Abstract
Objective
To determine if Healthy Choices, a motivational interviewing intervention targeting multiple risk behaviors, improved human immunodeficiency virus (HIV) viral load.
Design
A randomized, 2-group repeated measures design with analysis of data from baseline and 6- and 9-month follow-up collected from 2005 to 2007.
Setting
Five US adolescent medicine HIV clinics.
Participants
A convenience sample with at least 1 of 3 risk behaviors (nonadherence to HIV medications, substance abuse, and unprotected sex) was enrolled. The sample was aged 16 to 24 years and primarily African American. Of the 205 enrolled, 19 did not complete baseline data collections, for a final sample size of 186. Young people living with HIV were randomized to the intervention plus specialty care (n = 94) or specialty care alone (n = 92). The 3- and 6-month follow-up rates, respectively, were 86% and 82% for the intervention group and 81% and 73% for controls.
Intervention
Healthy Choices was a 4-session individual clinic-based motivational interviewing intervention delivered during a 10-week period. Motivational interviewing is a method of communication designed to elicit and reinforce intrinsic motivation for change.
Outcome Measure
Plasma viral load.
Results
Youth randomized to Healthy Choices showed a significant decline in viral load at 6 months postintervention compared with youth in the control condition (β = −0.36, t = −2.15, P = .03), with those prescribed antiretroviral medications showing the lowest viral loads. Differences were no longer significant at 9 months.
Conclusion
A motivational interviewing intervention targeting multiple risk behaviors resulted in short-term improvements in viral load for youth living with HIV.
Young people aged 15 TO 24 years represent almost half of new human immunodeficiency virus (HIV) infections globally, with more than 5 million young people currently living with HIV.1 While efforts have focused on increased access to antiretroviral (ARV) medications, poor adherence to HIV treatment is a primary cause of treatment failure in young people.2 Antiretroviral medication adherence has been shown to be suboptimal among young people living with HIV in the United States3,4 and in developing countries.5,6 In fact, several researchers have noted that young people with HIV have worse adherence than both children and adults.2–7 This is consistent with trends for other risk behaviors, such as sexual risk and substance use,8 which tend to cluster in adolescence and young adulthood. These other risk behaviors can also have deleterious effects on the health status of youth with HIV. Substance use has been shown to negatively affect immune system function in persons with HIV.9,10 Young people living with HIV having unprotected sex, in addition to possibly transmitting the disease, may also show increases in viral load as a result of contracting other sexually transmitted infections11,12 or superinfection with new strains of the virus.13
Concern about risk behaviors in young adults with HIV has prompted recommendations for multidisciplinary care that includes adolescent medicine specialists, integrated behavioral health services, adherence support, and peer outreach.14 However, these programs have not sufficiently reduced risk behaviors or improved health outcomes for this group of people in the United States.2–4,7 In adult populations, interventions based on motivational interviewing have been shown to improve adherence to ARV drugs15–17 and to reduce other risk behaviors associated with increased viral load.18–20 Motivational interviewing is a patient-centered, goal-oriented method of communication for eliciting and strengthening intrinsic motivation for health behavior change.21 Extensive research in social psychology22 has shown that positive behaviors in children and adults are more strongly associated with motivation based on intrinsic factors (eg, values and satisfaction) than on extrinsic factors (eg, rewards and guilt). Motivational interviewing has been shown to improve health behaviors in a variety of adult populations when delivered by a range of treatment providers, including physicians, health educators, and mental health professionals.23 Motivational interviewing is a flexible intervention tailored to the individual needs of the participant and has thus been successful with diverse populations, including individuals of minority ethnicity23 and sexual orientation.17 The flexibility of motivational interviewing to address multiple behaviors in diverse populations suggests its utility for addressing the array of risk behaviors present in diverse samples of youth with HIV.
Although use of motivational interviewing to improve pediatric/adolescent health behaviors has been recommended, 24 few studies have tested it in addressing adherence concerns in young people,25 and only 1 has addressed multiple risk behaviors.26 This pilot study tested a manualized 4-session motivational interviewing intervention (delivered during 10 weeks) that targeted 3 risk behaviors (ARV drug nonadherence, substance use, and sexual risk behavior) in a small sample of teenagers and young adults with HIV. The purpose of the present study was to test this intervention, Healthy Choices, in a large-scale, multisite, randomized clinical trial of young people with HIV with risk behaviors. Because of possible viral load reductions associated with improvements in substance use and sexual risk behaviors as well as ARV adherence, we hypothesized that participants with at least 1 of the 3 problem-level risk behaviors who were randomized to Healthy Choices plus multidisciplinary specialty care (intervention arm) would show greater reductions in viral load compared with those receiving specialty care alone (control arm). Viral load was chosen as the primary outcome because it was relevant to all participants regardless of risk behavior and was an objective measure not influenced by biases of the commonly used self-report measures of these behaviors.
