Abstract
Activation of the inflammasome generates the pro-inflammatory cytokines interleukin-1β and -18, which are important mediators of inflammation. Abnormal activation of the inflammasome leads to many inflammatory diseases, including gout, silicosis, neurodegeneration, and genetically inherited periodic fever syndromes. Therefore, identification of small molecule inhibitors that target the inflammasome is an important step toward developing effective therapeutics for the treatment of inflammation. Here, we show that the herbal NF-κB inhibitory compound parthenolide inhibits the activity of multiple inflammasomes in macrophages by directly inhibiting the protease activity of caspase-1. Additional investigations of other NF-κB inhibitors revealed that the synthetic IκB kinase-β inhibitor Bay 11-7082 and structurally related vinyl sulfone compounds selectively inhibit NLRP3 inflammasome activity in macrophages independent of their inhibitory effect on NF-κB activity. In vitro assays of the effect of parthenolide and Bay 11-7082 on the ATPase activity of NLRP3 demonstrated that both compounds inhibit the ATPase activity of NLRP3, suggesting that the inhibitory effect of these compounds on inflammasome activity could be mediated in part through their effect on the ATPase activity of NLRP3. Our results thus elucidate the molecular mechanism for the therapeutic anti-inflammatory activity of parthenolide and identify vinyl sulfones as a new class of potential therapeutics that target the NLRP3 inflammasome.
Keywords: Immunology/ Innate Immunity, Immunology/Toll Receptors, Proteases/Caspase, Proteases/Death Protease, Protease/Inhibitor, Signal Transduction, Toxins/Drugs
Introduction
The inflammasome collectively refers to oligomeric molecular platforms or assemblies that recruit and activate caspase-1 (1), which processes inactive pro-IL-1β3 and pro-IL-18 into the active pro-inflammatory cytokines IL-1β and IL-18, respectively (2, 3). These assemblies are formed in the cytoplasm of innate immune cells in response to danger signals (1). The NLRP3 (cryopyrin, NALP3) inflammasome (4) is particularly important because it is activated by diverse signals, including bacteria, viruses, purified microbial products, components of dying cells, small molecule immune activators, and crystalline or aggregated materials (1). Inappropriate activation of the NLRP3 inflammasome has been implicated in the pathogenesis of a number of human diseases, including gouty arthritis, silicosis, and neurodegeneration (5–8). In addition, abnormal activation of the NLRP3 inflammasome due to mutations in NLRP3 is responsible for several autoinflammatory diseases (9–12). Consequently, identification of small molecule inhibitors of the NLRP3 inflammasome is important for understanding its mechanism of activation and for the design of effective therapeutics for the treatment of inflammasome-associated diseases.
Here, we demonstrate that the NF-κB pathway inhibitors parthenolide and Bay 11-7082 are potent inhibitors of the inflammasome independent of their inhibitory effect on the NF-κB pathway. We show that parthenolide is a direct inhibitor of caspase-1 and NLRP3, whereas Bay 11-7082 and several structurally related vinyl sulfone compounds are selective inhibitors of NLRP3. This study identifies parthenolide as the first natural product that directly targets caspase-1 and the NLRP3 inflammasome and vinyl sulfones as the first small molecules that selectively inhibit activation of the NLRP3 inflammasome, possibly by targeting its ATPase activity. The ability of vinyl sulfones to cross the cell membrane and inhibit the NLRP3 inflammasome in macrophages should enable future development of related compounds as anti-inflammatory therapeutics for the treatment of inflammatory diseases and the development of vinyl sulfone-based probes to investigate the mechanism of activation of the NLRP3 inflammasome in macrophages.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
The anti-IL-1β monoclonal antibody (32D) was obtained from the NCI Preclinical Repository, Biological Resources Branch. Antibodies against human caspase-1 p30 (polyclonal anti-human caspase-1, residues 100–404; our laboratory), mouse NLRP3 (polyclonal anti-NLRP3 PYD; our laboratory), mouse ASC (polyclonal anti-mouse ASC; from Dr. Junji Sagara), and mouse caspase-1 p20 (monoclonal anti-mouse caspase-1 p20; from Dr. Junying Yuan) were described previously (13–15). Anti-FLAG® M2-agarose affinity gel, polyethyleneimine-cellulose TLC plates, ATP, nigericin, 1-(3-chloroprop-1-enylsulfonyl)-4-methylbenzene, phenyl vinyl sulfide, phenyl vinyl sulfone, phenyl vinyl sulfonate, and glutathione were from Sigma. Parthenolide, Bay 11-7082, Bay 11-7085, CA-074-Me, and E-64 were from BIOMOL. MG132 was from Calbiochem. IMD0354 and SC514 were from Tocris. Ultrapure LPS was from InvivoGen. Anthrax lethal factor and protective antigen were from List Biological Laboratories. Disuccinimidyl suberate was from Thermo Fisher Scientific. 3-[(4-Methylbenzene)sulfonyl]propanenitrile was from Enamine. [α-32P]ATP (3000 Ci/mmol) was from PerkinElmer Life Sciences. 1-(Ethenylsulfonyl)-4-methylbenzene and (E)-2-iodo-3-(4-methylphenyl)sulfonylprop-2-enenitrile were from the Developmental Therapeutics Program at NCI, National Institutes of Health.
Cell Culture and Generation of Stable NG5 Cell Line
Mouse bone marrow cells were isolated from mouse femurs with sterile Dulbecco's modified Eagle's medium (Invitrogen) and cultured in 6-well plates for 5–7 days in Dulbecco's modified Eagle's medium supplemented with 10% L929 cell-conditioned medium, 10% fetal bovine serum, and 1% penicillin/streptomycin. The differentiated macrophages were treated with or without ultrapure LPS (500 ng/ml) in serum-free Opti-MEM® I medium for 5 h and subsequently treated with drugs for 15 min, followed by NLRP3 stimuli as indicated. The immortalized Nlrp3−/− macrophages were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 100 units/ml penicillin and streptomycin. The stable NLRP3-reconstituted NG5 cell line, which expresses a C-terminally FLAG-tagged mouse NLRP3 protein, was generated by infecting immortalized Nlrp3−/− macrophages with the pMSCVgfp-mNLRP3-FLAG retroviral vector as described previously (14). All cells were grown at 37 °C and 5% CO2.
Immunoblot Analysis of Active Caspase-1
Cell culture supernatants from treated macrophages were precipitated and analyzed by immunoblotting as described (16).
