Abstract
MAPK and Akt pathways are predominant mediators of trophic signaling for many neuronal systems. Among the vasoactive intestinal peptide/secretin/glucagon family of related peptides, pituitary adenylate cyclase-activating polypeptide (PACAP) binding to specific PAC1 receptor isoforms can engage multiple signaling pathways and promote neuroprotection through mechanisms that are not well understood. Using a primary sympathetic neuronal system, the current studies demonstrate that PACAP activation of PAC1HOP1 receptors engages both MAPK and Akt neurotrophic pathways in an integrated program to facilitate neuronal survival after growth factor withdrawal. PACAP not only stimulated prosurvival ERK1/2 and ERK5 activation but also abrogated SAPK/JNK and p38 MAPK signaling in parallel. In contrast to the potent and rapid effects of PACAP in ERK1/2 phosphorylation, PACAP stimulated Akt phosphorylation in a late phase of PAC1HOP1 receptor signaling. From inhibitor and immunoprecipitation analyses, the PACAP/PAC1HOP1 receptor-mediated Akt responses did not represent transactivation mechanisms but appeared to depend on Gαq/phosphatidylinositol 3-kinase γ activity and vesicular internalization pathways. Phosphatidylinositol 3-kinase γ-selective inhibitors blocked PACAP-stimulated Akt phosphorylation in primary neuronal cultures and in PAC1HOP1-overexpressing cell lines; RNA interference-mediated knockdown of the receptor effectors attenuated PACAP-mediated Akt activation. Similarly, perturbation of endocytic pathways also blocked Akt phosphorylation. Between ERK and Akt pathways, PACAP-stimulated Akt signaling was the primary cascade that attenuated cultured neuron apoptosis after growth factor withdrawal. The partitioning of PACAP-mediated Akt signaling in endosomes may be a key mechanism contributing to the high spatial and temporal specificity in signal transduction necessary for survival pathways.
Keywords: G Proteins/Coupled Receptors (GPCR), Neurochemistry, Neurobiology/Neuroscience, Peptides/Neuropeptide, Receptors/Endocytosis, Signal Transduction
Introduction
Pituitary adenylate cyclase-activating polypeptides (PACAP)3 belong to the vasoactive intestinal peptide (VIP)/secretin/glucagon family of related peptides and have well described trophic properties for a number of neural and endocrine systems. Two α-amidated forms of PACAP arise from alternative post-translational processing of the 175-amino acid rat precursor molecule; PACAP38 has 38 amino acid residues (proPACAP-(131–168)), whereasPACAP27 corresponds to the amino terminus of PACAP38 (proPACAP-(131–157)). As PACAP and VIP appear to stem from the same molecular cladistic tree during evolution (1), the two peptides not only share considerable structural homology but also function through three distinct G protein-coupled receptors (2–8). Only PACAP peptides exhibit high affinity for the PAC1 receptor, whereas VIP and PACAP have similar high affinities for the VPAC1 and VPAC2 receptors. Although VPAC receptors appear to be coupled predominantly to adenylyl cyclase, PAC1 receptor isoforms can display unique patterns of second messenger activation through the alternative splicing of HIP and/or HOP cassettes in the region encoding the third cytoplasmic loop (2). Accordingly, the PAC1 receptor isoforms with neither HIP nor HOP (PAC1null receptor), HIP (PAC1HIP), HOP1 (PAC1HOP1), a shortened form of HOP1 termed HOP2, or HIPHOP (PAC1HIPHOP) cassette inserts can engage distinct or multiple intracellular signaling cascades. The PAC1null receptor expressed in rat cortical neuroblasts, for example, is coupled primarily to Gs for adenylate cyclase activation (9). PAC1HIP receptor expression in LLC-PK1 porcine kidney epithelial cells or NIH 3T3 fibroblasts results in adenylate cyclase activation but with impaired phospholipase C coupling. The PAC1HOP1 receptor, by contrast, is dually coupled to Gαs and Gαq/11 to potently and efficaciously stimulate adenylate cyclase, phospholipase C, mitogen-activated protein kinase (MAPK), and other intracellular effectors that in aggregate have the potential of generating diverse cellular response (2, 10–12). Recent studies, for example, have shown that the PAC1HOP receptor isoform coupling to phospholipase C for calcium mobilization and influx is essential for neurotransmitter/neuropeptide release (13, 14). PAC1HOP1 receptor overexpression in embryonic cortical neurons results in phospholipase C activation, PKC translocation, and cellular calcium flux to facilitate proliferation (9, 15). In addition to developmental trophic functions (16–23), PACAP/PAC1 receptor expression and signaling have well described abilities to abrogate apoptotic signals and promote central and peripheral neuron survival in vitro and in vivo under diverse proapoptotic challenges (1, 10, 24–36). The PAC1HOP1 receptor is a significant variant in nearly all neural tissues, and dominant PAC1HOP1 receptor expression and signaling in primary cerebellar granule and sympathetic cells appear key in promoting neuronal survival from apoptosis after growth factor or serum withdrawal, cessation of depolarizing conditions, or cytotoxic challenges (10, 25, 26, 28).
Despite these well studied prosurvival effects, the dynamic events underlying PACAP/PAC1 neurotrophic signaling are still not well understood. To examine the abilities for PACAP/PAC1 receptor signaling to engage and coordinate these events in one neuronal system, we have employed primary sympathetic neurons that we have shown previously to preferentially express the PAC1HOP1 receptor isoform (13, 37, 38). All of the highly conserved members of the MAPK family, including ERK1 and ERK2 (p44 and p42, respectively), ERK5, JNK, and p38 MAPK, have been well described to participate in neuronal development, differentiation, cell cycle progression, and survival; how PAC1HOP1 receptor signaling impacts one or all of the MAPKs is not known. Moreover, prosurvival mechanisms for many central and peripheral neuronal systems can also stem from PI3K/Akt signaling. PI3K facilitates neurotrophin-mediated survival in cerebellar, sympathetic, sensory, cortical, and motor neurons and targets Akt serine/threonine kinase as a central mediator of the trophic response (39–42). Although the potent neuroprotective effects of PACAP in a variety of injury paradigms strongly implicate PACAP/PAC1 receptor convergence on PI3K/Akt pathways, the mechanisms and contributions of Akt activation with MAPK cascades in PAC1HOP1 receptor-mediated neurotrophic signaling have not been well examined. Using an in vitro growth factor withdrawal paradigm, the current studies demonstrated that PACAP can regulate multiple sympathetic MAPKs in distinct temporal patterns consistent with neuronal survival processes. Furthermore, PACAP also stimulated late phase Akt signaling in a Gαq/PI3Kγ- and vesicular internalization-dependent manner that did not appear to rely on tyrosine receptor kinase mechanisms. Although PACAP stimulated both ERK1/2 and Akt pathways, comparative studies showed that the prosurvival responses of sympathetic PACAP/PAC1HOP1 receptor signaling rested predominantly with PI3K/Akt activation. These studies show that sympathetic PAC1HOP1 receptor neurotrophic signaling reflects a complex, coordinate, and multifaceted strategy to facilitate neuronal survival, repair, and/or development after injury.
EXPERIMENTAL PROCEDURES
Cell Culture
Late gestation female Sprague-Dawley rats were obtained from Charles Rivers Canada; all animal procedures were approved by the University of Vermont Institutional Animal Care and Use Committees. Superior cervical ganglia from mixed sex neonatal (postnatal day 1–2) rat litters were dissected for primary sympathetic neuronal cultures as previously described (13, 37, 43). The pooled ganglia were enzymatically dispersed, and the resulting cells were plated at a density of 1.5 × 104 neurons/cm2 on double-collagen coated dishes, treated with cytosine β-d-arabinofuranoside to eliminate nonneuronal cells, and maintained in defined complete serum-free medium containing 50 ng/ml nerve growth factor (NGF) for 8–10 days before treatments. In PACAP signaling studies NGF was acutely withdrawn from mature sympathetic neuronal cultures as described under “Results.” The cultures were rinsed once and maintained in complete serum-free medium without NGF supplements for the times shown; an NGF neutralizing antiserum (1 μg/ml; monoclonal 27/21, Chemicon, Temecula, CA) was also included in some experiments. Cultures were treated with PACAP27 or PACAP38 (American Peptide Co., Sunnyvale, CA) at the indicated concentrations prepared from 100 μm stocks. All inhibitors were prepared from 100- or 1000-fold stocks in DMSO or methanol, and the efficacy of these reagents was tested in previous work (37, 38). Inhibitors H89 (25 μm), PD98059 (25 μm), LY294002 (25 μm), LY30311 (25 μm), bisindolylmaleimide I (15 μm), PP1 (10 μm), K252a (200 nm), cycloheximide (5 μg/ml), brefeldin A (5 μg/ml), chlorpromazine (10 μg/ml), and nystatin (10 μg/ml) were all from Calbiochem. Monodansylcadaverine (200 μm) and filipin (5 μg/ml) were from Sigma. AS252424 and AS605240 were from Cayman Chemical Co. (Ann Arbor, MI). Dynasore reagent was from Thomas Kirchhausen (Harvard Medical School).
Survival Assays
For cell viability assessments, replicate sympathetic neuronal cultures were fixed in 4% paraformaldehyde for 20 min, rinsed in 0.1 m sodium phosphate buffer, pH 7.5, and incubated in bisbenzimide 33258 (1 μg/ml, Hoechst reagent, Sigma) for 10 min before mounting in anti-fade medium and visualization by fluorescence microscopy using 4′,6-diamidino-2-phenylindole/Hoechst/AMCA filter set (Chroma, Brattleboro, VT). For routine measurements, 10 random fields per culture were enumerated for total and condensed nuclei staining. Biochemical viability assays were also performed to complement the morphological analyses. After treatments, 20 μl of the MTS tetrazolium salt/phenazine methosulfate solution (AQueous Assay, Promega, Madison, WI) was added directly to sympathetic neuronal cultures with 100 μl of serum-free medium in 96-well tissue culture plates. The dishes were returned to the humidified tissue culture incubator at 37 °C, 5% CO2 for 4–6 h before spectrophotometric quantitation of the metabolized MTS formazan reaction product in the medium at 490 nm using an absorbance microplate reader (Molecular Devices, Sunnyvale, CA). Data represent the mean of 5–6 culture replicates ±S.E. One-way analysis of variance and Newman-Keuls post hoc analyses were used to determine differences among treatment; p < 0.05 was considered significant.
