Abstract
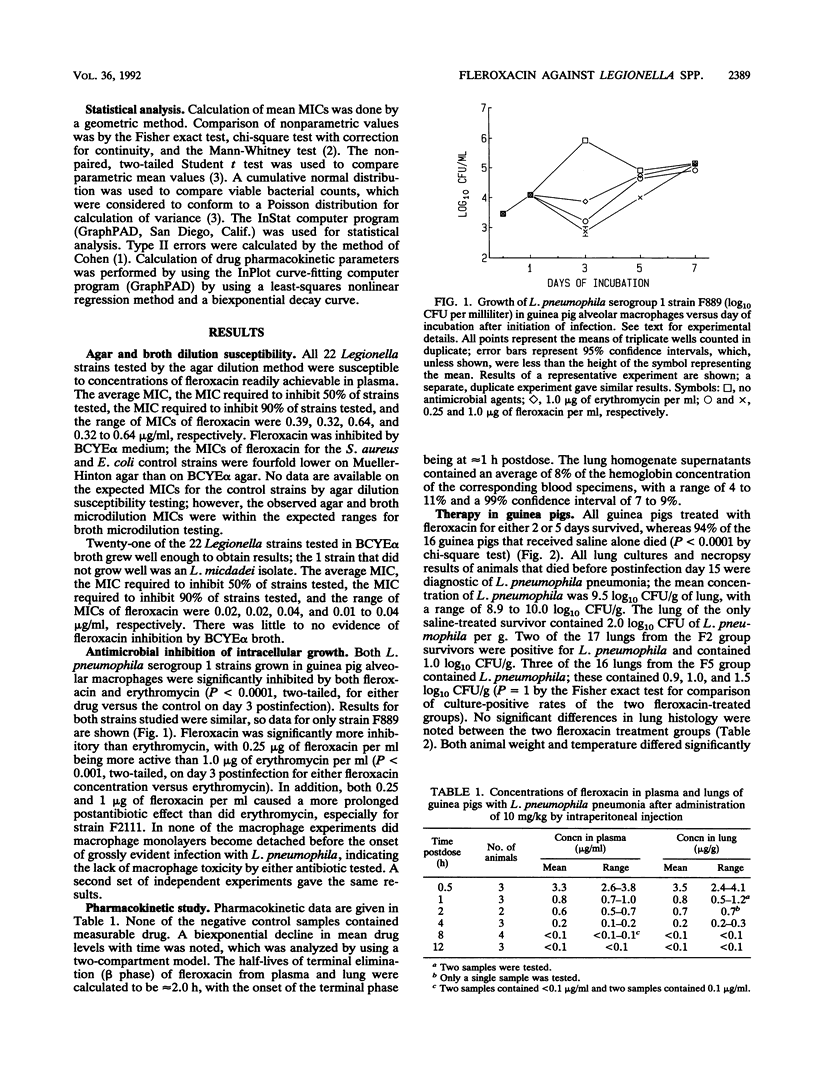
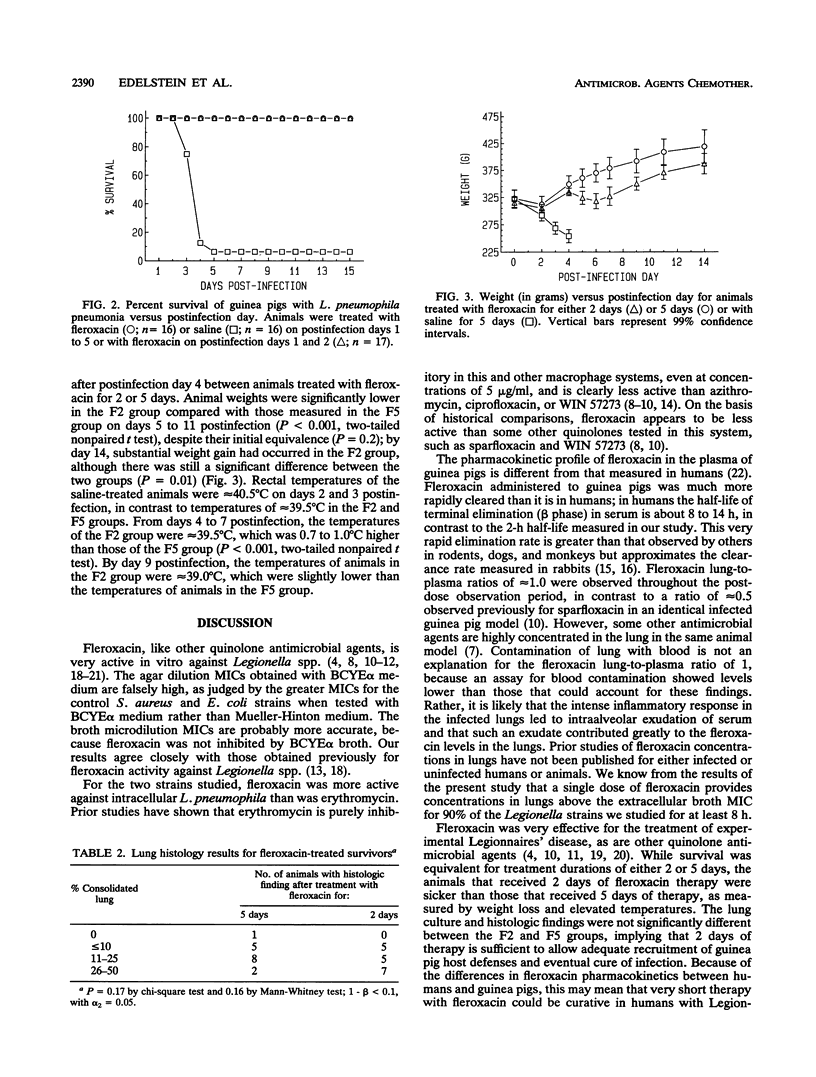
The activities of fleroxacin against 22 clinical Legionella isolates were determined by agar and broth microdilution susceptibility testing. The fleroxacin MIC required to inhibit 90% of strains tested on buffered charcoal yeast extract agar medium supplemented with 0.1% alpha-ketoglutarate was 0.64 micrograms/ml and was 0.04 microgram/ml when testing was done with buffered yeast extract broth supplemented with 0.1% alpha-ketoglutarate. Fleroxacin (0.25 microgram/ml) reduced the bacterial counts of two L. pneumophila strains grown in guinea pig alveolar macrophages by 1 log10 CFU/ml, but regrowth occurred over a 3-day period; fleroxacin was significantly more active than erythromycin in this assay. Single-dose (10 mg/kg of body weight given intraperitoneally) pharmacokinetic studies performed in guinea pigs with L. pneumophila pneumonia revealed peak levels in plasma and lungs to be 3.3 micrograms/ml and 3.5 micrograms/g, respectively, at 0.5 h and 0.8 microgram/ml and 0.8 microgram/g, respectively, at 1 h. The half-life of the terminal phase of elimination from plasma and lung was approximately 2 h. All 17 infected guinea pigs treated with fleroxacin (10 mg/kg/day) for 2 days survived for 14 days post-antimicrobial therapy, as did all 16 guinea pigs treated with the same dose of fleroxacin for 5 days. Only 1 of 16 animals treated with saline survived. The animals treated with fleroxacin for 2 days lost more weight and had higher temperatures than those treated with the antibiotic for 5 days. Fleroxacin is effective against L. pneumophila in vitro and in a guinea pig model of Legionnaires' disease. Fleroxacin should be evaluated as a treatment for human Legionnaires' disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dournon E., Rajagopalan P., Vilde J. L., Pocidalo J. J. Efficacy of pefloxacin in comparison with erythromycin in the treatment of experimental guinea pig legionellosis. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):41–48. doi: 10.1093/jac/17.suppl_b.41. [DOI] [PubMed] [Google Scholar]
- Edelstein P. H., Calarco K., Yasui V. K. Antimicrobial therapy of experimentally induced Legionnaires' disease in guinea pigs. Am Rev Respir Dis. 1984 Nov;130(5):849–856. doi: 10.1164/arrd.1984.130.5.849. [DOI] [PubMed] [Google Scholar]