In Vitro Caspase-1 Activation and IL-1β Cleavage Assays
Hypotonic THP-1 cell lysates were prepared in CHAPS buffer (20 mm HEPES-KOH, pH 7.5, 5 mm MgCl2, 0.5 mm EGTA, 0.1% CHAPS, 0.1 mm phenylmethylsulfonyl fluoride, and protease inhibitor mixture) as described (17). Cell lysates were incubated in the presence or absence of Bay 11-7082 (12 μm) or parthenolide (10 μm) at 37 °C for 60 min. The lysates were then analyzed by SDS-PAGE, followed by immunoblotting with anti-human caspase-1 p30 antibody. In some experiments, purified ASC pyroptosomes were incubated with FLAG-tagged procaspase-1 and pro-IL-1β in CHAPS buffer in the presence or absence of parthenolide at 37 °C for 60 min as described previously (17). The reaction mixtures were then fractionated by SDS-PAGE and analyzed by Western blotting with anti-FLAG and anti-IL-1β antibodies.
To assess the effect of parthenolide on caspase-1 activity, recombinant active caspase-1 was purified from a 293T-FLAG-caspase-1 stable cell line by immunoprecipitation with anti-FLAG antibody-agarose. Purified caspase-1 was incubated with pro-IL-1β in CHAPS buffer in the absence or presence of increasing concentrations of parthenolide at 37 °C for 60 min and analyzed by immunoblotting as described above.
Assay of ASC Pyroptosome Formation in Macrophages
Macrophages were seeded in 6-well plates (2 × 106 cells/well) and then treated with vehicle or 5 mm ATP for different periods of time in serum-free Opti-MEM® I for 45 min. In some experiments, macrophages were treated with vehicle or inhibitors for 15 min, followed by 5 mm ATP in serum-free OPTI-MEM® I for 45 min. The culture supernatants were collected and used for immunoblot analyses of secreted caspase-1 p20 as described above. The cells were pelleted by centrifugation and resuspended in 0.5 ml of ice-cold buffer containing 20 mm HEPES-KOH, pH 7.5, 150 mm KCl, 1% Nonidet P-40, 0.1 mm phenylmethylsulfonyl fluoride, and protease inhibitor mixture and lysed by shearing 10 times through a 21-gauge needle. The cell lysates were centrifuged at 6000 rpm for 10 min at 4 °C. The pellets were washed twice with phosphate-buffered saline and then resuspended in 500 μl of phosphate-buffered saline. The resuspended pellets were cross-linked with fresh disuccinimidyl suberate (2 mm) for 30 min as described (17) and pelleted by centrifugation at 6000 rpm for 10 min. The cross-linked pellets were resuspended in 30 μl of SDS sample buffer and fractionated on 12% SDS-polyacrylamide gel, followed by immunoblotting with anti-mouse ASC antibody.
Assay of NLRP3 ATPase Activity
NLRP3 was purified from NG5 cells by immunoprecipitation with anti-FLAG® M2- agarose affinity gel. Anti-FLAG immunoprecipitate from Nlrp3−/− cells was used as a negative control. NLRP3 ATPase activity was measured by assaying the hydrolysis of [α-32P]ATP to [α-32P]ADP by NLRP3 using TLC. NLRP3 was preincubated with different concentrations of Bay 11-7082, parthenolide, or vehicle control (DMSO) in a total volume of 40 μl of reaction buffer (25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10 mm MgCl2, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, and protease inhibitor mixture) for 30 min prior to addition of 0.1 μm [α-32P]ATP (3000 Ci/mmol) and 1 mm dithiothreitol (Research Products International) for an additional 2 h. The reactions were quenched by addition of an equal volume of TLC development solvent (1 m formic acid and 0.5 m LiCl). A total of 2 μl from each reaction mixture was spotted on a polyethyleneimine-cellulose TLC plate and developed with TLC development solvent in a TLC chamber. The hydrolysis of [α-32P]ATP to [α-32P]ADP was visualized by exposing the TLC plate to x-ray film.
Mass Spectrometry
Recombinant active caspase-1 was expressed in bacteria with an N-terminal His6 tag and subsequently purified according to established procedures on Talon metal affinity resin. The purified protein was incubated with parthenolide (5 μm) in buffer containing 20 mm HEPES-KOH, pH 7.5, 5 mm MgCl2, and 0.5 mm EGTA for 1 h at 37 °C. The parthenolide-modified protein was then fractionated by SDS-PAGE. Gel bands corresponding to caspase-1 p20 were excised and destained in 25 mm ammonium bicarbonate in 50% acetonitrile. After destaining, gel pieces were dehydrated with acetonitrile, reduced with 10 mm dithiothreitol, and alkylated with 55 mm iodoacetamide (GE Healthcare). Gel pieces were once more dehydrated before being digested with trypsin overnight at 37 °C. The tryptic peptides were extracted twice with 50% acetonitrile and 5% formic acid. The combined peptides were then dried on a centrifuge evaporator and reconstituted in 0.1% trifluoroacetic acid. Tryptic peptides were analyzed by mass spectrometry according to established procedures (see supplemental “Experimental Procedures”).
LDH Release Assay
NG5 macrophages were treated with parthenolide and Bay 11-7082-related compounds for 10 min, followed by ATP (5 mm) for 30 min. The culture supernatants were collected and assayed for LDH activity with the CytoTox 96 LDH release kit (Promega) following the manufacturer's protocols.
RESULTS
Parthenolide and Bay 11-7082 Are Potent Inhibitors of the Inflammasome
We have shown recently that NF-κB activation in bone marrow macrophages by ligands of Toll-like or cytokine receptors is required for induction of NLRP3 protein expression but not sufficient for its activation (15). A second stimulus such as ATP or nigericin is required for NLRP3 inflammasome activation. Consistent with these observations, inhibition of NLRP3 up-regulation by pretreatment of macrophages with the protein synthesis inhibitor cycloheximide or the NF-κB inhibitor Bay 11-7082 (or Bay 11-7085) before LPS priming abrogates caspase-1 activation by ATP (15, 18). Surprisingly, additional investigations of the effect of different NF-κB inhibitors on NLRP3 inflammasome activation revealed that two NF-κB inhibitors, parthenolide and Bay 11-7082, which are thought to selectively block the IKKβ kinase activity, were still able to block the activation of the NLRP3 inflammasome even when these inhibitors were added to the cells after LPS priming and just before ATP stimulation (Fig. 1A, left panels). This effect was not observed with other NF-κB inhibitors that selectively inhibit the IKKβ kinase activity (IMD0354 and SC514) or with MG132, which inhibits NF-κB by blocking the degradation of IκB (Fig. 1A, right panels), indicating that inhibition of IKKβ kinase activity or NF-κB activity is not sufficient to block activation of the NLRP3 inflammasome by ATP in LPS-primed macrophages. These results indicate that parthenolide and Bay 11-7082 inhibit activation of the NLRP3 inflammasome in LPS-primed macrophages independent of their effect on NF-κB activation.
FIGURE 1.