METHODS
PARTICIPANTS
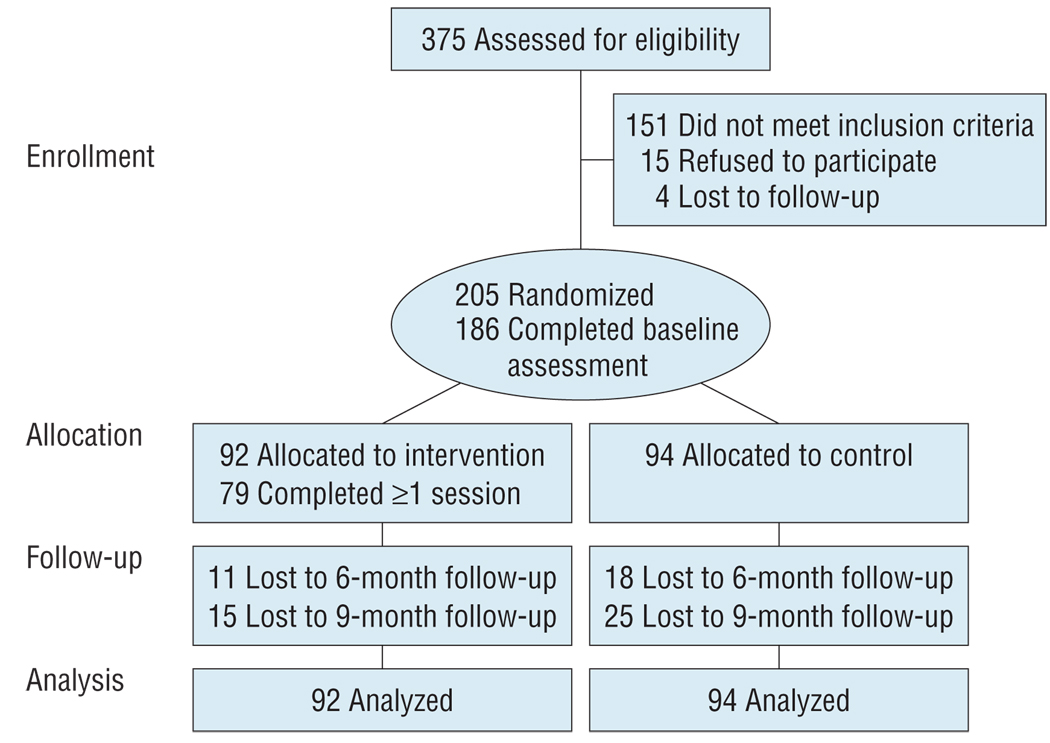
Participants were recruited from 5 adolescent medicine HIV clinics in Los Angeles, California, Philadelphia, Pennsylvania, Baltimore, Maryland, Fort Lauderdale, Florida, and Detroit, Michigan. Inclusion criteria included positive HIV status, age of 16 to 24 years, and ability to complete questionnaires in English. Participants also had to report having a problem in at least 1 of 3 HIV risk behaviors—substance use identified via an adolescent medicine screener,27 self-report of at least 1 unprotected act of intercourse in the last 3 months, or self-report of less than 90% adherence to ARV drugs in the last month. Because the intervention focused on multiple risk behaviors, youth had to have engaged in a second behavior (ever tried alcohol or illicit drugs, ever had sexual intercourse, or were ever prescribed HIV medications) that could be discussed in the intervention, even if the focus was on maintenance or preventing escalation to a problematic level. Exclusion criteria included having an active psychosis that resulted in an inability to complete questionnaires, being currently involved in research targeting any of the 3 behaviors, and being currently involved in a formal substance abuse treatment program. The Figure demonstrates participant flow through the trial. Because of the brevity of the intervention and the comprehensive support services available in each clinic setting, participants were randomized to intervention plus specialty care (n = 94) or specialty care alone (n = 92).
Figure.
Flowchart of study participants throughout the trial.
PROCEDURES
The protocol was approved by each clinic’s associated institutional review board, and a certificate of confidentiality was obtained from the National Institutes of Health. Data were collected between May 2005 and August 2007. Clinic care providers gave a general description of the study to potential participants. If they were interested, a researcher obtained verbal consent for screening. Upon determination of eligibility, written informed consent was obtained and a waiver of parental consent was permitted for youth younger than 18 years. Participants were then randomized so that intervention sessions could be scheduled immediately after the baseline assessment to promote intervention retention.
Randomization was carried out using a permuted block design, with randomly determined block sizes of 4 and 6. Randomization was stratified by site and targeted problem behavior. An automated clinical trial management tool based on telephone interactive voice-response technology was used to randomize subjects to their treatment arm. Using state-of-the-art technology, this tool allows users to send and receive randomization information from any telephone. Only study coordinators were unblinded to treatment condition so that they could assist in scheduling intervention visits. However, because of computer-assisted personal interviewing administration of questionnaires and the focus on a biological outcome, bias was thought to be minimal.
