Abstract
Anorexia and weight loss are prevalent in infectious diseases. To investigate the molecular mechanisms underlying these phenomena, we established animal models of infection-associated anorexia by administrating bacterial and viral products, lipopolysaccharide (LPS) and human immunodeficiency virus-1 transactivator protein (Tat). In these models, we found that the nuclear factor-κB (NF-κB), a pivotal transcription factor for inflammation-related proteins, was activated in the hypothalamus. In parallel, administration of LPS and Tat increased hypothalamic pro-inflammatory cytokine production, which was abrogated by inhibition of hypothalamic NF-κB. In vitro, NF-κB activation directly stimulated the transcriptional activity of pro-opiomelanocortin (POMC), a precursor of anorexigenic melanocortin, and mediated the stimulatory effects of LPS, Tat, and pro-inflammatory cytokines on POMC transcription, implying the involvement of NF-κB in controlling feeding behavior. Consistently, hypothalamic injection of LPS and Tat caused a significant reduction in food intake and body weight, which was prevented by blockade of NF-κB and melanocortin. Furthermore, disruption of IκB kinase-β, an upstream kinase of NF-κB, in POMC neurons attenuated LPS- and Tat-induced anorexia. These findings suggest that infection-associated anorexia and weight loss are mediated via NF-κB activation in hypothalamic POMC neurons. In addition, hypothalamic NF-κB was activated by leptin, an important anorexigenic hormone, and mediates leptin-stimulated POMC transcription, indicating that hypothalamic NF-κB also serves as a downstream signaling pathway of leptin.
Keywords: Diseases, Diseases/Metabolic, Hormones, Metabolism, Metabolism/Energy, Metabolism/Metabolic Syndrome
Introduction
Anorexia and wasting are common symptoms associated with infectious diseases (1, 2). Infection-associated anorexia is not merely the consequence of fever and general weakness (3), but it appears to occur due to an alteration in the motivation to eat (4). The suppression of food intake during acute infection may constitute a host defense mechanism as poor nutritional states might limit the proliferation of infectious organisms (5). However, a prolonged reduction in food intake will deplete body fat and protein reserves, resulting in malnutrition, impaired host immune functions, and increased morbidity and mortality (6). Therefore, elucidating the mechanism of infection-associated anorexia and weight loss should aid in improving the quality of life in patients with chronic debilitating infections.
Cumulating evidence suggests that pro-inflammatory cytokines produced in the periphery and brain play a role in infection-associated anorexia and weight loss (7). Because appetite is regulated by the central nervous system, infection-associated anorexia may be mediated via the central mechanisms. Consistent with this hypothesis, a recent study has demonstrated that central inflammatory signals are important for bacterial endotoxin lipopolysaccharide (LPS)3-induced anorexia (8). On the other hand, a number of studies suggest an important role of the hypothalamic melanocortin system in infection-associated anorexia (9). However, the molecular mechanisms triggering anorexia during infection are yet to be fully addressed.
Nuclear factor-κB (NF-κB) transcription factors were originally identified as critical regulators of genes involved in inflammation and innate immunity (10). In the quiescent state, NF-κB dimers exist in an inactive form in the cytoplasm bound to the IκBα inhibitory protein. Inflammatory stimuli activate the IκB kinase (IKK) complex, which phosphorylates IκB, leading to its ubiquitination and subsequent degradation. IκB degradation facilitates translocation of NF-κB to the nucleus, thereby regulating the transcription of genes involved in human disorders, including cancer, neurodegenerative disease, and obesity-related metabolic disease (11–13). NF-κB is also involved in cancer-induced cachexia, because intratumoral injection of NF-κB inhibitory oligonucleotides prevents cachexia in a mouse tumor model (14). In the present study, we present evidence that, during infection, NF-κB is activated in the hypothalamus, a center for appetite and body weight regulation, and stimulates the local production of pro-inflammatory cytokines and anorexigenic pro-opiomelanocortin (POMC), thereby causing anorexia and weight loss.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice 8–10 weeks of age were obtained from Orient Bio Inc. (Seoul, Korea). Mice were fed standard chow (Samyang Co, Seoul, Korea) ad libitum. Animals were housed under controlled temperature (22 °C) and a 12-h light-dark cycle, with light from 7:00 a.m. to 7:00 p.m. All procedures were approved by the Institutional Animal Care and Use Committee at the Asian Institute for Life Sciences.
Cell Culture
AtT-20 and SH-SY5Y cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. Hypothalamic neurons were primarily cultured as previously described (15).
Preparation of Recombinant Tat Protein
Tat1–72 cDNA was cloned and ligated into pGex4T3 vector (Amersham Biosciences). The resulting gene product was overexpressed in Escherichia coli BL21. Recombinant glutathione S-transferase-Tat was purified by affinity chromatography using glutathione-agarose 4B (Sigma).
Cannulation and Injection
Cannulation and injection into the third ventricle (ICV: 0.8 mm caudal to the bregma and 5 mm ventral to the sagittal sinus) or the mediobasal hypothalamus (intra-mediobasal hypothalamic: 1.8 mm caudal to the bregma, and 5.5 mm ventral and 0.3 mm lateral to the sagittal sinus) were performed according to a previous report (16). LPS (Sigma), Tat1–72, and leptin (R&D Systems) were dissolved in 0.9% saline and administered via intra-mediobasal hypothalamic- or ICV-implanted cannulae in 0.5 and 2 μl of volume, respectively, at 8:00–10:00 a.m. in mice following an overnight fast, unless otherwise indicated. For the inhibition study, Agouti-related protein (AGRP, Phoenix Pharmaceuticals), IKK inhibitory peptide (Calbiochem, #401477), and Bay 11-7085 (Sigma) were dissolved in either saline or 1% DMSO before use and injected 30 min before injection of LPS or Tat1–72. The doses of inhibitors used were determined from the preliminary studies with a wide range of doses and small sized groups, which alone did not significantly change food intake and body weight and blocked the effects of LPS or Tat.