Parthenolide and Bay 11-7082 inhibit the NLRP3 inflammasome. A, immunoblots of caspase-1 and IL-1β in culture supernatants (Sup; upper and middle panels) or caspase-1 in cell lysates (lower panels) of LPS-primed primary wild-type bone marrow-derived macrophages treated with Bay 11-7082 (Bay; 12 μm), parthenolide (Parth; 10 μm), MG132 (10 μm), IMD0354 (IMD; 10 μm), or SC514 (10 μm) for 15 min, followed by 5 mm ATP for 45 min as indicated. B, immortalized NLRP3 knock-out (NLRP3-KO; left panels) or NLRP3-reconstituted NG5 macrophages (right panels) were left untreated or stimulated with LPS (500 ng/ml) for 5 h and subsequently treated with 5 mm ATP for 45 min as indicated. Immunoblots of caspase-1 in culture supernatants (upper panels) or cell lysates (middle panels) are shown. Immunoblots of NLRP3 in cell lysates are shown in the lower panels. C, immunoblots of caspase-1 in culture supernatants of NG5 macrophages treated with vehicle (DMSO), Bay 11-7082 (12 μm), parthenolide (10 μm), MG132 (10 μm), IMD0354 (10 μm), SC514 (10 μm), or cycloheximide (Cyclo; 20 μm) for 15 min, followed by 5 mm ATP for 45 min as indicated. Procasp1, procaspase-1.
To further demonstrate that parthenolide and Bay 11-7082 inhibit caspase-1 activation by the NLRP3 inflammasome independent of their effect on NF-κB activation, we tested the effect of these drugs on ATP-induced caspase-1 activation in a stable Nlrp3−/− bone marrow macrophage cell line (designated NG5) that constitutively expresses NLRP3 under the control of a murine stem cell virus promoter. As observed previously (15), stimulation of NG5 cells with ATP alone activated the NLRP3 inflammasome without prior priming with LPS (Fig. 1B). Consistent with the results obtained with primary macrophages, treatment with parthenolide or Bay 11-7082 completely inhibited ATP-induced caspase-1 activation in NG5 cells (Fig. 1C). In contrast, treatment with the NF-κB inhibitors IMD0354, SC514, and MG132 or the protein synthesis inhibitor cycloheximide had no effect on ATP-induced caspase-1 activation in these cells (Fig. 1C), further confirming that inhibition of IKKβ kinase activity, NF-κB, or protein synthesis does not affect activation of the NLRP3 inflammasome by ATP. Parthenolide and Bay 11-7082 also inhibited nigericin-induced and MSU-induced caspase-1 activation by the NLRP3 inflammasome in NG5 cells (Fig. 2A and supplemental Fig. 1), indicating that these compounds target a common signaling component(s) downstream of the P2X7 receptor, which mediates NLRP3 activation by ATP. Parthenolide and Bay 11-7082 were also able to inhibit MSU-induced caspase-1 activation in human THP-1 macrophages (Fig. 2B), indicating that their inflammasome inhibitory effect is not restricted to mouse macrophages.
FIGURE 2.
Bay 11-7082 is a selective inhibitor of the NLRP3 inflammasome, whereas parthenolide can inhibit multiple inflammasomes. A and B, immunoblots of caspase-1 in culture supernatants of NG5 macrophages (A) and human THP-1 macrophages (B) treated with vehicle (DMSO), Bay 11-7082 (Bay; 12 μm), or parthenolide (Parth; 10 μm) for 15 min, followed by nigericin (Nig; 10 μm) for 45 min or MSU for 5 h as indicated. C and D, immunoblots of caspase-1 in culture supernatants of Nlrp3−/− macrophages treated with vehicle (DMSO), Bay 11-7082 (12 μm), or parthenolide (10 μm) for 15 min, followed by anthrax lethal toxin protective antigen/lethal factor (PA/LF) or Salmonella infection (Sal; multiplicity of infection of 20) for 3 h as indicated. Procasp1, procaspase-1.
Bay 11-7082 Is a Selective Inhibitor of the NLRP3 Inflammasome, whereas Parthenolide Can Inhibit Multiple Inflammasomes
To determine whether the effect of parthenolide and Bay 11-7082 on caspase-1 activation is specific for the NLRP3 inflammasome or whether these drugs could inhibit other inflammasome signaling pathways, we stimulated Nlrp3−/− macrophages with anthrax lethal toxin (protective antigen/lethal factor), which activates the NLRP1 (NALP1) inflammasome (19), in the presence or absence of these drugs. As shown in Fig. 2C, parthenolide inhibited protective antigen/lethal factor-induced caspase-1 activation, whereas Bay 11-7082 had no effect. Interestingly, parthenolide completely inhibited Salmonella-induced caspase-1 activation through the NLRC4 (IPAF) inflammasome, whereas Bay 11-7082 showed a partial inhibitory effect (Fig. 2D). This partial inhibition might be due to possible side effect of the drug on Salmonella uptake by macrophages. These results suggest that Bay 11-7082 selectively inhibits the NLRP3 inflammasome pathway, whereas parthenolide inhibits the activity of multiple inflammasome pathways.
Parthenolide Is a Direct Inhibitor of Caspase-1
Parthenolide is a potent natural anti-inflammatory drug derived from the medicinal plant feverfew. The anti-inflammatory activity of parthenolide has been attributed to its ability to alkylate critical cysteine residues in IKKβ and p65 (20–23), but its ability to inhibit the activity of multiple inflammasomes at low micromolar concentration (Figs. 1 and 2) suggests that it may act on a target that is common to all inflammasome signaling pathways. Because caspase-1 is the only component that is common to all inflammasomes, we explored whether parthenolide might directly inhibit in vitro caspase-1 activation in THP-1 lysates. As shown in Fig. 3A, incubation of THP-1 cell lysates at 37 °C resulted in caspase-1 activation, which was inhibited by parthenolide but not by Bay 11-7082. Activation of caspase-1 in this assay is mediated by self-oligomerization of ASC and formation of the ASC pyroptosome (13), so we incubated preformed ASC pyroptosomes with procaspase-1 to assess whether parthenolide blocks caspase-1 activation directly or indirectly via inhibition of ASC oligomerization. As expected, ASC pyroptosomes activated caspase-1, which cleaved pro-IL-1β to produce the active IL-1β cytokine, in the absence of parthenolide but not in its presence (Fig. 3B), indicating that parthenolide inhibits caspase-1 activity downstream of ASC oligomerization under these conditions. To provide additional support for the direct effect of parthenolide on caspase-1 activity, we incubated purified active caspase-1 with pro-IL-1β in the presence of increasing amounts of parthenolide. The upward shift in the migration of the p20 subunit of caspase-1 on a denaturing gel demonstrates that parthenolide directly alkylates the p20 subunit of caspase-1, which likely accounts for its ability to inhibit proteolysis of pro-IL-1β (Fig. 3C). This parthenolide-induced shift in the migration of caspase-1 p20 was also observed in Salmonella-infected cells when parthenolide was added 30 min after infection (Fig. 3D), indicating that parthenolide can cross the plasma membrane and covalently inactivate the p20 subunit of caspase-1, as was observed in the in vitro cell-free system. Together, these results suggest that parthenolide inactivates caspase-1 by alkylation of the p20 subunit.