Western Blot Analyses
Control and treated cultures were extracted with 200 μl of radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 8.0, 120 mm NaCl, 5 mm EDTA, 1% Nonidet P-40, 0.1% SDS) containing 0.3 mg/ml phenylmethylsulfonyl fluoride, protease inhibitors (16 μg/ml benzamidine, 2 μg/ml leupeptin, 50 μg/ml lima bean trypsin inhibitor, 2 μg/ml pepstatin A), and phosphatase inhibitor mix (5 mm EDTA, 5 mm EGTA, 1 mm sodium orthovanadate, 10 mm sodium pyrophosphate, 50 mm sodium fluoride) (44, 45). Total sample proteins were determined using the BCA reagent (Pierce). For Western analyses, protein samples (30 μg) were fractionated on 4–12% SDS-PAGE gels, transferred onto Immobilon-P polyvinylidene difluoride membranes (Millipore, Billerica, MA), blocked, and incubated with primary antisera for enhanced chemiluminescence detection (44) or quantitative infrared imaging (LiCor Biosciences, Lincoln, NE). Pan and phospho-specific antisera to ERK1/2, ERK5, JNK, p38, and Akt were all from Cell Signaling Technology (Danvers, MA). Pan and phosphorylated TrkA antisera were from Upstate Biotechnology (Charlottesville, VA); actin antiserum was from Sigma. For reprobing, the blots were stripped in 62.5 mm Tris-HCl, pH 6.7, containing 2% SDS and 100 mm β-mercaptoethanol at 55 °C for 30 min before the readdition of antisera. For these analyses the blots were hybridized first with antibodies that generated weaker signals before reprobing with those that produced stronger bands. In all instances, antibody to actin or total Akt was applied to the blots at the conclusion of the experiment.
ERK activity assays were performed using an Elk-1 fusion protein substrate (p44/42 mitogen-activated protein kinase assay; Cell Signaling). Briefly, treated sympathetic cultures in 6-well culture plates were harvested in 500 μl of lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100) containing phosphatase and protease inhibitors. After sample incubation with p44/42 mitogen-activated protein kinase antibody-coupled agarose beads for 24 h at 4 °C, the beads were recovered and incubated in 50 μl of kinase buffer (25 mm Tris-HCl, pH 7.5, 5 mm β-glycerophosphate, 2 mm dithiothreitol, 0.1 mm sodium orthovanadate, 10 mm MgCl2) containing 200 μm ATP and 2 μg Elk-1 fusion protein. The reaction was terminated after 30 min, and the samples were fractionated on 4–12% SDS-PAGE gels for Western blotting using a 1:1000 phospho-Elk-1 antibody and enhanced chemiluminescence detection.
Immunoprecipitations were performed as in previous work (46). Cleared cell lysate supernatants were incubated with 1:100 anti-TrkA overnight at 4 °C, and the samples were incubated subsequently with 20 μl of 50% slurry of protein A-agarose (Sigma) for an additional 1 h at 4 °C on a rocking platform. The immune complexes were washed in several changes in lysis buffer, resuspended in sample buffer, and boiled before SDS-PAGE fractionation and Western blotting as above using a phosphotyrosine antibody (pY-100; Cell Signaling).
Immunocytochemistry
Control and treated sympathetic neurons were cultured on collagen-coated Aclar dishes for immunocytochemistry as described (37). The cultures were fixed in 4% paraformaldehyde, permeabilized, and incubated in 1:500 anti-phosphoERK1/2 (Promega) for 24 h at 4 °C. Phosphorylated ERK was localized by culture incubation with 1:500 indocarbocyanine (Cy3)-labeled donkey anti-rabbit IgG and imaged with a Leica DMRB fluorescence microscope equipped with a Cy3 filter set (37). Mouse Gβ(1–4) monoclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA; sc-166123, 1:50); rabbit monoclonal antibody to phosphoAkt (Ser-473) was from Cell Signaling (D9E, 1:50).
GFP-tagged PAC1HOP1 Receptor Expression Plasmid Construction
The construction scheme for the PAC1HOP1-GFP expression plasmid is shown in supplemental Fig. 1. To create the carboxyl-terminal GFP-tagged receptor construct, the human PAC1HOP1 receptor cDNA clone (EM4 (47)) was amplified using synthetic oligonucleotide primer pairs hPRD03 (5′-CCCAGAGACACATTGGGGCTGAC-3′) and hPAC1AgeCT.rp (5′-GGGAGACCGGTCCGGTGGCCAG-3′) and the KOD DNA polymerase system (Novagen, Merck Biosciences UK); the PAC1HOP1 stop codon was replaced with a Gly codon, and an AgeI restriction site was engineered into the sequence. After agarose gel electrophoresis, the 1.4-kb PCR amplification product was purified using Qiaex II silica particles (Qiagen Ltd, Crawley, UK) and subcloned into the pCR4Blunt-TOPO cloning vector (Invitrogen). Clones with the appropriate insert size and restriction patterns were then digested with EcoRI/AgeI, and the PAC1HOP1 cDNA was subcloned into the EcoRI/AgeI site of pAcGFP-N1 (Clontech, Mountain View, CA). The reading frames and sequence integrity of selected clones were verified by restriction digest and by nucleotide sequence analysis; clone 4t26 was used for all expression studies.
Transfection and RNAi
AtT-20 cells were transfected with the full-length PAC1HOP1 receptor expression plasmid by electroporation and cultured in Dulbecco's modified Eagle's medium/F-12 medium containing 10% fetal bovine serum, 10% NuSerum, and 500 μg/ml Geneticin for stable cell selection. Functional expression of the PAC1HOP1 receptor was verified by PCR analyses and PACAP-induced stimulation of MAPK and calcium signaling. For knockdown studies, 70% confluent cells in 24-well plates were transfected with siRNAs to mouse Gαq or PI3K p110γ subunit (Santa Cruz Biotechnology) using Lipofectamine 2000 (10 pmol of siRNA/2 μl of transfection reagent; Invitrogen). The medium was replaced after 8 h, and the cells were maintained in culture for an additional 48 h before treatments. Adenovirus for wild type and dominant negative (K44A) dynamin were obtained from Yoram Altschuler (Hebrew University of Jerusalem). For biolistic gene transfer, PAC1HOP1-GFP DNA coated onto 1.6-μm gold particles were delivered to primary sympathetic neuronal cultures at 180 p.s.i. Overexpression or knockdown of the effector targets was verified by Western analyses or immunocytochemistry. Mock and control siRNA transfections did not alter transcript expression in all cases.
RESULTS
PAC1HOP1 Receptor Signaling Regulates Multiple MAPK Pathways with Distinct Temporal Dynamics
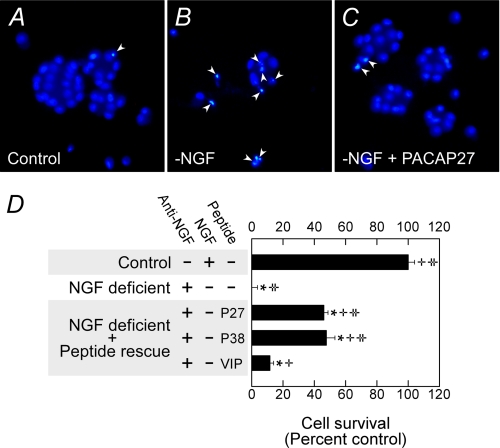
Previous studies have demonstrated the potent trophic properties of PACAP in many central and peripheral neuronal systems (1, 34, 36). Nearly all sympathetic superior cervical ganglion neurons express the PACAP-selective PAC1HOP1 receptor isoform, and receptor activation with PACAP peptides promoted neuronal survival after NGF withdrawal from growth factor-dependent immature sympathetic neurons (Fig. 1). Whereas NGF withdrawal and/or the addition of NGF-neutralizing antibodies to primary neonatal sympathetic cultures induced cell death to more than 50% that of the neurons within 18–24 h, the addition of 100 nm PACAP27 or PACAP38 to the cultures abrogated apoptotic signals and facilitated survival of most neurons (Fig. 1, A–C). The extent of neuronal cell death was accentuated by 48 h when >90% of the NGF-deprived neurons underwent apoptosis as measured by biochemical survival assays (Fig. 1D). PACAP-mediated neuroprotection was ∼50% over the same 48-h period, which appeared congruent with previous work, suggesting that the protective properties of PACAP were transient (28). By contrast, VIP rescued only 15% of the neurons in the chronic NGF withdrawal paradigm, which was consistent with the preferential expression of sympathetic PAC1HOP1 receptor selectivity for PACAP peptides.
FIGURE 1.
PACAP peptides promote sympathetic neuron survival after growth factor withdrawal. A–C, immature 5-day primary sympathetic neuronal cultures were withdrawn from NGF-containing serum-free medium for 24 h before fixation and Hoechst 33258 nuclear staining as described under “Experimental Procedures.” Neurons undergoing apoptosis after growth factor withdrawal (B) demonstrated nuclear condensation profiles (arrowheads), whereas cultures receiving 100 nm PACAP replacement presented far fewer nuclear apoptotic features (C). D, neuronal apoptosis after NGF deprivation was accentuated after 48 h as measured by MTS survival assays. Even under these circumstances, PACAP27 (P27) and PACAP38 (P38) demonstrated similar efficacy in protecting ∼50% of the population. VIP was less effective consistent with the prominence in PAC1 receptor expression in sympathetic neurons. Data represent a mean of 5–6 culture replicates for each treatment ±S.E. *, different from control; +, different from NGF-deficient cultures; ‡, different from VIP-treated cultures.