EMSA
Medial hypothalami were harvested at indicated times following intraperitoneal injection of LPS (200 μg/kg) and leptin (3 mg/kg) as previously described (15). These doses of LPS and leptin was determined from our preliminary feeding study and found to produce a significant decrease in food intake. Primary-cultured hypothalamic neurons were treated with LPS, Tat1–72, tumor necrosis factor-α, IL-1β, and IL-6 (Sigma) for 4 h before harvest. Nuclear protein extracts (10 μg) were incubated with 50,000 cpm of 32P-labeled general κB probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′), POMC-specific κB-1 probe (5′-GCCACAGGGAAAGCACTTCAGCTG-3′), or κB-2 probe (5′-GCTCTCTGGGGCTTTCCAGGAAG-3′), as previously described (15). For inhibition electromobility shift assay (EMSA), 10-fold excess cold oligonucleotides or antibodies against p50 and p65 (10–20 μg each, Santa Cruz Biotechnology) were added prior to the 32P-labeled κB probe.
Promoter Activity
The 1-kb promoter region (nucleotide positions −877 to +320) and the first intron (+1705 to +4134) of the human POMC gene or the promoter region (nucleotide positions −1000 to −1) of the human AGRP gene were cloned and ligated into a luciferase reporter (POMC-luc1, POMC-luc2, and AGRP-luc reporters). The κB2 mutant of POMC-luc2 was generated by PCR-based site-directed mutagenesis using the following primers: 5′-GCAGCTGAAATGCTTACACTGTGGCC-3′ and 5′-GGCCACAGTGTAAGCATTTCAGCTGC-3′. AtT-20 or SH-SY5Y cells were cultured in 6-well plates and transfected with POMC or AGRP reporters (200 ng), p50 (10–100 ng), p65 (10–100 ng), and CMV-β-galactosidase (20 ng) genes using Lipofectamine (Invitrogen). At 48 h after transfection, cells were treated with LPS (10 ng/ml), IL-1β (20 ng/ml), tumor necrosis factor-α (200 ng/ml), Tat1–72 (1 nm), and leptin (100–1000 nm) with or without NF-κB inhibitors Bay 11-7085 or pyrrolidine dithiocarbamate (100 μm) for 4 h. Luciferase activity was normalized to β-galactosidase activity. Data are shown as -fold increase compared with controls. Transfections were performed in duplicate, and the experiments were repeated at least three times.
Chromatin Immunoprecipitation
SH-SY5Y cells were treated with LPS (10 ng/ml) for 1 h, fixed with formaldehyde, lysed, and sonicated. Soluble chromatin was coimmunoprecipitated with anti-p50 rabbit serum (Santa Cruz Biotechnology) or an equivalent amount of rabbit immunoglobulin-γ (IgG). After de-cross-linking of DNA, samples were subjected to PCR to amplify the first intron region of the human POMC gene (nucleotide positions +2123 to +2430) using the primers (5′-TGAAGTTTTAGCAATAGCAGC-3′ and 5′-TGGTAGTTACGTATGTCACCAA-3′).
Determination of mRNA Expression
Total RNA was extracted from the mediobasal hypothalamus and AtT-20 cells using TRIzol reagent (Invitrogen). The mRNA levels of pro-inflammatory cytokines, neuropeptides, and NF-κB were determined by real-time PCR (PerkinElmer Life Sciences) or semi-quantitative reverse transcription-PCR using the primers specified in supplemental Fig. S1. Expression of each mRNA was normalized to that of glyceraldehyde-3-phosphate dehydrogenase.
Small Interfering RNA
The murine sequences of p50 (positions +2624 to +2643) were targeted for RNA interference (supplemental Fig. S2). AtT-20 cells were transfected with control or p50-specific small interfering RNA (100 nm) using Lipofectamine 36 h before promoter assay.
Immunofluorescence
Mice were sacrificed 45 min after intraperitoneal injection of saline or LPS (200 μg/kg). Whole brains were immediately frozen in isopentene-prechilled liquid nitrogen and maintained at −70 °C. Coronal brain sections (15-μm thickness) were fixed with 4% paraformaldehyde for 15 min at room temperature. Brain sections were incubated with primary antibodies against p50 (1:500, rabbit, Santa Cruz Biotechnology), IKKβ (1:200, rabbit, Abcam), MAP2 (1:500, mouse, Sigma), or αMSH (1:15000, sheep, Chemicon) at 4 °C for 48 h. After washing, slides were incubated with Alexa-Fluor-555-conjugated donkey anti-rabbit antibody, Alexa-Fluor-488-conjugated donkey anti-mouse or anti-sheep antibodies (Invitrogen) at room temperature for 1 h. For nuclear staining, slides were treated with 4′,6-diamidino-2-phenylindole (1:20,000, Invitrogen) for 10 min before mounting. Triple immunofluorescence was examined using confocal microscopy (Leica). The p50 and IKKβ immunoreactivities in the nuclei of MAP2- and α-MSH-labeled neurons were counted in three arcuate nucleus sections per mouse using the program ImageTool (The University of Texas Health Science Center at San Antonio, n = 3).
Generation of POMC-specific IKKβ Knock-out Mice
To generate POMC neuron-specific IKKβ knock-out (IkkβΔPOMC) mice, IKKβ-flox/flox (IkkβF/F) mice were crossed with Pomc-Cre transgenic mice (17, 18). Successful knockdown of IKKβ was confirmed by estimating IKKβ expression in hypothalamic POMC neurons via double immunohistochemistry with antibodies against IKKβ (Abcam) and αMSH (Chemicon).
Measurement of Body Temperature
Rectal temperature was measured using a thermometer (Harvard Apparatus, Holliston, MA).
Measurement of Serum Corticosterone
Trunk blood was collected 45 min after intraperitoneal administration of LPS. Serum corticosterone levels were measured using a radioimmunoassay kit (DPC, Deerfield, IL).
Data Analysis
All data are presented as mean ± S.E. Groups were compared using Student's t test or analysis of variance, followed by a post hoc least significant difference test. Significance was defined as p < 0.05.