FIGURE 3.
Parthenolide is a direct inhibitor of caspase-1. A, lysates from THP-1 cells (10 mg/ml) were incubated at 4 °C or activated at 37 °C in the presence of DMSO, Bay 11-7082 (Bay; 12 μm), or parthenolide (Parth; 10 μm) for 60 min. The lysates were analyzed by Western blotting with anti-human caspase-1 antibody. B, purified preformed ASC pyroptosomes were incubated with N-terminally FLAG-tagged procaspase-1 (Procasp1) and pro-IL-1β in the presence of DMSO or the indicated concentrations of parthenolide at 37 °C for 1 h. The total reaction mixtures were fractionated by SDS-PAGE, followed by Western blotting with anti-FLAG (upper panel) and anti-IL-1β (lower panel) antibodies. C, purified recombinant mature human caspase-1 was incubated with pro-IL-1β in the presence of the indicated concentrations of parthenolide at 37 °C for 1 h. The total reaction mixtures were fractionated by SDS-PAGE, followed by Western blotting with anti-human caspase-1 (upper panel) and anti-IL-1β (lower panel) antibodies. D, shown is an immunoblot of caspase-1 in culture supernatants of Nlrp3−/− macrophages treated with vehicle (DMSO), Bay 11-7082 (12 μm), or parthenolide (10 μm) for 30 min following Salmonella infection (Sal) as indicated. The infection was continued for a total of 3 h. E, shown is a Coomassie Blue-stained SDS-polyacrylamide gel of purified recombinant caspase-1 left untreated (lane 1) or treated with 10 μm parthenolide (lane 2) for 1 h at 37 °C. The gel shows the processed p20 subunit of caspase-1.
To further investigate the mechanism of caspase-1 inhibition by parthenolide, we incubated recombinant bacterially produced wild-type caspase-1 with or without parthenolide, fractionated the protein by SDS-PAGE, excised the modified caspase-1 band (Fig. 3E, lane 2), and subjected it to tryptic digestion, followed by mass spectrometry. This analysis revealed that tryptic fragments containing the catalytic Cys285 of caspase-1 p20 are covalently modified by parthenolide (supplemental Fig. 2A). In addition, Cys136, Cys169, and Cys244 of caspase-1 p20 were also modified by parthenolide. These results provide direct evidence that parthenolide can inactivate caspase-1 by direct alkylation of the active site (Cys285) and most of the conserved cysteines on the p20 subunit. Consistent with these results, site-directed mutagenesis of the parthenolide-modified cysteines to alanines abolished the parthenolide-induced upward shift in the migration of the p20 subunit of caspase-1 on a denaturing SDS-polyacrylamide gel (supplemental Fig. 2B).
Bay 11-7082 and Parthenolide Inhibit NLRP3-induced ASC Pyroptosome Formation by Inhibiting NLRP3 ATPase Activity
Because Bay 11-7082 can inhibit caspase-1 activation by NLRP3 stimuli in macrophages but does not inhibit the autocatalytic activation of caspase-1 in vitro or in cells (Fig. 3, A and D), the compound must interfere with NLRP3-mediated ASC oligomerization or a component of the upstream signaling pathway. To demonstrate this directly, we devised a simple biochemical assay that allows the determination of NLRP3-induced ASC oligomerization in stimulated macrophages. This assay is based on our previous observation that activation of the NLRP3 inflammasome by NLRP3 stimuli triggers formation of a large ASC oligomer termed the ASC pyroptosome (see Fig. 7 and Ref. 13), which is composed of oligomerized ASC dimers. The ASC pyroptosome can be isolated from stimulated cells by low speed centrifugation and quantified by immunoblotting. As shown in Fig. 4A (left and middle panels), ATP stimulation induced ASC pyroptosome formation within 5 min in NG5 macrophages, which express NLRP3, but not in parental Nlrp3−/− macrophages, indicating that NLRP3 activity is required for ASC pyroptosome formation. Similar results were obtained in LPS-primed wild-type macrophages (supplemental Fig. 3) (13). Pretreatment of NG5 cells with Bay 11-7082 inhibited ASC pyroptosome formation (Fig. 4A, right panels), Similarly, pretreatment of NG5 cells with parthenolide also inhibited ASC pyroptosome formation (Fig. 4A, right panels). These results indicate that Bay 11-7082 and parthenolide inhibit the NLRP3 inflammasome at the level of NLRP3 or upstream of it.
FIGURE 7.
Model illustrating the signaling pathways involved in activation of the NLRP3 inflammasome. See “Discussion” for more details. TLR, Toll-like receptor; ROS, reactive oxygen species; Casp, caspase; LRR, leucine-rich repeat.
FIGURE 4.
Parthenolide and Bay 11-7082 inhibit the NLRP3 inflammasome by targeting its ATPase activity. A, NLRP3 knock-out (NLRP3-KO) and NG5 macrophages were treated with ATP for the indicated periods of time (left and middle panels) or were treated with vehicle (DMSO), Bay 11-7082 (Bay; 12 μm), or parthenolide (Parth; 10 μm) for 15 min, followed by ATP (right panels) as indicated. The culture media were immunoblotted (IB) with anti-caspase-1 antibody (lower panels). The cells were lysed, and the resulting 6000-rpm cell pellets were cross-linked with disuccinimidyl suberate. The cross-linked pellets (upper panels) and cell lysates (middle panels) were immunoblotted with anti-ASC antibody. B, shown is the ATPase activity of immunoprecipitated recombinant NLRP3 and control immunoprecipitates in the presence of increasing concentrations of Bay 11-7082 or parthenolide as indicated. Procasp1, procaspase-1.
NLRP3 is an ATPase, and its ATPase activity is required to recruit and oligomerize ASC and activate caspase-1 (24). To test whether Bay 11-7082 or parthenolide can inhibit NLRP3 ATPase activity, we preincubated purified NLRP3 with Bay 11-7082 or parthenolide and then assayed its ability to hydrolyze [α-32P]ATP to [α-32P]ADP. As shown in Fig. 4B, both parthenolide and Bay 11-7082 inhibited the ATPase activity of NLRP3 in a dose-dependent manner. Collectively, our results suggest that Bay 11-7082 inhibits activation of the NLRP3 inflammasome, possibly by inhibiting its ATPase activity. Interestingly, in addition to its ability to directly inhibit the catalytic activity of caspase-1, parthenolide also inhibited NLRP3-induced ASC oligomerization by inhibiting NLRP3 ATPase activity, indicating that parthenolide inhibits multiple components of inflammasome pathways, which helps to rationalize its potent anti-inflammatory activity in vivo.