Youth had to complete the baseline assessment, including demographics and behavioral measures, within 30 days of screener completion using computer-assisted personal interviewing. Responses to computer-assisted personal interviewing questions were entered into the computer by a researcher in a confidential manner. Once entered, all responses were coded with a unique identifier, and no personal identifying information was recorded during the interview session. The analysis included information available for 3 study visits: at baseline and 6 and 9 months. All assessments and intervention sessions occurred at the clinic. Retention strategies included reminder calls and collaboration with clinic staff to contact hard-to-reach youth. Participants received $30 for the baseline visit, $40 for the 6-month visit, and $45 for the 9-month visit. Transportation, snacks, and child care were available.
MULTIDISCIPLINARY SPECIALTY CARE
All clinics provided HIV primary care with an adolescent medicine specialist and reported offering the following onsite services: adherence counseling, risk-reduction counseling, mental health services, case management, HIV support groups, peer advocacy and outreach, and transportation. At baseline, participants reported a mean use of support services of 9.8 sessions during the previous 3 months, with no difference between those assigned to the treatment or control condition (P > .05).
HEALTHY CHOICES
The 4-session individual intervention has been previously described. 26,28 Youth could work on 2 of 3 possible problem behaviors based on their entry screening: substance use, sexual risk, or HIV medication nonadherence. If they had problems in all 3 behaviors (n = 19), a random selection of 2 of them determined the intervention target. If they had reached a problematic level in 2 behaviors, both were selected for intervention. If the participant had only 1 behavior that had reached a problematic level, the second targeted behavior would be that which the subject had merely engaged in; however, if the subject had engaged in both of the remaining 2 behaviors, 1 of the 2 was chosen as the second possible target for the intervention using a random selection process (28 participants).
In session 1, participants chose which of the 2 behaviors to discuss first, and the interventionist elicited their views using standard motivational interviewing techniques. The remainder of the session focused on structured personalized feedback on risk behaviors based on the baseline assessment (normative data were not provided, as they were not available for youth with HIV), building motivation to initiate/maintain changes, decisional balance exercises to clarify the perceived pros and cons of behavior changes, and consideration of a plan to change his or her behavior. The plan was presented as an option, and the youth set his or her plan goal. The second session (week 2) followed the same format but focused on the second target behavior. In the subsequent 2 sessions (weeks 6 and 10), the interventionist reviewed the personalized behavior change plan, continued to monitor and encourage progress, problem-solved barriers, and elicited strategies to maintain health behaviors and to prevent relapse.
The interventionists were doctoral students in psychology or trained clinicians. They participated in a 2-day motivational interviewing training by members of the Motivational Interviewing Network of Trainers. They received weekly telephone supervision and case feedback from 1 of the supervising trainers. Interventionists submitted videotaped recordings of each session to the research team both for supervisor review and for coding with Motivational Interviewing Treatment Integrity codes.29 The Motivational Interviewing Treatment Integrity codes produce specific feedback to the supervisor and therapist on use of motivational interviewing techniques. A 20-minute portion of each tape was selected at random and coded by a member of the Motivational Interviewing Treatment Integrity coding team, a group of 7 trained raters whose reliability was assessed using the intraclass correlation statistic. To ensure reliability, each rater coded the same 20-minute portion of a taped session. The intraclass correlation for this coded segment was shown to be highly reliable (Cronbach α = .97; intraclass correlation average = 0.97).
OUTCOME MEASURES
Demographic variables that we investigated included age, biological sex, self-identified race/ethnicity, and self-identified sexual orientation. The primary outcome measure was viral load. Quantitative plasma HIV RNA testing was required at baseline and each follow-up visit. For purposes of the analysis, RNA measures below the level of detection were set to the lower limit of detection for the assay used: 25 copies per milliliter for the nucleic acid sequence base amplification assay; 50 copies per milliliter for the Roche Ultrasensitive assay; 75 copies per milliliter for branched DNA; and 400 copies per milliliter for the Roche Amplicor assay. These limits were verified by the study site representatives. The same assay was used for baseline and follow-up visits so that the primary outcome (viral load change score) would not be affected by different assays. As expected, preliminary analyses indicated that the distribution of the viral load was skewed, so a logarithmic (log10) transformation of this measure was carried out and used in subsequent analyses. Approximately one-third of the participants in each arm of the trial met clinical criteria to be prescribed ARV drugs. A dummy variable (1 = taking medication) was created and used as a covariate in the statistical analysis assessing the effect of behavioral intervention.