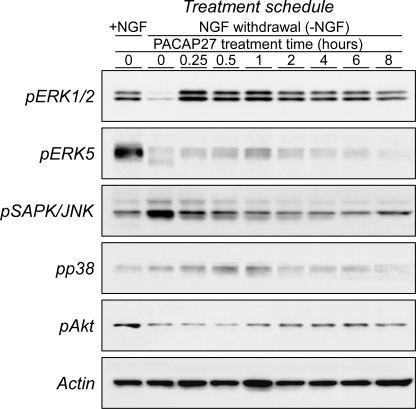
The PAC1HOP1 receptor can engage several signaling cascades, and in evaluating the relevant mechanisms for neuroprotection, PACAP stimulated multiple MAPK pathways with different temporal dynamics (Fig. 2). Although PAC1HOP1 receptor-mediated p44/p42 MAPK (ERK1/2) signaling has been implicated in neuroprotection (10), other MAPK pathways, especially ERK5, have also been associated with trophic responses to various challenges. Accordingly, the abilities for PACAP to stimulate to different MAPK pathways were investigated. For these studies, 9-day sympathetic neuronal cultures were acutely withdrawn from NGF for 4 h to simulate growth factor deprivation in injury. Under these conditions, MAPK phosphorylation levels were altered and attained steady levels; the paradigm had no effects on neuronal viability during the entire experimental duration as monitored by Hoechst nuclear staining and MTS survival assays. Although NGF withdrawal from the cultures significantly reduced ERK1/2 phosphorylation from diminished TrkA activation, the subsequent addition of either PACAP27 or PACAP38 dramatically induced ERK1/2 phosphorylation for sustained periods of time (Fig. 2). PACAP-mediated ERK1/2 phosphorylation was first detected within 1 min of peptide addition (data not shown); the response increased steadily and appeared maximal by 15–60 min before declining to a sustaining plateau by 6–8 h (Fig. 2). TrkA-mediated ERK5 activation has also been shown to be critical to NGF neurotrophic signaling, and similar to ERK1/2, NGF withdrawal diminished endogenous sympathetic ERK5 phosphorylation levels. PACAP addition to the growth factor-deprived cultures also maximally increased ERK5 phosphorylation during the first hour of treatment, but unlike ERK1/2 phosphorylation, ERK5 activation did not appear as robust and was transient, declining to control NGF-deprived levels within 2–4 h of peptide treatment (Fig. 2).
FIGURE 2.
PACAP regulates MAPK and Akt neurotrophic pathways with distinct temporal profiles. Mature primary sympathetic neuronal cultures (9 days in vitro) were acutely withdrawn from NGF for 4 h before 100 nm PACAP27 replacement for the times shown. The cultures were extracted in the presence of phosphatase/protease inhibitors for SDS-PAGE fractionation and Western blotting analyses as described under “Experimental Procedures.” In separate experiments the order of the different phospho-specific antibody applications to the blots was varied, and the results were identical. Acute NGF withdrawal diminished endogenous ERK1/2, ERK5, and Akt phosphorylation levels, whereas JNK and p38 MAPK activation was enhanced. The PACAP-induced changes in effector phosphorylation demonstrated distinct temporal profiles and in patterns consistent with neuronal survival and abrogation of proapoptotic signals. Blots were hybridized for actin immunoreactivity as a final step to demonstrate near equal sample loading. Data are representative of three independent experiments.
JNK and p38 MAPK pathways are complex and have been associated with diverse cellular responses including growth/differentiation and stress-related events. To the latter, growth factor withdrawal from the neurons induced both phosphorylated JNK and to a lesser degree p38 levels compared with cultures maintained in NGF (Ref. 48 and Fig. 2). In contradistinction to ERK1/2 and ERK5 signaling, sympathetic PAC1HOP1 receptor activation attenuated JNK phosphorylation in the NGF deprivation paradigm, returning JNK phosphorylation states to the low basal levels observed under optimal NGF-supplemented conditions. The effects of PACAP in diminishing JNK activation were rapid and detected in minutes, commensurate with induced ERK1/2 phosphorylation. By contrast, the responses for p38 MAPK were protracted; the modest increase in p38 phosphorylation after NGF withdrawal appeared to be heightened in the first hour after PACAP treatment, after which the levels declined to the low basal state found in growth factor-supplemented cultures. In stimulating ERK1/2 and ERK5 phosphorylation and abrogating JNK/p38 MAPK stress activation, sympathetic PACAP/PAC1HOP1 receptor signaling affected multiple MAPK pathways in a coherent program consistent with neuroprotection and survival.
The robust PACAP/PAC1HOP1 receptor effects on ERK1/2 activation were further characterized (see supplemental Fig. 2). Similar to sympathetic PAC1HOP1 receptor-mediated cyclic AMP activation, PACAP potently activated ERK1/2 phosphorylation and kinase activity at subnanomolar concentrations (supplemental Fig. 2, A and B); the increase in ERK1/2 phosphorylation was correlated directly with nuclear ERK translocation (supplemental Fig. 2, D and E). Preincubation of neurons with the MEK inhibitor PD98059 (25–50 μm) completely abrogated all endogenous and stimulated ERK1/2 phosphorylation in the primary cultures (supplemental Fig. 2C). The addition of PKA inhibitor H89 (10 μm) or PI3K inhibitor LY294002 (25–50 μm) blocked PACAP-mediated ERK phosphorylation; PKC inhibitor bisindolylmaleimide I (15 μm), TrkA tyrosine kinase inhibitor K252a (200 nm), and phospholipase C inhibitor U73122 (10 μm, not shown) had no effects. Both PKA and PI3K have well described interactions with MEK cascades (49–52), and PACAP/PAC1HOP1 receptor signaling may engage both pathways coordinately to stimulate ERK1/2 activation.
PACAP Stimulates Late Phase Sympathetic Akt Neurotrophic Signaling
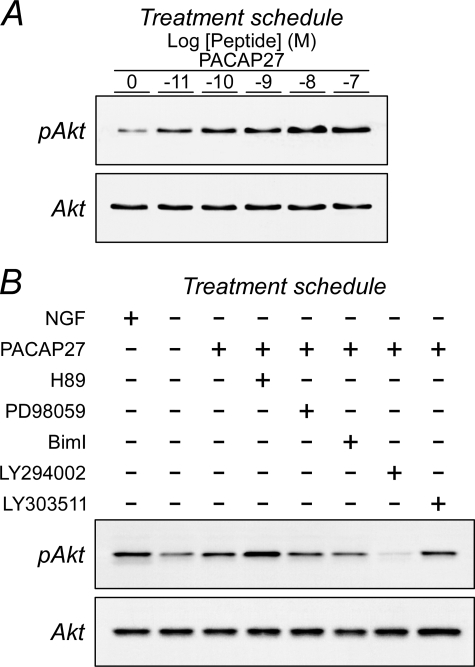
Because Trk neurotrophic signaling in sympathetic neurons is mediated largely by PI3K/Akt activation and downstream regulation of Bad/Bcl proteins, the ability for PACAP/PAC1HOP1 receptor to promote neuronal survival through Akt signaling was anticipated. Under the same experimental paradigm described above, the withdrawal of NGF from the cultures diminished Trk-induced phosphorylated Akt levels ∼80% from quantitative Western analyses (Fig. 2). In sharp contrast to the rapid phosphorylation of ERK (minutes), PAC1HOP1 receptor-mediated Akt phosphorylation in the same experimental paradigm appeared at a later phase. From low basal levels after growth factor withdrawal, PACAP-stimulated Akt phosphorylation was first detected 2 h after treatment, and a maximal 2.5-fold increase in phosphorylation levels was attained by 4–6 h of peptide exposure (Fig. 2 and supplemental Fig. 3). Similar to PAC1HOP1 receptor activation of ERK and other signaling pathways, the effects of PACAP peptides were potent in stimulating Akt phosphorylation at subnanomolar concentrations (Fig. 3A). From inhibitor studies, the PAC1HOP1 receptor-mediated Akt response was downstream of PI3K activation (Fig. 3B and supplemental Fig. 4). PACAP-mediated Akt phosphorylation was completely blocked by PI3K inhibitor LY294002 (25–50 μm) and not by its control LY303511 analog (25–50 μm), demonstrating that the response did not involve NFκB pathways (53). Culture pretreatment with inhibitors to MEK (50 μm PD 98059) or PKA (25 μm H89) did not attenuate PACAP/PAC1HOP1 receptor-stimulated Akt activation (Fig. 3B and supplemental Fig. 4); to the contrary, inhibition of PKA consistently potentiated the response through mechanisms that remain to be fully elucidated. Activation of PKA has been shown to abrogate Akt phosphorylation in HEK298 cells (54), and the increased level of PACAP-stimulated Akt phosphorylation after H89 treatment appeared consistent with these observations. H89 can also inhibit Rho kinase/ROCK-II, suggesting that the response may be mediated through inhibition of other protein kinases (55).
FIGURE 3.
PACAP potently increases sympathetic Akt phosphorylation. A, primary sympathetic neuronal cultures were withdrawn from NGF (as in Fig. 2) and treated with different concentrations of PACAP for 2 h before extraction and Western blot analyses. As with ERK, PACAP potently increased Akt phosphorylation. B, for studies with signaling pathway inhibitors, NGF-deprived cultures were pretreated with the indicated inhibitors for 15 min before peptide addition for 2 h. PI3K inhibitor LY294002 (25 μm) blocked PACAP-mediated Akt phosphorylation. MEK and PKC inhibitors, PD98059 (25 μm), and bisindolylmaleimide I (BimI, 15 μm) and the inactive LY294002 analog LY303511 (25 μm) had little or no effects. Application of the PKA inhibitor H89 (25 μm) consistently potentiated PACAP-stimulated Akt phosphorylation. Data are representative of four independent experiments.