The Vinyl Sulfone Group of Bay 11-7082 Is Required for Inhibition of the NLRP3 Inflammasome
On the basis of reports of covalent modification of proteins and nucleic acids with vinyl sulfones and acrylonitriles (25–27), we hypothesized that the trans-vinyl sulfone nitrile functional group in Bay 11-7082 might irreversibly inhibit activation of the NLRP3 inflammasome via alkylation of one or more nucleophilic residues. To assess whether alkylation is important for inhibitory activity, we first preincubated Bay 11-7082 with free glutathione or l-cysteine and then tested the ability of both pretreated and untreated Bay 11-7082 to inhibit caspase activation. Preincubation of Bay 11-7082 with glutathione or l-cysteine abrogated NLRP3 inhibitory activity (Fig. 5A, left panel), which is consistent with an inhibition mechanism involving target alkylation.
FIGURE 5.
The vinyl sulfone of Bay 11-7082 is required for inhibition of the NLRP3 inflammasome. A, immunoblots of caspase-1 in culture supernatants of NG5 macrophages treated with Bay 11-7082 (Bay) and related compounds containing phenyl vinyl sulfone (B) in the presence or absence of glutathione (Glut) or l-cysteine for 15 min, followed by ATP (5 mm) for 45 min. B, structures of Bay 11-7082 and related compounds used in the experiments in C and D. C, immunoblots of caspase-1 in culture supernatants of NG5 macrophages treated with the indicated concentrations of Bay 11-7082 and related compounds for 15 min, followed by ATP (5 mm) for 45 min. D, immunoblots of NF-κB-regulated pro-IL-1β in cell lysates of NG5 macrophages treated with DMSO, Bay 11-7082, CPSMB, or MBSPN compounds (left panels) or MBSPN, IMPSPN, or ESMB compounds (right panels) for 15 min, followed by LPS for 5 h. β-Actin was used as an internal loading control (lower panels). Procasp1, procaspase-1.
To explore structure-activity relationships governing target alkylation by Bay 11-7082, we assayed caspase activation by ATP (Fig. 5C) and activation of NF-κB by LPS (Fig. 5D) in NG5 macrophages in the presence of seven structurally related compounds (Fig. 5B): (2E)-3-[(4-tert-butylbenzene)sulfonyl]prop-2-enenitrile (Bay 11-7085), (E)-2-iodo- 3-(4-methylphenyl)sulfonylprop-2-enenitrile (IMPSPN), 3-[(4-methylbenzene)sulfonyl]propanenitrile (MBSPN), 1-(3-chloroprop-1-enylsulfonyl)-4-methylbenzene (CPSMB), 1-(ethenylsulfonyl)-4-methylbenzene (ESMB), phenyl vinyl sulfone (PV-sulfone), and phenyl vinyl sulfide (PV-sulfide). This series of compounds provides insight into the role of the olefin in alkylation (MBSPN), the relative significance of the sulfone and nitrile substituents in determining the site of nucleophilic attack (ESMB, CPSMB, PV-sulfone, and PV-sulfide), and the effect of steric bulk on inhibitory activity (IMPSPN and Bay 11-7085). In MBSPN, the prop-2-enenitrile of Bay 11-7082 is reduced, which eliminates unsaturation between the sulfone and nitrile functional groups. The resulting sulfonylpropanenitrile fails to inhibit caspase-1 and NF-κB activation as measured by procaspase-1 processing and pro-IL-1β induction, respectively (Fig. 5, C and D), demonstrating that the olefin is essential for inhibition and suggesting that alkylation results from Michael addition at either C-2 or C-3 of Bay 11-7082. To further clarify the site of nucleophilic attack, the inhibitory potencies of two compounds lacking the nitrile substituent were assessed. Replacing the nitrile in Bay 11-7082 with either hydrogen (ESMB and PV-sulfone) or a chloromethyl substituent (CPSMB) resulted in compounds that were still able to inhibit caspase-1 and NF-κB activation, albeit with reduced potency. However, reducing the benzylic sulfone (O=S=O) to a thioether produced a compound (PV-sulfide) that failed to inhibit caspase-1 activation, indicating that the sulfone group is also essential for inhibition. Taken together, these results strongly suggest that Bay 11-7082 inhibits the NLRP3 inflammasome by alkylation via Michael addition of target nucleophiles at C-2 of Bay 11-7082. Addition of steric bulk at C-2 (IMPSPN) or on the benzyl substituent (Bay 11-7085) had no effect on inhibition, suggesting that neither of these positions is important for productive interaction with the target.
Parthenolide and Phenyl Vinyl Sulfone Compounds Inhibit NLRP3-induced Pyroptotic Cell Death
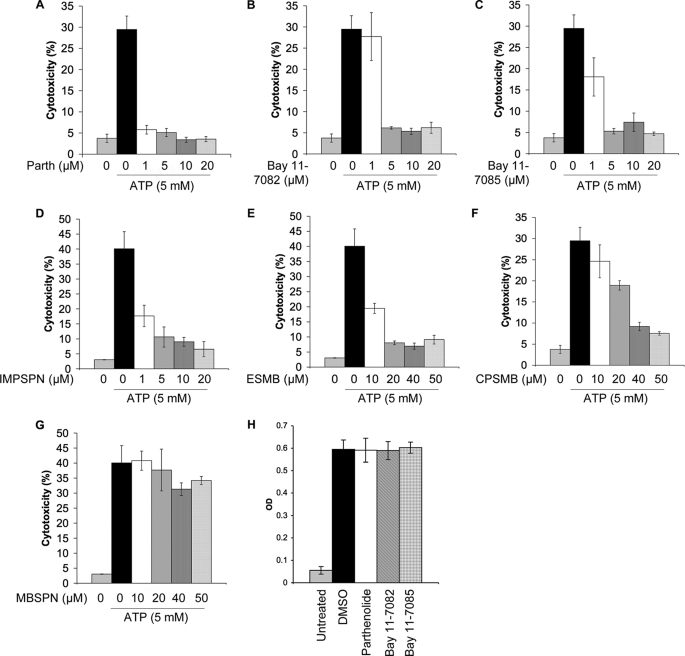
Treatment of LPS-primed macrophages with NLRP3 stimuli such as ATP induces rapid caspase-1-dependent pyroptotic cell death, which can be inhibited by extracellular KCl and the type 2 diabetes drug glyburide (13, 28). This (LPS plus ATP)-induced cell death is dependent on caspase-1 (13) and NLRP3 (28), as macrophages lacking caspase-1 or NLRP3 remain viable in the presence of LPS plus ATP. To determine the effect of parthenolide and phenyl vinyl sulfone compounds on the viability of NG5 cells, we assayed the activity of LDH released in the culture supernatants of cells after pretreatment with drugs for 10 min, followed by ATP treatment for 30 min (Fig. 6). Consistent with their ability to inhibit NALP3-mediated caspase-1 activation, parthenolide and the phenyl vinyl sulfone-related compounds Bay 11-7082, Bay 11-7085, IMPSPN, CPSMB, and ESMB all inhibited LDH release in a dose-dependent manner (Fig. 6, A–F). Additionally, cells treated with these drugs looked morphologically normal by microscopic examination (data not shown). In contrast, drugs such as MBSPN (Fig. 6G), which does not target the NLRP3 inflammasome, did not inhibit LDH release. The observed decrease in LDH activity in the presence of parthenolide or phenyl vinyl sulfone derivatives (Bay 11-7082 and Bay 11-7085) is not due to inhibition of LDH activity by these drugs because LDH activity in the culture supernatants of ATP-treated NG5 cells was not changed when these compounds were added to the assay reactions (Fig. 6H). Together, these results indicate that parthenolide and phenyl vinyl sulfone-related compounds not only inhibit caspase-1 activation but also block macrophage cell death, which provides further support for previous genetic findings that rapid ATP-induced cell death in primed macrophages is dependent on NALP3 and caspase-1 (13, 28).