STATISTICAL ANALYSIS
A preintervention/postintervention intent-to-treat evaluation strategy was used to test the hypothesis that young people with HIV randomized to Healthy Choices would have greater reductions in viral load than control group participants. Because there were no significant differences in attrition between the 2 groups, we first used the t test to compare the average differences in the logarithm of the viral load between the intervention and control participants, assessed at baseline and the 6- and 9-month follow-up. To avoid potential for bias due to regression to the mean and to control for difference in viral load at baseline and according to other covariates (eg, age, race/ ethnicity, taking ARV drugs, and sexual orientation), findings from the bivariate t test comparison were further verified using the multiple linear regression model to assess the effect of the intervention30: Yt=α + β0Y0 + β1(Healthy Choices) + ΣβiXi. In this equation, Yt represents the log viral load assessed at the t follow-up visit, Y0 represents viral load assessed at baseline, and Xi represents a group of i covariates. A significant negative coefficient for the intervention, β1 (equal to the adjusted reductions in log viral load), at P < .05 was used as evidence to support the effect of Healthy Choices in reducing viral loads. Although participants were randomized into intervention and control conditions, we first assessed the equality of the participants in the intervention and control groups for a number of key variables, including age, race/ethnicity, biological sex, sexual orientation, and taking ARV drugs. The variables that significantly differed between intervention arms were included in multivariate models to control for any potential confounding on program effect evaluation. Study sites (4 dummy variables for 5 sites, controlling for study site heterogeneity) were included to control for effect modification. Missing viral load (dummy variable, controlling for confounding), number of problem behaviors, ARV medication, sexual orientation, and chronological age were also included as covariates. Because sexual orientation is more significant than biological sex for HIV infection and prevention and both are statistically highly correlated, we included only sexual orientation as a covariate in the multiple regression models. All statistical analyses were conducted using SAS, version 9.1 (SAS Institute Inc, Cary, North Carolina), with α <.05 used to define statistical significance.
RESULTS
SAMPLE CHARACTERISTICS
Of the 186 participants, 122 (65.6%) had reached a problematic level for substance use, 82 (44.1%) had for HIV medication adherence, and 100 (53.8%) had for sexual risk. Table 1 presents sample characteristics at baseline. There were no significant baseline differences between the 2 groups with regard to mean age, race/ethnicity, sexual orientation, or treatment with ARV drugs based on treatment guidelines at the time of the study (P > .1). Although the proportion of subjects with different sexual orientations did not differ between the 2 groups, there were significantly fewer biological males in the intervention group than in the control group (44.7% vs 60.9%, , P = .03). Follow-up rates were 86% and 82% in the intervention group and 81% and 73% in the control group at the 6- and 9-month visits, respectively. Participants who were retained at 6 months did not differ significantly from those who were not in terms of demographic characteristics or baseline viral load (P > .05). The same was true of those retained at 9 months (P > .05).
Table 1.
Sample Characteristics
| No. (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Total (N=186) |
Intervention Group (n=94) |
Control Group (n=92) |
P Value | ||
| Age, y | ||||||
| Range | 16–24 | 16–24 | 16–24 | t184 = 0.06 | .95 | |
| Mean (SD) | 20.5 (2.3) | 20.5 (2.4) | 20.5 (2.3) | |||
| Race/ethnicity | ||||||
| African American | 155 (83.3) | 80 (85.1) | 75 (81.5) | 0.43 | .51 | |
| Other | 31 (16.7) | 14 (14.9) | 17 (18.5) | |||
| Male sex | 98 (52.7) | 42 (44.7) | 56 (60.9) | 4.89 | ||
| Heterosexual orientation | 103 (56.6) | 56 (61.5) | 47 (51.6) | 1.81 | ||
| No. of problematic behaviors | ||||||
| 1 | 86 (46.2) | 50 (53.2) | 36 (39.1) | .16 | ||
| 2 | 82 (44.1) | 36 (38.3) | 46 (50.0) | |||
| 3 | 18 (9.7) | 8 (8.5) | 10 (10.9) | |||
| Participants taking antiretroviral drugs | ||||||
| Baseline | 64 (34.4) | 31 (33.0) | 33 (35.9) | 0.17 | .68 | |
| At 6 mo | 63 (33.9) | 35 (37.2) | 28 (30.4) | 0.96 | .33 | |
| At 9 mo | 63 (33.9) | 32 (34.0) | 31 (33.7) | 0.03 | .96 | |
VIRAL LOAD IN THE INTERVENTION AND CONTROL GROUPS
Table 2 summarizes the differences in viral load between the intervention and control groups. There were no significant differences in log of the viral load at baseline. At 6 months, the overall mean log of the viral load differed significantly between the intervention and the control groups (3.37 − 3.78 = −0.41, df = 155, P =. 04, t test).
Table 2.