RESULTS
Activation of Hypothalamic NF-κB by LPS and Tat
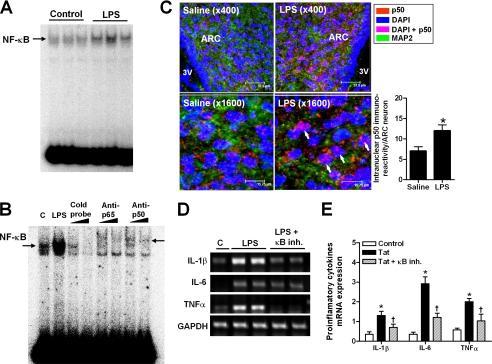
Initially, we investigated whether NF-κB is activated in the hypothalamus during systemic infection. LPS (200 μg/kg) was administered intraperitoneally in freely fed C57BL/6J mice in the early light phase and the mediobasal hypothalamus was collected to assess NF-κB activity at 1 and 6 h following LPS injection. NF-κB activity was determined with the EMSA using the NF-κB binding consensus sequence (19). A single intraperitoneal administration of LPS led to NF-κB activation in the hypothalamus at 1 h post injection (Fig. 1A). However, this effect was not sustained at 6 h (data not shown). Binding of NF-κB to the corresponding DNA sequences was inhibited and supershifted in the presence of antibodies specific for the NF-κB subunits, p50 and p65 (Fig. 1B), suggesting that the canonical NF-κB pathway was activated (20).
FIGURE 1.
Administration of LPS and Tat causes NF-κB activation and pro-inflammatory cytokine production in the hypothalamus. A, hypothalamic NF-κB activation following intraperitoneal administration of LPS. Mediobasal hypothalami were collected 1 h after intraperitoneal injection in the early light phase. B, LPS-induced NF-κB binding to the oligonucleotides was inhibited and supershifted in the presence of p50- and p65-specific antibodies, suggesting that canonical NF-κB pathway is activated. “C” represents saline-injected controls. C, triple immunohistochemistry with p50, neuron marker (MAP2), and nuclear marker (4′,6-diamidino-2-phenylindole (DAPI)) in mouse hypothalamic arcuate nucleus (ARC). Brains were collected at 45 min after intraperitoneal administration of saline or LPS. The intranuclear p50 immunoreactivity in MAP2-positive neurons of hypothalamic ARC were counted from three different slides per mice (n = 3). p < 0.05 versus saline. D and E, hypothalamic mRNA expression of pro-inflammatory cytokines following intraperitoneal administration of saline, LPS, or Tat1–72 with or without prior ICV administration of NF-κB inhibitor (κB inh.) (n = 5–6 per group). Mediobasal hypothalami were collected 3 h after intraperitoneal injection. Pro-inflammatory cytokine mRNA levels were determined using semiquantitative reverse transcription-PCR and normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each bar represents mean ± S.E. *, p < 0.005 versus saline-injected control (C); †, p < 0.05 versus Tat alone group.
NF-κB is expressed in neurons and glial cells in the central nervous system. Neuronal NF-κB plays an important role in both normal and pathologic conditions (20). To determine whether LPS activates NF-κB in hypothalamic neurons, we performed double immunofluorescence staining using antibodies specific to p50 and the neuron marker, MAP2. We used anti-p50-antibody, because commercially available anti-p65 antibodies were all nonspecific, as previously mentioned (21). Intraperitoneal administration of LPS (200 μg/kg) significantly increased the nuclear translocation of p50 in neurons of the hypothalamic arcuate nucleus at 45 min after injection (Fig. 1C). Treatment with LPS (10–1000 nm) in primary cultured hypothalamic neurons activated neuronal NF-κB (supplemental Fig. S3A), suggesting that LPS can directly activate NF-κB in hypothalamic neurons.
Tat, a viral protein produced by the human immunodeficiency virus, type 1, has originally been implicated in the transcription initiation and elongation of human immunodeficiency virus-encoding genes (22). In addition to its role as a transcriptional regulator, Tat is released from infected cells and acts like an extracellular cytokine (23). Extracellular Tat causes neuron toxicity through N-methyl-d-aspartic acid receptor (22). Therefore, we used Tat protein for establishment of viral infection model. Similar to LPS, biologically active Tat1–72 increased IκB phosphorylation and nuclear translocation of NF-κB in primary-cultured hypothalamic neurons (supplemental Fig. S3, B and C). These findings clearly demonstrate that the Tat1–72 protein also causes NF-κB activation in hypothalamic neurons.
LPS and Tat Stimulate Hypothalamic Cytokine Production via NF-κB
During systemic infection, pro-inflammatory cytokines are produced in the meninges, choroid plexus, and brain, which may provoke inflammatory responses within the central nervous system in a paracrine and autocrine manner (7). Consistent with this notion, intraperitoneal administration of LPS (200 μg/kg) and Tat1–72 (4 mg/kg) enhanced the mRNA expression of pro-inflammatory cytokines, including IL-1β, IL-6, and tumor necrosis factor-α in the mediobasal hypothalamus at 3 h post-injection (Fig. 1, D and E).
To explore a role for NF-κB in LPS- and Tat-induced hypothalamic cytokine production, we administered the NF-κB inhibitor (24), Bay 11-7085 (500 nmol), into the third ventricle, 30 min prior to intraperitoneal administration of LPS and Tat1–72. ICV administration of Bay 11-7085 significantly inhibited the increase in hypothalamic cytokine expression following LPS and Tat treatment (Fig. 1, D and E). These data suggest that hypothalamic NF-κB mediates LPS- and Tat-induced increase in hypothalamic cytokine expression. On the other hand, enhanced pro-inflammatory cytokine production may further amplify NF-κB activation in the hypothalamus, as evidenced by the finding that treatment of IL-1β and tumor necrosis factor-α caused NF-κB activation in primary cultured hypothalamic neurons (supplemental Fig. S4).