- Edelstein P. H., Edelstein M. A. In vitro activity of azithromycin against clinical isolates of Legionella species. Antimicrob Agents Chemother. 1991 Jan;35(1):180–181. doi: 10.1128/aac.35.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H., Edelstein M. A. WIN 57273 is bactericidal for Legionella pneumophila grown in alveolar macrophages. Antimicrob Agents Chemother. 1989 Dec;33(12):2132–2136. doi: 10.1128/aac.33.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H., Edelstein M. A., Weidenfeld J., Dorr M. B. In vitro activity of sparfloxacin (CI-978; AT-4140) for clinical Legionella isolates, pharmacokinetics in guinea pigs, and use to treat guinea pigs with L. pneumophila pneumonia. Antimicrob Agents Chemother. 1990 Nov;34(11):2122–2127. doi: 10.1128/aac.34.11.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981 Sep;14(3):298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgeorge R. B., Gibson D. H., Jepras R., Baskerville A. Studies on ciprofloxacin therapy of experimental Legionnaires' disease. J Infect. 1985 May;10(3):194–203. doi: 10.1016/s0163-4453(85)92438-7. [DOI] [PubMed] [Google Scholar]
- Havlichek D., Saravolatz L., Pohlod D. Effect of quinolones and other antimicrobial agents on cell-associated Legionella pneumophila. Antimicrob Agents Chemother. 1987 Oct;31(10):1529–1534. doi: 10.1128/aac.31.10.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl P., von Graevenitz A., Zollinger-Iten J. Fleroxacin (Ro 23-6240): activity in vitro against 355 enteropathogenic and non-fermentative gram-negative bacilli and Legionella pneumophila. J Antimicrob Chemother. 1987 Sep;20(3):373–378. doi: 10.1093/jac/20.3.373. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Intracellular multiplication of Legionnaires' disease bacteria (Legionella pneumophila) in human monocytes is reversibly inhibited by erythromycin and rifampin. J Clin Invest. 1983 Jan;71(1):15–26. doi: 10.1172/JCI110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusajima H., Ooie T., Kawahara F., Uchida H. High-performance liquid chromatographic determination of 6,8-difluoro-1-(2-fluoroethyl)-1,4- dihydro-7-(4-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid and its metabolites in laboratory animals. J Chromatogr. 1986 Aug 22;381(1):137–148. doi: 10.1016/s0378-4347(00)83572-0. [DOI] [PubMed] [Google Scholar]