Differences in the Levels of Log10 Viral Load Between the Intervention and Control Groupsa
| Intervention | Control | Differences | |||||
|---|---|---|---|---|---|---|---|
| Time | No. | Mean (SD) | No. | Mean (SD) | Mean (SD) | df | P Value |
| Baseline | 94 | 3.62 (1.14) | 91 | 3.79 (1.21) | −0.16 (1.18) | 183 | .35 |
| 6 mo | 81 | 3.37 (1.13) | 76 | 3.78 (1.18) | −0.41 (1.16) | 155 | .04 |
| 9 mo | 77 | 3.48 (1.14) | 69 | 3.61 (1.21) | −0.13 (1.17) | 144 | .50 |
Differences in log10 viral load were assessed as the average log10 viral load of the intervention subjects minus that of the control subjects, assessed at the baseline and the 2 follow-ups.
Table 3 presents the 2 constructed multiple regression models to test intervention effects (R2 = 0.37, 6-month model; and R2 = 0.44, 9-month model). The model was significant for the 6-month (F12,141 = 6.99, P < .001) and 9-month (F12,129 = 8.33, P < .001) follow-ups and demonstrated that the Healthy Choices intervention was associated with significant decline in viral load at 6-month follow-up (β = −0.36, t1 = −2.15, P = .03) after covariate adjustment (including the between-group differences in the baseline viral load, number of problem behaviors, study sites, ARV treatment, loss to follow-up, and demographic variables).
Table 3.
Effect of Motivational Interviewing on Viral Load Evaluated Using Multiple Regression Models
| 6-Month Follow-up (n = 157) |
9-Month Follow-up (n = 146) |
|||||
|---|---|---|---|---|---|---|
| Independent Variable | β1a | t1 | P Value | β1a | t1 | P Value |
| Intervention | −0.38 | −2.21 | .03 | −0.11 | −0.70 | .48 |
| Baseline viral load | 0.47 | 6.44 | <.001 | 0.45 | 6.76 | <.001 |
| Participating site | ||||||
| Los Angeles, CA | 0.53 | 1.81 | .07 | 0.53 | 1.93 | .06 |
| Philadelphia, PA | 0.08 | 0.32 | .75 | 0.24 | 0.96 | .34 |
| Baltimore, MD | 0.73 | 2.67 | .008 | 0.65 | 2.38 | .02 |
| Ft Lauderdale, FL | 0.40 | 1.38 | .17 | 0.55 | 2.02 | .05 |
| Detroit, MI | Reference | NA | NA | Reference | NA | NA |
| No. of problem behaviors | −0.08 | −0.58 | .56 | −0.09 | −0.70 | .49 |
| Taking ARV drugs | 0.05 | 0.27 | .79 | −0.81 | −4.82 | <.001 |
| Data missing on viral load | 0.25 | 1.07 | .29 | −0.01 | −0.04 | .97 |
| Sexual orientation | 0.00 | −0.01 | .99 | −0.12 | −0.64 | .52 |
| Non–African American race | −0.47 | −1.76 | .08 | 0.00 | 0.02 | .98 |
| Age | −0.05 | −1.46 | .15 | −0.08 | −2.14 | .03 |
Abbreviations: ARV, antiretroviral drugs; NA, not applicable.
Nonstandardized regression coefficients.
Table 4 demonstrates the mean viral load among groups defined by both randomization status and ARV medication. The group randomized to intervention and prescribed ARV drugs showed a decline in viral loads from 3.03 (SD, 0.87) at baseline to 2.39 (SD, 1.01) at the 6-month follow-up, the greatest among all 4 groups, suggesting the existence of an interaction between the behavioral intervention and ARV treatment. Reductions in viral load within the ARV group were greater than a half log, suggesting clinical significance of the intervention. 31 Results from the same multiple regression model plus an interaction term indicated that the interaction was statistically significant (β1 = −1.072, P < .001). In the intervention group, 33% had undetectable viral loads at 6 months (42% of those prescribed ARV drugs) compared with 22% of the control group (30% of those prescribed ARV drugs).
Table 4.
Mean Viral Load by Intervention Group and ARV Statusa
| Intervention Group | Control Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Taking ARV Drugs | Not Taking ARV Drugs | Taking ARV Drugs | Not Taking ARV Drugs | |||||
| Time | No. | Mean (SD) | No. | Mean (SD) | No. | Mean (SD) | No. | Mean (SD) |
| Baseline | 31 | 3.03 (0.87) | 63 | 3.92 (1.08) | 32 | 3.27 (1.34) | 55 | 4.07 (0.96) |
| 6 mo | 34 | 2.39 (1.01) | 47 | 3.83 (0.99) | 27 | 3.50 (1.46) | 49 | 3.94 (0.98) |
| 9 mo | 32 | 2.89 (1.11) | 45 | 3.90 (0.97) | 31 | 3.37 (1.41) | 38 | 3.81 (0.99) |
Abbreviation: ARV, antiretroviral.
Multiple regression analysis indicated that the interaction between the behavioral intervention and the ARV treatment was statistically significant (β1 = −1.072, P < .001) after controlling for baseline differences in viral load, study site heterogeneity, number of risk behaviors, loss to follow-up, and demographic variables.