NF-κB Directly Stimulates POMC Transcriptional Activity
The melanocortins, α-, β-, and γ-melanocyte-stimulating-hormone (MSH), are produced from the precursor protein, POMC (25). Central melanocortin and its receptors (melanocortin 3 and 4 receptors, MC3R and MC4R) play an important role in the maintenance of normal food intake and body weight in rodents and humans (25). Several lines of evidence suggest that the hypothalamic melanocortin system is a potential mediator in illness-associated anorexia (26–28). Hypothalamic αMSH levels are increased in pathologic conditions, such as human immunodeficiency virus infection (26). Consistently, we found that intraperitoneal administration of LPS (200 μg/kg) and Tat1–72 (4 mg/kg) increased hypothalamic POMC mRNA expression at 3 h after intraperitoneal administration (Fig. 2A). Moreover, ICV administration of αMSH augments LPS-induced anorexia and cachexia, whereas the melanocortin antagonist, SHU9119, attenuates it (27). LPS- and cancer-induced anorexia and weight loss are also diminished in MC4R-null mice (28). These findings collectively suggest that hypothalamic melanocortins cause anorexia and weight loss through the MC4R during the illness. However, the mechanisms via which infectious products or pro-inflammatory cytokines activate the hypothalamic melanocortin system remain to be established.
FIGURE 2.
NF-κB regulates POMC transcriptional activity. A, hypothalamic neuropeptide mRNA expression after intraperitoneal administration of LPS and Tat1–72 (n = 5–6). *, p < 0.05 versus saline-injected control. B, potential κB binding sites in the POMC genes. NF-κB consensus sequences are underlined. The shaded region indicates conserved sequences in promoter variants of human, mouse, and rat POMC genes. C, the promoter activity of the POMC region, including κB-2, was increased by expression of p50 and/or p65 in AtT-20 cells (n = 3). *, p < 0.01 versus mock vector-expressing controls; †, p < 0.05 versus p50 (50 ng) alone group. D, EMSA using oligonucleotides, including κB-1 and κB-2, with or without p50 antibody. Hypothalami were collected 1 h after intraperitoneal injection of saline (control) or LPS (200 μg/kg). E, nucleotide substitution within κB-2 blocked p50-induced increase in POMC promoter activity. *, p < 0.05 versus mock vector-expressing control. F, a chromatin immunoprecipitation assay revealed that LPS enhanced endogenous NF-κB binding to κB-2 sites in SH-SY5Y neuron cells. G, treatment with LPS, Tat1–72, IL-1β, and tumor necrosis factor-α increased POMC promoter activities in AtT-20 cells. Cotreatment of the NF-κB inhibitor, Bay 11-7085 suppressed these effects (n = 3). *, p < 0.005 versus LPS-, Tat-, and cytokines-untreated controls; †, p < 0.005 versus Bay-untreated groups. H, depletion of p50 using murine p50-specific small interfering RNA completely inhibited LPS (10 ng/ml)- and Tat1–72 (1 nm)-induced increase in POMC promoter activity in AtT-20 cells (n = 3). *, p < 0.05 versus LPS- and Tat-untreated and control small interfering RNA-treated controls; NS, not significant. Each bar represents mean ± S.E.
We hypothesized that POMC is a downstream target of NF-κB. Putative NF-κB binding motifs were identified within the 1-kb nucleotide sequence upstream of the coding sequence (κB-1) and the first intron (κB-2) of the human, mouse, and rat POMC genes (Fig. 2B). To investigate the effects of NF-κB on POMC transcriptional activity, we generated two luciferase reporter constructs containing κB-1 (nucleotides −877 to +320, POMC-luc1) and κB-2 (nucleotides +1705 to +4134, POMC-luc2) of the human POMC gene. Human p50 and/or p65 were coexpressed with POMC-luc1 or POMC-luc2 in POMC-producing murine AtT-20 cells. Expression of p50 increased POMC-luc2 activity up to ∼8-fold (Fig. 2C), and POMC-luc1 activity only by 2-fold (supplemental Fig. S5). Interestingly, expression of p65 alone did not affect POMC-luc2 activity, whereas coexpression with p50 further enhanced POMC-luc2 activity in a dose-dependent manner (Fig. 2C). These findings, taken together with EMSA data (Fig. 1B), indicate that p50:p50 and p50:p65 dimers, but not p65:p65 dimers, may be involved in transcriptional regulation of POMC. EMSA using oligonucleotides compatible with the potential κB binding sites 1 and 2 (κB-1 and κB-2 probes) demonstrated that the κB-2 probe was better than κB-1 probe for the detection of LPS-induced hypothalamic NF-κB activation (Fig. 2D). Consistently, mutation of κB-2 site completely abolished p50-induced stimulation of POMC transcriptional activity, suggesting that κB-2 site is critical for NF-κB-mediated stimulation of POMC transcription (Fig. 2E). Finally, we confirmed that endogenous p50 binds to κB-2 of the human POMC gene, using a chromatin immunoprecipitation assay. Chromatin immunoprecipitation data revealed that binding of endogenous p50 to this region was low in the basal state, but significantly increased by LPS treatment (10 ng/ml) in SH-SY5Y human neuroblastoma cells (Fig. 2F). Collectively, NF-κB p50 stimulates POMC transcriptional activity through direct interactions at κB-2 site.
Because Tat functions as a transcriptional coactivator (22), we also examined the effects of Tat gene expression on POMC promoter activity. Expression of the Tat gene modestly increased POMC-luc2 activity in AtT-20 cells (supplemental Fig. S6A). Moreover, coexpression of Tat and p50 led to a synergistic increase in POMC transcriptional activity (supplemental Fig. S6B), suggesting that NF-κB-induced POMC transcription is enhanced by endogenous Tat expression.
We additionally determined the direct effects of LPS, Tat1–72, and pro-inflammatory cytokines on POMC transcriptional activity. Treatment with these agents significantly stimulated POMC-luc2 transcriptional activity in both AtT-20 and SH-SY5Y cells (Fig. 2G and supplemental Fig. S6C), which was inhibited by pretreatment with a NF-κB inhibitor, Bay 11-7085 (Fig. 2G). Moreover, depletion of p50 expression using a small inhibitory RNA-mediated gene-silencing system completely abolished the effects of LPS and Tat on POMC-luc2 activity (Fig. 2H). Our results strongly suggest that NF-κB is an important downstream mediator in the regulation of POMC transcription by LPS, Tat, or pro-inflammatory cytokines.
