of 30 drugs related to amiloride were examined for their ability to inhibit ASIC-1 models. Furthermore, models of ASIC-3 were computationally screened against a group of 58 amiloride derivatives to examine whether our in silico approach could recapitulate the functional observations of Kuduk et al. (22).
For the first part, four drugs were compared against amiloride for their ability to interact with ASIC-1 function in whole-cell patch clamp. The functional results repeated the trends saw in computational results, with benzamil, 5-(N,N-hexamethylene) amiloride, and 3′,4′-dichlorobenzamil all found to inhibit the channel, while benzamidine was found to have no effect (Fig. 3). An important point often overlooked is the pleiotropic effects of amiloride and its analogs. Although it is well known that amiloride affects ENaC (1–100 nm) and ASIC (1–100 μm) at differing doses (1), its capabilities to interact with the endogenously expressed NHE1 (5–50 μm) and NCX (1 mm) transport proteins is less well appreciated (10). Importantly for this work and others, intracellular pH (41), which could be perturbed by NHE inhibition, has been shown to modulate ASIC function. As at least one of the drugs is characterized as an NHE inhibitor, the functional results were recapitulated and validated once more with a cell line lacking endogenous NHE activity (Fig. 4). It is important to consider that some of the functional effects of amiloride seen in experiments on ASICs are due to proteins other than those in the ENaC/Deg family. For example, the paradoxical stimulation of ASIC-2 by 0.1–1 mm amiloride (5) could be due to effects on the NHE rather than direct interactions with the channel. Our results do not preclude a stimulatory site for amiloride binding, as the computational results are currently incapable of differentiating between stimulatory and inhibitory interactions.
To further examine the capabilities of this technique, models of ASIC-3 were created and were tested in silico. The 58 ligands created using medicinal chemistry by Kuduk et al. (22) were recreated in silico using PRODRG (33), which allows for the de novo generation of a three-dimensional structure of small molecules. These ligands were blindly docked to the ASIC-3 models, and a similar analysis protocol to that of the ASIC-1 models was applied. Dose response data for only 3 of the drugs and amiloride were available, and a significant correlation between experimental and computational results was found for the best docked pose of the 2QTS-based models (Fig. 7). It should be noted, these experiments were performed using an automated patch clamp protocol where cells stably expressing ASIC3 were pretreated with drugs for 120 s, and thus it is possible that there may be off-target effects of these analogs leading to the functional inhibition of currents (22). The use of this methodology to find novel small molecules that interact with specific ENaC/ASIC-containing channels could greatly speed the search for therapeutics involving these channels.
Although this study defines amiloride binding residues/domains, describes the competition of sodium and amiloride for ASIC-1, exhibits the ability of two unexpected amiloride analogs to inhibit ASIC-1, and shows the capabilities of the blind docking approach to recapitulate high throughput electrophysiological data, it is still limited by the initial crystal structures and the rigidity of the models. By defining smaller pockets, it is possible that future studies can use molecular dynamics to better explore localized flexibility in the ligand/model interaction as well as accounting for water molecules. This should better define residues involved in the amiloride interaction with the ASIC proteins. Docking to smaller regions of the channel, such as the pore or the Cs+ binding sites in the extracellular domains, should also reduce the computational load from weeks to days and remove many outliers, increasing the number of drugs that can be screened and the validity of the results.
The experiments to test these results functionally with mutagenesis should be performed, but is fraught with the complexities of a system where mutations affecting amiloride binding also alter conductance and permeability (1). Ideally one would be able to co-crystallize amiloride and cASIC-1 or soak cASIC-1 crystals with amiloride to obtain a structure (50) that would allow for verification of docking data without the variables introduced by the complex conduction and function of the channel.
Perhaps the most promising aspect of this work is the predictive capability of this technique to calculate the ability of small molecule drugs to interact with the channel models. Using this homology modeling and docking technique, it may be feasible to design inhibitors of specific ASIC-containing channels, such as the homomeric ASIC-1b channel whose inhibition appears to be neuroprotective (12) or the putative heteromeric ENaC/ASIC channel whose inhibition could be useful in the treatment of malignant gliomas (13). It could also be used to create novel inhibitors of mutants of ASICs as a protective mechanism if the channels are used as tumor therapeutics (51). These possibilities show the impact of this work, and also the large step that the crystal structures have allowed in the understanding of and search for inhibitors of the ASIC/ENaC proteins.
Supplementary Material
Acknowledgments
We thank Drs. Eric Gonzales and Eric Gouaux at for supplying the coordinates of cesium cations bound to ASIC-1, Drs. Jonathan Plumb and Sergio Grinstein for the gift of the CHO-AP1 cell line, and Melissa McCarthy for cell culture assistance. We also thank Niren Kapoor, Edlira Bashari, Arun Rooj, and Dr. Bakhrom Berdiev for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant DK37206. This work was also supported by the AMA foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3 and Fig. S1.