COMMENT
A motivational interviewing intervention, Healthy Choices, targeting multiple risk behaviors, resulted in short-term improvements in viral load for young people living with HIV. Lower viral load is associated with slowed disease progression and mortality even for those not taking ARV drugs.32 The intervention was successful with a primarily African American sample, suggesting that motivational interviewing should be considered as a behavioral strategy to reduce racial disparities in HIV survival, as African Americans have lower survival rates than other racial groups.33 However, more than half of youth in the intervention condition, even among those prescribed ARV drugs, were still not achieving optimal viral suppression (undetectable viral load). This is similar to findings that showed adherence intervention effects among adult patients though not enough for virologic control. 34 While brief interventions may be more easily implemented in clinic settings, a more intensive intervention may be needed to sufficiently halt viral replication.17,35
Furthermore, reductions in viral load were not maintained at 9 months of follow-up. Other adherence interventions have also failed to find significant effects over long-term follow-up36,37; the absence of significant intervention effects for viral load at the 9-month follow-up could be attributable to a host of other clinical and biologic factors, including the development of resistance, preexisting resistance, or length of time taking ARV drugs. Studies of the long-term effects of substance abuse and sexual risk reduction interventions on viral load have not yet been conducted. Research on maintenance of health behavior change is in its infancy,38 but it is possible that more intensive intervention is necessary or that frequent repetitions of brief interventions (eg, booster sessions) may be needed to promote long-term adherence. It may also be that youth with HIV require opportunities for skills-building to achieve long-term change in their risk behaviors. Motivational interviewing combined with cognitive-behavioral skills-building has been shown to confer greater benefit than motivational interviewing alone.16 Future studies are critically needed to address these issues in child and adolescent health.
Study limitations include the use of a convenience sample, the lack of an attention control, and the unblinding of study coordinators. In addition, the lack of controlling for clustering effect (due to the few study sites) may affect the precision of the program effect evaluation. Further analyses are necessary to determine intervention effects on substance use and sexual risk, and a larger sample of youth with HIV prescribed ARV drugs at baseline is necessary to determine the specific effects of the intervention on adherence behaviors. Future studies should consider performing a cost-effectiveness analysis.
Previous pilot work26 suggested that the Healthy Choices intervention could reduce viral load among young people living with HIV, but the sample size was small and drawn from a single site. This is the first multisite randomized behavioral intervention trial in the literature to demonstrate a significant impact on health outcomes (viral load) among young HIV patients. The intervention could be easily integrated into existing HIV clinic settings that provide care to young people, as studies have shown that providers from a range of backgrounds (physicians, nurses, social workers, health educators, paraprofessionals, and even peer-outreach workers) can effectively deliver motivational interviewing.23,39 Integration of motivational interviewing into standard care of young HIV patients may prove even more effective, as youth would continue to receive the motivational interviewing at each contact, thus providing ongoing boosters, which may serve to promote long-term risk reduction and better virologic outcomes.
Motivational interviewing is a flexible method of communication that can be used to address health behaviors in HIV-infected youth of primarily minority ethnicities in the United States but who are diverse in terms of gender and sexual orientation. Larger samples are needed to determine differential effects based on these demographic characteristics. The motivational interviewing clinical text has been translated into 17 languages, and there are members of the Motivational Interviewing Network of Trainers in 28 countries.40 Future research adapting motivational interventions to countries with high rates of HIV infection in young people is necessary to determine the impact of this behavioral intervention on the global burden of HIV.
Acknowledgments
Funding/Support: The Adolescent Trials Network for HIV/AIDS Interventions (ATN) supported this work through grant U01-HD040533 from the National Institutes of Health through the National Institute of Child Health and Human Development (B. Kapogiannis and S. Lee), with supplemental funding from the National Institutes on Drug Abuse (N. Borek) and Mental Health (P. Brouwers and S. Allison).
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00103532
Author Contributions: Drs Naar-King and Chen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Naar-King, Parsons, and Murphy. Acquisition of data: Naar-King and Belzer. Analysis and interpretation of data: Naar-King, Murphy, Chen, Harris, and Belzer. Drafting of the manuscript: Naar-King, Chen, and Belzer. Critical revision of the manuscript for important intellectual content: Naar-King, Parsons, Murphy, Chen, Harris, and Belzer. Statistical analysis: Chen and Harris. Obtained funding: Naar-King and Murphy. Administrative, technical, and material support: Belzer. Study supervision: Parsons.
Financial Disclosure: None reported.