Hypothalamic NF-κB Mediates LPS- and Tat-induced Anorexia and Weight Loss
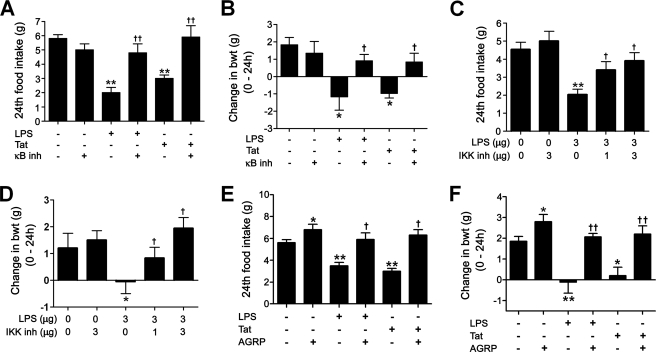
Consistent with a previous report (29), central administration of LPS and Tat caused a reduction in food intake and body weight (supplemental Fig. S7). Next, we investigated a role for hypothalamic NF-κB in LPS- and Tat-induced anorexia and weight loss. For this, we injected an NF-κB inhibitor, Bay 11-7085 (500 nmol) or IKK inhibitory peptide (30) (1 and 3 μg), into the mediobasal hypothalamus 30 min prior to administration of LPS (3 ng) or Tat1–72 (0.1 nmol). Injection of NF-κB inhibitor or IKK inhibitor alone did not significantly alter food intake and body weight but blocked the effects of LPS and Tat1–72 (Fig. 3, A–D). These findings indicate that hypothalamic NF-κB activation is an important signaling event for anorexia and weight loss induced by LPS and Tat.
FIGURE 3.
Inhibition of hypothalamic NF-κB and POMC prevents LPS- and Tat-induced anorexia and weight loss. A and B, prior intrahypothalamic administration of the NF-κB inhibitor Bay 11-7085 inhibited LPS- and Tat1–72-induced decrease in food intake and body weight (n = 6–7). C and D, intrahypothalamic administration of IKK inhibitory peptide prevented LPS-induced anorexia and weight loss (n = 5–6). E and F, prior ICV administration of the melanocortin antagonist, AGRP, blocked the effects of LPS and Tat1–72 on food intake and body weight (n = 5–6). *, p < 0.05, **, p < 0.01 versus vehicle-injected controls, †, p < 0.05; ††, p < 0.01 versus Tat or LPS alone groups. Each bar represents mean ± S.E.
Inhibition of NF-κB in POMC Neurons Attenuates LPS- and Tat-induced Anorexia
We further investigated the relationship of NF-κB and the hypothalamic melanocortin system in feeding regulation. In line with a previous report (27), prior ICV administration of an endogenous melanocortin antagonist, AGRP (4 μg), blocked the effects of LPS (10 ng) and Tat1–72 (1 nmol) on food intake and body weight (Fig. 3, E and F). Although treatment with AGRP alone increased food intake and body weight, the blocking effect of AGRP on LPS- and Tat-induced anorexia and weight loss was much greater than that seen in AGRP-alone treatment, supporting an involvement of the central melanocortin system in illness-associated anorexia.
Given that POMC is a target gene of NF-κB, we hypothesized that the central melanocortin system acts as a downstream of NF-κB in infection-associated anorexia and weight loss. To define the specific role of NF-κB in hypothalamic POMC neurons in illness-associate anorexia, we selectively disrupted the IKKβ gene, a key upstream signaling pathway leading to NF-κB activation (31), in POMC-producing neurons using the Cre-lox system. The IkkβF/F mice (17) were crossed with Pomc-Cre transgenic mice (18) to generate POMC-specific IKKβ-depleted mice (IkkβΔPomc). In IkkβΔPOMC mice, IKKβ expression in αMSH-expressing neurons was significantly less than the level of IkkβF/F mice (Fig. 4A). The IkkβΔPomc mice showed normal food intake and body weight under a standard chow diet at young adult ages compared with Pomc-Cre mice and IkkβF/F mice (supplemental Fig. S8, A and B). There were no differences in plasma glucose, insulin, and leptin concentrations between IkkβF/F and IkkβΔPomc mice (supplemental Fig. S8, C–E). Basal hypothalamic NF-κB activity and POMC mRNA expression tended to be lower in IkkβΔPomc mice than in IkkβF/F (Fig. 4, B and C). Furthermore, LPS-induced nuclear translocation of p50 was significantly decreased in the hypothalamic POMC neurons (Fig. 4D). In IkkβΔPomc mice, LPS (3 ng)- and Tat (0.1 nmol)-induced reduction in food intake and weight loss was significantly attenuated, which was reversed by intrahypothalamic coadministration of α-MSH (Fig. 4, E–H). These findings further support the hypothesis that NF-κB activation in POMC neurons plays a critical role in infection-associated anorexia and weight loss.
FIGURE 4.
NF-κB inactivation in POMC neurons blocks LPS- and Tat-induced anorexia. A, double immunofluorescence staining using antibodies against IKKβ and αMSH in IkkβF/F mice and IkkβΔPOMC mice. B and C, basal hypothalamic NF-κB activity and POMC mRNA expression in IkkβΔPOMC and IkkβF/F mice (n = 4–5). The mediobasal hypothalami were collected following a 5-h fast. D, LPS-induced nuclear translocation of p50 in POMC neurons was reduced in IkkβΔPOMC mice. A representative figure and the counts of intranuclear p50-immunoreactive spots in ARC POMC-producing neurons are presented. *, p < 0.01 versus saline-injected controls. NS: not significant. E–H, intrahypothalamic administration of LPS and Tat1–72 caused anorexia and weight loss in IkkβF/F mice. In IkkβΔPOMC mice, these effects were significantly attenuated (n = 5–6), which was recovered by coadministration of α-MSH. *, p < 0.05; **, p < 0.01 versus saline-injected IkkβF/F and IkkβΔPOMC mice; †, p < 0.05 versus LPS- or Tat1–72-injected IkkβF/F mice. I, the LPS-induced increase in plasma cortisol concentrations was reduced in Ikkβ ΔPOMC mice, compared with Ikkβ F/F mice (n = 5–6). *, p < 0.05; **, p < 0.005 versus saline-injected groups; †, p < 0.05 versus LPS-injected IkkβF/F mice. J, LPS-induced elevation in body temperature was greater in Ikkβ ΔPOMC mice than in IkkβF/F mice (n = 5–6). *, p < 0.05; **, p < 0.01 versus saline-injected groups; †, p < 0.05 versus LPS-injected IkkβF/F mice. Each bar represents mean ± S.E.