FIGURE 6.
Inhibition of ATP-induced cell death by parthenolide and Bay 11-7082-related compounds in NG5 macrophages. A–G, cell death was assayed by measuring the percentages of LDH activity released in culture supernatants of NG5 macrophages (% cytotoxicity) treated with the indicated concentrations of parthenolide (Parth; A), Bay 11-7082 (B), and the indicated Bay 11-7082-related compounds (C–G) for 10 min, followed by ATP (5 mm) for 30 min. Each point represents the average of three experiments (n = 3). H, shown is the LDH activity in culture supernatants of NG5 cells treated with ATP for 1 h. The LDH activity (A490 units) was assayed in the presence of DMSO, parthenolide (20 μm), Bay 11-7082 (20 μm), or Bay 11-7085 (20 μm). Note that these drugs did not affect LDH enzymatic activity (H).
DISCUSSION
The NLRP3 inflammasome is an important component of the innate immune response against a broad range of microbial and self-generated danger signals, including bacterial pore-forming toxins, ATP, and MSU crystals (Fig. 7) (1). The molecular mechanism of activation of the NLRP3 inflammasome by these diverse stimuli is still unclear, but evidence suggests that NLRP3 is activated by a two-step mechanism. In the first step, known as the priming step, NLRP3 is transcriptionally up-regulated by NF-κB-inducing stimuli such as ligands of the Toll-like receptors (15). However, the induction of NLRP3 protein expression is not sufficient for its activation, and another transcription-independent step (activation step) is required for NLRP3 activation (15). This step is triggered by stimuli such as ATP (which activates the P2X7 receptor), pore-forming toxins such as nigericin, or phagocytosis of MSU crystals. Several studies have shown that these stimuli lead to the generation of reactive oxygen species, potassium efflux, and disruption of lysosomes, which results in the release of lysosomal cathepsin B into the cytoplasm (reviewed in Ref. 1). A recent study provided evidence that reactive oxygen species generated by NLRP3 stimuli activate NLRP3 by promoting its association with TXNIP (thioredoxin-interacting protein) (29). Once activated, NLRP3 undergoes self-oligomerization to form a molecular platform (24), which recruits the adaptor protein ASC. This step leads to self-oligomerization of ASC into a very large supramolecular structure called the pyroptosome (13), which recruits and activates procaspase-1, leading to the processing of pro-IL-1β and pro-IL-18 into the active pro-inflammatory cytokines IL-1β and IL-18, respectively. Activated caspase-1 also triggers a form of cell death called pyroptosis (30).
The NLRP3 inflammasome activity is abnormally elevated in many human inflammatory diseases, including gout, silicosis, neurodegeneration, and genetically inherited periodic fever syndromes (5–8). Therefore, there is considerable interest in the identification of effective therapeutics that selectively inhibit the NLRP3 inflammasome pathway. In this work, we have identified parthenolide, Bay 11-7082, and structurally related vinyl sulfones as the first potential small molecule inhibitors that target the NLRP3 inflammasome pathway at multiple levels (Fig. 7). Parthenolide, a plant sesquiterpene lactone, has been used extensively as an herbal remedy for treatment of multiple inflammatory diseases, including fever, migraine, arthritis, psoriasis, and atherosclerosis, with few mild side effects (31–33). Recent studies have shown that treatment with parthenolide improves the survival of mice challenged with LPS, retards atherosclerotic lesions in apoE mice, and ameliorates bladder inflammation in a rat cystitis model (34–36). These therapeutic effects have been attributed mostly to inhibition of NF-κB by parthenolide. However, in addition to its anti-NF-κB activity, our results now clearly demonstrate that parthenolide is a potent inhibitor of multiple inflammasomes. We have shown for the first time that parthenolide is a direct inhibitor of the protease activity of caspase-1 and is also an inhibitor of the ATPase activity of NLRP3. The change in migration of the p20 subunit of caspase-1 on SDS-PAGE following incubation with parthenolide indicates that the compound inhibits caspase-1 activity by alkylating critical cysteine residues in the p20 subunit. Indeed, mass spectrometry identified the active-site Cys285 of caspase-1 as one of the p20 cysteine residues that are modified by parthenolide. Similarly, alkylation is likely to be responsible for the inhibitory effect of parthenolide on NLRP3-dependent ATPase activity.
Unlike parthenolide, Bay 11-7082 and related compounds appear to be more selective for the NLRP3 inflammasome pathway compared with other inflammasome pathways because Bay 11-7082 did not inhibit caspase-1 activity in vitro and did not affect activation of the NLRP1 inflammasome by anthrax lethal toxin. Because NLRP1 also possesses an ATPase activity that is important for its ability to activate caspase-1 (37), these observations indicate that Bay 11-7082 does not inhibit the NLRP1 ATPase activity. The partial inhibitory effect of Bay 11-7082 on Salmonella-induced NLRC4 inflammasome activation could be due to a toxic effect of Bay 11-7082 on Salmonella itself or might be due to other possibilities such as inhibition of bacterial uptake by macrophages, partial inhibition of NLRC4 ATPase activity itself, or partial inhibition of a signaling component upstream of NLRC4. However, like parthenolide, Bay 11-7082 and related phenyl vinyl sulfones inhibit their target proteins most likely through alkylation mediated by attack of essential nucleophilic residues such as cysteines on the vinyl sulfone group. Indeed, the inhibition of ATP-induced caspase-1 activation in NG5 macrophages by parthenolide or Bay 11-7082 could not be reversed by washing off the drugs, which is consistent with an irreversible covalent inactivation mechanism (supplemental Fig. 4). Furthermore, preincubation of Bay 11-7082 and the related phenyl vinyl sulfone compounds IMPSPN and ESMB with glutathione abrogated their NLRP3 inhibitory activity (Fig. 5A), which is consistent with an inhibition mechanism involving target alkylation.