Additional Contributions: The study was scientifically reviewed by the ATN’s Behavioral Leadership Group. Network, scientific, and logistical support was provided by the ATN coordinating center (C. Wilson and C. Partlow) at the University of Alabama at Birmingham. Network operations and data management support was provided by the ATN Data and Operations Center at Westat Inc (J. Korelitz, J. Davidson, and B. Harris). We acknowledge the contribution of the investigators and staff at the following ATN 004 sites that participated in this study: Children’s Diagnostic and Treatment Center (Ana Puga, MD, Esmine Leonard, BSN, and Zulma Eysallenne, RN); Children’s Hospital of Los Angeles (Marvin Belzer, MD, Cathy Salata, RN, and Diane Tucker, RN, MSN); University of Maryland (Ligia Peralta, MD, Leonel Flores, MD, and Esther Collinetti, BA); University of Pennsylvania and the Children’s Hospital of Philadelphia (Bret Rudy, MD, Mary Tanney, MPH, MSN, CPNP, Naini Seth, BSN, and Kelly Lannutti, BA); University of Southern California (Andrea Kovacs, MD); and Wayne State University Horizons Project (K. Wright, DO, P. Lam, MA, V. Conners, BA). We sincerely thank the youth who participated in this project.
REFERENCES
- 1.Joint United Nations Programme on HIV AIDS (UNAIDS) Geneva, Switzerland: UNAIDS; 2008 Report on the Global AIDS Epidemic. 2008
- 2.Flynn PM, Rudy BJ, Lindsey JC, et al. PACTG 381 Study Team. Long-term observation of adolescents initiating HAART therapy: three-year follow-up. AIDS Res Hum Retroviruses. 2007;23(10):1208–1214. doi: 10.1089/aid.2006.0290. [DOI] [PubMed] [Google Scholar]
- 3.Murphy DA, Wilson CM, Durako SJ, Muenz LR, Belzer M. Adolescent Medicine HIV/AIDS Research Network. Antiretroviral medication adherence among REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001;13(1):27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- 4.Kolmodin K, Naar-King S, Murphy D, Parson J, Harper G. Predictors of medication adherence in high risk youth living with HIV. J Pediatr Psychol. doi: 10.1093/jpepsy/jsp080. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rongkavilit C, Naar-King S, Wang TCB, Wright K, Phanuphak P. Health risk behaviors among HIV-infected youth in Bangkok, Thailand. J Adolesc Health. 2007;40(4):358.e1–358.e8. doi: 10.1016/j.jadohealth.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Charles M, Noel F, Leger P, et al. Survival, plasma HIV-1 RNA concentrations and drug resistance in HIV-1-infected Haitian adolescents ang young adults on antiretrovirals. Bull World Health Organ. 2008;86(12):970–977. doi: 10.2471/BLT.07.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy DA, Sarr M, Durako S, Moscicki AB, Wilson CM, Muenz LR. Adolescent Medicine HIV/AIDS Research Network. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157(3):249–255. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 8.Park MJ, Paul Mulye T, Adams SH, Brindis CC, Irwin CE., Jr The health status of young adults in the United States. J Adolesc Health. 2006;39(3):305–317. doi: 10.1016/j.jadohealth.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Carrico AW, Johnson MO, Moskowitz JT, et al. NIMH Healthy Living Project Team. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosom Med. 2007;69(8):785–792. doi: 10.1097/PSY.0b013e318157b142. [DOI] [PubMed] [Google Scholar]
- 10.Samet JH, Horton JN, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: are they related? Alcohol Clin Exp Res. 2003;27(5):862–867. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- 11.Palacios R, Jimenez-Onate F, Aguilar M, et al. Impact of syphilis infection on HIV viral load and CD4 cell counts in HIV-infected patients. J Acquir Immune Defic Syndr. 2007;44(3):356–359. doi: 10.1097/QAI.0b013e31802ea4c6. [DOI] [PubMed] [Google Scholar]
- 12.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186(12):1718–1725. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 13.Smith DM, Richman DD, Little SJ. HIV superinfection. J Infect Dis. 2005;192(3):438–444. doi: 10.1086/431682. [DOI] [PubMed] [Google Scholar]
- 14.D’Angelo LJ, Samples C, Rogers AS, Peralta L, Friedman L. HIV infection and AIDS in adolescents: an update of the position of the Society for Adolescent Medicine. J Adolesc Health. 2006;38(1):88–91. doi: 10.1016/j.jadohealth.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Dilorio C, McCarty F, Resnicow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20(3):273–283. doi: 10.1080/09540120701593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golin CE, Earp J, Tien HC, Stewart P, Porter C, Howie L. A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006;42(1):42–51. doi: 10.1097/01.qai.0000219771.97303.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgenstern J, Irwin TW, Wainberg ML, et al. A randomized controlled trial of goal choice interventions for alcohol use disorders among men-who-have-sex-with-men. J Consult Clin Psychol. 2007;75(1):72–84. doi: 10.1037/0022-006X.75.1.72. [DOI] [PubMed] [Google Scholar]