Because melanocortins have antipyretic and anti-inflammatory effects in the central and peripheral nervous systems (26), we compared the effects of LPS on body temperature and circulating levels of anti-inflammatory hormone corticosteroid. IkkβΔPOMC mice displayed lower corticosterone levels and higher body temperatures in response to intraperitoneal LPS (200 μg/kg), compared with IkkβF/F mice (Fig. 4, I and J).
Leptin Causes Hypothalamic NF-κB Activation
Previous studies have shown that leptin, an important anorexigenic hormone, activates NF-κB in cortical neurons, microglia, and astrocytes from the brain (32–34). Therefore, we investigated whether leptin also causes hypothalamic NF-κB activation. Similarly to LPS, intraperitoneal administration of leptin (3 mg/kg) significantly increased nuclear translocation of NF-κB in the mediobasal hypothalamus at 1 h post-injection (Fig. 5A).
FIGURE 5.
Leptin-induced hypothalamic NF-κB activation mediates the effects of leptin on food intake, body weight, and POMC transcription. A, hypothalamic NF-κB activation following intraperitoneal administration of leptin (3 mg/kg) and LPS (200 μg/kg). Mediobasal hypothalami were collected 1 h after intraperitoneal injection following a 5-h fast. B and C, ICV administration of IKK inhibitory peptide inhibited leptin-induced anorexia and weight loss (n = 6–7). Injection was carried out in the early light phase following an overnight fast. *, p < 0.01 versus saline-injected controls; †, p < 0.05 versus leptin-alone groups. D and E, effects of NF-κB inhibitor, pyrrolidine dithiocarbamate (100 μm) on leptin-stimulated POMC promoter activity and POMC mRNA expression in AtT-20 cells. *, p < 0.005 versus untreated control; †, p < 0.05 versus leptin alone groups. F and G, intrahypothalamic administration of leptin caused anorexia and weight loss in IkkβF/F mice. These effects were significantly attenuated in IkkβΔPOMC mice (n = 5–6). *, p < 0.05; **, p < 0.01 versus saline-injected controls; †, p < 0.05 versus leptin-injected IkkβF/F mice. Each bar represents mean ± S.E. H, diagram summarizing our data. Hypothalamic NF-κB is activated by infectious agents, LPS and Tat, and leptin to stimulate hypothalamic cytokine and POMC production, thereby contributing to anorexia and weight loss.
To examine if hypothalamic NF-κB may serve as a downstream signaling of leptin, we administered leptin (3 μg) with or without IKK inhibitor (3 μg) in overnight-fasted mice via ICV-implanted cannulae. ICV administration of IKK inhibitor significantly inhibited the effects of leptin on food intake and body weight (Fig. 5, B and C), suggesting that hypothalamic NF-κB activation is essential for leptin-induced anorexia and weight loss.
Hypothalamic melanocortins are considered as an important downstream mediator of the anorexigenic actions of leptin (35, 36). Thus we investigated an involvement of NF-κB in the regulation of POMC transcription by leptin. Leptin treatment in AtT-20 cells significantly increased the promoter activity and mRNA levels of POMC, but cotreatment of NF-κB inhibitor, pyrrolidine dithiocarbamate, blocked these effects, suggesting that NF-κB mediates the effects of leptin on POMC transcription (Fig. 5, D and E).
To further confirm a role for NF-κB activation of POMC neuron in leptin-induced anorexia, leptin (0.1 μg) was administered into the mediobasal hypothalamus of IkkβF/F and IkkβΔPOMC mice following an overnight fast. Intrahypothalamic administration of leptin induced a marked decrease in food intake and body weight in IkkβF/F mice (Fig. 5, F and G). However, these effects were significantly attenuated in IkkβΔPOMC mice, suggesting that leptin-induced anorexia and weight loss depends on NF-κB activation in POMC neurons.
DISCUSSION
Our findings are summarized in Fig. 5H. Hypothalamic NF-κB is activated by infectious agents, LPS and Tat, or by leptin. Hypothalamic NF-κB activation leads to increased production of anorexigenic cytokines and POMC in the hypothalamus. In particular, NF-κB activation in hypothalamic POMC neurons is an important molecular mechanism for anorexia and weight loss as evidenced by the findings that POMC neuron-specific NF-κB inhibition significantly reduced LPS, Tat, or leptin-induced anorexia and weight loss.
Although a critical role for the hypothalamic melanocortin system in illness-associated anorexia has been suggested (26–28), it was unclear how central melanocortin system was regulated in the state of illness. In this study, we demonstrated that NF-κB directly bound the human POMC gene to stimulate POMC transcription. Therefore, hypothalamic NF-κB may be a missing link between illness and central melanocortin system.
On the other hand, we have found that hypothalamic NF-κB is activated by leptin, a critical anorexigenic hormone. Inhibition of hypothalamic NF-κB blocks the anorexigenic and weight-reducing effects of leptin, suggesting an important role for hypothalamic NF-κB in normal feeding regulation.
Consistently, Zhang et al. demonstrated that IKKβ and NF-κB are abundantly expressed in hypothalamic neurons (37). However, in contrast to our data, they showed that overnutrition induced activation of hypothalamic NF-κB through elevated endoplasmic reticulum stress (37). Furthermore, hypothalamic NF-κB activation interrupted leptin and insulin signaling by increasing the suppressor of cytokine signaling-3. Finally, depletion of IKKβ in mediobasal hypothalamus and AGRP neurons had a protective effect against diet-induced obesity. From these data, they concluded that hypothalamic NF-κB activation in obese state causes hypothalamic resistance to leptin and insulin.