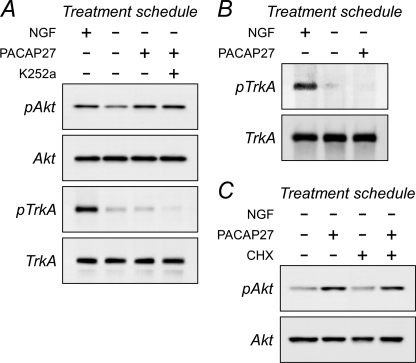
There were several potential mechanisms for G protein-coupled receptor activation of Akt, including transactivation of tyrosine kinase receptors and direct G protein interactions with PI3K. Because sympathetic neurons express significant levels of TrkA, the former was an important consideration. By all measures, sympathetic PACAP-mediated Akt signaling was not dependent on TrkA expression or activation. First, sympathetic TrkA-mediated Akt activation with saturating levels of NGF (50 ng/ml) did not occlude the ability for PACAP to stimulate Akt phosphorylation. The high Akt phosphorylation levels with NGF/TrkA signaling was augmented reliably nearly 1.4-fold upon PACAP treatment (supplemental Fig. 5). Second, exposure of sympathetic cultures with 100 nm PACAP27 over short term (minutes) or long term (hours) treatment paradigms did not increase neuronal TrkA transcript levels by semiquantitative or quantitative PCR (data not shown), total TrkA receptor protein expression by Western blot analyses, or TrkA phosphorylation in Western or immunoprecipitation assays. Essentially all TrkA in sympathetic neurons was represented by the 140-kDa mature protein; little immature TrkA was observed by Western analysis. When sympathetic cultures were placed in the NGF-withdrawal paradigm, neuronal TrkA and Akt phosphorylation levels were diminished as anticipated without affecting total cellular 140-kDa TrkA or 60-kDa Akt proteins (Fig. 4A). Although subsequent treatment with 100 nm PACAP27 for 4 h increased Akt activation, PACAP did not augment the TrkA phosphorylation state (Fig. 4A). These results coincided with TrkA immunoprecipitation studies that also failed to demonstrate increased TrkA tyrosine phosphorylation after PACAP addition (Fig. 4B). Furthermore, when 200 nm K252a was added to replicate PACAP27-treated cultures, the Trk tyrosine kinase inhibitor diminished total TrkA phosphorylation without attenuating PACAP/PAC1HOP1 receptor-stimulated Akt phosphorylation (Fig. 4A). The PACAP-mediated Akt response was not dependent on new protein synthesis (Fig. 4C); the addition of cycloheximide to the cultures did not block PACAP/PAC1HOP1 receptor-stimulated Akt phosphorylation, which also correlated with previous data suggesting that PACAP did not stimulate Trk protein levels to augment neurotrophic Akt signaling.
FIGURE 4.
Inhibition of sympathetic receptor tyrosine kinase activity or protein synthesis does not block PACAP-stimulated Akt phosphorylation. A, mature primary sympathetic cultures were acutely withdrawn from NGF and pretreated with 200 nm K252a for 15 min before 100 nm PACAP addition for 3 h. The cultures were harvested in lysis buffer containing phosphatase/protease inhibitors for SDS-PAGE and Western analyses using antibodies for phosphorylated TrkA or Akt. The major form of TrkA in sympathetic neurons is the 140-kDa mature protein. Acute NGF deprivation diminished endogenous TrkA phosphorylation levels. Although the addition of PACAP27 activated Akt, TrkA phosphorylation in the same samples was not enhanced from the low basal levels. Although treatments with the Trk tyrosine kinase inhibitor further diminished culture TrkA phosphorylation, co-treatment with K252a had no effects on PACAP-mediated Akt phosphorylation. B, these results were corroborated in immunoprecipitation experiments. Sympathetic neuronal cultures were acutely withdrawn from NGF to attenuate endogenous TrkA phosphorylation levels (4 h) before PACAP addition for 2 h. The cultures lysed in buffer containing phosphatase/protease inhibitors and the extracts were immunoprecipitated with TrkA antibodies for Western analyses using a phosphotyrosine antibody. PACAP did not stimulate TrkA phosphorylation from the low basal levels. C, similar to experimental designs in panel A, the presence of 5 μg/ml cycloheximide (CHX) for the entire 3-h PACAP treatment period did not abrogate PACAP-stimulated Akt activation. Data are representative of three independent experiments.
Sympathetic Akt Phosphorylation Relies on PI3Kγ Function
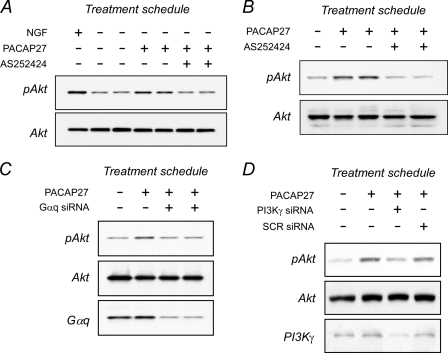
Growth factor Akt signaling is mediated predominantly by Class 1A PI3K, in which the catalytic p110α, p110β, or p110δ subunits form a complex with SH2/SH3-containing regulatory p85 subunit for interactions with tyrosine-phosphorylated receptor kinases. By contrast, the catalytic p110γ subunit of Class 1B PI3K dimerizes with the regulatory p101 protein, which contains no discernible protein-protein interaction motifs but can be markedly stimulated by Gβγ after release from activated G protein-coupled receptors. Unlike the pan PI3K inhibitor LY294002, the recent availability of several furan-2-ylmethylene thiazolidinedione compounds as PI3Kγ-specific inhibitors allowed evaluations of G protein/Akt signaling pathways (56, 57). Peripheral neurons express PI3Kγ transcript and proteins, and in the absence of growth factors, treatment of sympathetic cultures with PI3Kγ-selective inhibitor AS252424 (10 μm) completely blocked PACAP-induced Akt phosphorylation (Fig. 5A). Consistent with previous inhibitor characterization studies (57), the same concentration of AS252424 had no effects on NGF/TrkA PI3Kα/β-stimulated Akt activation; identical results were obtained with a related PI3Kγ inhibitor AS605240 (data not shown).
FIGURE 5.
Inhibition of Gαq and PI3Kγ signaling attenuates PACAP-stimulated Akt activation. A, sympathetic neuronal cultures were acutely withdrawn from NGF for 4 h to attenuate cellular Akt phosphorylation levels, then pretreated with 10 μm AS252424 (15 min) to selectively inhibit PI3Kγ activity before 100 nm PACAP27 addition for 4 h. AS252424 blocked PACAP/PAC1 receptor-stimulated Akt activation but did not attenuate NGF/TrkA-stimulated Akt phosphorylation (not shown). Identical results were obtained with PI3Kγ-selective inhibitor AS605240. B, treatment of stable AtT-20/PAC1HOP1 cultures with 100 nm PACAP27 for 4 h stimulated Akt phosphorylation; as in panel A with primary sympathetic neurons, the response was blocked upon inhibition with PI3Kγ-selective AS252424 (10 μm) treatment. AtT-20/PAC1HOP1 cultures were transfected at 70% confluency with mouse siRNA oligonucleotides to affect Gαq (C) or PI3Kγ (D) knockdown. After 48 h, the cultures were treated with 100 nm PACAP27 for 4 h. PACAP-stimulated Akt phosphorylation was attenuated when Gαq or PI3Kγ expression was diminished. Culture total Akt levels demonstrate loading equivalency across all samples. Representative data are from 2–4 experiments.
As transfection efficiency for primary sympathetic neurons was invariably low and not amenable for molecular studies, stable AtT-20 and SH-SY5Y cell lines expressing PAC1HOP1 receptors were produced. The addition of PACAP to these cell lines stimulated ERK phosphorylation and calcium flux consistent with PAC1HOP1 receptor activation. Similar to sympathetic neurons, treatment of AtT-20/PAC1HOP1 or SH-SY5Y/PAC1HOP1 cultures with PACAP for 2–6 h increased late-phase Akt phosphorylation 2-fold. When evaluated in AtT-20/PAC1HOP1 cells, the Akt response was again completely abrogated with AS252424, implicating PI3Kγ activation (Fig. 5B and supplemental Fig. 6). As PACAP-stimulated Akt phosphorylation appeared more robust in AtT-20/PAC1HOP1 cultures, these cells were used in the remaining studies.
PAC1HOP1 receptors are coupled to both Gαs and Gαq, and previous work suggested that Gβγ from Gαq, rather than Gαs, can target PI3Kγ for Akt activation (58, 59). The knockdown of the many β and/or γ subunits was unwieldy, but as Gαq release of Gβγ subunits may be compartmentalized and/or dependent on the appropriate scaffold context for PI3Kγ signaling, the disruption of Gαq and its associated Gβγ activities may attenuate PI3Kγ activation. When Gαq expression in AtT-20/PAC1HOP1 cells was decreased 60–70% using siRNA approaches, Akt phosphorylation was also diminished after PACAP stimulation (Fig. 5C and supplemental Fig. 6). Similar changes were seen with knockdown of PI3Kγ (Fig. 5D). Again, as Trk proteins were not detected in control AtT-20 and AtT-20/PAC1HOP1 cells and the cell lines did not respond to neurotrophin treatments, the PACAP-induced Akt phosphorylation was not secondary to Trk signaling.
Sympathetic PACAP/PAC1HOP1 Receptor Activation of Akt Is Dependent on Endocytic Mechanisms
The delayed temporal parameters for sympathetic PACAP-stimulated Akt phosphorylation may reflect receptor internalization. Thus, several experiments were undertaken to examine the possibility that PAC1HOP1 receptor internalization and distribution with signaling molecules after ligand binding was required for Akt activation. The primary sympathetic neurons were transfected with the PAC1HOP1-GFP construct by biolistic gene transfer, and after 36–48 h, the receptor was localized almost exclusively to the plasma membrane (supplemental Fig. 7A). However, if the same cultures were subsequently treated with 100 nm PACAP27 for 4 h, as in the signaling assays, a large fraction of the PAC1HOP1 receptor was internalized into 200–350-nm vesicular structures (supplemental Fig. 7, A versus D). A similar staining profile was observed for Gβ except a significant portion of the immunoreactivity remained on the cell surface even after PACAP stimulation (compare supplemental Fig. 7, B with D). No staining for phosphorylated Akt was evident in sympathetic cultures without NGF; by contrast, after 4 h of PACAP treatment, fine punctate vesicular immunoreactivity for pAkt was widely distributed in the cytoplasm (supplemental Fig. 7C). When the PAC1HOP1-GFP-transfected sympathetic neurons were NGF-deprived, treated with PACAP for 4 h, and processed for pAkt immunoreactivity using a Cy3-secondary antibody conjugate, PAC1HOP1 and pAkt were colocalized in a significant fraction of the deep vesicular structures (supplemental Fig. 7, D–F). Hence, the distribution patterns for PAC1HOP1, G proteins, and pAkt appeared overlapping, suggesting that the endocytosis and routing of these signaling components may be similar and intersect for activation.