A recent study clearly demonstrated inhibition of the NLRP3 inflammasome by the type 2 diabetes drug glyburide (28). However, glyburide was ∼10-fold less effective than Bay 11-7082 and did not affect the ATPase activity of NLRP3, indicating that it is not a direct inhibitor of NLRP3 but rather acts upstream of NLRP3 and downstream of the P2X7 receptor to block NLRP3 inflammasome activation. In contrast to glyburide, we have provided in vitro biochemical evidence that Bay 11-7082 inhibits the ATPase activity of NLRP3, which is required for its activation (24), suggesting that the inflammasome inhibitory effect of Bay 11-7082 and related vinyl sulfone compounds might be the result of inhibition of the ATPase activity of NLRP3 by these compounds. However, it is still possible that these compounds, like glyburide, inhibit a signaling component upstream of NLRP3. Recent structural studies showed that phenyl vinyl sulfones or phenyl vinyl sulfonates are irreversible mechanism-based inhibitors of protein-tyrosine phosphatases (38). Because of their structural similarity to phosphotyrosines, these compounds bind to the active site of protein-tyrosine phosphatases with high affinity and inactivate their active-site cysteine by a mechanism involving a Michael addition of the γ-thiol to the terminal carbon of the vinyl group (38). Remarkably, we found that phenyl vinyl sulfonate is as potent as Bay 11-7082 in inhibiting the NLRP3 inflammasome activity (supplemental Fig. 5), suggesting the possibility that these drugs might also target critical, still unidentified protein-tyrosine phosphatases in the NLRP3 and NF-κB pathways. Phenyl vinyl sulfone derivatives are also irreversible inhibitors of cysteine proteases such as cathepsins (39–41), raising the possibility that these compounds might also target a critical cysteine protease upstream of NLRP3. Notably, the lysosomal cysteine protease cathepsin B has been previously suggested to be involved in the activation of the NLRP3 inflammasome (6, 42–44). However, our results using the specific cathepsin B inhibitor CA-074-Me or the broad-spectrum cysteine protease inhibitor E-64 showed that neither of these two compounds was able to inhibit caspase-1 activation by ATP (supplemental Fig. 6). Consistent with these results, a recent study demonstrated that a pan-cathepsin inhibitor, as well as specific cathepsin B inhibitors, did not impair caspase-1 activation induced by LPS plus ATP (45). Based on these observations, the inhibition of the NLRP3 inflammasome by Bay 11-7082 and related vinyl sulfones/sulfonates is not likely to be due to inhibition of cathepsins.
Finally, recent preclinical trials in mice and dogs using vinyl sulfone derivatives as antiparasitic agents (46) have shown that these drugs are non-mutagenic and well tolerated and display an acceptable pharmacokinetic profile. Therefore, the identification of Bay 11-7082 and related vinyl sulfone/sulfonate compounds as effective NLRP3 inflammasome inhibitors provides a framework for the future design of effective anti-inflammatory vinyl sulfone/sulfonate derivatives that selectively target the NLRP3 inflammasome pathway for the treatment of many inflammatory diseases, as well as for the design of Bay 11-7082 and vinyl sulfone/sulfonate-based probes to characterize the critical components involved in the activation of the NLRP3 inflammasome by various stimuli.
Supplementary Material
Acknowledgments
We thank M. McCormick for technical assistance, J. Sagara for the anti-mouse ASC antibody, J. Yuan for the anti-mouse caspase-1 antibody, and Gabriel Núñez for Salmonella.
This work was supported, in whole or in part, by National Institutes of Health Grants AG14357 and AR055398 (to E. S. A.) and Grants 1R01AI083713 and R01HL093262 (to E. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. 1–6.
- IL
- interleukin
- LPS
- lipopolysaccharide
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- DMSO
- dimethyl sulfoxide
- LDH
- lactate dehydrogenase
- IKKβ
- IκB kinase-β
- MSU
- monosodium urate
- IMPSPN
- (E)-2-iodo-3-(4-methylphenyl)sulfonylprop-2-enenitrile
- MBSPN
- 3-[(4-methylbenzene)sulfonyl]propanenitrile
- CPSMB
- 1-(3-chloroprop-1-enylsulfonyl)-4-methylbenzene
- ESMB
- 1-(ethenylsulfonyl)-4-methylbenzene
- PV
- phenyl vinyl.