- 19.Parsons JT, Rosof E, Punzalan JC, Dimaria L. Integration of motivational interviewing and cognitive behavioral therapy to improve HIV medication adherence and reduce substance use among HIV-positive men and women: results of a pilot project. AIDS Patient Care STDS. 2005;19(1):31–39. doi: 10.1089/apc.2005.19.31. [DOI] [PubMed] [Google Scholar]
- 20.Velasquez MM, von Sternberg KV, Johnson DH, Green C, Carbonari J, Parsons JT. Reducing sexual risk behaviors and alcohol use among HIV-positive men who have sex with men: a randomized clinical trial. J Consult Clin Psychol. 2009;77(4):657–667. doi: 10.1037/a0015519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller WR, Rollnick S. Ten things that Motivational Interviewing is not. Behav Cogn Psychother. 2009;37(2):129–140. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 22.Vallerand RJ, Ratelle CF. Intrinsic and extrinsic motivation: a hierarchical model. In: Deci EL, Ryan RM, editors. Handbook of Self-Determination. Rochester, NY: NY University of Rochester Press; 2002. [Google Scholar]
- 23.Hettema J, Steele J, Miller WR. Motivational interviewing. Ann Rev Clin Psychol. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 24.Sindelar HA, Abrantes AM, Hart C, Lewander W, Spirito A. Motivational interviewing in pediatric practice. Curr Probl Pediatr Adolesc Health Care. 2004;34(9):322–339. doi: 10.1016/j.cppeds.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Suarez M, Mullins S. Motivational interviewing and pediatric health behavior interventions. J Dev Behav Pediatr. 2008;29(5):417–428. doi: 10.1097/DBP.0b013e31818888b4. [DOI] [PubMed] [Google Scholar]
- 26.Naar-King S, Wright K, Parsons J, et al. Healthy choices: motivational enhancement therapy for health risk behaviors in HIV-positive youth. AIDS Educ Prev. 2006;18(1):1–11. doi: 10.1521/aeap.2006.18.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Knight JR, Shrier LA, Bravender TD, Farrell M, Vander Bilt J, Shaffer HJ. A new brief screen for adolescent substance abuse. Arch Pediatr Adolesc Med. 1999;153(6):591–596. doi: 10.1001/archpedi.153.6.591. [DOI] [PubMed] [Google Scholar]
- 28.Naar-King S, Lam P, Wang B, Wright K, Parsons JT, Frey MA. Brief report: maintenance of effects of motivational enhancement therapy to improve risk behaviors and HIV-related Health in a randomized controlled trial of youth living with HIV. J Pediatr Psychol. 2008;33(4):441–445. doi: 10.1093/jpepsy/jsm087. [DOI] [PubMed] [Google Scholar]
- 29.Moyers TB, Martin T, Manuel JK, Miller WR. The motivational interviewing treatment integrity (MITI) code: version 2.0. 2004:1–19. [Google Scholar]
- 30.Twisk WR. Applied Longitudinal Data Analysis for Epidemiology. Cambridge, England: Cambridge University Press; 2003. [Google Scholar]
- 31.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed January 10, 2009];Department of Health and Human Services; 2008 November 3;:1–139. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 32.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344(10):720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 33.National Center for Health Statistics. Hyattsville, MD: National Center for Health Statistics; United States: With Chartbook on Trends in the Health of Americans. 2007
- 34.Murphy DA, Marelich WD, Rappaport NB, Hoffman D, Farthing C. Results of an antiretroviral adherence intervention: STAR (Staying Healthy: Taking Antiretrovirals Regularly) J Int Assoc Physicians AIDS Care (Chic Ill) 2007;6(2):113–124. doi: 10.1177/1545109707301243. [DOI] [PubMed] [Google Scholar]
- 35.Ellis DA, Naar-King S, Cunningham PB, Secord E. Use of multisystemic therapy to improve antiretroviral adherence and health outcomes in HIV-infected pediatric patients: evaluation of a pilot program. AIDS Patient Care STDS. 2006;20(2):112–121. doi: 10.1089/apc.2006.20.112. [DOI] [PubMed] [Google Scholar]
- 36.Wagner GJ, Kanouse DE, Golinelli D, et al. Cognitive-behavioral Intervention to enhance adherence to antiretroviral therapy: a randomized controlled trial (CCTG 578) AIDS. 2006;20(9):1295–1302. doi: 10.1097/01.aids.0000232238.28415.d2. [DOI] [PubMed] [Google Scholar]
- 37.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19(8):807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 38.Orleans CT. Promoting the maintenance of health behavior change: recommendations for the next generation of research and practice. Health Psychol. 2000;19(1) suppl:76–83. doi: 10.1037/0278-6133.19.suppl1.76. [DOI] [PubMed] [Google Scholar]
- 39.Naar-King S, Outlaw A, Green-Jones A, Wright K, Parsons J. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care. 2009;21(7):868–873. doi: 10.1080/09540120802612824. [DOI] [PubMed] [Google Scholar]
- 40.Wagner C, Conners W. Motivational Interviewing: Resources for Clinicians, Researchers, and Trainers. [Accessed January, 22, 2008]; http://www.motivationalinterview.org.