- Leibovitz E., Keren G., Shabtai M., Barzilai A., Rubinstein E. The pharmacokinetics and therapeutic efficacy of fleroxacin and pefloxacin in a rat abscess model. J Antimicrob Chemother. 1989 Sep;24(3):375–385. doi: 10.1093/jac/24.3.375. [DOI] [PubMed] [Google Scholar]
- Meyer R. D., Edelstein P. H., Kirby B. D., Louie M. H., Mulligan M. E., Morgenstein A. A., Finegold S. M. Legionnaires' disease: unusual clinical and laboratory features. Ann Intern Med. 1980 Aug;93(2):240–243. doi: 10.7326/0003-4819-93-2-240. [DOI] [PubMed] [Google Scholar]
- Pohlod D. J., Saravolatz L. D., Somerville M. M. Inhibition of Legionella pneumophila multiplication within human macrophages by fleroxacin. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):49–54. doi: 10.1093/jac/22.supplement_d.49. [DOI] [PubMed] [Google Scholar]
- Saito A., Koga H., Shigeno H., Watanabe K., Mori K., Kohno S., Shigeno Y., Suzuyama Y., Yamaguchi K., Hirota M. The antimicrobial activity of ciprofloxacin against Legionella species and the treatment of experimental Legionella pneumonia in guinea pigs. J Antimicrob Chemother. 1986 Aug;18(2):251–260. doi: 10.1093/jac/18.2.251. [DOI] [PubMed] [Google Scholar]
- Saito A., Sawatari K., Fukuda Y., Nagasawa M., Koga H., Tomonaga A., Nakazato H., Fujita K., Shigeno Y., Suzuyama Y. Susceptibility of Legionella pneumophila to ofloxacin in vitro and in experimental Legionella pneumonia in guinea pigs. Antimicrob Agents Chemother. 1985 Jul;28(1):15–20. doi: 10.1128/aac.28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vildé J. L., Dournon E., Rajagopalan P. Inhibition of Legionella pneumophila multiplication within human macrophages by antimicrobial agents. Antimicrob Agents Chemother. 1986 Nov;30(5):743–748. doi: 10.1128/aac.30.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidekamm E., Portmann R., Suter K., Partos C., Dell D., Lücker P. W. Single- and multiple-dose pharmacokinetics of fleroxacin, a trifluorinated quinolone, in humans. Antimicrob Agents Chemother. 1987 Dec;31(12):1909–1914. doi: 10.1128/aac.31.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]