- ENaC
- epithelial sodium channel
- ASIC
- acid-sensing ion channel
- Deg
- degenerin
- NHE
- sodium hydrogen exchanger
- NCX
- sodium calcium exchanger
- MES
- 4-morpholineethanesulfonic acid
- ANOVA
- analysis of variance
- COM
- center of mass.
REFERENCES
- 1.Kellenberger S., Schild L. (2002) Physiol. Rev. 82, 735–767 [DOI] [PubMed] [Google Scholar]
- 2.Bicking J. B., Mason J. W., Woltersdorf O. W., Jones J. H., Kwong S. F., Robb C. M., Cragoe E. J. (1965) J. Med. Chem. 8, 638–642 [Google Scholar]
- 3.Benos D. J. (1982) Am. J. Physiol. 242, C131–C145 [DOI] [PubMed] [Google Scholar]
- 4.Benos D. J., Stanton B. A. (1999) J. Physiol. 520, 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams C. M., Snyder P. M., Welsh M. J. (1999) J. Biol. Chem. 274, 15500–15504 [DOI] [PubMed] [Google Scholar]
- 6.Li X. J., Xu R. H., Guggino W. B., Snyder S. H. (1995) Mol. Pharmacol. 47, 1133–1140 [PubMed] [Google Scholar]
- 7.Kieber-Emmons T., Lin C., Foster M. H., Kleyman T. R. (1999) J. Biol. Chem. 274, 9648–9655 [DOI] [PubMed] [Google Scholar]
- 8.Ismailov, Kieber-Emmons T., Lin C., Berdiev B. K., Shlyonsky V. G., Patton H. K., Fuller C. M., Worrell R., Zuckerman J. B., Sun W., Eaton D. C., Benos D. J., Kleyman T. R. (1997) J. Biol. Chem. 272, 21075–21083 [DOI] [PubMed] [Google Scholar]
- 9.Kashlan O. B., Sheng S., Kleyman T. R. (2005) J. Biol. Chem. 280, 26206–26215 [DOI] [PubMed] [Google Scholar]
- 10.Teiwes J., Toto R. D. (2007) Am. J. Hypertens. 20, 109–117 [DOI] [PubMed] [Google Scholar]
- 11.Hirsh A. J., Zhang J., Zamurs A., Fleegle J., Thelin W. R., Caldwell R. A., Sabater J. R., Abraham W. M., Donowitz M., Cha B., Johnson K. B., St George J. A., Johnson M. R., Boucher R. C. (2008) J. Pharmacol. Exp. Ther. 325, 77–88 [DOI] [PubMed] [Google Scholar]
- 12.Xiong Z. G., Pignataro G., Li M., Chang S. Y., Simon R. P. (2008) Curr. Opin. Pharmacol. 8, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoor N., Bartoszewski R., Qadri Y. J., Bebok Z., Bubien J. K., Fuller C. M., Benos D. J. (2009) J. Biol. Chem. 284, 24526–24541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bondarava M., Li T., Endl E., Wehner F. (2009) Pflugers Arch. 458, 675–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye J. H., Gao J., Wu Y. N., Hu Y. J., Zhang C. P., Xu T. L. (2007) Biochem. Biophys. Res. Commun. 355, 986–992 [DOI] [PubMed] [Google Scholar]
- 16.Gonzales E. B., Kawate T., Gouaux E. (2009) Nature 460, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Nature 449, 316–323 [DOI] [PubMed] [Google Scholar]
- 18.Qadri Y. J., Berdiev B. K., Song Y., Lippton H. L., Fuller C. M., Benos D. J. (2009) J. Biol. Chem. 284, 17625–17633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietra F. (2009) J. Chem. Inf. Model 49, 972–977 [DOI] [PubMed] [Google Scholar]
- 20.Trott O., Olson A. J. (2009) J. Comput. Chem. 30, 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eswar N., John B., Mirkovic N., Fiser A., Ilyin V. A., Pieper U., Stuart A. C., Marti-Renom M. A., Madhusudhan M. S., Yerkovich B., Sali A. (2003) Nucleic Acids Res. 31, 3375–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuduk S. D., Di Marco C. N., Chang R. K., Dipardo R. M., Cook S. P., Cato M. J., Jovanovska A., Urban M. O., Leitl M., Spencer R. H., Kane S. A., Bilodeau M. T., Hartman G. D., Bock M. G. (2009) Bioorg. Med. Chem. Lett. 19, 2514–2518 [DOI] [PubMed] [Google Scholar]
- 23.Kelly O., Lin C., Ramkumar M., Saxena N. C., Kleyman T. R., Eaton D. C. (2003) Am. J. Physiol. Renal Physiol. 285, F1279–F1290 [DOI] [PubMed] [Google Scholar]
- 24.Li J., Sheng S., Perry C. J., Kleyman T. R. (2003) J. Biol. Chem. 278, 13867–13874 [DOI] [PubMed] [Google Scholar]