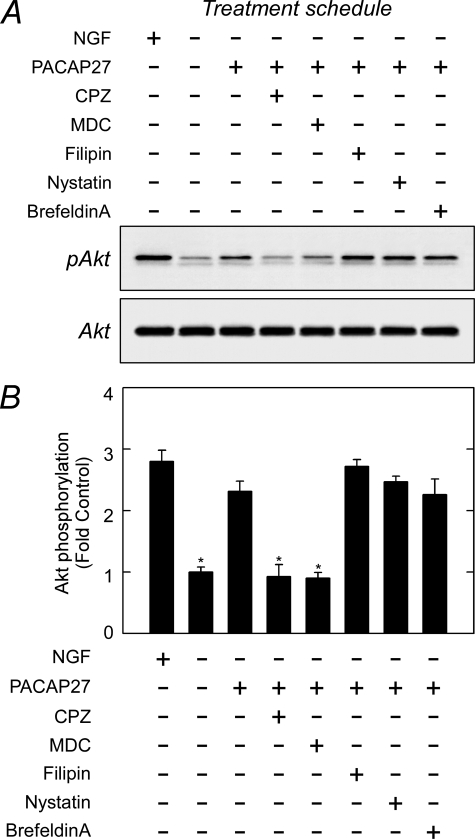
To determine whether these internalization mechanisms may account for the temporal dynamics in Akt phosphorylation after PACAP treatment, several well studied inhibitors for vesicular endocytosis were evaluated (Fig. 6 and supplemental Fig. 8). As before, the addition of PACAP27 to NGF-deprived cultures stimulated Akt phosphorylation. Among the reagents tested in parallel cultures, chlorpromazine (10 μg/ml), a cationic amphilic drug that induces a redistribution of clathrin adaptor AP-2 component, and monodansylcadaverine (100 μm), an inhibitor of transglutaminases and clathrin assembly/receptor invagination processes, blocked most if not all PACAP/PAC1HOP1 receptor-stimulated Akt phosphorylation (Fig. 6). In good agreement, the addition of the dynamin 1/2 inhibitor dynasore (20–80 μm) also completely blocked PACAP-induced Akt activation (data not shown). The addition of filipin (2–5 μg/ml) and nystatin (10 μg/ml) to disrupt lipid rafts and caveolae integrity, by contrast, had little or modest effects on PACAP-mediated Akt response. Disruption of Golgi-derived endosomes with brefeldin A (5 μg/ml) also had no apparent effects on PACAP-stimulated Akt phosphorylation levels in sympathetic neurons.
FIGURE 6.
PACAP-stimulated Akt phosphorylation is blocked by clathrin pathway inhibitors. A, acute NGF-deprived cultures as described in Fig. 2 were pretreated with inhibitors for clathrin (chlorpromazine (CPZ), monodansylcadaverine (MDC)) or caveolae (filipin, nystatin) for 30 min before the addition of 100 nm PACAP27 for 3 h. As before, the cultures were harvested for SDS-PAGE and Western analyses for activated Akt. B, quantitative Western analyses of data in panel A were normalized to total Akt and expressed as -fold change from −NGF control. Reagents associated with clathrin-mediated vesicle internalization blocked PACAP-stimulated Akt phosphorylation. n = 3, data represent the mean ± S.E. *, different from PACAP-treated cultures at p < 0.05.
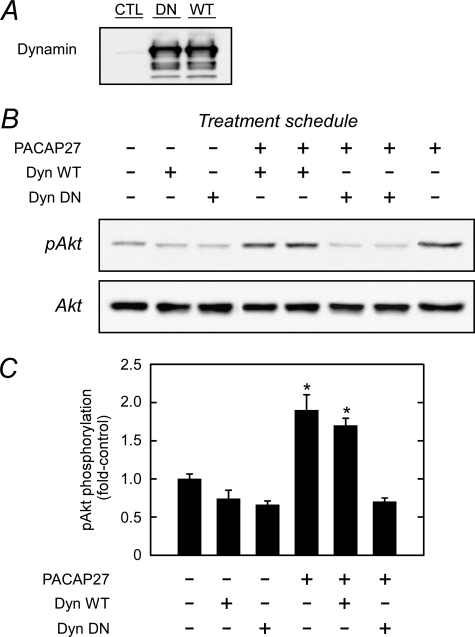
The dynamin GTPases are principal mediators of vesicle scission, and as a different means of evaluation, the sympathetic neuronal culture was infected with either wild type or dominant negative (DN) dynamin constructs (Fig. 7). The dynamin proteins were well expressed, but only DN dynamin blocked PACAP-induced Akt phosphorylation. In aggregate, these results suggested that the delayed Akt signaling events were related to PAC1 receptor/effector internalization and endosome complex assembly and insensitive to Golgi vesicle production.
FIGURE 7.
Sympathetic neuron expression of dominant negative dynamin inhibits PACAP-stimulated Akt phosphorylation. A, control sympathetic neurons (CTL) and cultures infected with wild type (WT) or dominant negative dynamin K44A (DN) were extracted for Western analyses of dynamin protein expression. The two dynamin preparations yielded comparable levels of protein expression. B, sympathetic neuronal cultures infected with either dynamin (Dyn) WT or dynamin DN (48 h) were deprived of NGF for 4 h before the addition of 100 nm PACAP27 (4 h) for Western analyses of phosphorylated Akt levels. Cultures expressing dominant negative dynamin blocked PACAP-stimulated Akt activation. C, quantitative Western analyses of data in panel B were normalized to total culture Akt levels and expressed as -fold change from untreated control. n = 3, data represent the mean ± S.E. *, different from untreated controls at p < 0.05.
PACAP Activation of Akt Is the Primary Means of Neuroprotection in Sympathetic Neurons
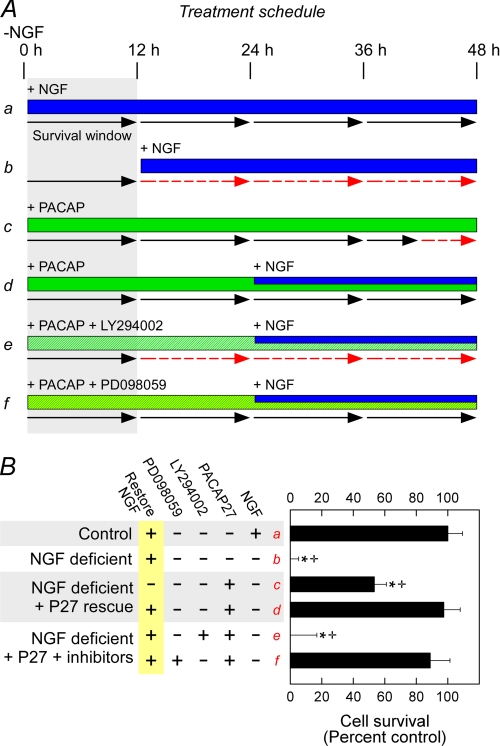
The events that precede sympathetic neuron apoptosis have been well studied and allow evaluations of the relative contributions of MEK/ERK and PI3K/Akt signaling in PACAP-mediated neuronal survival (60). After growth factor withdrawal from the cultures, sympathetic neurons do not undergo apoptosis immediately but subsist within a 12–15-h survival window during which the readdition of trophic factors to the medium can rescue the neurons (Fig. 8, A and B, lane a, black arrows). However, once this survival window is exceeded before the return of NGF or other trophic factors, the neurons are committed to the apoptosis cascade and undergo cell death regardless of subsequent NGF addition (Fig. 8, A and B, lane b, broken red arrows). In illustration, NGF replacement to growth factor-deprived cultures outside of the survival window (>15 h) did not promote survival assessed 48 h later (Fig. 8B, lane b). However, if NGF was withdrawn and replaced with PACAP during the survival window, the window was effectively extended for the next 24–48 h (Figs. 8, A and B, lane c, note the length of the black arrows) such that the readdition of NGF within this period rescued all neurons (Figs. 8, A and B, lane d). When PI3K inhibitor LY294002 was added simultaneously with PACAP27 within the window (Figs. 8, A and B, lane e), the neurons were not protected in this paradigm. By contrast, the concomitant addition of MEK inhibitor PD98059 with PACAP27 did not attenuate its protective properties (Fig. 8, A and B, lane f). From these results, the abilities of PACAP/PAC1HOP1 receptor activation to promote neuronal survival appeared to be mediated largely by downstream sympathetic PI3K/Akt signaling.
FIGURE 8.
PACAP-mediated Akt signaling is neuroprotective in sympathetic neuronal cultures. A, shown is an experimental schematic for PACAP-mediated sympathetic neuronal survival. After NGF withdrawal (0 h) from immature sympathetic neuronal cultures, there is a 12–15-h survival window delaying neuronal commitment to apoptosis. If NGF is returned to the culture within the survival window (a), the cultures continue to live (black solid arrows) and do not undergo apoptosis programs. But if NGF is returned to the cultures outside of window parameters (b), the neurons are committed to apoptosis, and subsequent NGF addition is unable to affect rescue (red broken arrows). The addition of PACAP in place of NGF during the survival window extends the survival period (c) such that a much delayed addition of NGF (blue bar) can fully protect the neurons (d). Accordingly, the addition of signaling inhibitors (hatched bars, e and f) with PACAP during the window period will block the extended survival period and can be diagnostic of PACAP neurotrophic pathways. B, in this paradigm using the MTS survival assay (lanes a–f correspond to those in panel A), NGF replacement outside of the survival window did not offer neuroprotection when survival was assessed 48 h later (b). PACAP alone offered 50% protection (c) in NGF-deprived cultures, whereas the sequential addition of PACAP + NGF as described above was fully protective (d). Between ERK and Akt pathways, only LY294002 blocked the abilities for PACAP to promote neuronal survival in this experimental paradigm (e). As the MEK inhibitor PD98059 had no apparent effects (f), these results suggested that PI3K/Akt signaling may be more immediate in mediating PACAP survival signaling. Data represent the mean of 5–6 culture replicates ± S.E.; *, significantly different from control; +, different from PACAP-rescued cultures at p < 0.05.