Taking these data and our data together, hypothalamic NF-κB plays a pivotal role in controlling feeding behaviors under the two opposite metabolic situations, undernutrition and overnutrition. Leptin-induced activation of NF-κB acts as a downstream mediator of leptin in feeding regulation. On the other hand, NF-κB activation may induce suppressor of cytokine signaling-3 expression, which would constitute a negative feedback pathway of leptin signaling, as is the case for the signal transduction-activated transcript-3 signaling pathway (38). It is also possible that NF-κB activation in POMC neurons may have a differential role from that in AGRP neurons. Indeed, NF-κB inhibition in POMC neurons did not show a protective effect against diet-induced obesity (supplemental Fig. S9).
The central melanocortin receptor activities are determined by the relative amounts of melanocortins and endogenous antagonist, AGRP at the receptors (25). Thus activation of melanocortin receptors also results from the decrease levels of AGRP. Indeed, hypothalamic AGRP mRNA expression showed a tendency of decrease following LPS and Tat administration (Fig. 2A). Furthermore, treatment of LPS, Tat, and pro-inflammatory cytokines suppressed AGRP promoter activity in SH-SY5Y cells (supplemental Fig. S10). Thus NF-κB-mediated suppression of AGRP may also contribute to infection-associated anorexia and weight loss, as suggested previously (39).
Chronic activation of hypothalamic NF-κB and the melanocortin system during illness may have a detrimental effect on the host by causing a negative energy balance. However, cumulating evidence indicates that melanocortins have antipyretic, anti-inflammatory, and neuroprotective effects on both the central and peripheral nervous systems (26). In line with these notions, POMC-specific IKKβ knock-out mice showed decreased activation of the hypothalamo-pituitary-adrenal axis and enhanced febrile reaction in response to LPS. Therefore, NF-κB-mediated POMC activation may have both positive and negative effects on the hosts during infection.
Although we focused on hypothalamic events during infection, illness-associated anorexia and cachexia may be also mediated by peripheral or non-hypothalamic mechanisms. Indeed, LPS- and Tat-induced reduction in food intake and body weight was partially blocked by hypothalamic administration of NF-κB and IKK inhibitors or in POMC-specific NF-κB knock-out mice, when LPS and Tat were injected peripherally (data not shown). On the other hand, we did not expand our study to include the cancer-anorexia model. However, cancer-associated anorexia may be through a similar molecular mechanism, because hypothalamic IL-1β expression was increased in the hypothalamus from tumor-bearing rats (40). Furthermore, blockade of hypothalamic melanocortins prevented IL-1β- or cancer-induced anorexia (41–43), indicating that hypothalamic NF-κB and melanocortin may be a general molecular mediator causing anorexia and weight loss during chronic illness state.
In conclusion, we show that NF-κB activation in the hypothalamic melanocortin system is essential for anorexia and weight loss induced by infectious agents and leptin. Therefore, chemicals having NF-κB inhibitory activity or MC4R antagonistic activity may have a therapeutic potential on anorexia and cachexia.
Supplementary Material
Acknowledgments
We are grateful to J. K. Elmquist and B. B. Lowell for kindly providing Pomc-Cre mice and Steve Shoelson, Jae-Hyuk Shim, and Berndbau Baumann for helpful suggestions.
This work was supported by the Korean Ministry of Health & Welfare (Grant A06-2363), the National Research Foundation of Korea funded by the Korea government (Grants 2006-0050225, 2007-0056866, 2009-0079566, KRF-2008-313-E00083, and 2009-0084114), and the Asan Institute for Life Science (Grant 06-326).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S10.
- LPS
- lipopolysaccharide
- NF-κB
- nuclear factor-κB
- IKK
- IκB kinase
- POMC
- pro-opiomelanocortin
- Tat
- transactivator protein
- EMSA
- electrophoretic mobility shift assay
- IL-1
- interleukin-1
- CMV
- cytomegalovirus
- AGRP
- Agouti-related protein
- ICV
- intracerebroventricular
- MAP2
- microtubule-associated protein 2
- α-MSH
- α-melanocyte-stimulating hormone.
REFERENCES
- 1.Hart B. L. (1988) Neurosci. Biobehav. Rev. 12, 123–137 [DOI] [PubMed] [Google Scholar]
- 2.Grunfeld C., Feingold K. R. (1992) N. Engl. J. Med. 327, 329–337 [DOI] [PubMed] [Google Scholar]
- 3.Aubert A., Goodall G., Dantzer R. (1995) Physiol. Behav. 57, 869–873 [DOI] [PubMed] [Google Scholar]
- 4.McCarthy D. O., Kluger M. J., Vander A. J. (1984) Am. J. Clin. Nutr. 40, 310–316 [DOI] [PubMed] [Google Scholar]
- 5.Murray M. J., Murray A. B. (1979) Am. J. Clin. Nutr. 32, 593–596 [DOI] [PubMed] [Google Scholar]
- 6.Grinspoon S., Mulligan K. (2003) Clin. Infect. Dis. 36, S69–78 [DOI] [PubMed] [Google Scholar]
- 7.Plata-Salaman C. R. (2001) Int. J. Obes. Relat. Metab. Disord. 25 Suppl5, S48–52 [DOI] [PubMed] [Google Scholar]
- 8.Wisse B. E., Ogimoto K., Tang J., Harris M. K., Jr., Raines E. W., Schwartz M. W. (2007) Endocrinology 148, 5230–5237 [DOI] [PubMed] [Google Scholar]