- 25.Schild L., Schneeberger E., Gautschi I., Firsov D. (1997) J. Gen. Physiol. 109, 15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji H. L., Bishop L. R., Anderson S. J., Fuller C. M., Benos D. J. (2004) J. Biol. Chem. 279, 8428–8440 [DOI] [PubMed] [Google Scholar]
- 27.Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 28.Stothard P. (2000) BioTechniques 28, 1102–1104 [DOI] [PubMed] [Google Scholar]
- 29.Shen M. Y., Sali A. (2006) Protein Sci. 15, 2507–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sali A., Blundell T. L. (1993) J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 31.Van Der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A. E., Berendsen H. J. (2005) J. Comput. Chem. 26, 1701–1718 [DOI] [PubMed] [Google Scholar]
- 32.Hess B., Kutzner C., van der Spoel D., Lindahl E. (2008) J. Chem. Theory Comput. 4, 435–447 [DOI] [PubMed] [Google Scholar]
- 33.Schüttelkopf A. W., van Aalten D. M. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 34.Heyer L. J., Kruglyak S., Yooseph S. (1999) Genome Res. 9, 1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphrey W., Dalke A., Schulten K. (1996) J. Mol. Graph 14, 33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- 36.Team R. D. C. (2008) R: A Language and Environment for Statistical Computing [Google Scholar]
- 37.Kleyman T. R., Cragoe E. J., Jr. (1988) J. Membr. Biol. 105, 1–21 [DOI] [PubMed] [Google Scholar]
- 38.Jernigan N. L., Paffett M. L., Walker B. R., Resta T. C. (2009) Am. J. Physiol. Lung Cell Mol. Physiol. 297, L271–L285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garnovskaya M. N., Mukhin Y. V., Vlasova T. M., Raymond J. R. (2003) J. Biol. Chem. 278, 16908–16915 [DOI] [PubMed] [Google Scholar]
- 40.Garnovskaya M. N., Gettys T. W., van Biesen T., Prpic V., Chuprun J. K., Raymond J. R. (1997) J. Biol. Chem. 272, 7770–7776 [DOI] [PubMed] [Google Scholar]
- 41.Wang W. Z., Chu X. P., Li M. H., Seeds J., Simon R. P., Xiong Z. G. (2006) J. Biol. Chem. 281, 29369–29378 [DOI] [PubMed] [Google Scholar]
- 42.Rotin D., Grinstein S. (1989) Am. J. Physiol. 257, C1158–C1165 [DOI] [PubMed] [Google Scholar]
- 43.Palmer L. G. (1985) J. Membr. Biol. 87, 191–199 [DOI] [PubMed] [Google Scholar]
- 44.Benos D. J., Simon S. A., Mandel L. J., Cala P. M. (1976) J. Gen. Physiol. 68, 43–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer L. G., Andersen O. S. (1989) Biophys. J. 55, 779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segal A., Awayda M. S., Eggermont J., Van Driessche W., Weber W. M. (2002) Pflugers Arch. 443, 882–891 [DOI] [PubMed] [Google Scholar]
- 47.Reddy Ch S., Vijayasarathy K., Srinivas E., Sastry G. M., Sastry G. N. (2006) Comput. Biol. Chem. 30, 120–126 [DOI] [PubMed] [Google Scholar]
- 48.Stockand J. D., Staruschenko A., Pochynyuk O., Booth R. E., Silverthorn D. U. (2008) IUBMB Life 60, 620–628 [DOI] [PubMed] [Google Scholar]
- 49.Kleyman T. R., Carattino M. D., Hughey R. P. (2009) J. Biol. Chem. 284, 20447–20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassell A. M., An G., Bledsoe R. K., Bynum J. M., Carter H. L., 3rd, Deng S. J., Gampe R. T., Grisard T. E., Madauss K. P., Nolte R. T., Rocque W. J., Wang L., Weaver K. L., Williams S. P., Wisely G. B., Xu R., Shewchuk L. M. (2007) Acta Crystallogr. D Biol. Crystallogr. 63, 72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tannous B. A., Christensen A. P., Pike L., Wurdinger T., Perry K. F., Saydam O., Jacobs A. H., García-Añoveros J., Weissleder R., Sena-Esteves M., Corey D. P., Breakefield X. O. (2009) Mol. Ther. 17, 810–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.