DISCUSSION
Classical neurotrophic signaling pathways engage MAPK and Akt signaling to promote neuroprotection and regeneration after injury. Although PACAP peptides have well described prosurvival attributes, how G protein-coupled PAC1 receptor signaling intersects with MAPK and/or Akt effectors to elicit these neurotrophic responses is still not well understood. Primary sympathetic neurons are particularly well suited to address these questions from a number of considerations. Sympathetic neurons can be maintained in primary culture under well prescribed serum-free defined conditions that well mimic the morphological and functional properties of neurons in vivo (13, 61). The majority of sympathetic neurons preferentially express the PAC1HOP1 receptor subtype associated with neurotrophic signaling (15, 37). Furthermore, neonatal sympathetic neuronal cultures demonstrate NGF dependence and have been well studied with respect to apoptosis after growth factor deprivation (60). Because the temporal and molecular sequences leading to neuronal death have been defined, primary sympathetic cultures are particularly amenable for PACAP neurotrophic studies.
PACAP/PAC1HOP1 receptor signaling promotes neuronal survival after NGF withdrawal from sympathetic cultures (Fig. 1). In an acute NGF withdrawal paradigm, the addition of PACAP peptides to the culture regulated not one but multiple MAPK effectors in patterns that reflected a concerted program to promote neuronal survival. PACAP stimulated survival signals yet abrogated concurrently several pathways associated with cellular stress and apoptotic events. Expectedly, the withdrawal of NGF attenuated endogenous TrkA-mediated ERK activation; the addition of PACAP rapidly induced ERK phosphorylation to levels that appeared comparable with those from endogenous TrkA activation within the first hour of treatment. From dose dependence and kinase activity studies, the abilities for PACAP to stimulate ERK1/2 phosphorylation at subnanomolar concentrations were consistent with previous work demonstrating the high potency of PACAP peptides in neuronal signaling (62); moreover, the ERK1/2 responses were durable, which has been associated with trophic differentiation in PC12 pheochromocytoma cells (63, 64). These effects most likely reflected potent PACAP/PAC1 receptor activation of cAMP/PKA cascades in sympathetic neurons (37), and in agreement, PKA inhibitors blocked PACAP-stimulated ERK1/2 activation. Although several kinases have been shown to be H89-sensitive (55), the experimental conditions and results were consistent with previous signaling studies in PC12 cultures, a widely used sympathetic neuronal model, which in aggregate implicate PACAP activation of ERK through a PKA-dependent mechanism (52, 65). B-Raf is the major Raf isoform in neural crest-derived neurons and cAMP/PKA activation of Rap1 may represent a potential means of participating or initiating B-Raf-mediated ERK1/2 phosphorylation (51, 66, 67). Although cAMP binding to Epac, as an alternative to PKA, can also directly stimulate Rap1 and lead to ERK phosphorylation in the appropriate cellular context, crest-derived cells appear to express low levels of Epac1/Epac2 (52, 65). Although the coordinate cAMP/PKA signaling with PKC and other effectors has been implicated in ERK activation in some cell systems (68), the inability for bisindolylmaleimide I (PKC-selective inhibitor) or U73122 (phospholipase C inhibitor, data not shown) to blunt PACAP-stimulated ERK phosphorylation suggested that these mechanisms may not be required in primary sympathetic neurons (supplemental Fig. 2). Because ERK5 is not stimulated by cAMP or depolarization mechanisms (69), PACAP-mediated MKK5/ERK5 signaling may not be as robust compared with ERK1/2 phosphorylation. Accordingly, although ERK5 plays a significant role in Trk neurotrophic signaling, the mechanisms and significance of PACAP-mediated ERK5 activation remain to be elucidated. Although PI3K/Akt and MAPK are frequently considered to represent separate pathways, the current studies implicated PI3K in PACAP/PAC1HOP1 receptor-stimulated ERK activation, in good agreement with other work (50, 70, 71). Accordingly, PACAP/PAC1HOP1 receptor-mediated ERK activation represents the integration and summation of multiple downstream effector pathways.
Importantly, in coordination with survival signal activation, PACAP can abrogate stress-induced proapoptotic pathways. Enhanced JNK and p38 MAPK signaling in response to environmental stresses, including growth factor deprivation, UV irradiation heat shock, osmotic stress, lipopolysaccharide, protein synthesis inhibitors, and proinflammatory cytokines have been well studied (48, 72). The stresses induced in sympathetic neurons after acute growth factor withdrawal increased culture-activated JNK levels significantly, but the addition of PACAP27 to the cultures rapidly diminished phosphorylated JNK1 and JNK2/3 in temporal patterns that were reciprocal to those for phosphorylated ERK1/2 induction. The abilities for PACAP to reduce activated p38 MAPK were also apparent but on a more protracted time scale compared with that for JNK. Whether the overall PACAP/PAC1HOP1 receptor-mediated decreases in JNK and p38 MAPK activation are components of common pathways that concurrently stimulate ERK phosphorylation remain to be evaluated, but in aggregate the MAPK responses demonstrate the abilities for PACAP/PAC1 receptor signaling to integrate mechanisms and mitigate injury responses.
However, for many neuronal systems, PI3K/Akt signaling is the more dominant survival pathway. G protein-coupled PAC1 receptor signaling also stimulated Akt phosphorylation, but unlike the more immediate PACAP-mediated ERK/JNK responses, which may have reflected PAC1HOP1 receptor activation of plasma membrane-associated effectors, the onset of the Akt responses was apparent only after longer peptide treatment periods. There are several potential mechanisms, and previous studies using PC12 overexpression system have suggested that the protracted increase in Akt activation was secondary to Golgi-derived endosome TrkA transactivation after PAC1 receptor internalization (73, 74). Although G protein-coupled receptor transactivation of receptor tyrosine kinases has been well described for many systems, our current studies implicate other pathways in primary sympathetic neurons. Contrary to previous work, PACAP did not increase TrkA or the expression of other Trk receptors with the temporal dynamics, consistent with Akt activation. PACAP did not increase TrkA transcript expression under any of the current treatment paradigms,4 and treatment of the sympathetic cultures with PACAP peptides under conditions that maximally stimulated Akt activation did not increase TrkA protein or phosphorylation levels by Western or immunoprecipitation analyses. PACAP-mediated Akt phosphorylation was not K252a- or cycloheximide-sensitive; furthermore, reagents that disrupted Golgi derived-endosome formation also did not block PACAP-stimulated Akt activation. From these considerations, sympathetic PAC1 receptor activation of Akt appears to be Trk-independent. Many G protein-coupled receptors, including the 5-HT2A and 5-HT7A receptors have been shown to activate Akt (75). Although Akt may be phosphorylated directly by PKA (76) from potent PAC1HOP1 receptor stimulation of cAMP, the ability for LY294002, rather than PKA inhibitors, to attenuate PACAP-stimulated Akt phosphorylation demonstrated that PI3K was the upstream kinase.
After agonist binding to G protein-coupled receptors, the liberated Gβγ subunit from Gα may stimulate plasma membrane Akt phosphorylation directly through its interactions with PI3Kγ. But more recently there has been increasing appreciation that G protein-coupled receptor activation can also result in heterometric Gα and/or Gβγ protein internalization and vesicular translocation/trafficking to stimulate PI3Kγ-mediated Akt phosphorylation in endosomal compartments (77). Although many signaling effector proteins including PI3K have been identified in endosomes (78), Gβγ association with Rab11 has been suggested to allow Gβγ routing to these compartments for enhanced endosome PI3Kγ/Akt recruitment and assembly for signaling complex activation (77). Our current results in primary neurons and neuroendocrine cells appear entirely consistent with this scheme. The immunocytochemical studies demonstrated that PAC1HOP1 receptor, G proteins, and activated Akt can be localized to vesicular structures, consistent with the concept of effector assembly and interactions for signaling. PI3Kγ protein and transcripts are prevalent in sympathetic neurons (data not shown), and specific PI3Kγ inhibitors and PI3Kγ pathway knockdown studies completely blocked PACAP-stimulated Akt phosphorylation. Perhaps more importantly, various manipulations to inhibit intracellular vesicular trafficking also dramatically attenuated PACAP-stimulated Akt activation. Our results with chlorpromazine and monodansylcadaverine, two reagents frequently used to perturb clathrin-dependent vesicular pathways, implicated clathrin-mediated endocytosis and routing of activated receptor components to mature signaling endosomes. By contrast, filipin and nystatin, compounds typically used to disrupt lipid rafts and caveolae structure, had no apparent effects on PACAP-stimulated Akt activation. Although pharmacological disruption cannot unequivocally identify specific mechanisms, these results coupled with data using DN dynamin emphasize the importance of endocytic mechanisms for long term signaling. Again, the inability for brefeldin A to block the response suggested that Akt phosphorylation did not rely on trafficking to new Golgi-derived compartments. Although other studies have suggested that heterometric G protein internalization may proceed through caveolae or other clathrin-independent mechanisms (79), whether this reflects cell-specific differences or the transfer of vesicular Gα/Gβγ proteins into endosomal compartments in the clathrin pathway remains to be established. Cellular vesicle internalization, transport, trafficking, and effector recruitment processes, especially from neuronal axonal termini and under serum-free conditions, may contribute to the apparent delay in Akt activation. Alternatively, other models including the potential binding of PI3K to the consensus amino acid motif found in the intracellular cytoplasmic tail of the PAC1 receptor and PAC1 receptor/Gαq-mediated Src signaling (80, 81) may be considered within the context of vesicle endocytosis. Both PI3K and Src, for example, have been shown to regulate vesicle molecular scaffold assembly and AP-2 recruitment in clathrin-mediated internalization (82–85).