- 9.DeBoer M. D. (2007) Curr. Opin. Clin. Nutr. Metab. Care 10, 457–462 [DOI] [PubMed] [Google Scholar]
- 10.Barnes P. J., Karin M. (1997) N. Engl. J. Med. 336, 1066–1071 [DOI] [PubMed] [Google Scholar]
- 11.Karin M., Greten F. R. (2005) Nat. Rev. Immunol. 5, 749–759 [DOI] [PubMed] [Google Scholar]
- 12.Yang L., Tao L. Y., Chen X. P. (2007) Neurosci. Bull 23, 307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai D., Yuan M., Frantz D. F., Melendez P. A., Hansen L., Lee J., Shoelson S. E. (2005) Nat. Med. 11, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura I., Morishita R., Tomita N., Lacey E., Aketa M., Tsujimoto S., Manda T., Tomoi M., Kida I., Higaki J., Kaneda Y., Shimomura K., Ogihara T. (1999) Gene Ther. 6, 91–97 [DOI] [PubMed] [Google Scholar]
- 15.Kim M. S., Pak Y. K., Jang P. G., Namkoong C., Choi Y. S., Won J. C., Kim K. S., Kim S. W., Kim H. S., Park J. Y., Kim Y. B., Lee K. U. (2006) Nat. Neurosci. 9, 901–906 [DOI] [PubMed] [Google Scholar]
- 16.Kim M. S., Park J. Y., Namkoong C., Jang P. G., Ryu J. W., Song H. S., Yun J. Y., Namgoong I. S., Ha J., Park I. S., Lee I. K., Viollet B., Youn J. H., Lee H. K., Lee K. U. (2004) Nat. Med. 10, 727–733 [DOI] [PubMed] [Google Scholar]
- 17.Park J. M., Greten F. R., Li Z. W., Karin M. (2002) Science 297, 2048–2051 [DOI] [PubMed] [Google Scholar]
- 18.Balthasar N., Coppari R., McMinn J., Liu S. M., Lee C. E., Tang V., Kenny C. D., McGovern R. A., Chua S. C., Jr., Elmquist J. K., Lowell B. B. (2004) Neuron 42, 983–991 [DOI] [PubMed] [Google Scholar]
- 19.Cummings R., Zhao Y., Jacoby D., Spannhake E. W., Ohba M., Garcia J. G. N., Watkins T., He D., Saatian B., Natarajan V. N. (2004) J. Biol. Chem. 279, 41085–41094 [DOI] [PubMed] [Google Scholar]
- 20.Meffert M. K., Baltimore D. (2005) Trends Neurosci. 28, 37–43 [DOI] [PubMed] [Google Scholar]
- 21.Meffert M. K., Chang J. M., Wiltgen B. J., Fanselow M. S., Baltimore D. (2003) Nat. Neurosci. 6, 1072–1078 [DOI] [PubMed] [Google Scholar]
- 22.Rice A. P., Mathews M. B. (1988) Nature 332, 551–553 [DOI] [PubMed] [Google Scholar]
- 23.Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. (1990) Nature 345, 84–86 [DOI] [PubMed] [Google Scholar]
- 24.Pierce J. W., Schoenleber R., Jesmok G., Best J., Moore S. A., Collins T., Gerritsen M. E. (1997) J. Biol. Chem. 272, 21096–21103 [DOI] [PubMed] [Google Scholar]
- 25.Ellacott K. L., Cone R. D. (2006) Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361, 1265–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catania A., Airaghi L., Colombo G., Lipton J. M. (2000) Trends Endocrinol. Metab. 11, 304–308 [DOI] [PubMed] [Google Scholar]
- 27.Huang Q. H., Hruby V. J., Tatro J. B. (1999) Am. J. Physiol. 276, R864–871 [DOI] [PubMed] [Google Scholar]
- 28.Marks D. L., Butler A. A., Turner R., Brookhart G., Cone R. D. (2003) Endocrinology 144, 1513–1523 [DOI] [PubMed] [Google Scholar]
- 29.Langhans W., Harlacher R., Balkowski G., Scharrer E. (1990) Physiol. Behav. 47, 805–813 [DOI] [PubMed] [Google Scholar]
- 30.Traenckner E. B., Pahl H. L., Henkel T., Schmidt K. N., Wilk S., Baeuerle P. A. (1995) EMBO J. 14, 2876–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto Y., Gaynor R. B. (2004) Trends Biochem. Sci 29, 72–79 [DOI] [PubMed] [Google Scholar]
- 32.Valerio A., Dossena M., Bertolotti P., Boroni F., Sarnico I., Faraco G., Chiarugi A., Frontini A., Giordano A., Liou H. C., De Simoni M. G., Spano P., Carruba M. O., Pizzi M., Nisoli E. (2009) Stroke 40, 610–617 [DOI] [PubMed] [Google Scholar]
- 33.Tang C. H., Lu D. Y., Yang R. S., Tsai H. Y., Kao M. C., Fu W. M., Chen Y. F. (2007) J. Immunol. 179, 1292–1302 [DOI] [PubMed] [Google Scholar]
- 34.Mattace Raso G., Esposito E., Iacono A., Pacilio M., Coppola A., Bianco G., Diano S., Di Carlo R., Meli R. (2006) Neurosci. Lett. 396, 121–126 [DOI] [PubMed] [Google Scholar]
- 35.Seeley R. J., Yagaloff K. A., Fisher S. L., Burn P., Thiele T. E., van Dijk G., Baskin D. G., Schwartz M. W. (1997) Nature 390, 349. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz M. W., Seeley R. J., Woods S. C., Weigle D. S., Campfield L. A., Burn P., Baskin D. G. (1997) Diabetes 46, 2119–2123 [DOI] [PubMed] [Google Scholar]
- 37.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. (2008) Cell 135, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjørbaek C., Elmquist J. K., Frantz J. D., Shoelson S. E., Flier J. S. (1998) Mol. Cell 1, 619–625 [DOI] [PubMed] [Google Scholar]
- 39.Wisse B. E., Schwartz M. W., Cummings D. E. (2003) Ann. N. Y. Acad. Sci. 994, 275–281 [DOI] [PubMed] [Google Scholar]
- 40.Plata-Salamán C. R., Ilyin S. E., Gayle D. (1998) Am. J. Physiol. 275, R566–R573 [DOI] [PubMed] [Google Scholar]
- 41.Lawrence C. B., Rothwell N. J. (2001) J. Neuroendocrinol. 13, 490–495 [DOI] [PubMed] [Google Scholar]
- 42.Joppa M. A., Ling N., Chen C., Gogas K. R., Foster A. C., Markison S. (2005) Peptides 26, 2294–2301 [DOI] [PubMed] [Google Scholar]
- 43.Joppa M. A., Gogas K. R., Foster A. C., Markison S. (2007) Peptides 28, 636–642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.