REFERENCES
- 1.Martinon F., Mayor A., Tschopp J. (2009) Annu. Rev. Immunol. 27, 229–265 [DOI] [PubMed] [Google Scholar]
- 2.Dinarello C. A. (1998) Ann. N.Y. Acad. Sci. 856, 1–11 [DOI] [PubMed] [Google Scholar]
- 3.Dinarello C. A. (2009) Annu. Rev. Immunol. 27, 519–550 [DOI] [PubMed] [Google Scholar]
- 4.Pétrilli V., Dostert C., Muruve D. A., Tschopp J. (2007) Curr. Opin. Immunol. 19, 615–622 [DOI] [PubMed] [Google Scholar]
- 5.Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. (2008) Nat. Immunol. 9, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008) Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. (2006) Nature 440, 237–241 [DOI] [PubMed] [Google Scholar]
- 8.Cassel S. L., Eisenbarth S. C., Iyer S. S., Sadler J. J., Colegio O. R., Tephly L. A., Carter A. B., Rothman P. B., Flavell R. A., Sutterwala F. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9035–9040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmann J., Prieur A. M., Quartier P., Berquin P., Certain S., Cortis E., Teillac-Hamel D., Fischer A., de Saint Basile G. (2002) Am. J. Hum. Genet. 71, 198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman H. M., Mueller J. L., Broide D. H., Wanderer A. A., Kolodner R. D. (2001) Nat. Genet. 29, 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brydges S. D., Mueller J. L., McGeough M. D., Pena C. A., Misaghi A., Gandhi C., Putnam C. D., Boyle D. L., Firestein G. S., Horner A. A., Soroosh P., Watford W. T., O'Shea J. J., Kastner D. L., Hoffman H. M. (2009) Immunity 30, 875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng G., Zhang F., Fuss I., Kitani A., Strober W. (2009) Immunity 30, 860–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes-Alnemri T., Wu J., Yu J. W., Datta P., Miller B., Jankowski W., Rosenberg S., Zhang J., Alnemri E. S. (2007) Cell Death Differ. 14, 1590–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J. W., Fernandes-Alnemri T., Datta P., Wu J., Juliana C., Solorzano L., McCormick M., Zhang Z., Alnemri E. S. (2007) Mol. Cell 28, 214–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., Hornung V., Latz E. (2009) J. Immunol. 183, 787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes-Alnemri T., Yu J. W., Datta P., Wu J., Alnemri E. S. (2009) Nature 458, 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes-Alnemri T., Alnemri E. S. (2008) Methods Enzymol. 442, 251–270 [DOI] [PubMed] [Google Scholar]
- 18.Kahlenberg J. M., Lundberg K. C., Kertesy S. B., Qu Y., Dubyak G. R. (2005) J. Immunol. 175, 7611–7622 [DOI] [PubMed] [Google Scholar]
- 19.Boyden E. D., Dietrich W. F. (2006) Nat. Genet. 38, 240–244 [DOI] [PubMed] [Google Scholar]
- 20.Kwok B. H., Koh B., Ndubuisi M. I., Elofsson M., Crews C. M. (2001) Chem. Biol. 8, 759–766 [DOI] [PubMed] [Google Scholar]
- 21.Hehner S. P., Hofmann T. G., Dröge W., Schmitz M. L. (1999) J. Immunol. 163, 5617–5623 [PubMed] [Google Scholar]
- 22.García-Piñeres A. J., Lindenmeyer M. T., Merfort I. (2004) Life Sci. 75, 841–856 [DOI] [PubMed] [Google Scholar]
- 23.García-Piñeres A. J., Castro V., Mora G., Schmidt T. J., Strunck E., Pahl H. L., Merfort I. (2001) J. Biol. Chem. 276, 39713–39720 [DOI] [PubMed] [Google Scholar]
- 24.Duncan J. A., Bergstralh D. T., Wang Y., Willingham S. B., Ye Z., Zimmermann A. G., Ting J. P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 8041–8046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meadows D. C., Gervay-Hague J. (2006) Med. Res. Rev. 26, 793–814 [DOI] [PubMed] [Google Scholar]
- 26.Masri M. S., Friedman M. (1988) J. Protein Chem. 7, 49–54 [DOI] [PubMed] [Google Scholar]
- 27.Farooqui M. Y., Ahmed A. E. (1983) Chem. Biol. Interact. 47, 363–371 [DOI] [PubMed] [Google Scholar]
- 28.Lamkanfi M., Mueller J. L., Vitari A. C., Misaghi S., Fedorova A., Deshayes K., Lee W. P., Hoffman H. M., Dixit V. M. (2009) J. Cell Biol. 187, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. (2010) Nat. Immunol. 11, 136–140 [DOI] [PubMed] [Google Scholar]
- 30.Bergsbaken T., Fink S. L., Cookson B. T. (2009) Nat. Rev. Microbiol. 7, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schinella G. R., Giner R. M., Recio M. C., Mordujovich de Buschiazzo P., Ríos J. L., Máñez S. (1998) J. Pharm. Pharmacol. 50, 1069–1074 [DOI] [PubMed] [Google Scholar]
- 32.Heinrich M., Robles M., West J. E., Ortiz de Montellano B. R., Rodriguez E. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 539–565 [DOI] [PubMed] [Google Scholar]
- 33.Hall I. H., Starnes C. O., Jr., Lee K. H., Waddell T. G. (1980) J. Pharm. Sci. 69, 537–543 [DOI] [PubMed] [Google Scholar]
- 34.Sheehan M., Wong H. R., Hake P. W., Malhotra V., O'Connor M., Zingarelli B. (2002) Mol. Pharmacol. 61, 953–963 [DOI] [PubMed] [Google Scholar]
- 35.López-Franco O., Hernández-Vargas P., Ortiz-Muñoz G., Sanjuán G., Suzuki Y., Ortega L., Blanco J., Egido J., Gómez-Guerrero C. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1864–1870 [DOI] [PubMed] [Google Scholar]
- 36.Kiuchi H., Takao T., Yamamoto K., Nakayama J., Miyagawa Y., Tsujimura A., Nonomura N., Okuyama A. (2009) J. Urol. 181, 2339–2348 [DOI] [PubMed] [Google Scholar]
- 37.Faustin B., Lartigue L., Bruey J. M., Luciano F., Sergienko E., Bailly-Maitre B., Volkmann N., Hanein D., Rouiller I., Reed J. C. (2007) Mol. Cell 25, 713–724 [DOI] [PubMed] [Google Scholar]
- 38.Liu S., Zhou B., Yang H., He Y., Jiang Z. X., Kumar S., Wu L., Zhang Z. Y. (2008) J. Am. Chem. Soc. 130, 8251–8260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer J. T., Rasnick D., Klaus J. L., Brömme D. (1995) J. Med. Chem. 38, 3193–3196 [DOI] [PubMed] [Google Scholar]
- 40.Scheidt K. A., Roush W. R., McKerrow J. H., Selzer P. M., Hansell E., Rosenthal P. J. (1998) Bioorg. Med. Chem. 6, 2477–2494 [DOI] [PubMed] [Google Scholar]
- 41.Frankel B. A., Bentley M., Kruger R. G., McCafferty D. G. (2004) J. Am. Chem. Soc. 126, 3404–3405 [DOI] [PubMed] [Google Scholar]
- 42.Duncan J. A., Gao X., Huang M. T., O'Connor B. P., Thomas C. E., Willingham S. B., Bergstralh D. T., Jarvis G. A., Sparling P. F., Ting J. P. (2009) J. Immunol. 182, 6460–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joly S., Ma N., Sadler J. J., Soll D. R., Cassel S. L., Sutterwala F. S. (2009) J. Immunol. 183, 3578–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu J., Thomas L. M., Watkins S. C., Franchi L., Núñez G., Salter R. D. (2009) J. Leukocyte Biol. 86, 1227–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franchi L., Chen G., Marina-Garcia N., Abe L., Qu Y., Bao S., Shayman J. A., Turk J., Dubyak G. R., Nunez G. (2009) J. Innate Immun. 1, 607–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerr I. D., Lee J. H., Farady C. J., Marion R., Rickert M., Sajid M., Pandey K. C., Caffrey C. R., Legac J., Hansell E., McKerrow J. H., Craik C. S., Rosenthal P. J., Brinen L. S. (2009) J. Biol. Chem. 284, 25697–25703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.