Nevertheless, Akt signaling through endocytic pathways in several systems has been increasingly appreciated to serve as a key mechanism contributing to the high spatial and temporal specificity in signal transduction for cell survival (86–88). Akt has well described roles in orchestrating cell proliferation, differentiation, and survival from apoptosis during development or injury, and one means of conferring that specificity among the seemingly diverse and opposing physiological signals has been suggested to be dependent on vesicle trafficking and endosomal signaling (86). In evaluating that possibility, receptor effector internalization and Akt activation on endosomal platforms has been suggested to represent one potential means of discriminating survival signaling from growth or proliferation (86). From the many PACAP/PAC1 receptor-activated signaling pathways, our studies on the role of Akt activation in sympathetic neurons was consistent with that interpretation. Whereas MEK inhibitors had no apparent effects on PACAP-mediated survival in a sympathetic NGF withdrawal paradigm, PACAP neuroprotection was sensitive to PI3K inhibition. In support, the clathrin adapter β-arrestin can be co-immunoprecipitated with the PAC1 receptor5; furthermore, clathrin inhibitors also blocked PACAP-stimulated neuronal survival and BAD phosphorylation, a step downstream of Akt activation (data not shown). In summary, PACAP/PAC1HOP1 receptor activation regulates dual MAPK and Akt signaling in a complementary multifaceted strategy that integrates the induction of neurotrophic pathways with the abrogation of stress-induced proapoptotic signals (Fig. 9). In contrast to rapid membrane signaling, PACAP-stimulated Akt activation through PI3Kγ and vesicle endocytosis may represent spatial and temporal mechanisms to distinguish Akt-mediated survival from cellular proliferation, differentiation, or other Akt-related trophic functions.
FIGURE 9.
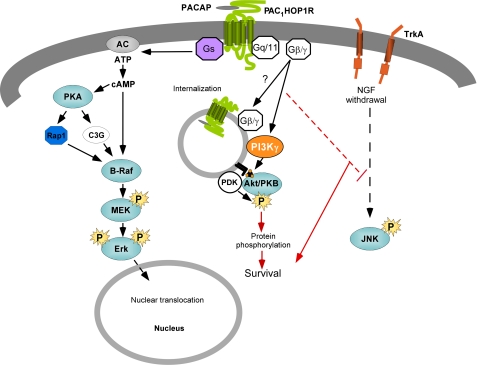
Schematic of coordinate PACAP receptor signaling for sympathetic neuronal survival. The ability for the PAC1 receptor to engage Gαs/Gαq signaling pathways represents a multifaceted but coordinate means for neurotrophic signaling. PAC1 receptor Gαs signaling in sympathetic neurons can stimulate cAMP mechanisms to promote sustained ERK activation. In addition to rapid PAC1 receptor-mediated Gαq activation of phospholipase C /PKC pathways, the internalization of PAC1 receptors through clathrin-coated vesicles may facilitate scaffold assembly to engage PI3kγ/Akt signaling for survival mechanisms. Whether ERK, Akt, or other PAC1 receptor-stimulated mechanisms attenuate JNK/p38 MAPK activation after NGF-deprivation remains to be elucidated. PDK, phosphoinositide-dependent protein kinase; AC, adenylyl cyclase.
Supplementary Material
Acknowledgment
The use of the Molecular Biology Core Facility, supported by National Institutes of Health National Center for Research Resources Grant P20RR16435, is also gratefully acknowledged.
This work was supported, in whole or in part, by National Institutes of Health Grants HD27468 and NS37179.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–8.
B. M. Girard, K. M. Braas, and V. May, unpublished observations.
E. M. Lutz, unpublished observations.
- PACAP
- pituitary adenylate cyclase-activating polypeptide
- MAPK
- mitogen-activated protein kinase
- ERK
- extracellular signal-regulated kinase
- JNK
- c-Jun N-terminal kinase
- MEK
- MAPK/ERK kinase
- NGF
- nerve growth factor
- PI3K
- phosphatidylinositol 3-kinase
- PKA
- cAMP-dependent protein kinase A
- PKC
- protein kinase C
- MTS
- (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- PAC1
- G protein-coupled receptor selective for PACAP
- VIP
- vasoactive intestinal peptide
- VPAC
- G protein-coupled receptor exhibiting similar binding affinities for VIP and PACAP
- DN
- dominant negative
- SAPK
- stress-activated protein kinase
- GFP
- green fluorescent protein
- siRNA
- small interfering RNA.
REFERENCES
- 1.Sherwood N. M., Krueckl S. L., McRory J. E. (2000) Endocr. Rev. 21, 619–670 [DOI] [PubMed] [Google Scholar]
- 2.Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. (1993) Nature 365, 170–175 [DOI] [PubMed] [Google Scholar]
- 3.Lutz E. M., Sheward W. J., West K. M., Morrow J. A., Fink G., Harmar A. J. (1993) FEBS Lett. 334, 3–8 [DOI] [PubMed] [Google Scholar]
- 4.Ishihara T., Shigemoto R., Mori K., Takahashi K., Nagata S. (1992) Neuron. 8, 811–819 [DOI] [PubMed] [Google Scholar]
- 5.Inagaki N., Yoshida H., Mizuta M., Mizuno N., Fujii Y., Gonoi T., Miyazaki J.., Seino S. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 2679–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosoya M., Onda H., Ogi K., Masuda Y., Miyamoto Y., Ohtaki T., Okazaki H., Arimura A., Fujino M. (1993) Biochem. Biophys. Res. Commun. 194, 133–143 [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto H., Ishihara T., Shigemoto R., Mori K., Nagata S. (1993) Neuron. 11, 333–342 [DOI] [PubMed] [Google Scholar]
- 8.Laburthe M., Couvineau A., Marie J. C. (2002) Receptors Channels 8, 137–153 [PubMed] [Google Scholar]
- 9.Nicot A., DiCicco-Bloom E. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 4758–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villalba M., Bockaert J., Journot L. (1997) J. Neurosci. 17, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrie A. P., Clohessy A. M., Buensuceso C. S., Rogers M. V., Allen J. M. (1997) J. Biol. Chem. 272, 19666–19671 [DOI] [PubMed] [Google Scholar]
- 12.McCulloch D. A., MacKenzie C. J., Johnson M. S., Robertson D. N., Holland P. J., Ronaldson E., Lutz E. M., Mitchell R. (2002) Biochem. Soc. Trans. 30, 441–446 [DOI] [PubMed] [Google Scholar]
- 13.May V., Braas K. M. (1995) J. Neurochem. 65, 978–987 [DOI] [PubMed] [Google Scholar]
- 14.Mustafa T., Grimaldi M., Eiden L. E. (2007) J. Biol. Chem. 282, 8079–8091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu N., Zhou R., DiCicco-Bloom E. (1998) J. Neurosci. Res. 53, 651–662 [DOI] [PubMed] [Google Scholar]
- 16.Masuo Y., Tokito F., Matsumoto Y., Shimamoto N., Fujino M. (1994) Neurosci. Lett. 170, 43–46 [DOI] [PubMed] [Google Scholar]
- 17.Shuto Y., Uchida D., Onda H., Arimura A. (1996) Regul. Pept. 67, 79–83 [DOI] [PubMed] [Google Scholar]
- 18.Waschek J. A., Casillas R. A., Nguyen T. B., DiCicco-Bloom E. M., Carpenter E. M., Rodriguez W. I. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 9602–9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waschek J. A. (1996) Ann. N. Y. Acad. Sci. 805, 290–301 [DOI] [PubMed] [Google Scholar]
- 20.Carey R. G., Li B., DiCicco-Bloom E. (2002) J. Neurosci. 22, 1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu N., DiCicco-Bloom E. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 3357–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basille M., Gonzalez B. J., Fournier A., Vaudry H. (1994) Brain Res. Dev. Brain Res. 82, 81–89 [DOI] [PubMed] [Google Scholar]
- 23.Vaudry D., Gonzalez B. J., Basille M., Fournier A., Vaudry H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 9415–9420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lioudyno M., Skoglösa Y., Takei N., Lindholm D. (1998) J. Neurosci. Res. 51, 243–256 [DOI] [PubMed] [Google Scholar]
- 25.DiCicco-Bloom E., Deutsch P. J., Maltzman J., Zhang J., Pintar J. E., Zheng J., Friedman W. F., Zhou X., Zaremba T. (2000) Dev. Biol. 219, 197–213 [DOI] [PubMed] [Google Scholar]
- 26.Vaudry D., Gonzalez B. J., Basille M., Anouar Y., Fournier A., Vaudry H. (1998) Neuroscience 84, 801–812 [DOI] [PubMed] [Google Scholar]
- 27.Przywara D. A., Kulkarni J. S., Wakade T. D., Leontiev D. V., Wakade A. R. (1998) J. Neurochem. 71, 1889–1897 [DOI] [PubMed] [Google Scholar]
- 28.Chang J. Y., Korolev V. V. (1997) Neurochem. Int. 31, 161–167 [DOI] [PubMed] [Google Scholar]
- 29.Tabuchi A., Funaji K., Nakatsubo J., Fukuchi M., Tsuchiya T., Tsuda M. (2003) J. Neurosci. Res. 71, 504–515 [DOI] [PubMed] [Google Scholar]
- 30.Kienlen Campard P., Crochemore C., René F., Monnier D., Koch B., Loeffler J. P. (1997) DNA Cell Biol. 16, 323–333 [DOI] [PubMed] [Google Scholar]
- 31.Silveira M. S., Costa M. R., Bozza M., Linden R. (2002) J. Biol. Chem. 277, 16075–16080 [DOI] [PubMed] [Google Scholar]
- 32.Hansel D. E., May V., Eipper B. A., Ronnett G. V. (2001) J. Neurosci. 21, 4625–4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morio H., Tatsuno I., Hirai A., Tamura Y., Saito Y. (1996) Brain Res. 741, 82–88 [DOI] [PubMed] [Google Scholar]
- 34.Arimura A. (1998) Jpn. J. Physiol. 48, 301–331 [DOI] [PubMed] [Google Scholar]
- 35.Ohtaki H., Nakamachi T., Dohi K., Aizawa Y., Takaki A., Hodoyama K., Yofu S., Hashimoto H., Shintani N., Baba A., Kopf M., Iwakura Y., Matsuda K., Arimura A., Shioda S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7488–7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaudry D., Gonzalez B. J., Basille M., Yon L., Fournier A., Vaudry H. (2000) Pharmacol. Rev. 52, 269–324 [PubMed] [Google Scholar]
- 37.Braas K. M., May V. (1999) J. Biol. Chem. 274, 27702–27710 [DOI] [PubMed] [Google Scholar]
- 38.Beaudet M. M., Parsons R. L., Braas K. M., May V. (2000) J. Neurosci. 20, 7353–7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franke T. F., Kaplan D. R., Cantley L. C. (1997) Cell 88, 435–437 [DOI] [PubMed] [Google Scholar]
- 40.Datta S. R., Brunet A., Greenberg M. E. (1999) Genes Dev. 13, 2905–2927 [DOI] [PubMed] [Google Scholar]
- 41.Kaplan D. R., Miller F. D. (2000) Curr. Opin. Neurobiol. 10, 381–391 [DOI] [PubMed] [Google Scholar]
- 42.Brunet A., Datta S. R., Greenberg M. E. (2001) Curr. Opin. Neurobiol. 11, 297–305 [DOI] [PubMed] [Google Scholar]
- 43.Brandenburg C. A., May V., Braas K. M. (1997) J. Neurosci. 17, 4045–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.May V., Schiller M. R., Eipper B. A., Mains R. E. (2002) J. Neurosci. 22, 6980–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girard B. M., Keller E. T., Schutz K. C., May V., Braas K. M. (2004) Regul. Pept. 123, 107–116 [DOI] [PubMed] [Google Scholar]
- 46.May V., Stoffers D. A., Eipper B. A. (1989) Endocrinology 124, 157–166 [DOI] [PubMed] [Google Scholar]
- 47.Lutz E. M., Ronaldson E., Shaw P., Johnson M. S., Holland P. J., Mitchell R. (2006) Mol. Cell. Neurosci. 31, 193–209 [DOI] [PubMed] [Google Scholar]
- 48.Xia Z., Dickens M., Raingeaud J., Davis R. J., Greenberg M. E. (1995) Science 270, 1326–1331 [DOI] [PubMed] [Google Scholar]
- 49.Hawes B. E., Luttrell L. M., van Biesen T., Lefkowitz R. J. (1996) J. Biol. Chem. 271, 12133–12136 [DOI] [PubMed] [Google Scholar]
- 50.Gutkind J. S. (2000) Sci. STKE 2000, RE1. [DOI] [PubMed] [Google Scholar]
- 51.Stork P. J., Schmitt J. M. (2002) Trends Cell Biol. 12, 258–266 [DOI] [PubMed] [Google Scholar]
- 52.Wang Z., Dillon T. J., Pokala V., Mishra S., Labudda K., Hunter B., Stork P. J. (2006) Mol. Cell. Biol. 26, 2130–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi E. K., Park H. J., Ma J. S., Lee H. C., Kang H. C., Kim B. G., Kang I. C. (2004) FEBS Lett. 559, 141–144 [DOI] [PubMed] [Google Scholar]
- 54.Mei F. C., Qiao J., Tsygankova O. M., Meinkoth J. L., Quilliam L. A., Cheng X. (2002) J. Biol. Chem. 277, 11497–11504 [DOI] [PubMed] [Google Scholar]
- 55.Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camps M., Rückle T., Ji H., Ardissone V., Rintelen F., Shaw J., Ferrandi C., Chabert C., Gillieron C., Françon B., Martin T., Gretener D., Perrin D., Leroy D., Vitte P. A., Hirsch E., Wymann M. P., Cirillo R., Schwarz M. K., Rommel C. (2005) Nat. Med. 11, 936–943 [DOI] [PubMed] [Google Scholar]
- 57.Pomel V., Klicic J., Covini D., Church D. D., Shaw J. P., Roulin K., Burgat-Charvillon F., Valognes D., Camps M., Chabert C., Gillieron C., Françon B., Perrin D., Leroy D., Gretener D., Nichols A., Vitte P. A., Carboni S., Rommel C., Schwarz M. K., Rückle T. (2006) J. Med. Chem. 49, 3857–3871 [DOI] [PubMed] [Google Scholar]
- 58.Kerchner K. R., Clay R. L., McCleery G., Watson N., McIntire W. E., Myung C. S., Garrison J. C. (2004) J. Biol. Chem. 279, 44554–44562 [DOI] [PubMed] [Google Scholar]
- 59.Murga C., Laguinge L., Wetzker R., Cuadrado A., Gutkind J. S. (1998) J. Biol. Chem. 273, 19080–19085 [DOI] [PubMed] [Google Scholar]
- 60.Edwards S. N., Tolkovsky A. M. (1994) J. Cell Biol. 124, 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.May V., Brandenburg C. A., Braas K. M. (1995) J. Neurosci. 15, 4580–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li M., David C., Kikuta T., Somogyvari-Vigh A., Arimura A. (2005) J. Mol. Neurosci. 27, 91–105 [DOI] [PubMed] [Google Scholar]
- 63.Kao S., Jaiswal R. K., Kolch W., Landreth G. E. (2001) J. Biol. Chem. 276, 18169–18177 [DOI] [PubMed] [Google Scholar]
- 64.Hisata S., Sakisaka T., Baba T., Yamada T., Aoki K., Matsuda M., Takai Y. (2007) J. Cell Biol. 178, 843–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C., Takahashi M., Li Y., Song S., Dillon T. J., Shinde U., Stork P. J. (2008) Mol. Cell. Biol. 28, 7109–7125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stork P. J. S. (2003) Trends Biochem. Sci. 28, 267–275 [DOI] [PubMed] [Google Scholar]
- 67.Vossler M. R., Yao H., York R. D., Pan M. G., Rim C. S., Stork P. J. (1997) Cell 89, 73–82 [DOI] [PubMed] [Google Scholar]
- 68.Bouschet T., Perez V., Fernandez C., Bockaert J., Eychene A., Journot L. (2003) J. Biol. Chem. 278, 4778–4785 [DOI] [PubMed] [Google Scholar]
- 69.Cavanaugh J. E., Ham J., Hetman M., Poser S., Yan C., Xia Z. (2001) J. Neurosci. 21, 434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopez-Ilasaca M., Crespo P., Pellici P. G., Gutkind J. S., Wetzker R. (1997) Science 275, 394–397 [DOI] [PubMed] [Google Scholar]
- 71.Gayer C. P., Chaturvedi L. S., Wang S., Craig D. H., Flanigan T., Basson M. D. (2009) J. Biol. Chem. 284, 2001–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson G. L., Lapadat R. (2002) Science 298, 1911–1912 [DOI] [PubMed] [Google Scholar]
- 73.Lee F. S., Rajagopal R., Kim A. H., Chang P. C., Chao M. V. (2002) J. Biol. Chem. 277, 9096–9102 [DOI] [PubMed] [Google Scholar]
- 74.Rajagopal R., Chen Z. Y., Lee F. S., Chao M. V. (2004) J. Neurosci. 24, 6650–6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson-Farley N. N., Kertesy S. B., Dubyak G. R., Cowen D. S. (2005) J. Neurochem. 92, 72–82 [DOI] [PubMed] [Google Scholar]
- 76.Filippa N., Sable C. L., Filloux C., Hemmings B., Van Obberghen E. (1999) Mol. Cell. Biol. 19, 4989–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.García-Regalado A., Guzmán-Hernández M. L., Ramírez-Rangel I., Robles-Molina E., Balla T., Vázquez-Prado J., Reyes-Cruz G. (2008) Mol. Biol. Cell 19, 4188–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Christoforidis S., Miaczynska M., Ashman K., Wilm M., Zhao L., Yip S. C., Waterfield M. D., Backer J. M., Zerial M. (1999) Nat. Cell Biol. 1, 249–252 [DOI] [PubMed] [Google Scholar]
- 79.Hynes T. R., Mervine S. M., Yost E. A., Sabo J. L., Berlot C. H. (2004) J. Biol. Chem. 279, 44101–44412 [DOI] [PubMed] [Google Scholar]
- 80.Macdonald D. S., Weerapura M., Beazely M. A., Martin L., Czerwinski W., Roder J. C., Orser B. A., MacDonald J. F. (2005) J. Neurosci. 25, 11374–11384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma Y. C., Huang X. Y. (2002) Cell. Mol. Life Sci. 59, 456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naga Prasad S. V., Laporte S. A., Chamberlain D., Caron M. G., Barak L., Rockman H. A. (2002) J. Cell Biol. 158, 563–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fessart D., Simaan M., Laporte S. A. (2005) Mol. Endocrinol. 19, 491–503 [DOI] [PubMed] [Google Scholar]
- 84.Luttrell L. M., Ferguson S. S., Daaka Y., Miller W. E., Maudsley S., Della Rocca G. J., Lin F., Kawakatsu H., Owada K., Luttrell D. K., Caron M. G., Lefkowitz R. J. (1999) Science 283, 655–661 [DOI] [PubMed] [Google Scholar]
- 85.Waters C. M., Connell M. C., Pyne S., Pyne N. J. (2005) Cell. Signal. 17, 263–277 [DOI] [PubMed] [Google Scholar]
- 86.Schenck A., Goto-Silva L., Collinet C., Rhinn M., Giner A., Habermann B., Brand M., Zerial M. (2008) Cell 133, 486–497 [DOI] [PubMed] [Google Scholar]
- 87.Hunker C. M., Kruk I., Hall J., Giambini H., Veisaga M. L., Barbieri M. A. (2006) Arch. Biochem. Biophys. 449, 130–142 [DOI] [PubMed] [Google Scholar]
- 88.Su X., Lodhi I. J., Saltiel A. R., Stahl P. D. (2006) J. Biol. Chem. 281, 27982–27990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.